Abstract
We investigated whether, during maximal exercise, intercostal muscle blood flow is as high as during resting hyperpnoea at the same work of breathing. We hypothesized that during exercise, intercostal muscle blood flow would be limited by competition from the locomotor muscles. Intercostal (probe over the 7th intercostal space) and vastus lateralis muscle perfusion were measured simultaneously in ten trained cyclists by near-infrared spectroscopy using indocyanine green dye. Measurements were made at several exercise intensities up to maximal (WRmax) and subsequently during resting isocapnic hyperpnoea at minute ventilation levels up to those at WRmax. During resting hyperpnoea, intercostal muscle blood flow increased linearly with the work of breathing (R2= 0.94) to 73.0 ± 8.8 ml min−1 (100 g)−1 at the ventilation seen at WRmax (work of breathing ∼550–600 J min−1), but during exercise it peaked at 80% WRmax (53.4 ± 10.3 ml min−1 (100 g)−1), significantly falling to 24.7 ± 5.3 ml min−1 (100 g)−1 at WRmax. At maximal ventilation intercostal muscle vascular conductance was significantly lower during exercise (0.22 ± 0.05 ml min−1 (100 g)−1 mmHg−1) compared to isocapnic hyperpnoea (0.77 ± 0.13 ml min−1 (100 g)−1 mmHg−1). During exercise, both cardiac output and vastus lateralis muscle blood flow also plateaued at about 80% WRmax (the latter at 95.4 ± 11.8 ml min−1 (100 g)−1). In conclusion, during exercise above 80% WRmax in trained subjects, intercostal muscle blood flow and vascular conductance are less than during resting hyperpnoea at the same minute ventilation. This suggests that the circulatory system is unable to meet the demands of both locomotor and intercostal muscles during heavy exercise, requiring greater O2 extraction and likely contributing to respiratory muscle fatigue.
During maximal exercise in fit humans, competition for blood flow between the locomotor and the respiratory muscles exists, such that respiratory muscle blood flow may increase at the expense of blood flow to working limb muscles (Harms et al. 1997, 1998). Such a suggestion came from experiments where an increase in the force output of the respiratory muscles (via added inspiratory resistance) caused a reduction in limb blood flow during maximal exercise that was attributed to increased sympathetic vasoconstrictor outflow to the limb muscles (Sheel et al. 2001). As only limb and not respiratory muscle blood flow measurements were performed in those and other studies (Harms et al. 1997, 1998) it remains unknown whether at maximal exercise the respiratory muscle vasculature also vasoconstricts in response to increased global sympathetic outflow, thus limiting blood flow to respiratory muscles as well (Romer & Polkey, 2008).
Measuring blood flow to the several respiratory muscles is difficult owing to their complex anatomical arrangement, their extensive vascular network and the large variation in muscular recruitment with varying degrees of ventilation. Guenette et al. (2008) and Vogiatzis et al. (2008) reported changes in respiratory muscle blood flow during hyperpnoea and submaximal exercise, respectively in fit subjects using near-infrared spectroscopy over the left 7th intercostal space and the light-absorbing tracer indocyanine green dye. Blood flow measurements over the left 7th intercostal space primarily reflected the activity of the internal and external intercostal muscles and only to a lesser extend the costal part of the diaphragm (Guenette et al. 2008). Whilst the diaphragm acts as the primary flow generator during exercise, the role of the intercostal muscles is also important as they develop the necessary pressure to move the rib cage (Aliverti et al. 1997). Vogiatzis et al. (2008) revealed that comparing voluntary resting hyperpnoea with sub-maximal exercise at essentially the same work of breathing, intercostal muscle blood flow was similar. It was suggested that intercostal muscle blood flow was not increased to higher levels than those demanded by a given amount of respiratory muscle work during submaximal exercise, most likely because the load placed on respiratory muscles was insufficient to cause a profound change in blood flow distribution (Wetter et al. 1999). Whether during maximal exercise intercostal muscle blood flow increased above that seen at the same work of breathing during resting hyperpnoea, as the basis for reduced leg blood flow described above, was not explored (Vogiatzis et al. 2008).
The purpose of the present study was to investigate whether at maximal levels of cycling exercise, and normally encountering maximal levels of work of breathing, intercostal muscle blood flow is different from that recorded at the same respiratory muscle load during resting hyperpnoea (i.e. when respiratory and locomotor muscles do not compete for the available blood flow). To accomplish our goal we performed simultaneous measurements of intercostal and quadriceps femoris muscle blood flow during (1) graded exercise up to maximal levels and (2) during resting hyperpnoea at the same levels of minute ventilation and work of breathing as during the different levels of exercise. We reasoned that if intercostal muscle blood flow during maximal exercise was greater than or equal to that recorded during resting hyperpnoea at the same work of breathing, this would support that the work of breathing normally experienced during maximal exercise causes redistribution of blood flow from the legs to the respiratory muscles (Harms et al. 1997, 1998, 2000; Dempsey et al. 2006). If, however, intercostal muscle blood flow during maximal exercise was lower than during resting hyperpnoea, this would suggest that intercostal muscles do not ‘steal’ blood flow from the exercising legs to support high levels of ventilation.
Methods
Ten competitive Greek male cyclists (Table 1) participated in the study, which was approved by the University Hospital Ethics Committee and was conducted in accordance with the guidelines of the Declaration of Helsinki. Prior to participation in the study, all subjects were informed of any risks and discomforts associated with the experiments and gave written, signed, informed consent.
Table 1.
Pulmonary function and maximal exercise data
| Age (years) | 35 ± 10 (23–45) |
| Height (cm) | 178 ± 5 (168–183) |
| Weight (kg) | 74 ± 8 (58–83) |
| WRmax (W) | 361 ± 31 (302–413) |
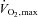 (ml kg−1 min−1) (ml kg−1 min−1) |
61.8 ± 8.3 (50.7–71.3) |
| HRmax (beats min−1) | 179 ± 8 (170–192) |
| RER at WRmax | 1.18 ± 0.05 (1.12–1.26) |
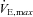 (l min−1) (l min−1) |
158.8 ± 25.7 (126–183) |
| VT,max (l min−1) | 2.97 ± 0.28 (2.52–3.44) |
| fmax (breaths min−1) | 53 ± 10 (36–65) |
Values are means ±s.d. (range) for 10 subjects. Exercise data depict the results of the incremental exercise test starting at 30 W and increasing by 30 W every minute, with the subjects maintaining a pedalling frequency of 70–90 r.p.m. WRmax, maximal work rate; 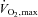 , maximal oxygen uptake; HRmax, maximal heart rate; RER, respiratory exchange ratio;
, maximal oxygen uptake; HRmax, maximal heart rate; RER, respiratory exchange ratio; 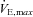 , maximal minute ventilation; VTmax, maximal tidal volume; fmax, maximal breathing frequency.
, maximal minute ventilation; VTmax, maximal tidal volume; fmax, maximal breathing frequency.
Experimental design
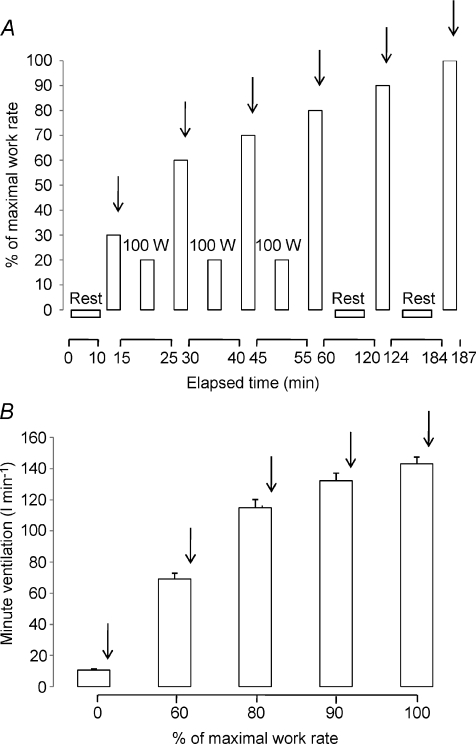
Experiments were conducted in two visits. In visit 1, subjects underwent an incremental preliminary exercise test to the limit of tolerance (WRmax). In visit 2, subjects initially undertook a graded exercise test (protocol 1), which was followed by resting isocapnic hyperpnoea trials (protocol 2). During the graded exercise test subjects completed six bouts of constant-load exercise corresponding to targeted intensities (Fig. 1A): (i) 30% WRmax for 5 min; (ii) 60% WRmax for 5 min; (iii) 70% WRmax for 5 min; (iv) 80% WRmax for 5 min; (v) 90% WRmax for 3–4 min and (vi) 100% WRmax for 2–3 min. Between exercise bouts at 30–80% WRmax, subjects cycled at 100 W for 10 min, whereas after completion of exercise bouts at 80 and 90% WRmax subjects rested for 60 min as diaphragmatic force generation is recovered almost completely by an average time of 60 min in highly trained athletes exercising at heavy work loads (Vogiatzis et al. 2006; 2007;). Two hours after completion of the exercise tests, subjects performed five 5 min bouts of isocapnic hyperpnoea at the same tidal volume, breathing frequency and thus minute ventilation recorded at rest and during exercise at 60, 80, 90 and 100% WRmax (Fig. 1B). Blood flow over the 7th intercostal space and over the vastus lateralis muscle as well as cardiac output were measured during the final minute of each of the exercise and the hyperpnoea bouts.
Figure 1. Experimental protocols.
A, protocol 1, exercise. Subjects performed six 5 min bouts of constant-load exercise at 30% (109 ± 8 w), 60% (218 ± 18 w), 70% (253 ± 20 w), 80% (290 ± 24 w), 90% (319 ± 26 w) and 100% (359 ± 29 w) of maximal work rate. Between 30 and 80%, they cycled at 100 W for 10 min, but after exercise at 80% and 90%, they rested for 60 min. Vertical arrows indicate when haemodynamic measurements were made (during the final minute of exercise). Ventilatory variables were recorded continuously. B, protocol 2, isocapnic, resting hyperpnoea. Two hours after completion of protocol 1, subjects performed 5 bouts of isocapnic hyperpnoea at the same minute ventilation values recorded during protocol 1 at rest and during exercise at 60, 80, 90 and 100% of maximal work rate. Vertical arrows indicate when haemodynamic measurements were made (during the final minute of hyperpnoea). Ventilatory variables were recorded continuously.
Preliminary testing
In visit 1, the incremental exercise tests were performed on an electromagnetically braked cycle ergometer (Ergoline 800; Sensor Medics, Anaheim, CA, USA) starting at 30 W and increasing by 30 W every minute, with the subjects maintaining a pedalling frequency of 70–90 r.p.m. Tests were preceded by a 3 min rest period, followed by 3 min of unloaded pedalling. The following pulmonary gas exchange and ventilatory variables were recorded breath by breath (Vmax 229; Sensor Medics, Anaheim, CA, USA): oxygen uptake 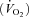 , carbon dioxide elimination
, carbon dioxide elimination 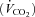 , minute ventilation
, minute ventilation 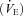 , tidal volume (VT), breathing frequency (f), and respiratory exchange ratio (RER). Heart rate (HR) was determined using the R-R interval from a 12-lead on-line electrocardiogram (Marquette Max; Marquette Hellige GmbH, Germany).
, tidal volume (VT), breathing frequency (f), and respiratory exchange ratio (RER). Heart rate (HR) was determined using the R-R interval from a 12-lead on-line electrocardiogram (Marquette Max; Marquette Hellige GmbH, Germany).
Subject preparation
Subjects were prepared first with arterial and venous catheters for blood flow measurements and blood sampling, and then with oesophageal balloons for assessment of the work of breathing. Using local anaesthesia (2% lidocaine) and sterile technique, identical catheters were introduced percutaneously into the right radial artery and a right antecubital forearm vein, both oriented in the proximal direction. The catheters were used to collect arterial and venous blood samples and also to inject indocyanine green dye (ICG) and sample blood after each injection for blood flow measurements. They were kept patent throughout the experiment by periodic flushing with heparinized (1 unit ml−1) saline.
Oesophageal pressure was assessed by thin-walled balloon catheters (Ackrad Laboratories, Inc., Crandford, NJ, USA) coupled to differential pressure transducers (MP-45, ±250 cmH2O; Validyne Corp., Northridge, CA, USA). The balloons were inserted by nasal intubation following the application of 2% lidocaine anaesthetic gel to the nose and with the assistance of continuous pressure monitoring. The balloon tip was positioned in the middle third of the oesophagus.
Protocol 1: graded exercise
During these tests recordings of pulmonary gas exchange and ventilatory variables were performed as mentioned above, whereas arterial blood pressure was measured by a sphygmomanometer during the final minute of exercise. Arterial and venous blood was taken during the last minute of exercise, while oesophageal pressure (Poes) was continuously monitored. Flow was measured with a hot wire pneumotachograph (Vmax 229; Sensor Medics) near the mouthpiece, and tidal volume changes were obtained by integrating the flow signal. Oesophageal pressure and air flow rates were displayed on a computer screen and digitized at 60 Hz using an analog-to-digital converter connected to the same computer used for optoelectronic plethysmography (OEP system, BTS, Milan, Italy). End-inspiratory and expiratory compartmental (rib cage and abdominal) chest wall volume changes during exercise were determined by OEP (Vogiatzis et al. 2005). In brief, the movement of 89 retro-reflective markers placed front and back over the chest wall from clavicles to pubis was recorded. Markers were tracked by six video cameras, three in front of the subject and three behind. Dedicated software recognised in real time the markers on each camera, reconstructed their 3D co-ordinates by stereophotogrametry and calculated volume changes.
Poes was averaged over 30 s breath samples in every minute of the exercise tests. The mechanical work of breathing (WOB) over a typical breath was determined at each exercise or hyperpnoea level by ensemble-averaging several breaths to integrate the averaged oesophagus pressure-tidal volume loop. The WOB was then multiplied by the breathing frequency to obtain the total amount of work done per minute by the respiratory system and expressed in J min−1 (Otis, 1964).
Protocol 2: resting isocapnic hyperpnoea trials
Subjects were asked to maintain targeted ventilation equal to their own mean ventilation recorded at rest and during exercise at 60, 80, 90 and 100% WRmax. Experimenters provided verbal guidance to adjust the rate and depth of their breathing such that the target ventilation was obtained and held constant to within 5%. Isocapnia was maintained by having subjects inspire from a Douglas bag containing 5% Co2, 21% O2, balance N2 that was connected to a two-way non-rebreathing valve (model 2700, Hans Rudolph) by a piece of tubing. Blood flow over the 7th intercostal space and over the vastus lateralis muscle as well as cardiac output and arterial blood pressure were measured during the final minute of each hyperpnoea bout. Compartmental chest wall volume regulation and the WOB were determined as described above for protocol 1, whereas recordings of all ventilatory variables were performed throughout each hyperpnoea bout.
Cardiac output
Cardiac output was determined by the dilution method (Dow, 1956), using known volumes of Indocyanine Green dye (ICG, range: 0.8–1.2 ml at 5 mg ml−1) injected into the right antecubital forearm vein followed by a rapid 10 ml flush of isotonic saline. Blood was withdrawn from the radial artery using an automated pump (Harvard Apparatus, USA) at 20 ml min−1 through a linear photodensitometer (Pulsion ICG, ViCare Medical, Denmark) connected to a cardiac output computer (Waters CO-10, Rochester, MN, USA) through a closed loop, sterile tubing system. The blood was re-infused into the antecubital forearm vein immediately upon completion of the measurements. The cardiac output computer was connected to a data acquisition system (DI-720, Dataq, OH, USA). Data were sampled at 100 Hz and stored on a computer for subsequent analysis. To remove the influence of dye recirculation, the downslopes of the dye concentration curves were linearly extrapolated using a semi-logarithmic scale in the conventional manner. Cardiac output was calculated as the ratio of ICG mass injected to the mean arterial ICG concentration over the time interval of the curve and expressed as litres per minute. ICG calibration curves were obtained following each experiment by measuring the raw voltage deflection from three 20 ml blood samples containing various concentrations of ICG. Calibrations at each concentration were performed 2–3 times to ensure linearity and consistency.
Intercostal and quadriceps muscle blood flow by NIRS
In order to measure intercostal and quadriceps muscle blood flow, two sets of near-infrared spectroscopy (NIRS) optodes were placed, one on the skin over the left 7th intercostal space and the other over the left vastus lateralis muscle 10–12 cm above the knee, both secured using double sided adhesive tape. NIRS signals were collected continuously during exercise.
The optode separation distance was 4 cm, corresponding to a penetration depth of ∼2 cm. The left intercostal space was used in order to avoid potential blood flow contributions from the liver on the right side of the body. Optodes were connected to a NIRO 200 spectrophotometer (Hamamatsu Photonics KK, Hamamatsu, Japan), which was used to measure ICG concentration following the same 4–5 mg bolus injection of ICG in the right antecubital forearm vein as used for cardiac output assessment. Tissue microcirculatory ICG was detected transcutaneously by measuring light attenuation with NIRS at 775, 813 and 850 nm wavelengths and analysed using an algorithm incorporating the Modified Beer–Lambert Law (van der Zee et al. 1992; Duncan et al. 1995; Boushel et al. 2000; Kalliokoski et al. 2006). Since the measured light attenuation in the tissue is influenced by ICG and oxy- and deoxyhaemoglobin, the independent contribution of ICG to the light absorption signal was isolated using a matrix operation (MATLAB). The matrix operation incorporates path length-specific extinction coefficients for each of the light absorbing chromophores [haemoglobin + myoglobin (Hb+Mb) and ICG] at each wavelength employed by the NIRS machine (Hamamatsu Photonics KK).
Intercostal and quadriceps muscle oxygenation by NIRS
Muscle oxygenation was assessed by the same NIRO 200 spectrophotometer as used for the measurement of muscle blood flow. High ICG tissue concentrations during the passage of the dye bolus through the muscle may interfere with Hb results. Therefore, to avoid any interference between ICG and Hb wavelengths tissue oxygenation data were averaged over 10 s immediately prior to ICG injection. The variables assessed by NIRS were the concentration changes of oxygenated, deoxygenated and total Hb. A derived parameter from NIRS studies in humans is the ratio of oxygenated Hb to total Hb, an index of changes in tissue O2 saturation 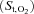 relative to rest (Chance et al. 1992; DeLorey et al. 2003). Among these variables, several laboratories have adopted the deoxy-Hb/Mb signal (deoxy-Hb) as the preferred indicator of changes in muscle microvascular oxygenation during exercise (DeLorey et al. 2004, 2005; Grassi et al. 2003). The deoxy-Hb response to exercise is then considered a proxy of O2 extraction in the microcirculation, reflecting the balance between O2 delivery and utilization.
relative to rest (Chance et al. 1992; DeLorey et al. 2003). Among these variables, several laboratories have adopted the deoxy-Hb/Mb signal (deoxy-Hb) as the preferred indicator of changes in muscle microvascular oxygenation during exercise (DeLorey et al. 2004, 2005; Grassi et al. 2003). The deoxy-Hb response to exercise is then considered a proxy of O2 extraction in the microcirculation, reflecting the balance between O2 delivery and utilization.
Statistical analysis
Data are reported as means ±s.e.m. unless otherwise stated. s.e.m. was used rather than s.d. because our interest is in mean differences between conditions (exercise, hyperpnoea) rather than in differences between subjects. Two-way ANOVA with repeated measures was used to identify statistically significant differences across different ventilation rates between the exercise and the hyperpnoea tests for the WOB, the blood flow measured over the 7th intercostal space, for intercostal muscle 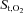 and deoxygenated Hb (deoxy Hb) as well as for compartmental (rib cage and abdominal) chest wall volume variations. One-way ANOVA with repeated measures was used to identify statistically significant differences across the mean values recorded during the exercise or the hyperpnoea tests for intercostal and quadriceps muscle blood flow and for the haemodynamic variables recorded during the preliminary incremental test on the cycle ergometer. When one- or two-way ANOVA detected statistical significance, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. The level of significance for all analyses was set at P < 0.05.
and deoxygenated Hb (deoxy Hb) as well as for compartmental (rib cage and abdominal) chest wall volume variations. One-way ANOVA with repeated measures was used to identify statistically significant differences across the mean values recorded during the exercise or the hyperpnoea tests for intercostal and quadriceps muscle blood flow and for the haemodynamic variables recorded during the preliminary incremental test on the cycle ergometer. When one- or two-way ANOVA detected statistical significance, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. The level of significance for all analyses was set at P < 0.05.
Results
Work of breathing and respiratory kinematics
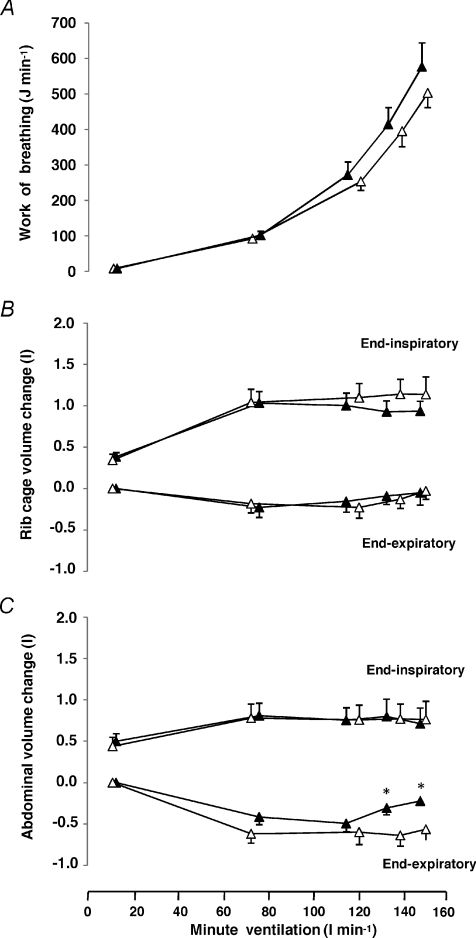
The work of breathing during exercise and hyperpnoea at equivalent rates of minute ventilation was not significantly different (Fig. 2A). Figure 2B and C displays end-inspiratory and end-expiratory volume variations of the rib cage and the abdominal chest wall compartments, respectively during the exercise and hyperpnoea tests. Volume variations of the rib cage compartment at the end of inspiration and expiration (reflecting the activity of the external and internal intercostal muscles, respectively) were not different between exercise and resting hyperpnoea (Fig. 2B). Similarly, volume variations of the abdominal compartment at the end of inspiration (reflecting the activity of the diaphragm) were not different between the exercise and the hyperpnoea trials (Fig. 2C). In contrast, volume variations of the abdominal compartment at the end of expiration (reflecting the activity of the expiratory muscles of the abdominal wall) were greater during exercise compared to the hyperpnoea trials (Fig. 2C).
Figure 2. Work of breathing and chest wall volume changes during exercise and isocapnic resting hyperpnoea.
Changes (relative to values at end-expiration at lowest ventilation) in work of breathing (A) rib cage (B) and the abdominal (C) volume at the end of inspiration and expiration at different levels of minute ventilation during exercise (open triangles) and isocapnic, resting hyperpnoea (filled triangles). Values are means ±s.e.m. for 10 subjects. Asterisks denote significant differences between the exercise and hyperpnoea tests (P < 0.05).
Cardiorespiratory and haemodynamic responses
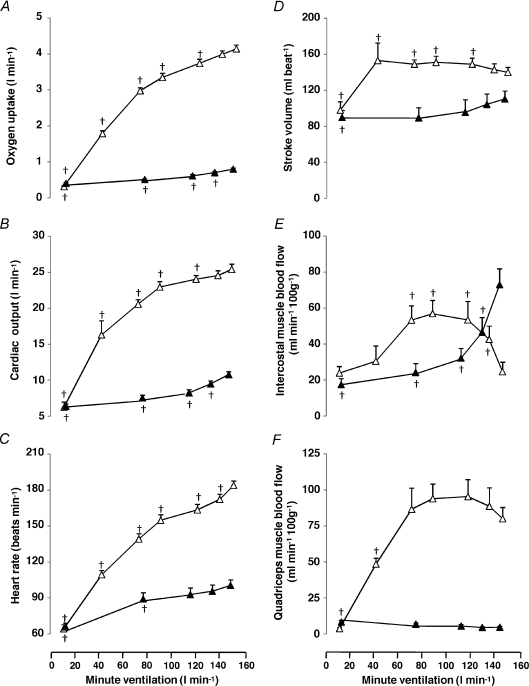
Whole body 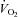 , systemic haemodynamics, and quadriceps and intercostal muscle blood flow are shown in Fig. 3 as a function of minute ventilation sustained during graded exercise and isocapnic resting hyperpnoea.
, systemic haemodynamics, and quadriceps and intercostal muscle blood flow are shown in Fig. 3 as a function of minute ventilation sustained during graded exercise and isocapnic resting hyperpnoea.
Figure 3. Metabolic and haemodynamic responses during exercise and isocapnic resting hyperpnoea.
Oxygen uptake (A), cardiac output (B), heart rate (C), stroke volume (D), intercostal muscle blood flow (E) and quadriceps muscle blood flow (F) at different levels of minute ventilation during exercise (open triangles) and isocapnic, resting hyperpnoea (filled triangles). Values are means ±s.e.m. for 10 subjects. Crosses denote significant differences compared to maximal ventilation, P < 0.05.
During exercise whole body 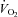 and cardiac output increased linearly to minute ventilation up to a work load corresponding to 80% WRmax (R2= 0.89 and 0.81, respectively) and levelled off thereafter (Fig. 3A and B). The plateau in cardiac output above 80% WRmax was due to a fall in stroke volume (Fig. 3D) because HR continued to increase linearly to maximal minute ventilation (Fig. 3C). The increase in whole body
and cardiac output increased linearly to minute ventilation up to a work load corresponding to 80% WRmax (R2= 0.89 and 0.81, respectively) and levelled off thereafter (Fig. 3A and B). The plateau in cardiac output above 80% WRmax was due to a fall in stroke volume (Fig. 3D) because HR continued to increase linearly to maximal minute ventilation (Fig. 3C). The increase in whole body 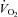 was linear to cardiac output during exercise (R2= 0.91) but not during hyperpnoea (R2= 0.70).
was linear to cardiac output during exercise (R2= 0.91) but not during hyperpnoea (R2= 0.70).
The pattern of change in intercostal muscle blood flow was different (P= 0.001) between the exercise and the hyperpnoea tests (Fig. 3E). Accordingly, during exercise mean intercostal muscle blood flow increased with increasing minute ventilation up to 60% WRmax reaching a plateau between 60 and 80% WRmax (Fig. 3E). At maximal exercise intercostal muscle blood flow decreased (P= 0.002) compared to exercise at 80% WRmax (Fig. 3E). In contrast, during isocapnic hyperpnoea the increase in mean intercostal muscle blood flow was linear (R2= 0.95) with respect to both cardiac output (from 6.4 ± 0.3 to 10.7 ± 0.4 l min−1) and the work of breathing (R2= 0.94), reaching maximal values at the highest levels of minute ventilation (Fig. 3E).
During exercise quadriceps muscle blood flow increased with increasing minute ventilation up to 60% WRmax and then plateaued at higher intensities. During hyperpnoea, quadriceps muscle blood flow did not change with increasing minute ventilation (Fig. 3F).
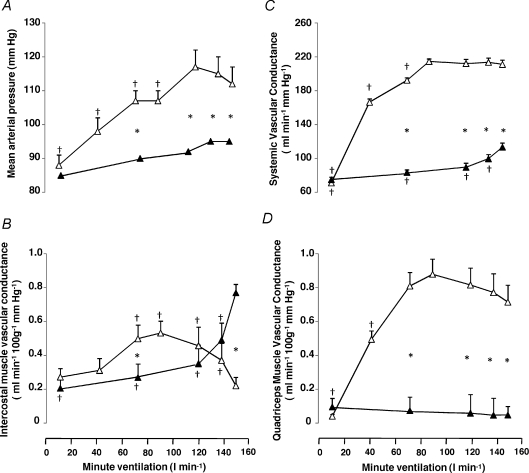
During exercise, mean arterial pressure (MAP) increased with increasing minute ventilation up to 80% WRmax (Fig. 4A). In contrast, during isocapnic hyperpnoea, MAP did not change significantly from rest. During exercise systemic vascular conductance increased to 70% WRmax, whereas during isocapnic hyperpnoea systemic vascular conductance increased to maximal ventilation (Fig. 4C).
Figure 4. Haemodynamic responses during exercise and isocapnic, resting hyperpnoea.
Mean arterial blood pressure (A), systemic vascular conductance (B), intercostal muscle vascular conductance (C) and quadriceps muscle vascular conductance (D) at different levels of minute ventilation during exercise (open triangles) and isocapnic, resting hyperpnoea (filled triangles). Values are means ±s.e.m. for 10 subjects. Asterisks denote significant differences between exercise and hyperpnoea tests; crosses denote significant differences compared to maximal ventilation, P < 0.05.
The pattern of change in intercostal muscle vascular conductance was different (P= 0.007) between the exercise and the hyperpnoea tests (Fig. 4B). During exercise intercostal muscle vascular conductance increased with increasing minute ventilation up to 60% WRmax reaching a plateau between 60 and 80% WRmax. At maximal minute ventilation intercostal muscle vascular conductance decreased (P= 0.005) compared to ventilation recorded at 80% WRmax (Fig. 4B). In contrast, during isocapnic hyperpnoea the increase in mean intercostal vascular conductance was linear with respect to minute ventilation (R2= 0.79). During exercise quadriceps muscle vascular conductance increased with increasing ventilation up to 60% WRmax. During hyperpnoea, quadriceps muscle vascular conductance did not change with increasing ventilation (Fig. 4D).
Intercostal and quadriceps muscle oxygenation by NIRS
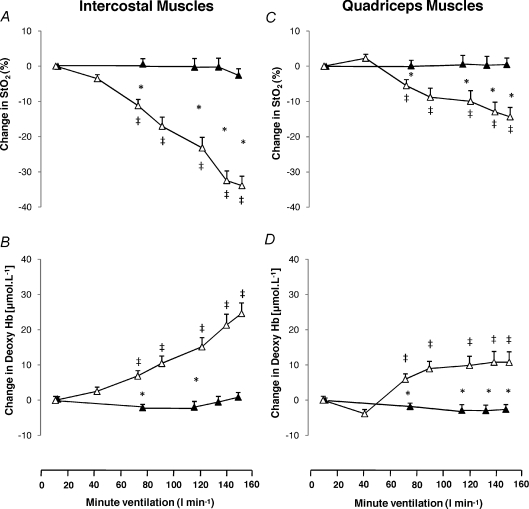
Figure 5 displays changes in intercostal and quadriceps muscle 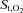 and deoxygenated Hb during the exercise and hyperpnoea trials sustained at similar levels of minute ventilation. During exercise intercostal and quadriceps muscle
and deoxygenated Hb during the exercise and hyperpnoea trials sustained at similar levels of minute ventilation. During exercise intercostal and quadriceps muscle 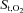 decreased from baseline, whereas
decreased from baseline, whereas 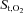 remained unchanged during resting hyperpnoea at equivalent levels of work of breathing (Fig. 5A and C). The decline in
remained unchanged during resting hyperpnoea at equivalent levels of work of breathing (Fig. 5A and C). The decline in 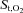 from baseline during exercise was smaller (P= 0.0001) in the quadriceps compared to the intercostal muscles. Intercostal and quadriceps muscle deoxygenated Hb increased from baseline during the exercise trials, but remained unchanged during resting hyperpnoea (Fig. 5B and D). The increase from baseline in deoxygenated Hb during exercise was smaller (P= 0.004) in the quadriceps compared to the intercostal muscles.
from baseline during exercise was smaller (P= 0.0001) in the quadriceps compared to the intercostal muscles. Intercostal and quadriceps muscle deoxygenated Hb increased from baseline during the exercise trials, but remained unchanged during resting hyperpnoea (Fig. 5B and D). The increase from baseline in deoxygenated Hb during exercise was smaller (P= 0.004) in the quadriceps compared to the intercostal muscles.
Figure 5. Changes in intercostal and quadriceps oxygenation during exercise and isocapnic, resting hyperpnoea.
Changes in oxygen saturation and deoxygenated haemoglobin concentration for the intercostal muscles (A and B) and the quadriceps muscles (C and D) at different levels of minute ventilation during exercise (open triangles) and isocapnic, resting hyperpnoea (filled triangles). Values are means ±s.e.m. for 10 subjects. Asterisks denote significant differences between the exercise and hyperpnoea tests; double crosses denote significant differences compared to baseline, P < 0.05.
Discussion
The present study investigated whether during maximal cycling exercise in athletes there is an increase in blood flow in favour of the respiratory muscles, at the expense of the exercising leg muscles. Since we were unable to measure diaphragmatic blood flow, respiratory muscle blood flow was assessed by measuring intercostal perfusion using indocyanine green and an NIRS probe positioned over the 7th intercostal space. Flow during resting hyperpnoea (when respiratory muscle perfusion was presumably the maximal attainable, which is, not limited by competition from other active muscle groups) was compared to intercostal muscle blood flow recorded during maximal exercise where limb and respiratory muscles had to compete for the available blood flow. There are two key findings of this study. Firstly, intercostal muscle blood flow and vascular conductance were lower during maximal exercise than during hyperpnoea at the same work of breathing as measured during maximal exercise. Secondly, intercostal muscle blood flow during maximal exercise was lower than during sub-maximal exercise, whereas cardiac output, quadriceps muscle perfusion and vascular conductance reached a plateau but did not significantly fall at heavy exercise. Collectively, these observations suggest that at maximal exercise restriction of intercostal muscle blood flow along with the attainment of a plateau in cardiac output and quadriceps muscle perfusion represent the inability of the circulatory system to satisfy the energy demands of both locomotor and intercostal muscles.
The concept of blood flow redistribution from the locomotor to the respiratory muscles during exercise was evolved from studies showing reductions in limb blood flow with respiratory muscle loading (Harms et al. 1997, 1998) and subsequently from investigations demonstrating that fatiguing levels of respiratory muscle work caused a reduction in resting limb blood flow (St Croix et al. 2000; Sheel et al. 2001, 2002). The mechanism orchestrating the interaction of respiratory and locomotor muscles is thought to depend on the stimulation of metaboreceptors innervating the respiratory muscles (Dempsey et al. 2002, 2006). Upon their discharge, sympathetric nerve efferent activity is believed to increase and vascular conductance in several vascular beds, including those in limb muscles, to decrease (Hussain et al. 1991; Hussain, 1996; Offner et al. 1992; Rodman et al. 2003). However, none of the studies reported by Harms et al. (1997, 1998) assessed respiratory muscle blood flow during exercise. The novelty of the present investigation is that simultaneous measurements of quadriceps and respiratory muscle blood flow and muscle vascular conductance were performed under conditions where the amount of both respiratory and leg muscle work concurrently increased during graded discontinuous exercise. We observed that cardiac output and quadriceps muscle blood flow plateaued at ∼80% WRmax (Fig. 3B and F) in parallel to a marked blunting of systemic and quadriceps muscle vascular conductance (Fig. 4C and D), thereby indicating enhanced sympathetic vasoconstrictor activity. Although our findings agree with those by Mortensen et al. (2005, 2008) in that peripheral muscle perfusion becomes restricted during maximal exercise, we also witnessed a reduction in intercostal muscle blood flow at maximal compared to submaximal exercise (Fig. 3E). The inability to preserve perfusion to locomotor muscles at maximal exercise appears associated with both the plateau in cardiac output and increased sympathetic vasoconstrictor activity (Gonzalez-Alonso & Calbet, 2003; Mortensen et al. 2005). The latter is thought to be secondary to fatigue of the respiratory muscles (Dempsey et al. 2002), taking place only during heavy exercise (Babcock et al. 1995; Johnson et al. 1993, 1996). Increased vasoconstriction could also account for the observed reduction in intercostal muscle blood flow as intercostals muscle vascular conductance was also reduced above ∼80% WRmax.
Intercostal muscle blood flow: technical strengths and limitations
Using NIRS and ICG to measure blood flow over the 7th intercostal space was employed during exercise in athletes (Vogiatzis et al. 2008). Blood flow measured over the 7th intercostal space is mainly attributed to blood flow perfusing the external and internal intercostals muscles and to a lesser extent the costal segment of the diaphragm owing to the distance encompassed between the sampling point of NIRS on the skin and the diaphragmatic appositional area (Guenette et al. 2008). Monitoring intercostal muscle blood flow during exercise has important implications for understanding circulatory regulation of respiratory muscles as these muscles provide the necessary pressures to displace the ribcage and increase end-inspiratory lung volume, whilst the diaphragm acts primarily as a flow generator (Aliverti et al. 1997). Although we compared respiratory muscle blood flow under the exact same NIR probe position during hyperpnoea and exercise, a limitation of the NIR measurement over the 7th intercostal space is that it does not allow partitioning of blood flow between the intercostal muscles and the diaphragm.
Intercostal and quadriceps muscle blood flow during hyperpnoea
During the hyperpnoea tests, blood flow to the intercostal muscles increased linearly with work of breathing and cardiac output. Since intercostal muscle oxygen saturation and deoxygenated haemoglobin (the latter a proxy of tissue fractional oxygen extraction; DeLorey et al. 2003, 2004, 2005; Grassi et al. 1996, 1998, 2003) did not change across increasing levels of respiratory muscle work (Fig. 5A and B), we suggest that increasing oxygen demand of the respiratory muscles was met by proportionally increased blood flow and oxygen delivery, thereby preventing respiratory muscle fatigue. Resting hyperpnoea at the levels of respiratory muscle work incurred during heavy exercise does not produce respiratory muscle fatigue (Babcock et al. 1995). In fact, respiratory muscle fatigue during hyperpnoea in the studies by Babcock et al. (1995) was seen only when subjects sustained levels of respiratory muscle work far in excess of those usually demanded by heavy exercise. Therefore, our finding that the amount of respiratory muscle work endured during resting hyperpnoea had no effect on quadriceps muscle blood flow (Fig. 3F) is consistent with findings by St Croix et al. (2000) and Sheel et al. (2001, 2002) showing lack of changes in limb blood flow in the absence of respiratory muscle fatigue.
Intercostal and quadriceps muscle blood flow during exercise
Intercostal muscle blood flow and vascular conductance during exercise were, however, different from those during hyperpnoea, as they increased with intensity before reaching a plateau at levels greater than those recorded during hyperpnoea, and then decreasing at maximal exercise (Figs 3E and 4B). The progressive reduction in intercostal muscle oxygen saturation and the increase in deoxygenated haemoglobin (Fig. 5A and B) against the background of essentially stable intercostal muscle blood flow and vascular conductance during graded exercise (Figs 3E and 4B) are consistent with a mismatch between oxygen delivery to, and demand of, the intercostal muscles, suggesting that respiratory muscle blood flow limitation may contribute to respiratory muscle fatigue (Babcock et al. 1995). Fatigue of the respiratory muscles would be expected to stimulate the discharge of the respiratory muscle metaboreceptors, thus reducing blood flow elsewhere (Sheel et al. 2001, 2002). We observed a restriction in quadriceps muscle blood flow at maximal exercise that is consistent with that reported by Mortensen et al. (2005, 2008). This finding is also in accordance with those by Harms et al. (1997) who concluded that the work of breathing incurred during maximal exercise in humans compromises locomotor muscle perfusion. Without actual respiratory muscle blood flow measurements, Harms et al. (1997, 1998) hypothesized that the reduction in limb blood flow was evidence of flow redistribution to the respiratory muscles. However, the results of the present study do not confirm the above hypothesis as flow measured over the 7th intercostal space at maximal exercise was decreased compared to submaximal levels of exercise (Fig. 3E).
The pattern of attenuation in intercostal and quadriceps muscles blood flow at high levels of simultaneous respiratory and leg muscle work during submaximal exercise was similar. Accordingly, both intercostal and quadriceps muscle blood flow increased with increasing exercise intensity to 60% WRmax and levelled off to 80–90% WRmax (Fig. 3E and F). The observed plateau in quadriceps muscle blood flow and vascular conductance (Fig. 4D) is compatible with findings showing that during graded exercise, leg blood flow levels off at intensities ranging from ∼70 to 90% of peak power output, whereas at maximal exercise leg vascular conductance and blood flow are restricted (Mortensen et al. 2005, 2008). As both cardiac output and mean arterial blood pressure levelled off at exercise intensities above 80% WRmax (Figs 3B and 4A), the reduction in intercostal muscle flow and vascular conductance at maximal exercise appears to be the result of the bunted cardiac output and the overriding muscle sympathetic vasoconstrictor activity (Mortensen et al. 2005; Calbet et al. 2007). Such an increase in muscle sympathetic nerve activity during intense whole body exercise (Saito et al. 1993) has a functional role in maintaining blood pressure by limiting blood flow to the exercising muscles (Saltin et al. 1998). In fact, the time course of the systemic, intercostal and quadriceps muscle vascular conductance responses recorded beyond 70–80% WRmax (Fig. 4) mirrors the normal exponential increases in muscle sympathetic nerve activity during heavy and exhaustive exercise where the vasomotor activity has been reported to increase severalfold compared to mild and moderate exercise (Ichinose et al. 2008).
When the intensity of exercise exceeds 80–85%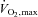 , inspiratory (Johnson et al. 1993, 1996) and expiratory (Taylor et al. 2006) muscle fatigue is exhibited in subjects with varying degrees of fitness levels including highly trained endurance athletes (Vogiatzis et al. 2006, 2007, 2008). Conversely, exercise at lower intensities attenuates respiratory muscle fatigue, thus preventing the activation of the sympathetic vasoconstrictor efferent output to the locomotor muscles (Wetter et al. 1999). Therefore, the restriction in both quadriceps and intercostal muscle blood flow beyond 80% WRmax could be due to mediation of a muscle chemoreflex that originates from type III and IV thin fibre phrenic afferents in respiratory muscles (Hussain, 1996), causing sympatho-excitation and vasoconstriction in several vascular beds including those of limb and respiratory muscles (Hussain, 1996). Secondary to respiratory muscle fatigue, limb vasoconstriction and reduced oxygen transport are expected to exacerbate locomotor muscle fatigue (Romer et al. 2006), thereby triggering the limb muscle metaboreflex known to impact on systemic blood flow (Romer & Polkey, 2008). In the present study during exercise at work loads not producing respiratory muscle fatigue (≤ 80% WRmax) intercostal muscle blood flow was greater than at equivalent levels of respiratory muscle work during hyperpnoea (Fig. 3E). This is likely to be due to greater vasodilatory capacity during whole body exercise as opposed to hyperpnoea alone where in the latter condition a relatively small muscle mass was activated and cardiac output (10.7 ± 0.4 l min−1) was roughly a third of that during exercise (Fig. 3B) (Saltin et al. 1998).
, inspiratory (Johnson et al. 1993, 1996) and expiratory (Taylor et al. 2006) muscle fatigue is exhibited in subjects with varying degrees of fitness levels including highly trained endurance athletes (Vogiatzis et al. 2006, 2007, 2008). Conversely, exercise at lower intensities attenuates respiratory muscle fatigue, thus preventing the activation of the sympathetic vasoconstrictor efferent output to the locomotor muscles (Wetter et al. 1999). Therefore, the restriction in both quadriceps and intercostal muscle blood flow beyond 80% WRmax could be due to mediation of a muscle chemoreflex that originates from type III and IV thin fibre phrenic afferents in respiratory muscles (Hussain, 1996), causing sympatho-excitation and vasoconstriction in several vascular beds including those of limb and respiratory muscles (Hussain, 1996). Secondary to respiratory muscle fatigue, limb vasoconstriction and reduced oxygen transport are expected to exacerbate locomotor muscle fatigue (Romer et al. 2006), thereby triggering the limb muscle metaboreflex known to impact on systemic blood flow (Romer & Polkey, 2008). In the present study during exercise at work loads not producing respiratory muscle fatigue (≤ 80% WRmax) intercostal muscle blood flow was greater than at equivalent levels of respiratory muscle work during hyperpnoea (Fig. 3E). This is likely to be due to greater vasodilatory capacity during whole body exercise as opposed to hyperpnoea alone where in the latter condition a relatively small muscle mass was activated and cardiac output (10.7 ± 0.4 l min−1) was roughly a third of that during exercise (Fig. 3B) (Saltin et al. 1998).
Intercostal and quadriceps muscle oxygenation during exercise
The decline in muscle oxygen saturation and the increase in de-oxygenated haemoglobin from baseline during exercise were smaller in the quadriceps compared to the intercostal muscles (Fig. 5). The difference in the rate of change in tissue oxygenation variables between muscle groups is attributed to the finding that during moderate and heavy exercise quadriceps muscle blood flow was not as compromised as intercostal muscle blood flow (Fig. 3E and F).
In addition, the progressive changes in tissue oxygenation variables suggest that the metabolic demand of the intercostal and quadriceps muscles was increased during graded exercise (Fig. 5). Thus, a reduced local muscle metabolic demand is unlikely to be a factor causing the attenuation in local muscle blood flow. However, the latter possibility cannot be ruled out as neither power production nor the metabolic demands of the intercostal and quadriceps muscles can be independently measured at present to conclusively reject this possibility.
Regulation of blood flow within the respiratory muscles
Since muscle blood flow measured by the NIRS–ICG over the 7th intercostal space mainly reflects perfusion of the external and internal intercostal muscles, it remains unknown whether, at maximal exercise, there is a corresponding reduction in blood flow of the diaphragm and/or the expiratory abdominal muscles as their activity is crucial in generating flow and the pressure to displace the abdomen, respectively during heavy exercise (Aliverti et al. 1997). Blood flow to these respiratory muscles cannot be assessed in intact exercising humans. Animal studies have shown different patterns of blood flow partitioning between the intercostal muscles and the diaphragm during exercise. In dogs during submaximal exercise (Fixler et al. 1976) and in ponies during maximal exercise (Manohar, 1986) diaphragm blood flow was twofold greater than that to the intercostal muscles owing to the greater vascular resistance in the latter. If human intercostal muscles are also more prone to vasoconstriction than the diaphragm, this may explain why intercostal muscle blood flow was lower at maximal ventilation during exercise compared to hyperpnoea as during whole body heavy exercise sympathetic outflow discharge would be expected to be more profound (Rowell, 2004).
Furthermore the lower intercostal muscle blood flow during maximal exercise compared to hyperpnoea occurred secondarily to blood flow redistribution from the intercostal muscles to the expiratory abdominal muscles. During maximal exercise the expiratory muscles of the abdominal compartment make a greater contribution to tidal volume expansion than the rib cage muscles (Vogiatzis et al. 2005). Volume variations of the rib cage and abdominal chest wall compartments during exercise revealed that whilst the activation of the external and internal intercostal muscles (represented by the volume changes of the rib cage compartment) were similar between exercise and hyperpnoea, volume variations of the abdominal compartment at the end of expiration (abdominal muscle recruitment) were greater during exercise compared to hyperpnoea (Fig. 2), thereby suggesting greater abdominal wall muscle energy and blood flow requirements.
The present study shows that during maximal exercise in healthy humans, blood flow does not increase in favour of the intercostal muscles, possibly owing to a globally increased sympathetic outflow that is triggered as respiratory muscle fatigue develops. It is likely that during maximal exercise, restriction of blood flow to the intercostal muscles reflects the inability of the circulatory system to meet the increasing energy demands of both locomotor and intercostal muscles.
Acknowledgments
This work was supported by Thorax Foundation and by grants from the ‘A. Perotti’ visiting Professorship fund of the Thorax Foundation. P.D. and H.W. were supported in part by the National Institutes of Health (NIH HL 84281 and 91830). We are grateful to Dr S. Golemati, Ms M. Vasilopoulou, Ms S. Spetsioti and Mr Z Louvaris for their assistance with the collection and analysis of the data acquired by optoelectronic plethysmography. We are also grateful to Mr Ludwig Schleinkofer from Hamamatsu Photonics Deutschland GmbH and Mr John Pastelas from Bio Pro L.T.D. Hellas for loaning to us the NIRO 200 spectrophotometer (Hamamatsu Photonics KK, Hamamatsu, Japan).
Authors contributions
I.V. participated in the conception and design of the study, the analysis and interpretation of data and writing up the article. D.A. participated in the analysis and interpretation of data and drafting the article. H.H. participated in the analysis and interpretation of data. W.M.K. participated in the analysis and interpretation of data. H.W. participated in the analysis and interpretation of data. C.R. participated in the interpretation of data. P.W. participated in the conception and design of the study, analysis and interpretation of data, and drafting the article. S.Z. participated in the conception and design of the study, analysis and interpretation of data and drafting the article. All authors participated in revising the article critically for important intellectual content and approved the final version for publication.
References
- Aliverti A, Cala SJ, Duranti R, Ferrigno G, Kenyon CM, Pedoti A, Scano G, Sliwinski P, Macklem PT, Yan S. Human respiratory muscle actins and control during exercise. J Appl Physiol. 1997;83:1256–1269. doi: 10.1152/jappl.1997.83.4.1256. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol. 1995;78:82–92. doi: 10.1152/jappl.1995.78.1.82. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Nowak M, Simonsen L, Bulow J, Kjaer M. Regional blood flow during exercise in humans measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol. 2000;89:1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- Calbet JA, González-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Chance B, Dait M, Zhang C, Hamaoka T, Hong L. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol Cell Physiol. 1992;262:C766–C775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Effects of prior heavy intensity exercise on pulmonary O2 uptake and muscle deoxygenation kinetics in young and older adult humans. J Appl Physiol. 2004;97:998–1005. doi: 10.1152/japplphysiol.01280.2003. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy intensity exercise in young and older adults. J Appl Physiol. 2005;98:1697–1704. doi: 10.1152/japplphysiol.00607.2004. [DOI] [PubMed] [Google Scholar]
- Dow P. Estimations of cardiac output and central blood volume by dye dilution. Physiol Rev. 1956;36:77–102. doi: 10.1152/physrev.1956.36.1.77. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Fixler DE, Atkins JM, Mitchell JH, Horwitz LD. Blood flow to respiratory, cardiac, and limb muscles in dogs during graded exercise. Am J Physiol. 1976;231:1515–1519. doi: 10.1152/ajplegacy.1976.231.5.1515. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JAL. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Vogiatzis I, Zakynthinos S, Athanasopoulos D, Koskolou M, Golemati S, Vasilopoulou M, Wagner HE, Roussos C, Wagner PD, Boushel R. Human respiratory muscle blood flow measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol. 2008;104:1202–1210. doi: 10.1152/japplphysiol.01160.2007. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Thomas A, Wetter J, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hussain SNA, Chattilon A, Comtois A, Roussos C, Magder S. Chemical activation of thin fibre phrenic afferents 2. Cardiovascular responses. J Appl Physiol. 1991;70:77–86. doi: 10.1152/jappl.1991.70.1.77. [DOI] [PubMed] [Google Scholar]
- Hussain SNA. Regulation of ventilatory muscle blood flow. J Appl Physiol. 1996;81:1455–1468. doi: 10.1152/jappl.1996.81.4.1455. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Aaron EA, Babcock MA, Dempsey JA. Respiratory muscle fatigue during exercise: implications for performance. Med Sci Sports Exerc. 1996;28:1129–1137. doi: 10.1097/00005768-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Scheede-Bergdahl C, Kjaer M, Boushel R. Muscle perfusion and metabolic heterogeneity: insights from non-invasive imaging techniques. Exerc Sport Sci Rev. 2006;34:164–170. doi: 10.1249/01.jes.0000240018.07502.48. [DOI] [PubMed] [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonzalez-Alonso Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Damsgaard R, Dawson EA, Secher NH, Gonzalez-Alonso Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2008;586:2621–2635. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner B, Dembowsky K, Czachurski J. Characteristics of sympathetic reflexes evoked by electrical stimulation of phrenic afferents. J Auton Nerv Syst. 1992;42:103–111. doi: 10.1016/0165-1838(92)90132-z. [DOI] [PubMed] [Google Scholar]
- Otis AB. The work of breathing. In: Fenn WO, Rahn H, editors. Handbook of Physiology, section 3, respiration, vol. 1. Washington, DC: American Physiological Society; 1964. pp. 463–476. [Google Scholar]
- Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle mataboreflexes in dogs: rest and exercise. J Appl Physiol. 2003;95:1159–1169. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol. 2008;104:879–888. doi: 10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to gratded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal muscle and cardiac blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Reflex effects from a fatiguing diaphragm increase sympathetic efferent activity (MSNA) to limb muscle in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, How SC, Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J Appl Physiol. 2006;100:1554–1562. doi: 10.1152/japplphysiol.01389.2005. [DOI] [PubMed] [Google Scholar]
- Van Der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO, et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol. 1992;316:143–153. doi: 10.1007/978-1-4615-3404-4_17. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Aliverti A, Golemati S, Georgiadou O, LoMauro A, Kosmas E, Kastanakis E, Roussos C. Respiratory kinematics by optoelectronic plethysmography during exercise in men and women. Eur J Appl Physiol. 2005;93:581–587. doi: 10.1007/s00421-004-1249-4. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Georgiadou O, Giannopoulou I, Koskolou M, Peraki E, Kostikas K, Kosmas E, Wagner H, Peraki E, Koutsoukou A, Koulouris N, Wagner PD, Roussos C. Effects of exercise-induced arterial hypoxaemia and work rate on diaphragmatic fatigue in highly trained endurance athletes. J Physiol. 2006;572:539–549. doi: 10.1113/jphysiol.2005.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Goergiadou O, Koskolou M, Athanasopoulos D, Kostikas K, Golemati S, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Effects of hypoxia on diaphragmatic fatigue in highly trained athletes. J Physiol. 2007;581:299–308. doi: 10.1113/jphysiol.2006.126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Boushel R, Guenette JA, Koskolou M, Vasilopoulou M, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Contribution of respiratory muscle blood flow to exercise-induced diaphragmatic fatigue in trained cyclists. J Physiol. 2008;586:5575–5587. doi: 10.1113/jphysiol.2008.162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on
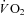 and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]