Abstract
Resistance exercise induces a hypertrophic response in skeletal muscle and recent studies have begun to shed light on the molecular mechanisms involved in this process. For example, several studies indicate that signalling by the mammalian target of rapamycin (mTOR) is necessary for a hypertrophic response. Furthermore, resistance exercise has been proposed to activate mTOR signalling through an upstream pathway involving the phosphoinositide 3-kinase (PI3K) and protein kinase B (PKB); however, this hypothesis has not been thoroughly tested. To test this hypothesis, we first evaluated the temporal pattern of signalling through PI3K–PKB and mTOR following a bout of resistance exercise with eccentric contractions (EC). Our results indicated that the activation of signalling through PI3K–PKB is a transient event (<15 min), while the activation of mTOR is sustained for a long duration (>12 h). Furthermore, inhibition of PI3K–PKB activity did not prevent the activation of mTOR signalling by ECs, indicating that PI3K–PKB is not part of the upstream regulatory pathway. These observations led us to investigate an alternative pathway for the activation of mTOR signalling involving the synthesis of phosphatidic acid (PA) by phospholipase D (PLD). Our results demonstrate that ECs induce a sustained elevation in [PA] and inhibiting the synthesis of PA by PLD prevented the activation of mTOR. Furthermore, we determined that similar to ECs, PA activates mTOR signalling through a PI3K–PKB-independent mechanism. Combined, the results of this study indicate that the activation of mTOR following eccentric contractions occurs through a PI3K–PKB-independent mechanism that requires PLD and PA.
It has been well recognized that the maintenance of skeletal muscle mass plays a critical role in health and issues associated with the quality of life, and engagement in resistance exercise is one of the most commonly employed interventions for maintaining/increasing muscle mass (Seguin & Nelson, 2003; Phillips, 2007). In addition to promoting gains in muscle mass and strength, resistance exercise also promotes metabolic health and reduces the risk of developing diabetes and cardiovascular disease (Lakka & Laaksonen, 2007; Phillips, 2007). For these, and many other reasons, there has been a great deal of research focused on understanding the mechanisms through which resistance exercise promotes an increase in skeletal muscle mass.
In general, skeletal muscle mass is regulated by the net balance between protein synthesis and protein breakdown. Following resistance exercise, there is a net increase in the balance between protein synthesis and protein breakdown and this is primarily driven by an increase in the rate of protein synthesis (Tipton & Wolfe, 2001; Hornberger & Esser, 2004; Coffey & Hawley, 2007; Kumar et al. 2009). Protein synthesis can be regulated through changes in the translational capacity (concentration of ribosomes) or translational efficiency (rate that a given ribosome translates proteins) and to date, most studies suggest that changes in translational efficiency are primarily responsible for the increase in protein synthesis that occurs following resistance exercise (Kimball et al. 2002).
Translational efficiency is regulated by a coordinated series of biochemical events, and a large proportion of these events have been shown to lie downstream of a molecule called phosphoinositide 3-kinase (PI3K) (Bader et al. 2005). One of the most highly studied molecules downstream from PI3K is a protein kinase called the mammalian target of rapamycin (mTOR). Signalling by mTOR has become a topic of particular interest for skeletal muscle biologists because signalling by mTOR has been shown to be necessary for resistance exercise-induced changes in protein synthesis and the concomitant hypertrophic response (Bodine et al. 2001; Hornberger et al. 2003; Kubica et al. 2005; Drummond et al. 2009). Thus, numerous studies have been aimed at defining how resistance exercise activates mTOR signalling.
To date, the most widely accepted hypothesis is that resistance exercise activates mTOR, and the concomitant hypertrophic response, via an upstream pathway involving PI3K and protein kinase B (PKB) (PI3K–PKB → mTOR → Growth) (Adams, 2002; Nader, 2005; Baar et al. 2006; Dreyer et al. 2006; Frost & Lang, 2007). This hypothesis has been supported by several observations such as: (i) increased signalling through PI3K–PKB following resistance exercise (Carlson et al. 2001; Spangenburg & McBride, 2006; Thomson et al. 2008), (ii) increased expression of insulin-like growth factor 1 (IGF-1) following resistance exercise (note: IGF-1 promotes the activation of the PI3K–PKB pathway) (Rommel et al. 2001; Adamo & Farrar, 2006), and (iii) constitutive activation of PI3K–PKB induces a robust hypertrophic response in skeletal muscle (Pallafacchina et al. 2002; Bodine, 2006). Combined, these observations provide a solid rationale for the hypothesis that resistance exercise activates mTOR signalling and growth through a mechanism involving IGF-1 and the PI3K → PKB → mTOR pathway; however, recent studies have challenged the validity of this hypothesis, and to date, no studies have directly tested whether the PI3K–PKB pathway is required for the activation of mTOR by resistance exercise.
The first study that challenges the above hypothesis comes from Spangenburg et al. (2008). In this study, a transgenic mouse that expresses a dominant negative IGF-1 receptor specifically in skeletal muscle was employed. It was demonstrated that skeletal muscles from these mice fail to show a signalling response (increase in PKB phosphorylation) following IGF-1 stimulation; however, the hypertrophic response induced by mechanical overload was fully preserved in muscles from these mice. These observations led to the conclusion that a functional IGF-1 receptor is not required for mechanically induced growth of skeletal muscle. Furthermore, we have previously determined that PI3K–PKB activity is not required for the activation of mTOR signalling in response to passive stretch (Hornberger & Chien, 2006). Although the models employed in the aforementioned studies do not accurately mimic resistance exercise, the results do cast doubt on the hypothesis that the PI3K–PKB pathway is necessary for the activation of mTOR and growth by this stimulus. Therefore, the primary goal of this study was to test the hypothesis that signalling through the PI3K–PKB pathway is required for the activation of mTOR following resistance exercise.
The results from this study indicate that mTOR signalling is elicited through a PI3K–PKB-independent mechanism following a bout of resistance exercise with eccentric contractions (EC). This observation led us to investigate an alternative pathway for the activation of mTOR signalling involving phospholipase D (PLD) and its lipid second messenger phosphatidic acid (PA). We were interested in this pathway because PLD is the primary enzyme that generates PA in cells, and previous studies have shown that phosphatidic acid can directly bind to mTOR, and in doing so, activate mTOR signalling (Fang et al. 2001; Veverka et al. 2008). Our results demonstrate that ECs induce a sustained elevation in [PA] and inhibiting the synthesis of PA by PLD prevents the EC-induced activation of mTOR signalling. Furthermore, we determined that similar to ECs, PA activates mTOR signalling through a PI3K–PKB-independent mechanism. Combined, these results indicate that ECs activate mTOR signalling through a PI3K–PKB-independent pathway that requires PLD and PA. The implications of these findings and the potential roles that PLD/PA might play in resistance exercise-induced hypertrophy will be discussed.
Methods
Materials
Phosphatidic acid (1,2-dioctanoyl-sn-glycero-3-phosphate) was used for cell stimulation experiments and purchased from Avanti Lipids (Alabaster, AL, USA). The PA was prepared by drying under nitrogen gas and then dissolved in phosphate buffered saline (PBS) with 3 min of bath sonication at a concentration of 200 μm. Insulin (Humalog) was purchased from Lilly (Indianapolis, IN, USA) and dissolved in PBS to a concentration of 2 μm before adding to culture media. Wortmannin was purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in DMSO at a concentration of 250 μm before adding to culture media. Rapamycin was purchased from Calbiochem (San Diego, CA, USA) and dissolved in DMSO at a concentration of 50 μm before adding to culture media. Methanol, acetic acid, ethyl acetate, trimethylpentane and l-butanol were purchased from Fisher Chemical (Fair Lawn, NJ, USA). 3H-Arachidonic acid was purchased from Perkin Elmer (Waltham, MA, USA). Egg l-α-phosphatidic acid was purchased from Avanti Lipids and used for the standard on TLC plates. Rabbit primary antibodies including anti-total p70S6k[Total p70], anti-phospho-PKB(Ser473) [P-PKB(473)], anti-phospho-p38 (Thr180/Tyr182) [P-p38] and anti-phospho-JNK(Thr183/Tyr185) [P-JNK2] were purchased from Cell Signalling (Danvers, MA, USA). Rabbit anti-phospho-p70S6k (Thr389) [P-p70(389)] was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Anti-rabbit peroxidase-conjugated IgG (H+L) was purchased from Vector Laboratories (Burlingame, CA, USA). Regular enhanced chemiluminescence (ECL) reagent was purchased from Pierce (Rockford, IL, USA) and ECL plus reagent was purchased from Amersham (Piscataway, NJ, USA).
Animal care and use
All experimental procedures were approved by the Animal Care Committee of the University of Wisconsin-Madison. A total of 96 male C57BL6 mice (Jackson Laboratories, Bar Harbor, MA, USA) 8–12 weeks of age, and 30 male Sprague–Dawley (Harlan, Indianapolis, IN, USA) 8–10 weeks of age, were randomly assigned to different experimental groups. All animals were allowed free access to food and water.
Prior to all surgical procedures, animals were anaesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg kg−1). An initial assessment for a proper level of analgesic administration was performed by pinching the pad of the foot and observing the animal for any signs of reactivity. If the animal showed signs of reactivity an additional dose of sodium pentobarbital (25 mg kg−1) was administered until a proper analgesic state was achieved. Upon the completion of experimental treatments, mice were killed by cervical dislocation under anaesthesia. Euthanasia was confirmed 2 min later by checking for indicators of respiration and a heartbeat. Anaesthetized rats were subjected to killing by an intracardiac injection of 2 mmol kg−1 potassium chloride. Euthanasia was confirmed 5 min after the injection by checking for indicators of respiration and a heartbeat.
Stimulation of skeletal muscle contractions in vivo
The model previously described by Baar & Esser (1999) was used to evoke contractions in the muscles of the lower hind limb (Baar & Esser, 1999). In this model, the dorsiflexors including the tibialis anterior (TA) and extensor digitorum longus muscle (EDL) are subjected to eccentric contractions while the plantar flexors, such as the soleus muscle, are subjected to concentric contractions. To induce these contractions, mice and rats were anaesthetized and then a small incision was made through the thigh to expose the sciatic nerve. Electrodes were attached to the sciatic nerve and then the body of the animal was secured to a stimulation apparatus which allowed for free movement of the right leg. Contractions were elicited by stimulating the sciatic nerve with a 100 Hz, 5–7 V pulse through a S48 Grass stimulator (Warwick, RI, USA). The contractions lasted 3 s and were followed by a 10 s rest, during which time the foot was returned to the neutral position. After the sixth repetition of contractions, there was an additional 50 s of rest. This pattern of stimulation was repeated for a total of 10 sets of six repetitions, resulting in 60 contractions over a 22 min period.
Stimulation of eccentric contractions ex vivo
An ex vivo model was developed to mimic the eccentric contraction stimulus that was applied in the in vivo contraction model. In the ex vivo model, mice were anaesthetized with sodium pentobarbital (100 mg kg−1) and the EDL muscle of the hind limb was attached to the lever arms of a force transducer/dynamometer and micromanipulator. The EDL muscle was then placed in an organ culture system at optimal length (Lo) as previously described (Hornberger & Chien, 2006). Krebs–Henseleit buffer (120 mm NaCl, 4.8 mm KCl, 25 mm NaHCO3, 2.5 mm CaCl2, 1.2 mm KH2PO4, 2 mm MgSO4, 5 mm Hepes) supplemented with 1× MEM amino acid mixture (HyClone, Logan, UT, USA) and 25 mm glucose was used for incubation media and maintained at 37°C with continuous 95% O2 and 5% CO2 gassing. The incubation media was replaced with fresh media every 30 min. Eccentric contractions were evoked by electrically field stimulating the EDL muscle with a 80 V 100 Hz pulse for 3 s. At the onset of this electrical stimulus, the EDL muscle was lengthened 15% using a 100 ms ramp and then held at this length for the remainder of the contraction. At the end of the contraction, the EDL muscle was returned to optimal length using a 100 ms ramp. The ramp speeds were selected from previous studies which have reported that the natural rate of elongation of dorsiflexors in the in vivo contraction model occur over a 100 ms duration (Burry et al. 2007). Similar to the in vivo model, each contraction was followed by a 10 s rest period during which time the muscle was maintained at Lo. After the sixth repetition of contractions, there was an additional 50 s of rest period. This pattern of stimulation was repeated for a total of 10 sets of six repetitions, resulting in 60 contractions over a 22 min period. Upon completion of the bout of eccentric contractions, the muscles were maintained at Lo for an additional 0–60 min and then collected for biochemical analysis. Signals from the force transducer/dynamometer and electrical stimulator were controlled and monitored with DMC/DMA software (Aurora Scientific, Aurora, ON, USA).
Cell culture
Mouse wild type C2C12 myoblasts were cultured in growth media consisting of high glucose DMEM (HyClone, Logan, UT, USA) supplemented with antibiotics and antimycotics (100 U ml−1 penicillin G, 100 μg ml−1 streptomycin and 0.25 μg ml−1 amphotericin) and 10% fetal bovine serum (Gibco, Grand Island, NY, USA). C2C12 myoblasts stably expressing a rapamycin-resistant mutant of mTOR (RR-mTOR) have been previously described (Hornberger et al. 2007) and were kindly provided by Dr Jie Chen (Department of Cell and Developmental Biology, University of Illinois, Urbana, IL, USA). The RR-mTOR myoblasts were maintained in growth media containing 0.2 mg ml−1 G418 (HyClone). G418 was not included in the media after the cells had been plated for stimulation experiments. For stimulation experiments, myoblasts were plated on 6-well dishes and grown to confluence. Upon confluence, the myoblasts were switched to serum-free high glucose DMEM for 18 h before being subjected to experimental treatments. All cell culture experiments were performed in a humidified 95% air, 5% CO2 incubator at 37°C.
Western blot analysis
Upon collection, skeletal muscles were immediately frozen in liquid nitrogen. Frozen muscles were homogenized with a Polytron in an ice-cold buffer containing 40 mm Tris (pH 7.5), 1 mm EDTA, 5 mm EGTA, 0.5% Triton X-100, 25 mmβ-glycerolphosphate, 25 mm NaF, 1 mm Na3VO4, 10 μg ml−1 leupeptin and 1 mm PMSF. The entire skeletal muscle homogenate was used for analysis. For cell culture experiments, myoblasts were lysed in the ice-cold buffer described above and then centrifuged at 500 g for 5 min and the supernatant was used for further analysis. Protein concentration was determined with DC protein assay kit (Bio-Rad, Hercules, CA, USA) and equivalent amounts of protein from each sample were subjected to Western blot analysis as previously described (Hornberger & Chien, 2006). Briefly, samples were dissolved in Laemmli buffer and subjected to electrophoretic separation by SDS-PAGE on 7.5% acrylamide gels. Following electrophoretic separation, proteins were transferred to a PVDF membrane, blocked with 5% powdered milk in TBST (Tris-buffered saline, 1% Tween 20) for 1 h followed by an overnight incubation at 4°C with primary antibody. After overnight incubation, the membranes were washed for 30 min in TBST and then probed with an anti-rabbit peroxidase-conjugated secondary antibody for 1 h at room temperature. Following 30 min of washing in TBST, the blots were developed on film using regular ECL or ECL plus. Once the appropriate image was captured, the membranes were stained with Coomassie Blue to verify equal loading in all lanes. Densitometric measurements were carried out using the public domain NIH Image program (ImageJ) developed at the US National Institutes of Health and available on the internet at http://rsb.info.nih.gov/nih-image/.
Analysis of phosphatidic acid concentration ex vivo
The method for measuring the concentration of phosphatidic acid ([PA]) in skeletal muscles ex vivo has been previously described (Hornberger et al. 2006). Briefly, in this procedure muscles were pre-labelled in the organ culture system with media containing 3H arachidonic acid (1 μCi ml−1) for 2 h and then subjected to experimental treatments. Upon collection, samples were homogenized in chloroform–methanol 2 : 1 (v/v) with a polytron, and lipids extracted according to Folch et al. (1957). A PA standard (10 μg) was added to the extracted lipids, and aliquots were used for the measurement of radioactivity in the total lipids or spotted on LK5D silica gel plates for separation of PA by thin layer chromatography (TLC). The plates were developed with a solvent system consisting of ethyl acetate–isooctane–acetic acid–water 13 : 2 : 3 : 10 (v/v). The PA spots were visualized by iodine staining and the spots containing the 3H-labelled PA were scraped off the TLC plate. The amount of radioactivity in the PA spots was measured by liquid scintillation spectrometry. Final calculations for [PA] were made by dividing the amount of radioactivity in the PA spot by the amount of radioactivity in the total lipid extract.
Analysis of phosphatidic acid concentration in vivo
Measurements of total PA concentration in the rat TA muscle and soleus muscle were performed with a modification of the procedure described by Cleland et al. (1989). Briefly, frozen muscles were crushed with a liquid nitrogen chilled mortar and pestle. A 160 mg portion of the sample was homogenized with a polytron in 1.2 ml of chloroform–methanol 1 : 2 (v/v). The lipids were extracted by adding 0.4 ml chloroform and 0.4 ml dH2O followed by 15 s of vortexing. Samples were centrifuged at 2000 g for 5 min and the lower organic phase was collected. The extraction procedure was repeated three times on the remaining aqueous phase to ensure maximal recovery. The organic phases were pooled and dried under nitrogen gas. The samples were resuspended with 100 μl chloroform–methanol 95 : 5 (v/v) and subjected to TLC for separation of PA as described above. A serial dilution of PA standard (0.5–5.0 μg) was also run on the TLC plate. The developed plate was stained (30% methanol, 0.03% (w/v) Coomassie Blue R250 and 100 mm NaCl) for 2 h, followed by 5 min of destaining (30% methanol and 100 mm NaCl). The plates were dried overnight at room temperature and then analysed by transmission densitometry. The concentration of PA was determined by comparing the density of PA in the samples to the density of PA in the standards.
Statistical analysis
All values are expressed as means ±s.e.m. Statistical significance was determined by using ANOVA, followed by Student–Newman–Keuls post hoc analysis. Differences between groups were considered significant if P≤ 0.05. All statistical analyses were performed on SigmaStat software (San Jose, CA, USA).
Results
Temporal response of various signalling events following eccentric contractions
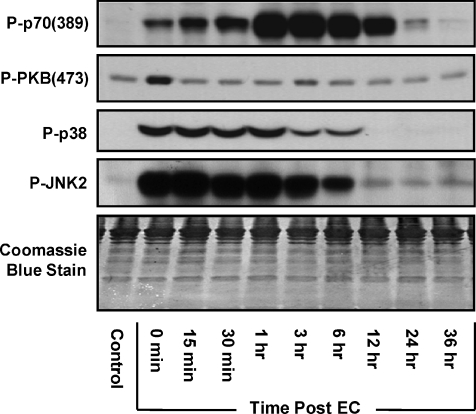
Markers for signalling through mTOR [P-p70(389)], PI3K [P-PKB(473)], p38 [P-p38] and JNK2 [P-JNK2] were evaluated at several time points in the TA muscle following a bout of eccentric contractions (EC) in vivo (Fig. 1 and Supplemental Fig. 1). The results identified three distinct temporal patterns: (i) a progressive increase in p70(389) phosphorylation during the first hour following ECs and p70(389) phosphorylation remained elevated above control values for more than 12 h, (ii) an immediate but transient increase in PKB(473) phosphorylation that was only detectable immediately following ECs, (iii) an increase in p38 and JNK2 phosphorylation that peaked immediately following ECs and then gradually returned to control levels between 6 and 12 h. The different temporal responses in PKB(473) and p70(389) phosphorylation was of particular interest and suggest that the EC-induced activation of signalling through PI3K and mTOR may occur through distinct upstream regulatory mechanisms.
Figure 1. Temporal response of signalling through various protein kinases following eccentric contractions in vivo.
Mouse tibialis anterior (TA) muscles of the right leg were subjected to a bout of eccentric contractions (EC) in vivo via stimulation of the sciatic nerve and the contralateral left TA muscle served as a control. A, muscles were collected at various time points following the bout of eccentric contractions (0 min–36 h) and subjected to Western blot analysis for markers of signalling through mTOR [P-p70(389)], PI3K [P-PKB(473)], p38 [P-p38] and JNK2 [P-JNK2]. The images are representative Western blots and a Coomassie Blue-stained membrane which verifies equal loading of proteins (n= 4–7 per group). Quantitative analyses of the Western blots from this figure are presented in Supplemental Fig. 1.
The role of PI3K and PLD–PA in the activation of mTOR by eccentric contractions
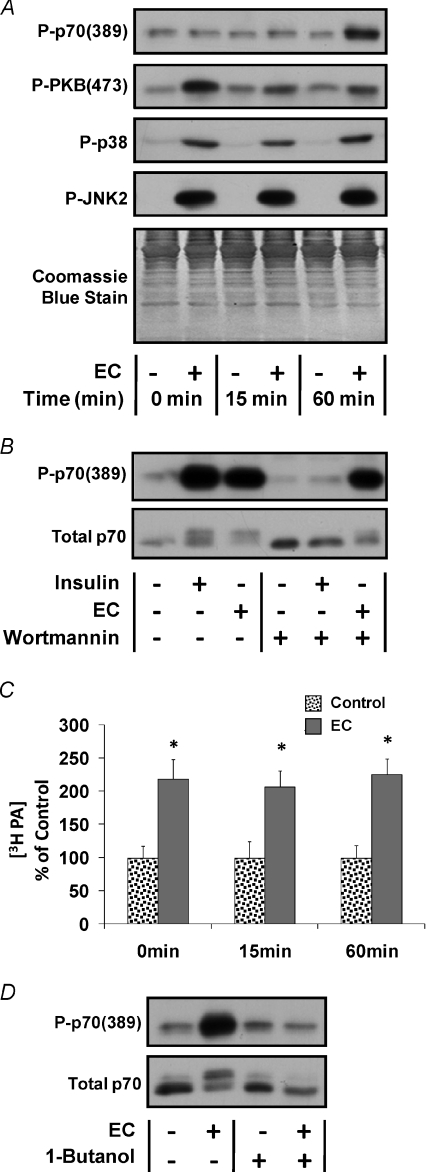
The different temporal response of signalling through PI3K and mTOR suggested that PI3K is not involved in the activation of mTOR following ECs. To further test this possibility we attempted to employ the well characterized PI3K inhibitor, Wortmannin, in our in vivo EC model (Crabbe et al. 2007). However, previous reports have demonstrated that Wortmannin is moderately unstable in aqueous solutions and therefore is not generally suitable for in vivo applications (Holleran et al. 2003; Yuan et al. 2007). Consistent with these reports, we were unable to successfully inhibit signalling by PI3K with Wortmannin in vivo. To overcome this limitation, we developed an ex vivo EC model to mimic the in vivo EC model (see Methods for details). To begin characterizing this model, the temporal response of markers for signalling through mTOR, PI3K, p38 and JNK2 were evaluated during the first hour following ECs (Fig. 2A and Supplemental Fig. 2A–D). The results indicated that, in general, the activation of signalling through these markers followed a similar temporal pattern to that observed following ECs in vivo.
Figure 2. The role of PI3K and PLD–PA in the activation of mTOR signalling by eccentric contractions ex vivo.
Mouse extensor digitorum longus (EDL) muscles were subjected to a bout of eccentric contractions (EC+) in an ex vivo organ culture system or held static at resting length as a control condition (EC−). A, EDL muscles were collected at various time points following the bout of eccentric contractions (0–60 min) and subjected to Western blot analysis for markers of signalling through mTOR [P-p70(389)], PI3K [P-PKB(473)], p38 [P-p38] and JNK2 [P-JNK2]. Western blot membranes were also stained with Coomassie Blue to verify equal loading of proteins in all lanes. B, EDL muscles were incubated in media containing 500 nm Wortmannin or the solvent vehicle (DMSO) for 30 min and then subjected to a bout of eccentric contractions or the control condition. Muscles were maintained in media containing 500 nm Wortmannin or the solvent vehicle for an additional 60 min after the bout of eccentric contractions. Where indicated, control muscles were stimulated with 100 nm insulin during the final 10 min of this 60 min incubation period. Samples were subjected to Western blot analysis for P-p70(389) and total p70. C, EDL muscles were pre-labelled with 3H arachidonic acid for 2 h and then subjected to a bout of eccentric contractions. Muscles were collected at various time points (0–60 min) after stimulation and the concentration of 3H-labelled phosphatidic acid (3H-PA) was determined. The 3H-PA concentration is expressed as a percentage of values obtained in time-matched control muscles. D, EDL muscles were incubated with control media for 25 min and then switched to fresh control media or media containing 1.0%l-butanol for 5 min before being subjected to a bout of eccentric contractions. Muscles were maintained in control media or media containing 1.0%l-butanol for an additional 60 min after stimulation and then subjected to Western blot analysis for P-p70(389) and total p70. All values are presented as the mean ±s.e.m. for each group (n= 3–6 per group). *Significantly different from the time-matched control (P≤ 0.05). Quantitative analyses of the Western blots from A, B and D are presented in Supplemental Fig. 2.
The ex vivo model was then used to determine if signalling through PI3K was necessary for the EC-induced activation of mTOR. To accomplish this, muscles were incubated with media containing Wortmannin or the solvent vehicle and then stimulated with ECs, or insulin as a positive control for signalling through PI3K. The results demonstrated that Wortmannin completely blocked the insulin-induced increase in p70(389) phosphorylation, thus verifying that Wortmannin inhibited signalling through PI3K. However, Wortmannin did not prevent the EC-induced increase in p70(389) phosphorylation (Fig. 2B and Supplemental Fig. 2E). Taken together, these results indicate that ECs activate mTOR through a PI3K-independent mechanism.
Similar to ECs, we have previously determined that passive intermittent stretch activates mTOR signalling through a PI3K-independent mechanism (Hornberger et al. 2006). Furthermore, we have determined that passive stretch utilizes a mechanism involving phospholipase D (PLD)-mediated synthesis of phosphatidic acid (PA) (Hornberger et al. 2006, 2007). To determine if a similar mechanism was involved in the EC-induced activation of mTOR, we first measured the [PA] in muscles following ECs ex vivo. The results indicated that ECs promoted a sustained increase in [PA] (Fig. 2C). Furthermore, inhibiting the synthesis of PA by PLD with l-butanol blocked the EC-induced increase in p70(389) phosphorylation (Fig. 2D and Supplemental Fig. 2F). Combined, these observations indicate that the synthesis of PA by PLD is necessary for the EC-induced activation of mTOR signalling.
Phosphatidic acid induces mTOR signalling via a PI3K–PKB-independent mechanism
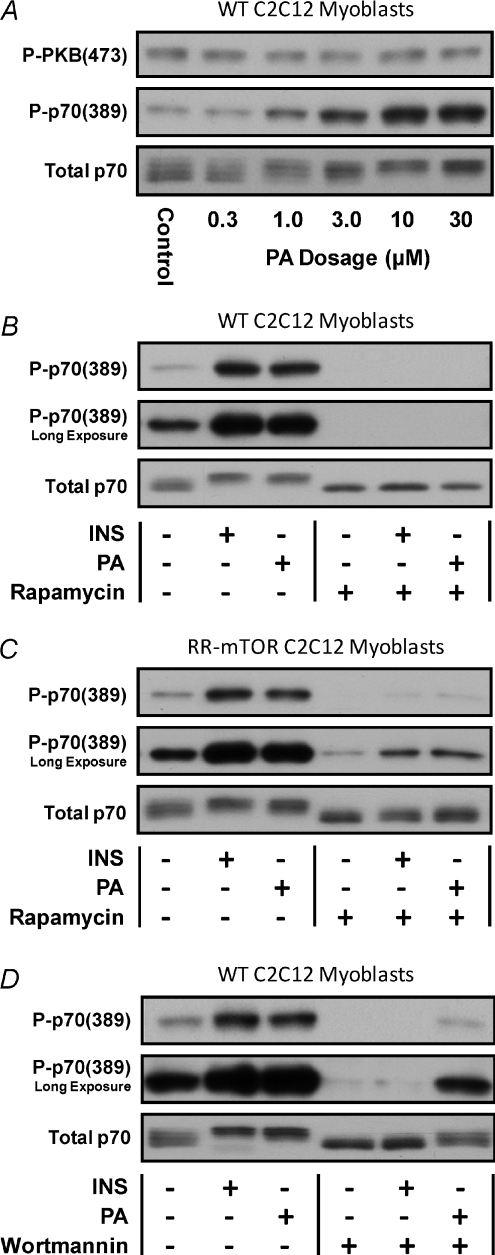
The results from the ex vivo EC model implicated PA as part of the PI3K-independent mechanism through which ECs activate mTOR. To further address this possibility we used a C2C12 myoblast culture system to determine if exogenous PA could induce mTOR signalling, and whether this would occur through a PI3K-independent mechanism. A cell culture system was deemed necessary for these experiments because previous reports have shown that exogenous PA has a very limited degree of cellular permeability (Schatter et al. 2003). Thus, sufficient penetration of exogenous PA into the depths of an intact muscle represents a limiting condition. Our results with the C2C12 myoblast culture system indicated that the addition of exogenous PA was sufficient to promote an increase in p70(389) phosphorylation, but not PKB(473) phosphorylation (Fig. 3A and Supplemental Fig. 3A–B). Furthermore, rapamycin completely blocked the PA-induced increase in p70(389) phosphorylation (Fig. 3B and Supplemental Fig. 3C). Combined, these results indicate that PA is sufficient for the induction of p70(389) phosphorylation and that this occurs through a rapamycin-sensitive mechanism.
Figure 3. Phosphatidic acid induces mTOR signalling via a PI3K–PKB-independent mechanism.
A, wild type (WT) C2C12 myoblasts were serum starved for 18 h and then stimulated with 0.3–30 μm phosphatidic acid (PA) or the solvent vehicle (PBS) for 20 min. B and C, WT C2C12 myoblasts (B) or C2C12 myoblasts stably expressing a rapamycin-resistant mutant of mTOR (RR-mTOR) (C) were serum starved for 18 h and then incubated with 50 nm rapamycin or the solvent vehicle (DMSO) for a total of 50 min. The myoblasts were stimulated with 10 μm PA during the final 20 min, or with 100 nm insulin during the final 10 min of the incubation period. D, WT C2C12 myoblasts were serum starved for 18 h and then incubated with 500 nm Wortmannin or the solvent vehicle (DMSO) for a total of 35 min. The myoblasts were stimulated with 10 μm PA during the final 20 min, or with 100 nm insulin during the final 10 min of the incubation period. Samples were subjected to Western blot analysis for markers of signalling through PI3K [P-PKB(473)], mTOR [P-p70(389)] and total p70. All images are representative of at least three independent experiments (n= 3–8 per group). Quantitative analyses of the Western blots from this figure are presented in Supplemental Fig. 3.
To determine if mTOR was the rapamycin-sensitive element that conferred the PA-induced increase in p70(389) phosphorylation, we employed C2C12 myoblasts that stably express a rapamycin-resistant mutant of mTOR (RR-mTOR) (Erbay & Chen, 2001). As shown in Fig. 3C and Supplemental Fig. 3D, expression of RR-mTOR rescued both insulin- and PA-induced signalling to p70(389) from the inhibitory effects of rapamycin. It should be noted that the RR-mTOR cells express both endogenous (wild-type) mTOR and RR-mTOR, and the expression levels of RR-mTOR is relatively low (∼50% of endogenous mTOR – data not shown). Thus, the reduction in the basal level of signalling to p70(389) in the presence of rapamycin was expected. Furthermore, when the data from the rapamycin-treated cells were normalized for the reduced basal level of p70(389) phosphorylation, the results indicated that the magnitude of insulin- and PA-induced signalling to p70(389) was similar to that observed in the vehicle-treated cells (Supplemental Fig. 3D). These results verified that mTOR is the rapamycin-sensitive element that confers both insulin- and PA-induced signalling to p70(389), and therefore demonstrate that PA is sufficient for the activation of mTOR signalling.
To determine if PI3K was necessary for the activation of mTOR signalling by PA, C2C12 myoblasts were incubated with media containing Wortmannin or the solvent vehicle and then stimulated with PA or insulin as a positive control for signalling through PI3K. The results demonstrated that Wortmannin completely blocked the insulin-induced increase in p70(389) phosphorylation, thus verifying that Wortmannin inhibited signalling through PI3K. However, Wortmannin did not prevent the PA-induced increase in p70(389) phosphorylation (Fig. 3D and Supplemental Fig. 3E). Combined, the results from this series of experiments demonstrate that similar to ECs, PA activates mTOR signalling through a PI3K-independent mechanism.
Elevations in phosphatidic acid and the activation of mTOR signalling occur specifically in response to contractions that induce growth
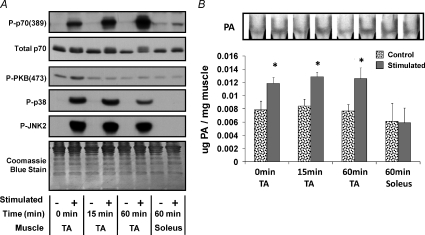
The results from the experiments described above led us to hypothesize that an increase in [PA] is responsible for the activation of mTOR, and potentially growth, following ECs. Based on this hypothesis, we also predicted that an increase in [PA] and mTOR signalling would occur specifically in response to the types of contractions that induce growth. To test this prediction, we stimulated the TA muscles from rats to ECs and the soleus muscles to concentric contractions in vivo. It is important to note that the rat model we employed to induce these contractions has previously been shown to induce growth of the TA muscle, but not the soleus muscle (Baar & Esser, 1999).
Consistent with the observations that we made in the mouse TA muscle following ECs (Fig. 1), stimulating the rat TA muscle with ECs promoted robust signalling through markers of mTOR, p38 and JNK2 (Fig. 4A). Of particular significance, signalling through mTOR was highly activated at 1 h after stimulation in the TA muscle; however, stimulation of the soleus muscle did not promote a significant activation of mTOR signalling (Fig. 4A). Furthermore, stimulating the TA muscle with ECs promoted an increase in [PA], but this effect was not detected following stimulation of the soleus muscle (Fig. 4B). Taken together, these results suggest that an increase in [PA] and the activation of mTOR signalling is an event that occurs specifically in response to contractions that induce growth.
Figure 4. Elevations in phosphatidic acid and the activation of mTOR signalling occur specifically in response to contractions that induce growth in vivo.
Rat lower hind-limb muscles of the right leg were subjected to a bout of contractions via stimulation of the sciatic nerve and the muscles from the contralateral limb were used as time-matched controls. Tibialis anterior (TA) and soleus muscles were collected at various time points following the bout of contractions (0–60 min). A, TA and soleus muscles were subjected to Western blot analysis for markers of signalling through mTOR [P-p70(389)], PI3K [P-PKB(473)], p38 [P-p38] and JNK2 [P-JNK2]. Western blots were also stained with Coomassie Blue to verify equal loading of proteins. B, TA and soleus muscles were subjected to measurements of total phosphatidic acid (PA) concentration by thin layer chromatography (TLC). The inset in B is a representative image of phosphatidic acid detected on a Coomassie Blue-stained TLC plate and samples were loaded in the order indicated by the graph below. The graph represents the mean ±s.e.m. for each group expressed as a percentage of the time-matched control group. *Significantly different from control (P≤ 0.05), (n= 3–5 per group). Quantitative analyses of the Western blots from this figure are presented in Supplemental Fig. 4.
Discussion
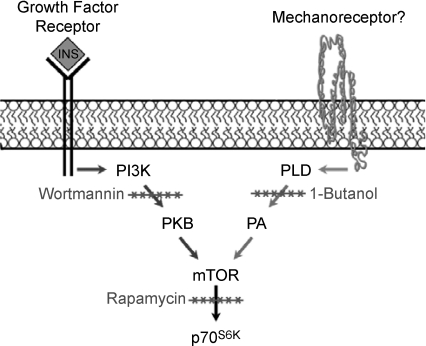
Signalling by mTOR has been shown to be necessary for resistance exercise-induced changes in protein synthesis and the concomitant hypertrophic response (Bodine et al. 2001; Hornberger et al. 2003; Kubica et al. 2005). As mentioned in the introduction, it has been widely proposed, and generally accepted, that resistance exercise contractions, such as ECs, activate mTOR and a concomitant hypertrophic response via an upstream pathway involving PI3K and PKB (PI3K–PKB → mTOR → Growth) (Adams, 2002; Nader, 2005; Baar et al. 2006; Frost & Lang, 2007). However, the results from this study demonstrate that the activation of mTOR following ECs is elicited through a PI3K–PKB-independent mechanism (Fig. 2). Furthermore, the results demonstrate that ECs only induce a very transient (<15 min) activation of PI3K–PKB (Fig. 1). Although this finding cannot exclude a role for PI3K–PKB in the EC-induced hypertrophic response, it seems unlikely that the transient activation of this pathway is sufficient to induce hypertrophy. On the contrary, ECs induced a prolonged activation of mTOR (>12 h) and the synthesis of PA by PLD was determined to be necessary for mTOR activation (Figs 1 and 2). Similar to ECs, the results from this study also demonstrate that PA can activate mTOR through a PI3K–PKB-independent mechanism (Fig. 3). Finally, it was determined that an increase in [PA] and activation of mTOR does not occur in response to all types of contractions, but instead appears to be specific to growth promoting contractions such as ECs (Fig. 4). Based on these findings, we propose that PLD and PA play a central role in the activation of mTOR and the concomitant hypertrophic response that is induced by ECs and other types of growth promoting contractions that occur during resistance exercise. A schematic which summarizes the findings from this study is provided in Fig. 5.
Figure 5. Schematic of the proposed signalling mechanisms involved in the activation of mTOR by growth factors and eccentric contraction.
Growth factors such as insulin activate mTOR through a PI3K-dependent mechanism. Eccentric contractions activate mTOR through a PI3K-independent mechanism involving PLD and PA. Eccentric contractions also activate signalling through p38 and JNK. Activation of signalling through mTOR, p38 and JNK might be elicited through a common mechanotransduction mechanism, but the activation of signalling through p38 and JNK does not appear to be involved in the activation of mTOR.
The proposition stated above raises the question of whether an increase in [PA] and the subsequent activation of mTOR is sufficient for the induction of hypertrophy. In support of this possibility, several studies have suggested that the activation of mTOR is sufficient for the induction of hypertrophy. For example, constitutive activation of PI3K and PKB induces mTOR signalling and hypertrophy, and signalling through mTOR is necessary for the hypertrophic response (Bodine et al. 2001; Pallafacchina et al. 2002; Lai et al. 2004). However, to date, no studies have determined whether activation of mTOR, independent of PI3K–PKB, is sufficient for the induction of skeletal muscle hypertrophy. Therefore, it remains possible that signalling through mTOR simply plays a permissive role in the PI3K–PKB-induced hypertrophic response. This is a particularly important point when considering reports which have shown that the activation of PI3K–PKB not only induces mTOR signalling, but also activates several other growth regulatory pathways including signalling through the forkhead box O class of transcription factors and glycogen synthase kinase 3 (Glass, 2005; Nader, 2005). Thus, an important area for future studies will be to determine whether the activation of mTOR, independent of PI3K–PKB, is sufficient for the induction of skeletal muscle hypertrophy.
Based on the findings from this study, another important area for future investigations will be to define how ECs promote the increase in [PA]. Our previous work using a passive intermittent stretch model has potentially shed some light on this mechanism and indicates that PLD activity is necessary for the stretch-induced increase in [PA] (Hornberger et al. 2006). However, this study also indicated that an increase in PLD activity alone was not sufficient for the increase in [PA]. Specifically, both [PA] and PLD activity were found to be elevated during the first 15 min of stretch. After this time point, PLD activity returned to basal levels but the [PA] continued to increase. This observation suggests that PLD may contribute to the increase in [PA] but additional PA regulatory mechanisms are also likely to be involved. These mechanisms could include activation of the enzymes which synthesize PA including the lysophosphatidic acid acyltransferases which convert lysophosphatidic acid (LPA) into PA, and the diacylglycerol kinases which convert diacylglycerol (DAG) into PA (Wang et al. 2006; Foster, 2007). Furthermore, inhibition of the enzymes that degrade PA including the A type phospholipases which convert PA into LPA, and the phosphatidic acid phosphatases which convert PA into DAG, could also be an important part of the mechanism through which ECs promote an increase in [PA] (Carman & Han, 2006; Wang et al. 2006; Aoki et al. 2007).
Another interesting observation that was made in this study is that, similar to mTOR, the activation of signalling through p38 and JNK did not occur in response to all types of contractions, but instead appeared to be specific to growth-promoting contractions such as ECs (Fig. 4). Furthermore, like mTOR, ECs induced a prolonged activation (>6 h) of signalling through p38 and JNK (Fig. 1). These similarities suggest that a common mechanotransduction mechanism may be involved in the activation of signalling through mTOR, p38 and JNK. However, if a common mechanism exists, it appears that this mechanism lies upstream of PLD. This argument is based on results from our previous studies which demonstrated that inhibition of PLD blocked the stretch-induced activation of mTOR, but not p38 or JNK (Hornberger et al. 2006). Thus, PLD activity does not appear to be required for the stretch-induced activation of signalling through p38 and JNK. Furthermore, this observation indicates that the activation of signalling through p38 and JNK is not sufficient for the stretch-induced activation of mTOR. Based on these observations, a role for p38 and JNK in the EC-induced activation of mTOR seems unlikely; however, the results from the current study cannot fully exclude a potential role for these molecules.
In summary, the results from this study indicate that resistance exercise contractions, such as ECs, activate mTOR through a PI3K–PKB-independent mechanism that involves PLD and PA. Since signalling through mTOR is required for resistance exercise-induced hypertrophy, these findings are likely to significantly advance our understanding of how the mechanical signals from resistance exercise are converted into the molecular events that induce skeletal muscle growth and remodelling.
Acknowledgments
This work was supported by a National Institutes of Health grant AR053280 to T.A.H. Special thanks are extended to Jie Chen for providing the C2C12 cell lines stably expressing RR-mTOR.
Glossary
Abbreviations
- DAG
diacylglycerol
- EC
eccentric contractions
- IGF-1
insulin-like growth factor 1
- JNK
c-jun n-terminal kinase
- LPA
lysophosphatidic acid
- mTOR
mammalian target of rapamycin
- p38
p38 mitogen-activated protein kinase
- p70S6k
ribosomal S6 kinase 1
- PA
phosphatidic acid
- PI3K
phosphoinositide 3-kinase
- PKB
protein kinase B
- PLD
phospholipase D
- TLC
thin layer chromatography
Author contributions
Tyriina K. O’Neil, analysis and interpretation of data and writing; Lilly R. Duffy, analysis and interpretation of data and writing; John W. Frey, conception, design and writing; Troy A. Hornberger, conception, design, analysis and interpretation of data and writing.
Supplemental material
References
- Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev. 2006;5:310–331. doi: 10.1016/j.arr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 2007;89:197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- Bodine SC. mTOR signalling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38:1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Burry M, Hawkins D, Spangenburg EE. Lengthening contractions differentially affect p70s6k phosphorylation compared to isometric contractions in rat skeletal muscle. Eur J Appl Physiol. 2007;100:409–415. doi: 10.1007/s00421-007-0444-5. [DOI] [PubMed] [Google Scholar]
- Carlson CJ, Fan Z, Gordon SE, Booth FW. Time course of the MAPK and PI3-kinase response within 24 h of skeletal muscle overload. J Appl Physiol. 2001;91:2079–2087. doi: 10.1152/jappl.2001.91.5.2079. [DOI] [PubMed] [Google Scholar]
- Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31:694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem. 1989;264:17704–17711. [PubMed] [Google Scholar]
- Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37:737–763. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- Crabbe T, Welham MJ, Ward SG. The PI3K inhibitor arsenal: choose your weapon! Trends Biochem Sci. 2007;32:450–456. doi: 10.1016/j.tibs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay E, Chen J. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem. 2001;276:36079–36082. doi: 10.1074/jbc.C100406200. [DOI] [PubMed] [Google Scholar]
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signalling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signalling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signalling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Holleran JL, Egorin MJ, Zuhowski EG, Parise RA, Musser SM, Pan SS. Use of high-performance liquid chromatography to characterize the rapid decomposition of wortmannin in tissue culture media. Anal Biochem. 2003;323:19–25. doi: 10.1016/j.ab.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signalling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;97:1207–1216. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signalling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Esser KA. Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc Nutr Soc. 2004;63:331–335. doi: 10.1079/PNS2004357. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, McLoughlin TJ, Leszczynski JK, Armstrong DD, Jameson RR, Bowen PE, Hwang ES, Hou H, Moustafa ME, Carlson BA, Hatfield DL, Diamond AM, Esser KA. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr. 2003;133:3091–3097. doi: 10.1093/jn/133.10.3091. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Sukhija KB, Wang XR, Chien S. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr(389) in p70(S6k) FEBS Lett. 2007;581:4562–4566. doi: 10.1016/j.febslet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bɛ mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280:7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- Nader GA. Molecular determinants of skeletal muscle mass: getting the ‘AKT’ together. Int J Biochem Cell Biol. 2005;37:1985–1996. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fibre type specification. Proc Natl Acad Sci U S A. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM. Resistance exercise: good for more than just Grandma and Grandpa's muscles. Appl Physiol Nutr Metab. 2007;32:1198–1205. doi: 10.1139/H07-129. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI3K/Akt/mTOR and PI3K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Schatter B, Walev I, Klein J. Mitogenic effects of phospholipase D and phosphatidic acid in transiently permeabilized astrocytes: effects of ethanol. J Neurochem. 2003;87:95–100. doi: 10.1046/j.1471-4159.2003.01971.x. [DOI] [PubMed] [Google Scholar]
- Seguin R, Nelson ME. The benefits of strength training for older adults. Am J Prev Med. 2003;25:141–149. doi: 10.1016/s0749-3797(03)00177-6. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenburg EE, McBride TA. Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70S6K activation. J Appl Physiol. 2006;100:129–135. doi: 10.1152/japplphysiol.00619.2005. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signalling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol. 2008;104:625–632. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Wolfe RR. Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab. 2001;11:109–132. doi: 10.1123/ijsnem.11.1.109. [DOI] [PubMed] [Google Scholar]
- Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene. 2008;27:585–595. doi: 10.1038/sj.onc.1210693. [DOI] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R. Signalling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Yuan H, Barnes KR, Weissleder R, Cantley L, Josephson L. Covalent reactions of wortmannin under physiological conditions. Chem Biol. 2007;14:321–328. doi: 10.1016/j.chembiol.2007.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.