Abstract
Striated muscle exhibits a pronounced structural–functional plasticity in response to chronic alterations in loading. We assessed the implication of focal adhesion kinase (FAK) signalling in mechano-regulated differentiation of slow-oxidative muscle. Load-dependent consequences of FAK signal modulation were identified using a multi-level approach after electrotransfer of rat soleus muscle with FAK-expression plasmid vs. empty plasmid-transfected contralateral controls. Muscle fibre-targeted over-expression of FAK in anti-gravitational muscle for 9 days up-regulated transcript levels of gene ontologies underpinning mitochondrial metabolism and contraction in the transfected belly portion. Concomitantly, mRNA expression of the major fast-type myosin heavy chain (MHC) isoform, MHC2A, was reduced. The promotion of the slow-oxidative expression programme by FAK was abolished after co-expression of the FAK inhibitor FAK-related non-kinase (FRNK). Elevated protein content of MHC1 (+9%) and proteins of mitochondrial respiration (+165–610%) with FAK overexpression demonstrated the translation of transcript differentiation in targeted muscle fibres towards a slow-oxidative muscle phenotype. Coincidentally MHC2A protein was reduced by 50% due to protection of muscle from de-differentiation with electrotransfer. Fibre cross section in FAK-transfected muscle was elevated by 6%. The FAK-modulated muscle transcriptome was load-dependent and regulated in correspondence to tyrosine 397 phosphorylation of FAK. In the context of overload, the FAK-induced gene expression became manifest at the level of contraction by a slow transformation and the re-establishment of normal muscle force from the lowered levels with transfection. These results highlight the analytic power of a systematic somatic transgene approach by mapping a role of FAK in the dominant mechano-regulation of muscular motor performance via control of gene expression.
Striated muscle exhibits a pronounced phenotypic plasticity in response to work-related stimuli (Loughna et al. 1990; Pette & Staron, 1990; Booth & Thomason, 1991; Fluck & Hoppeler, 2003). This malleability is exemplified by specific adjustments of muscle force versus fatigue resistance subsequent to strength as opposed to endurance training. Muscle conditioning by functional demand is dramatically visualized in the prolonged reductions in weight-bearing during bed-rest, when the force and metabolic capacity in anti-gravitational muscles are reduced (Desplanches et al. 1987; Fluck & Hoppeler, 2003). These deteriorations are reversible and the muscles recover with subsequent elevations in muscle loading and activity-induced energy consumption (Desplanches et al. 1987).
Muscle plasticity has its foundation in the capacity of the individual muscle cells, the muscle fibres, to remodel their contractile and metabolic makeup in response to neuronal, mechanical, metabolic and hormonal stimuli (Loughna et al. 1990; Pette & Staron 1990; Booth & Thomason, 1991; Fluck & Hoppeler, 2003; Dapp et al. 2006). Accordingly, the transformation of muscle fibres underlies the shift from a slow-oxidative to an atrophic, fast-glycolytic phenotype with unloading, and its reversion upon muscle loading (Desplanches et al. 1987; Fluck & Hoppeler, 2003).
Distinct signalling pathways have been implied in the control of muscle form and function (Chin et al. 1998; Pallafacchina et al. 2002; Wu et al. 2002; Puigserver & Spiegelman, 2003; Koulmann & Bigard, 2006; Sandri, 2008). The consensus is that AKT–mTOR–S6K signalling and calcium/calmodulin signalling separately integrate the hormone- and nerve-dependent control of muscle size and contractile proteins, respectively. In regards to metabolic perturbations, signalling pathways connecting the transcriptional regulators peroxisome proliferator-activated receptor γ coactivator (PGC)-1α and hypoxia-inducible factor (HIF)-1α appear to be central for the molecular regulation of muscle metabolism via the control of gene regulation (Dapp et al. 2006; Koulmann & Bigard 2006; Sandri, 2008). Currently, there is a distinct lack of understanding about the implication of signalling mechanisms in the conditioning of muscle structure and function by mechanical stimuli. Concerted control of gene ontology (GO) expression, which instructs the contractile and metabolic adjustments of muscle function with unloading and reloading (Stevenson et al. 2003; Fluck et al. 2005), implies the existence of a load-dependent master pathway. The upstream elements of this mechano-sensory pathway for muscle transcript differentiation, and the downstream consequences for contractile and metabolic protein expression and muscle function, are not known.
Sarcolemmal focal adhesion complexes (costameres) are key elements for the transmission of contraction force from muscle fibres to tendons and adjacent fibres (reviewed in Huijing 1999; Fluck et al. 2002; Bloch & Gonzalez-Serratos, 2003; Samarel, 2005; Quach & Rando, 2006; Durieux et al. 2007) and constitute potential sites for the conversion of contraction forces into mechano-sensitive signalling within the myocellular compartment (Huijing 1999; Bloch & Gonzalez-Serratos, 2003; Fonseca et al. 2005; Samarel, 2005; Quach & Rando, 2006). This idea is supported by the load-dependent post-translational regulation of the associated protein tyrosine kinase, focal adhesion kinase (FAK). Tyrosine phosphorylation of integrin-bound FAK at residue 397 (pY397) reflects the mechano-chemical coupling between mechanical stimulation of integrins and activation of intracellular signal transduction (Shyy & Chien, 1997; Parsons, 2003). Sarcolemmal FAK concentration corresponds to the degree of muscle fibre recruitment for contraction (Fluck et al. 2002). It is particularly high in slow-oxidative muscle (Gordon et al. 2001), suggesting an increasing biological relevance of FAK for mechano-regulation in this muscle type and the assembling muscle fibres. The activation of FAK is known to promote the growth and differentiation of cardiac and skeletal muscle cells in culture via a mechanism involving the translocation of FAK to costameres (reviewed in (Sastry et al. 1999; Pham et al. 2000; Kovacic-Milivojevic et al. 2001; Fonseca et al. 2005; Quach & Rando, 2006). The functional implication of focal adhesion signalling for the mechano-regulation of skeletal muscle function with regard to gene expression has not been addressed experimentally (Bloch & Gonzalez-Serratos, 2003; Durieux et al. 2007).
We hypothesized that myocellular FAK is a load-dependent switch controlling the expression programme underlying the structural–functional differentiation of the contractile machinery and energy metabolism in slow-oxidative muscle. To address this research question we deployed gene electrotransfer because this technology allows the overexpression of native molecules in striated muscle (Durieux et al. 2002) under the inclusion of inter-animal specificity controls. We monitored muscle transcript expression, structure and function after somatic overexpression of a FAK homologue and its competitor, FAK-related non-kinase (FRNK), in anti-gravitational muscle, which has a physiologically altered loading state (Gordon et al. 2001; Fluck et al. 2005). The effect of FAK overexpression was evaluated from the paired comparison to empty-transfected muscles in contralateral limbs. The inference of somatic gene transfer was assessed vs. non-transfected muscles of matched controls for the investigated loading conditions.
Methods
Ethical approval
The experiments were performed at the Universities of Berne (Switzerland), Lyon (France), and Pavia (Italy) with the permission of the local Animal Care Committee of the Canton of Berne (Switzerland) and following the recommendations provided by the European Convention for the protection of Vertebrate Animals used for Experimental and Scientific purposes (Strasbourg, 18.III.1986).
Experimental design
Three-month-old female pathogen-free Wistar rats (Charles River Laboratory, L’Arbresles, France) were used for this study. Focal adhesion signalling in soleus muscle was manipulated by overexpression of a FAK homologue via gene electrotransfer of expression constructs and by different loading protocols: hindlimb unloading–reloading or tenotomy (Fig. 1). A paired design was adopted to allow for intra-animal comparisons of the effects of FAK signal modulation on muscle and the interaction with muscle loading. Equal amounts of FAK construct pCMV-FAK and empty control plasmid pCMV were electro-transferred into the right and left soleus muscle, respectively. Another experimental group co-expressed FAK and FRNK in the left soleus muscle whereas contralateral muscles were double-transfected with FAK construct pCMV-FAK and empty pCMV plasmid. At least six biological replicas were analysed per experimental condition. In addition, the data were compared to published results on the effect of a hindlimb unloading–reloading protocol on non-transfected soleus muscle (Fluck et al. 2005).
Figure 1. Experimental design.
Sketch depicting the comparisons used to test the interaction effect of FAK overexpression (A) and loading (B) on molecular, cellular and functional variables of rat soleus muscle. A, drawings visualizing the approach to quantify the effect of FAK overexpression via (a) intra-animal gene transfer with pCMV-FAK and empty plasmid pCMV in contralateral (CTL-CTL) soleus muscles, (b) intra-animal competition experiments deploying co-transfection of FAK construct with empty plasmid pCMV or pCMV-FRNK construct (encoding the FAK inhibitor FRNK) in contralateral muscles, and (c) inter-animal assessment of the effect of transfection with empty plasmid vs. non-transfected muscle. B, summary of the loading protocols imposed on non-transfected and transfected muscles and time-points of sampling. The procedures included unloading-reloading and overload via bilateral tenotomy and normal cage activity. The number of biological replicas per transfection and loading protocol is indicated. The specific analyses being performed are indicated in abbreviation and detailed in Methods: T, transcript profiling; P, protein biochemistry; I, immunostaining; Q, quantitative confocal microscopy; M, myography.
Plasmid construction
Cytomegalovirus (CMV) promoter-driven plasmids for the constitutive overexpression of chicken FAK (pCMV-FAK) and FRNK homologues (pCMV-FRNK) were a gift from Tony Parsons (University of Virginia, Charlottesville, USA). The amino acid sequences are highly conserved between the chicken and rat FAK homologues (92%) with all major regulatory sites present. Empty pCMV plasmid was constructed by the excision of the DNA insert from pCMV-FRNK via BamHI restriction digestion and ligation. Plasmids were sent to plasmidfactory (Bielefeld, Germany, http://www.plasmidfactory.de) for propagation and isolation of endotoxin-free DNA.
Somatic transgenesis
Gene electrotransfer was carried out as previously described (Durieux et al. 2002) and optimized for the soleus muscle. Rats were anaesthetized by intra-peritoneal injection of sodium pentobarbital (60 mg (kg body weight)−1, Sanofi, France). The depth of anaesthesia during the intervention was checked by verifying the absence of muscle reflexes to pinching the digits with fine forceps and by monitoring the respiratory rhythm. Hindlimbs were shaved and cleaned with Betadine (Viatris, France) and soleus muscle was surgically exposed by a lateral split of the connective tissue sheet between the gastrocnemius and tibialis anterior muscles. Endotoxin-free plasmid in 0.9% NaCl solution (50 μg, 70 μl) was injected in the deep and superficial region of the belly portion of the soleus muscle and three trains of 80 100 μs pulses, each at 100 mA, were delivered using needle electrodes with the GET42 generator (E.I.P. Electronique et Informatique du Pilat, Jonzieux, France). The skin and fascia were closed with sutures and the animals transferred to single cages.
Muscle loading
The unloading of the soleus muscles by 7 day hindlimb suspension was performed as previously described (Fluck et al. 2005) at the Université Lyon 1 (France) and started 2 days after transfection. Subsequent reloading was provoked by allowing the animals to return to normal cage activity for 1 or 5 days.
For the tenotomy experiments, animals were anaesthetized 2 days after gene electrotransfer by intra-peritoneal injection of sodium pentobarbital 40 mg kg−1. An incision was made with a scalpel to the superficial gastrocnemius portion of the Achilles tendon of both limbs. The site was secured with stitches and the rats were allowed to re-establish in a quiet environment while signs of pain or distress were carefully monitored. Rats recovered rapidly and started to walk 2 h after the procedure. Correct healing was checked during the following days and favoured by topical application of Vulnamin (Errekappa Euroterapici, Milan, Italy). Subsequently animals were allowed free cage activity for 8 days. Gene electrotransfer was carried out at the University of Berne under anaesthesia with 2% isoflurane (Rhodia, France). The freshly transfected animals were transported to the University of Pavia (Italy) for tenotomy. Cage control groups were analysed 7–9 days after gene electrotransfer.
At the end of the respective protocol, the rats were weighed, and the m. solei of both hindlimbs were harvested under pentobarbital anaesthesia. Killing of the anaesthetized animals was carried out by dislocation of the cervical vertebrae and rapid exsanguination. For the molecular analysis, the transfected belly portion was rapidly dissected and frozen in melting-isopentane. Muscles for the contractile measures were excised from extracted legs into oxygenated Krebs solution (95% O2, 5% CO2). For single fibre analysis, muscles were stored for up to 3 weeks at −20°C in a 1 : 1 (v/v) mixture of skinning solution (150 mm potassium propionate, 5 mm KH2PO4, 5 mm magnesium acetate, 3 mm Na2ATP, 5 mm EGTA, pCa 9.0 and glycerol including 20 μm and 10 μm of the protease inhibitors leupeptin and E-64, respectively, and fibres chemical skinned as described (Rossi et al. 2001; D’Antona et al. 2006).
Transcript profiling
RNA isolation and microarray analysis for 1185 transcripts (ATLAS TM rat 1.2 cDNA array, BD Clontech, Basel, Switzerland) was carried out basically as described (Fluck et al. 2005). In brief, total mRNA was extracted from cryosections of the transfected soleus portions of contralateral muscle pairs with the RNA mini-kit (Qiagen, Basel, Switzerland) after homogenization with a rotor-stator homogenizer (Polytron PT1200, Kinematica, Lucerne, Switzerland) and digestion with proteinase K for 1.5 h at 45°C (600 mAU ml−1; Qiagen). RNA concentration was quantified with ribogreen (Molecular Probes, Eugene, OR, USA) and equal RNA amounts (2.5 μg) were subjected to reverse-transcription under standardized conditions. The reactions were carried out at 37°C using a specific set of primers for the cDNAs spotted on the array, nucleotide mix with calibrated radio-labelled [α-32P]dATP (3000 Ci mmol−1, 10 μCi μl−1, Hartmann Analytic, Braunschweig, Germany) and initiated with the addition of 200 units of Superscript II reverse transcriptase (Invitrogen). The radiolabelled cDNA was purified by column chromatography (ChromaSpin, supplied with the ATLAS TM kit) and subjected to array filter hybridization in ExpressHyb solution overnight at 68°C. The mean labelling efficiency of target cDNAs from pCMV-FAK and pCMV-transfected samples was 4.1 ± 0.8 × 106 c.p.m. and 4.3 ± 0.3 × 106 c.p.m. per μg total RNA, respectively. Subsequently, the filters were washed and exposed for 6 days to detect cDNA signals with a Phosphorimager no. 425E running under ImageQuant v. 3.3 (Molecular Dynamics, Sunnyvale, California, USA). The signals corresponding to the individual cDNA spots were identified and quantified as the sum of pixels using the AIDA Array Easy software (Raytest Schweiz A.G., Urdorf, Switzerland). The integrated signal and local background values were estimated by the ‘local dot ring’ mode.
Six biological replicates were run separately for all conditions of transfection and loading, except for the unloading experiments where two pairs of the six samples were pooled for reverse transcription. Significantly altered transcripts were identified from raw signals with statistical analysis for microarrays (SAM) for a two class paired design (Dapp et al. 2004). False discovery rate corresponded to the automatically computed value for the selected T-statistics. Expression ratios were calculated from the mean of pCMV-FAK vs. pCMV-transfected muscle pairs. Subsequently, the enrichment of the co-directional level changes of altered transcripts within a GO, i.e. up- or down, was analysed as described (Flück et al. 2008). The grouping of transcripts into muscle-relevant GOs was based on the information available for the microarray platform and the electronic literature (http://www.expasy.org/sprot/ and http://www.ncbi.nlm.nih.gov/sites/entrez). Changes in the RNA of sarcomere proteins, MHC1, 2A and 2X were separately assessed in cage control and 1-day-reloaded muscle using the reverse-transcriptase PCR against 28S RNA (Fluck et al. 2005) and combined with the microarray data. The PCR primers were MHC1: 5′-CAGCCTACCTCATGGGACTGA-3′, 5′-TGACATACTCGTTGCCCACTTT-3′; MHC2A: 5′-AGAATGACAACTCTTCACGATTTGG-3′, 5′-GGCGGATAGCACGAGATTTC-3′; MHC2X: 5′-GGCCAGGGTCCGTGAACT-3′, 5′-GCTTCAACATTGCGCTTCTG-3′ (Microsynth, Balgach, Switzerland). P-values were visualized via Cluster and Treeview (http://rana.lbl.gov/EisenSoftware.htm) and assembled with CorelDraw X3 (Corel Corporation) and Powerpoint (Microsoft Office for Windows XP). Data series were deposited under provisional accession codes GSE12743, GSE12744, GSE12745, GSE12746 and GSE12747 at Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo).
Protein biochemistry
Sample preparation in RIPA buffer, protein detection, and quantification by immunoblotting were carried out as previously described (Fluck et al. 1999, 2002). Equal loading (25 μg total protein) per lane was verified with Ponceau S staining of the nitrocellulose blot before immunodetection. The FAK-pY397-specific antibody was from BioSource International and the C-terminal FAK serum ‘Lulu’ was a gift of Dr Andrew Ziemiecki (University of Berne) (Fluck et al. 1999). Monoclonal antibodies against components of complex I–V of the mitochondrial respiratory chain were from Molecular probes (Molecular Probes/Invitrogen Ltd, Paisley, UK). Antibodies against type 1 and all type 2 MHC isoforms, and horseradish peroxidase-conjugated secondary antibodies were applied as described previously to visualize MHC expression (Fluck et al. 2005). The content of different MHC isoforms, MHC1, MHC2A, MHC2X and MHC2B, in the belly part of the muscle was quantified by additional highly resolving SDS-PAGE electrophoresis (D’Antona et al. 2006). The signal for each MHC was normalized to the total MHC signal per sample lane. The respective MHC signal per sample was determined from the mean of three technical replicas per sample. A Wilcoxon test was applied to test the effect of pCMV-FAK plasmid gene electrotransfer and the paired pCMV-transfected control on protein expression in the homogenate.
Immunostaining and morphometry
Microscopic measurements were carried out after the reaction of muscle cross-sections with polyclonal rabbit antibody A-17 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) against the FAK N-terminus and detection with a horseradish peroxidase-labelled secondary antibody (Fluck et al. 2002).
The percentage of fibres showing FAK overexpression was determined from visual fields of stained sections using a microscopic station (Leitz DMRBE, Vienna, Austria) running under Analysis 5.0 software (Olympus Soft Imaging Solutions GmbH, http://www.olympus-sis.com). Sections were visualized with a PL Fluotar 20× 0.50 or PL Fluotar 40× 0.70 objective and digitally recorded with a digital camera (Color view, Soft imaging system) through a 0.63× objective. The frequency distribution and mean of fibre cross-sectional area (CSA) in the targeted muscle portion was estimated by the circumference method from the recorded picture. For the measure of CSA in dependence of FAK localization, fibres were classified into those with exclusive sarcolemmal staining or those exhibiting staining of both the sarcoplasm and sarcolemma. The numbers were pooled for muscles with the same treatment.
Quantitative confocal microscopy
The analysis for co-expression of FAK and FAK-modulated factors in muscle fibres was carried out with a Leica TCS SP5 confocal microscope on a DMI6000 stage powered by Argon laser and He–Ne lasers (Leica Microsystem CMS, Mylton Keynes, UK). In brief, cryosections were reacted with a 1 : 100 dilution of rabbit FAK antibody A-17 (Santa Cruz) in 0.3% BSA in phosphate-buffered saline (PBS) as described (Fluck et al. 2002) but with the modification that a 1 : 200 dilution of a second primary antibody from mouse was added to detect MHC1 or MHC2 (Fluck et al. 2005) or subunits of complex I–V of mitochondrial respiration (Molecular Probes/Invitrogen). Sections were reacted with fluorescent-labelled secondary antibodies (Alexa488-conjugated anti-rabbit IgG, and Alexa555-conjugated anti-mouse IgG, Molecular Probes/Invitrogen) and embedded in fluorescence-compatible mounting medium (DAKO, Glostrup, Denmark). Quantification of the signal for FAK and the co-detected protein was carried out on digital images from the FAK-transfected region of double-stained sections. Fluorescence was excited at 458 nm, 476 nm and 488 nm with sampling in channels between 510–533 nm (Alexa 488) and 593–614 nm (Alexa 555). Four to five images were recorded from different visual fields for each section with a 10× objective (HCX PL APO CS 10.0 × 0.40 DRY UV) using the specific channel settings. Resolution was set to 521 pixels × 512 pixels (8 bit) with a scan speed of 400 Hz and a pinhole of 53 μm. Four to ten muscle fibres were selected from each image. Mean signal intensity per selected fibre was determined in separate channels with the intensity quantification tool of Leica Application Suite version 2.0.0. For each fibre, pixel intensity (in bits) was sampled in a systematic manner across 2–5 parallel optical slices at 5–10 μm distance. The raw data for the recording in the channel for FAK-staining (Alexa488) and the staining for the FAK-modulated factor (Alexa555) were exported as csv-format into Microsoft Excel. The signal intensity per fibre was calculated for each channel from the mean of integrated channel intensities per sampled pixels along the optical slices. Fibres were classified in FAK-positive and FAK-negative fibres based on the intensity of the Alexa488 channel. The cut-off for FAK staining was declared at a mean intensity of 50 bits pixels−1 in line with the optic discrimination on the screen. The fibre signals from the different fields from one muscle cryosection were related to the mean of pixel signals of FAK-negative fibres. Thereby normalized data were pooled to reveal the relative myocellular expression per FAK-positive and FAK-negative fibres. Statistical analysis was carried out with Wilcoxon's test.
FAK expression in single fibres of pCMV-FAK-transfected soleus muscle was evaluated after collection of the chemically skinned fibres on microscope slides by immunofluorescence using the A-17 antibody.
Myography
The contractile parameters in freshly isolated soleus muscle were evaluated at 25°C as previously described (Rossi et al. 2001; D’Antona et al. 2006).
Model considerations
We chose a somatic option for transgenesis due to the proven efficiency of electrotransfer for skeletal muscle (Durieux et al. 2002). This approach, unlike a complex germline approach, allows us to include a specificity control via the co-overexpression of the FAK inhibitor FRNK, which circumvents lethal effects of FAK's elimination (Ilic et al. 1995) or the labour and cost-intensive generation of conditional or muscle-specific knock-out for FAK or its activation site (Booth et al. 1998). A sizeable increase in FAK levels in muscle fibres within the pCMV-FAK transfected muscle portion by up to 40-fold indicates the effectiveness and myocellular specificity of our somatic approach. Due to the selective targeting of exogenous protein expression in the belly portion, only an approximate 4% of the total fibre mass was transfected, i.e. 20% of the muscle cross-section injected with plasmid times 20% of fibres transfected. Therefore, we limited the analysis of molecular consequences to the belly portion, which was targeted by the transfection, and followed the alterations at the cellular level by microscopy.
The contrast with non-transfected muscle implied that adjustments due to FAK overexpression occur in the context of de-differentiation of slow-type soleus muscle to a hybrid slow/fast type during the regeneration of muscle after gene transfer (Fig. 2A and online Supplemental Material Fig. S2A; Rizzuto et al. 1999; Durieux et al. 2002; Fluck & Hoppeler, 2003). We therefore adopted a paired approach that allowed subtracting the combined influence of surgery and transfection via quantitative intra-animal comparisons (Fig. 1). A high correlation (r2≥ 0.98) for mean transcript level differences between empty transfection of soleus muscle and non-transfected muscle in the cage control, unloaded and 1-day-reloaded groups indicated that transfection-related background is largely comparable for the studied samples.
Figure 2. Muscle fibre targeted FAK overexpression.
A, immunostaining of FAK protein (orange, red arrow) in cross-sections of a soleus muscle pair after gene electrotransfer. The right soleus muscle was transfected with FAK-expression construct (pCMV-FAK) and the contralateral (left) muscle was transfected with empty plasmid (pCMV). Nuclei appear in blue. Regenerating fibres are indicated with arrowheads. B, FAK immunostaining of an isolated muscle fibre from pCMV-FAK-transfected muscle. The bar denotes 50 μm. C, immunoblot visualizing FAK protein levels in homogenate from pCMV-FAK-transfected muscle compared to its empty-transfected contralateral control (CTL-CTL). D, immunoblot visualizing the induced expression of the endogenous competitor FRNK in soleus muscle after double transfection with FAK and FRNK expression construct compared to CTL-CTL muscle being transfected with FAK construct and empty pCMV plasmid. The positions of the FAK and FRNK proteins, and a 90 kDa FAK-gene product, pp90, are indicated (40).
Statistics
A paired design was adopted to test the effect of FAK overexpression in muscle compared to empty-transfected contralateral controls. Protein level changes were calculated from the expression ratio between contralateral muscle pairs. Probability-based statistical tests (Wilcoxon's Test, χ2 test) were performed with StatSoft v. 6 (Statistica, Inc., Tulsa, OK, USA http://www.statsoft.com) as indicated in the respective Methods paragraph and figure legends. Statistical significance was assumed at P < 0.05, with 0.05 ≤P < 0.10 being considered a trend.
Results
Muscle fibre targeted FAK overexpression
Electrotransfer of the constitutively active expression plasmid for FAK, pCMV-FAK, into soleus muscle induced the appearance of FAK-immunoreactive fibres in the transfected portion (Fig. 2A). The FAK protein localized exclusively to muscle fibres and was detected in both the sarcolemma and the sarcoplasm (Fig. 2A and B). Contralateral muscles being transfected with empty pCMV plasmid did not demonstrate notable FAK immunostaining. Eight days after electrotransfer, 18% of the total cross-sectional area (CSA) in the targeted portion corresponded to fibres overexpressing FAK. The total FAK protein content in the targeted portion was increased 2.6-fold between the pCMV-FAK and pCMV-transfected soleus muscle pair (Fig. 2C).
FAK modulates the muscle transcriptome
We analysed FAK-dependent muscle gene expression in rats housed under normal cage activity by comparing differences in transcript levels between six contralateral soleus muscles being transfected with empty plasmid or FAK construct (Fig. 1). Transcript profiling identified a general upregulation of mRNAs in soleus muscle after 8 days of FAK overexpression vs. empty transfected contralateral controls. This involved muscle-relevant GOs associated with energy metabolism, control of contraction and protein turnover (Table 1). The majority of RNA changes were less than 50% (see online Supplemental Material, Table S1).
Table 1.
FAK-dependent gene ontologies
| Effect FAK |
Effect FRNK |
|||
|---|---|---|---|---|
| Gene ontology | Trend | P | Trend | P |
| Energy | ||||
| Mitochondria | ↑ 38 | <0.001 | ↓ 31 | <0.001 |
| Mitochondrial oxidative metabolism | ↑ 50 | <0.001 | ↓ 46 | <0.001 |
| Carbohydrate metabolism | ↑ 7 | 0.029 | – 6 | 0.300 |
| Contraction | ||||
| Voltage-gated ion channels | ↑ 21 | <0.001 | ↓ 35 | <0.001 |
| Adhesion | ↑ 15 | <0.001 | ↓ 19 | 0.002 |
| Cytoskeleton/mobility | ↑ 8 | 0.014 | – 7 | 1.000 |
| Sarcomere | – 1 | 1.000 | – 1 | 1.000 |
| Protein turnover | ||||
| Protein synthesis | – 5 | 0.100 | – 3 | 1.000 |
| Proteases | ↑ 29 | <0.001 | – 10 | 0.476 |
| Proteasome | ↑ 11 | 0.002 | – 5 | 1.000 |
| Cell regulation | ||||
| Intracellular signalling | ↑ 151 | <0.001 | ↓ 87 | <0.001 |
| Extracellular signalling | ↑ 30 | <0.001 | ↓ 44 | <0.001 |
| G-protein | ↑ 16 | <0.001 | ↓ 44 | <0.001 |
| All | ↑ 594 | <0.001 | ↓ 729 | <0.001 |
GOs which transcripts showed unidirectional level alterations upon FAK overexpression in rat soleus muscle vs. contralateral controls as revealed by SAM for a two class paired design. n= 6 per biological replica. The trend of significant alterations per GO is indicated with arrows (↑, up; ↓, down, –, no effect) alongside the number of affected transcripts. Specificity of the FAK effect was controlled by the co-expression of FAK with its inhibitor FRNK. For a comprehensive list of the affected transcripts see Supplemental Material Table S1.
Co-expression of the FAK competitor FRNK in pCMV-FAK-transfected soleus muscles (Figs 1 and 2D) resulted in the down-regulation of the expression of FAK-regulated GOs related to mitochondrial oxidative metabolism, voltage-gated ion channels, adhesion and cell regulation compared to transfection with FAK alone in the contralateral muscle (Table 1). Within the many FRNK affected transcripts, the expression of two factors, ATP4B and CAMK2B, was altered above 1.5-fold with FAK overexpression.
Translation of FAK-regulated muscle gene expression
Key components of the GOs underpinning the energy metabolic and contractile muscle phenotype and for which transcripts were regulated by FAK were verified at the protein level in paired transfection experiments of cage control animals (Fig. 1). This included the mitochondrial components cytochrome c oxidase subunit 1 and 4 (COX1 and COX4), the factor ATP5A1 of oxidative phosphorylation, myosin heavy chain 1 (MHC1), and MHC2 isoforms MHC2A and MHC2X. The measures in the transfected muscle portion confirmed the FAK-dependent transcript level alterations of COX4 and MHC2A at the protein level and demonstrated a tentative increase in ATP5A1 after FAK overexpression vs. empty transfected contralateral controls (Fig. 3B). The 50% decrease in fast type MHC2A occurred concomitantly with a 9% elevation of slow type MHC1 content. Additional measures identified sizable elevations in protein content for subunits of complex I (NDUFA9) and complex III (UQCRC1) of the mitochondrial respiratory chain (Fig. 3B). The increase in MHC1 protein and combined subunits of mitochondrial respiration in pCMV-FAK transfected muscle was confined to FAK-overexpressing fibres (Fig. 3C). Total MHC2 protein content was not different between FAK-overexpressing and non-overexpressing fibres of pCMV-FAK transfected muscle. The number of hybrid slow/fast type fibres was reduced in pCMV-FAK transfected muscle vs. its empty-transfection control (Supplemental Material, Fig. S2). The alterations of sarcomeric and mitochondrial proteins after FAK overexpression in cage controls did not translate into functional changes of muscle contraction ex vivo (Fig. S2 and Table S2).
Figure 3. Expression of selected gene products in FAK-modulated GOs.
A, immunoblots visualizing the detection of selected mitochondrial proteins of oxidative phosphorylation (oxphos), i.e. complex I (NDUFA9), complex II (SDHA), complex III (UQCRC1), complex IV (COX1 and COX4) and complex V (ATP5A1), and sarcomeric proteins MHC1 and combined MHC2 isoforms in pCMV and pCMV-FAK transfected muscle pairs. B, mean ±s.e.m. of the expression ratio of transcripts and corresponding protein of the selected factors between pCMV-FAK-transfected soleus muscle and empty-transfected CTL-CTL. n= 6 per transfection. *P < 0.05 vs. CTL-CTL, SAM for a two class paired design of microarray data (COX1, COX4, ATP5A1) or a sign-test for PCR data (MHC1, MHC2A, MHC2X), respectively. The line of identity is indicated. C, mean ±s.e.m. of the relative myocellular expression of FAK-modulated proteins in FAK overexpressing (FAK+) and negative fibres (FAK–) of pCMV-FAK-transfected soleus muscle of cage controls. Fifty-eight FAK+ and 74 FAK– fibres were analysed on average from four different muscles. *P < 0.05, Wilcoxon's test. D, double panels presenting examples of confocal microscopic images of co-expression of FAK and the respectively FAK-modulated factor (i.e. MHCI, MHCII and combined oxphos subunits). A panel with a control reaction omitting the use of primary mouse antibody (2nd ab) is included as well. The bar denotes 50 μm.
A comparison with non-transfected muscle showed that the high content of hybrid slow/fast type fibres in cage controls was due to de-differentiation of slow type soleus muscle fibres with electrotransfer (Fig. 2A and Fig. S2).
FAK overexpression elevates fibre size
A trend of increased muscle weight with FAK overexpression was observed for the six soleus muscle pairs of cage controls being subjected to transcript profiling. There was a significant 12% increase when 14 muscles were pooled (Table S3). Microscopic analysis revealed a shift towards larger fibre callipers in pCMV-FAK-transfected muscle compared to the empty-transfected muscle (Fig. 4A and C). The mean CSA of muscle fibres was 6% higher in FAK-overexpressing muscle (Fig. 4B).
Figure 4. FAK overexpression and fibre size.
A, bar graph of the cross-sectional area (CSA) of soleus muscle fibres as a function of FAK overexpression in cage controls. *P < 0.05 between comparisons (χ2 test). B, mean ±s.e.m. of fibre CSA in FAK-overexpressing muscle and its contralateral control. *P < 0.05 vs. cage control (Wilcoxon's test). On average, 608 fibres were counted from six muscles per transfection. C, representation of two FAK overexpressing fibres with large callipers (circled) in pCMV-FAK transfected muscle. The bar denotes 100 μm.
Muscle loading modulates FAK-dependent muscle gene expression
We tested whether altered muscle loading modifies the FAK-dependent muscle transcriptome. Towards this end we deployed paired transfection experiments in combination with a protocol for muscle unloading and reloading (Fig. 1). The FAK protein level remained elevated in pCMV-FAK-transfected soleus muscle versus pCMV-transfected contralateral control, after 7 days of unloading and 1 day of subsequent reloading (Fig. 5A). Unloading resulted in a general suppression of RNA levels in the muscle overexpressing FAK vs. empty contralateral controls (Fig. 5B). Reloading reversed this trend; several GOs with a FAK-dependent reduction of transcript levels during unloading demonstrated up-regulated expression with reloading. The comparison to empty-transfected muscle identified that the effects of FAK overexpression resulted from a promotion of the load-dependent expression changes of GOs associated with mitochondria, oxidative metabolism, adhesion, protein turnover and signalling (Fig. 5A and B). The major exception to this general trend was the GO for voltage-gated ion channels.
Figure 5. Global control of the FAK-dependent muscle transcriptome by muscle loading.
A, FAK protein ratio (mean ±s.e.m.) between pCMV-FAK-transfected soleus muscle and empty-transfected CTL-CTL from cage control rats and rats after unloading and reloading. *P < 0.05 vs. CTL-CTL (sign test). n= 5–10 per intervention. The line of identity is indicated. B, gene ontology (GO) map visualizing RNA expression changes per muscle-relevant GO with altered loading in pCMV-FAK vs. pCMV transfected muscle (left) and in empty-transfected muscle (right). The significance level of co-directional transcript level alterations per GO for each condition is indicated by colour coding. Names in red denote those GOs which were co-directionally affected between both comparisons. CTL, cage control; HU7, 7 days of unloading; R1, 1 day reloaded; R5, 5 days reloaded. n= 6 per intervention except for HU7 where n= 4.
Post-translational regulation of FAK by muscle loading
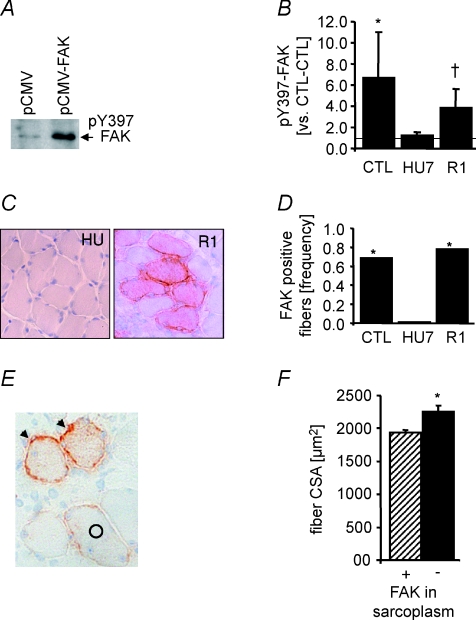
The load-modulated adjustments of the muscle transcriptome due to FAK overexpression corresponded to the post-translational regulation of FAK; FAK-pY397 content was higher in pCMV-FAK-transfected soleus muscle compared to contralateral muscle from cage controls. The ratio of phosphorylated FAK content decreased with unloading and was tentatively elevated after 1 day of reloading (Fig. 6A and B). Correspondingly, immunoreactivity against the FAK N-terminus was reversibly modified by muscle unloading and reloading (Fig. 6C and D).
Figure 6. Load-modulated post-translational regulation of FAK.
A, representative immunoblot detecting phosphorylated FAK (FAK-pY397) levels in pCMV-FAK-transfected soleus muscle and pCMV-transfected CTL-CTL from a cage control rat. B, FAK-pY397 ratio (mean ±s.e.m.) between pCMV-FAK-transfected soleus muscle and empty-transfected CTL-CTL as a function of unloading and reloading. n= 3–9 per time point. *P < 0.05 vs. CTL-CTL; †0.05 ≤P < 0.10 vs. CTL-CTL (Wilcoxon's test). Representative immunostaining (C) and frequency (D) of N-terminal FAK immunoreactivity (orange) in cross-sections of FAK-overexpressing soleus muscle of cage controls and after unloading and 1 day of reloading. *P < 0.05 between comparisons (χ2 test). E, representative picture depicting muscle fibres with exclusive FAK staining at the sarcolemma (arrowhead) or with additional staining in the sarcoplasm (circle). F, mean ±s.e.m. of CSA in FAK-overexpressing muscle fibres as a function of sarcoplasmic FAK abundance. On average, 67 FAK-positive fibres were counted from each of six biological replicas per comparison. *P < 0.05 vs. comparison (Wilcoxon's test).
Load-dependent effect of FAK on fibre size
The differences in MHC and COX protein levels in FAK overexpressing soleus muscle from empty-transfected contralateral controls were lost with unloading (data not shown). As well, the tendency for FAK-promoted muscle growth disappeared with unloading and was only transiently re-established with reloading (Table S3). The differences in muscle weight with 1 day of reloading were associated with FAK localization. Exclusive sarcolemmal FAK localization in 1-day-reloaded muscle coincided with a 16% increase in mean fibre CSA compared to fibres with exclusive sarcoplasmic FAK expression (Fig. 6E and F).
Functional overload promotes slow contractile muscle characteristics in muscle overexpressing FAK
We tested whether enhanced muscle loading in the context of FAK overexpression would provoke a functional manifestation of FAK-modulated molecular adjustments. We reasoned that overload of the soleus muscle by tenotomy (Gordon et al. 2001) would be a suitable alternative to avoid FAK overexpression fading during the prolonged experimental duration of the unloading–reloading model (Fig. 5A).
The results confirmed the reduction of fast MHC2A composition and elevated MHC1 content by FAK overexpression (Fig. S3). A concomitant slowing of muscle contraction and relaxation was evident in the FAK-transfected muscles (Fig. 7). Fatigue resistance and soleus mass were not altered by FAK overexpression during overload (Table S2). The specific force of tetanic contractions was increased 2-fold in pCMV-FAK-transfected m. solei reaching the levels in non-transfected controls (Fig. 7 and Fig. S2C).
Figure 7. Load-dependent shift to a slow type muscle phenotype after FAK overexpression.
Mean ±s.e. of contractile properties in pCMV-FAK- and pCMV-transfected soleus muscle after 8 days of overloading. A, time-to-peak and half relaxation time; B, force of a single twitch and tetanic contractions as quantified by myography. N= 4–7 per measure. *P < 0.05 vs. CTL-CTL (Wilcoxon's test).
Discussion
Several biochemical pathways have been proposed for the remodelling of the contractile and metabolic properties of striated muscle in response to use-related stimuli (Pallafacchina et al. 2002; Fluck & Hoppeler, 2003; Koulmann & Bigard 2006; Sandri, 2008). The functional implication of possible signalling routes for muscle conditioning by physiological stimuli is not understood. Thus, our muscle-targeted transgenic investigation focused on FAK, which complies with an active role in mechano-transduction within striated muscle (Fluck et al. 1999, 2002; Gordon et al. 2001; Quach & Rando, 2006) through post-transcriptional regulation and localization to sites of mechano-sensation. The multi-level approach analysing the consequences of muscle fibre-targeted overexpression of a FAK homologue delineated that the load-dependent functional differentiation of slow contractile features and fibre-growth in oxidative rat muscle is regulated by a FAK-mediated pathway to gene expression.
A main strength of this approach was that myocellular focal adhesion signalling was probed via a ‘native’ FAK homologue in the exceptionally load-dependent soleus muscle. We reasoned that the exogenous production of a native rather than a constitutively active FAK protein (Sastry et al. 1999) in muscle fibres would allow for the exposure of the physiologically motivated post-translational regulation of this signalling molecule. By adopting a paired transfection approach of intra-animal comparisons we took account for the inference caused by surgery and somatic gene transfer.
FAK promotes differentiation of slow-oxidative muscle characteristics
The significant drop in the number of hybrid slow/fast fibre types and fast MHC2A expression (Fig. 3B and Fig. S2A) in FAK transfected muscle of cage controls implies that FAK overexpression protects fibres from de-differentiation induced by electrotransfer. Microscopic examination indicates that this promotion of slow-type characteristics is not limited to the FAK overexpressing fibres but also involves reduced fast MHC2A expression in fibres which do show low FAK levels (Fig. 3C). The concurrent increase in slow MHC1 with FAK overexpression in the transfected muscle portion of cage controls was related to elevated protein content of key factors of mitochondrial respiration and corresponding alterations of the encoding transcripts (Fig. 3B). This observation extends previous findings in cardiomyocytes on a role of FAK in sarcomere organization (Kovacic-Milivojevic et al. 2001) to imply the differential control of both slow and fast myosin turnover and mitochondrial biogenesis by FAK. The concentration changes of major elements of muscle make-up in the transfected fibre population (Fig. 3C and D) is astonishing given that gene transfer with plasmid for a native (i.e. not constitutively active) FAK molecule was carried out only 9 days earlier. The present novel finding provides evidence that FAK is part of a myocellular pathway which mediates the expression of slow-oxidative muscle fibre characteristics.
The FAK-driven expressional adjustments of the metabolic and contractile phenotype occurred concomitantly with transcript up-regulation of both proteolytic and synthetic aspects of protein turnover and a trend for an increase in fibre cross-sectional area (Table 1 and Figs 4A and 5B). This observation with FAK overexpression in the slow tonic muscle under investigation relates to the elevated FAK content in muscle fibres with a high degree of load-bearing fibre activity (Fluck et al. 1999, 2002; Fluck & Hoppeler, 2003; Evans et al. 2008). This association of FAK with characteristics of the frequently recruited muscle is supported by the general reduction of FAK-induced transcript levels in soleus muscle after co-expression of the FAK inhibitor FRNK (Table 1; Taylor et al. 2001; Mansour et al. 2004; Quach & Rando, 2006). These considerations identify FAK as a broadly effective facilitator of the transcriptome programme, which promotes the activity-dependent re-establishment of the normal phenotype of anti-gravitational muscle after gene transfer.
Mechano-regulated pathway of slow-oxidative muscle differentiation
The load-dependent signature of FAK-dependent GOs complies with our general hypothesis that FAK is a major myocellular transducer of mechanical signals towards gene expression. Several of the combined FAK- and load-regulated transcript families (Fig. 5B) are associated with particularly developed features in soleus muscle such as mitochondrial metabolism, adhesion and protein turnover (Habets et al. 1999; Fluck & Hoppeler, 2003). The similarity in load-dependent control of these GOs by FAK overexpression with the effect of unloading and reloading in non-transfected and empty-transfected muscle (Fig. 5A and B; Stevenson et al. 2003; Fluck et al. 2005) supports the mechano-regulated expression of major transcripts in soleus muscle by FAK.
The contention of a functional implication of FAK in mechano-regulated muscle differentiation is supported by the reestablishment of specific tetanic force in FAK-transfected and overloaded muscle to normal levels (compare Fig. 7 and Fig. S2). This observation was paralleled by the load-dependent prolongation of half-relaxation time of muscle contraction in FAK-transfected muscle. Conversely, the upregulation of mitochondrial factors after FAK overexpression did not translate into alterations in the functional proxy of energy metabolism, such as fatigue, in either control or overloaded muscle (Table S2). The inspection of mitochondrial proteins indicates that this lack of adaptation relates to a dissonance in the up-regulation of nuclear and mitochondrially encoded factors of respiration. For instance, the nuclear-encoded NDFUA9, UQCRC1 and COX4 (and tentatively ATP5A1) show sizable elevations while this is not observed for the mitochondrially encoded proteins COX1 and SDHA (Fig. 3B). This suggests a lack of coordination during mitochondrial biogenesis after FAK overexpression due to the absence of elevations in energy consumption in our model (Desplanches et al. 1987; Fluck & Hoppeler, 2003). Collectively this indicates selectivity in FAK's involvement in the expressional regulation of contractile versus energetic aspects of the muscle phenotype.
Chemical mechano-transduction via FAK
The probing with non-constitutively active FAK revealed that the FAK modulated expression control corresponds to phosphorylation of FAK at Y397 (compare Figs 5B and 6B). This post-translational modification induces a conformation change in the FAK molecule which promotes the binding of signalling factors to FAK (Shyy & Chien, 1997; Parsons, 2003). Support for such a scenario was provided by the correspondence of elevated phospho-Y397 content and induced FAK immunoreactivity in muscle fibres when probed with an N-terminal antibody (Fig. 6C and D). Elevated pY397 content of FAK corresponds to the translocation of FAK from a myofibrillar pool to the sarcolemma during hypertrophy of cardiac cells in culture (Fonseca et al. 2005). Similarly, FAK activation close to the sarcolemma is related to control of myofibrillogenesis (Fluck et al. 1999, 2002; Gordon et al. 2001; Quach & Rando, 2006). Our findings in fully developed tissue on the association of elevated CSA of muscle fibres with enhanced sarcolemmal FAK localization (Fig. 6F) is compatible with a mechanism whereby FAK shuttles between a myofibre-associated and a sarcolemmal pool during radial growth of muscle fibres.
Fibre hypertrophy after FAK overexpression corresponded to the load-dependent up-regulation of transcripts being associated with protein synthesis (Fig. 5B). We and others have shown recently that tyrosine phosphorylation of FAK is an upstream event of the mechano-induced activation of the regulator of protein synthesis and ribosomal biogenesis, p70S6K, in rodent muscle (Jastrzebski et al. 2007; Klossner et al. 2009). These considerations suggest that mechano-regulated expression control of protein turnover via post-translational modification of FAK underpins FAK's load-dependent association with fibre hypertrophy and muscle protein synthesis (Gordon et al. 2001; de Boer et al. 2007). Mechano-regulated phosphorylation of FAK at Y397 evolves as upstream event of the physiological expression pathway that governs the size and differentiation of slow oxidative muscle fibres by muscle loading.
Conclusions
The findings identify focal adhesion kinase (FAK) translocation to the sarcolemma as an upstream signalling element of load-dependent contractile differentiation and growth in slow-oxidative muscle. Corresponding adjustments between molecules, muscle fibres and function point out that FAK is part of the signalling pathway that governs the mechano-regulation and repair of striated muscle.
Acknowledgments
This study was financially supported by grants from the Région Rhône-Alpes’, the Association Française contre les myopathies, and the Swiss National Science Foundation. The experiments were performed at the University of Berne (Switzerland), the University of Lyon (France), and the University of Pavia (Italy). The support of Prof. Hans Hoppeler is acknowledged.
Glossary
Abbreviations
- CSA
cross-sectional area
- FAK
focal adhesion kinase
- FRNK
FAK-related non-kinase
- MHC
myosin heavy chain
- GO
gene ontology
- SAM
statistical analysis for microarrays
Author contributions
Conception and design: A.C.D., G.D.A., D.F., D.D., R.B., M.F.; data analysis: A.C.D., G.D.A., S.K., M.F.; data interpretation: A.C.D., G.D.A., D.F., R.B., M.F.; article preparation: A.C.D., D.D., D.F., S.K., M.F.
Supplemental material
References
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Booth FW, Flück M, Carson JA. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol Scand. 1998;162:343–350. doi: 10.1046/j.1365-201X.1998.0326e.x. [DOI] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol. 2006;570:611–627. doi: 10.1113/jphysiol.2005.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapp C, Gassmann M, Hoppeler H, Fluck M. Hypoxia-induced gene activity in disused oxidative muscle. Adv Exp Med Biol. 2006;588:171–188. doi: 10.1007/978-0-387-34817-9_16. [DOI] [PubMed] [Google Scholar]
- Dapp C, Schmutz S, Hoppeler H, Fluck M. Transcriptional reprogramming and ultrastructure during atrophy and recovery of mouse soleus muscle. Physiol Genomics. 2004;20:97–107. doi: 10.1152/physiolgenomics.00100.2004. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplanches D, Mayet MH, Sempore B, Frutoso J, Flandrois R. Effect of spontaneous recovery or retraining after hindlimb suspension on aerobic capacity. J Appl Physiol. 1987;63:1739–1743. doi: 10.1152/jappl.1987.63.5.1739. [DOI] [PubMed] [Google Scholar]
- Durieux AC, Bonnefoy R, Manissolle C, Freyssenet D. High-efficiency gene electrotransfer into skeletal muscle: description and physiological applicability of a new pulse generator. Biochem Biophys Res Commun. 2002;296:443–450. doi: 10.1016/s0006-291x(02)00901-4. [DOI] [PubMed] [Google Scholar]
- Durieux AC, Desplanches D, Freyssenet D, Fluck M. Mechanotransduction in striated muscle via focal adhesion kinase. Biochem Soc Trans. 2007;35:1312–1313. doi: 10.1042/BST0351312. [DOI] [PubMed] [Google Scholar]
- Evans M, Morine K, Kulkarni C, Barton ER. Expression profiling reveals heightened apoptosis and supports fiber size economy in the murine muscles of mastication. Physiol Genomics. 2008;35:86–95. doi: 10.1152/physiolgenomics.00232.2007. [DOI] [PubMed] [Google Scholar]
- Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Cell Physiol. 1999;277:C152–162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity – from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- Flück M, Mund SI, Schittny JC, Klossner S, Durieux AC, Giraud MN. Mechano-regulated tenascin-C orchestrates muscle repair. Proc Natl Acad Sci U S A. 2008;105:13662–13667. doi: 10.1073/pnas.0805365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R4–14. doi: 10.1152/ajpregu.00833.2004. [DOI] [PubMed] [Google Scholar]
- Fluck M, Ziemiecki A, Billeter R, Muntener M. Fibre-type specific concentration of focal adhesion kinase at the sarcolemma: influence of fibre innervation and regeneration. J Exp Biol. 2002;205:2337–2348. doi: 10.1242/jeb.205.16.2337. [DOI] [PubMed] [Google Scholar]
- Fonseca PM, Inoue RY, Kobarg CB, Crosara-Alberto DP, Kobarg J, Franchini KG. Targeting to C-terminal myosin heavy chain may explain mechanotransduction involving focal adhesion kinase in cardiac myocytes. Circ Res. 2005;96:73–81. doi: 10.1161/01.RES.0000152390.99806.A5. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Fluck M, Booth FW. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174–1183. doi: 10.1152/jappl.2001.90.3.1174. discussion 1165. [DOI] [PubMed] [Google Scholar]
- Habets PE, Franco D, Ruijter JM, Sargeant AJ, Pereira JA, Moorman AF. RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem. 1999;47:995–1004. doi: 10.1177/002215549904700803. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Suda T, Atsumi T, Fujimoto J, Ikawa Y, Yamamoto T, Aizawa S. Focal adhesion kinase is not essential for in vitro and in vivo differentiation of ES cells. Biochem Biophys Res Commun. 1995;209:300–309. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- Klossner S, Durieux AC, Freyssenet D, Flück M. Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol. 2009;106(3):389–398. doi: 10.1007/s00421-009-1032-7. [DOI] [PubMed] [Google Scholar]
- Koulmann N, Bigard AX. Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch. 2006;452:1–15. doi: 10.1007/s00424-005-0030-9. [DOI] [PubMed] [Google Scholar]
- Kovacic-Milivojevic B, Roediger F, Almeida EA, Damsky CH, Gardner DG, Ilic D. Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol Biol Cell. 2001;12:2290–2307. doi: 10.1091/mbc.12.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Ce and focal adhesion kinase. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Pham CG, Harpf AE, Keller RS, Vu HT, Shai SY, Loftus JC, Ross RS. Striated muscle-specific β1D-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway. Am J Physiol Heart Circ Physiol. 2000;279:H2916–2926. doi: 10.1152/ajpheart.2000.279.6.H2916. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Quach NL, Rando TA. Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Dev Biol. 2006;293:38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, La Monica N, Fattori E. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. 1999;96:6417–6422. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Bottinelli R, Sorrentino V, Reggiani C. Response to caffeine and ryanodine receptor isoforms in mouse skeletal muscles. Am J Physiol Cell Physiol. 2001;281:C585–594. doi: 10.1152/ajpcell.2001.281.2.C585. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551:33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.