Abstract
Using the hyperphagic, obese, Otsuka Long–Evans Tokushima Fatty (OLETF) rat, we sought to determine if progression to type 2 diabetes alters visceral white adipose tissue (WAT) mitochondrial content and if these changes are modified through prevention of type 2 diabetes with daily exercise. At 4 weeks of age, OLETF rats began voluntary wheel running (OLETF-EX) while additional OLETF rats (OLETF-SED) and Long–Evans Tokushima Otsuka (LETO-SED) rats served as obese and lean sedentary controls, respectively, for 13, 20 and 40 weeks of age (n= 6–8 for each group at each age). OLETF-SED animals displayed insulin resistance at 13 and 20 weeks and type 2 diabetes by 40 weeks. OLETF-SED animals gained significantly (P < 0.001) more weight and omental fat mass compared with OLETF-EX and LETO-SED. Markers of WAT mitochondrial protein content (cytochrome c, COXIV-subunit I, and citrate synthase activity) significantly increased (P < 0.05) from 13 to 40 weeks in the LETO-SED, but were significantly attenuated in the OLETF-SED rats. Daily exercise normalized WAT cytochrome c and COXIV-subunit I protein content in the OLETF-EX to the healthy LETO-SED animals. In conclusion, increases in omental WAT mitochondrial content between 20 and 40 weeks of age in LETO control animals are attenuated in the hyperphagic, obese OLETF rat. These alterations occurred in conjunction with the progression from insulin resistance to type 2 diabetes and were prevented with daily exercise. Reduced ability to increase WAT mitochondrial content does not appear to be a primary cause of insulin resistance, but may play a key role in the worsening of the disease condition.
Adipose tissue is now recognized as more than just an energy reservoir for lipid storage; in fact, it is considered an active endocrine organ and an important regulator in glucose homeostasis. Overexpression of GLUT4 selectively in adipose tissue has been shown to prevent insulin resistance and type 2 diabetes in mice lacking skeletal muscle GLUT4 (Carvalho et al. 2005). High amounts of visceral white adipose tissue (WAT) are more closely associated with insulin resistance, cardiovascular disease, type 2 diabetes, and systemic low grade inflammation than subcutaneous WAT (Xu et al. 2003). In addition, reducing the accumulation of WAT is considered an important component in preventing the development of type 2 diabetes. This is due in part to intrinsic differences in metabolism that include lower anti-inflammatory leptin and adiponectin release (Van Harmelen et al. 1998; Motoshima et al. 2002), higher pro-inflammatory cytokine release (Fried et al. 1998), higher lipolytic response to catecholamines (Arner, 1995), and lower adipocyte proliferation capacities (Van Harmelen et al. 2004) in visceral versus subcutaneous adipose tissue.
Decreases in mitochondrial content are noted in various tissues and are associated with neurodegenerative, cardiovascular and metabolic diseases (Boveris & Navarro, 2008; Reeve et al. 2008; Ungvari et al. 2008). For instance, diabetic humans have reduced skeletal muscle mitochondria (He et al. 2001) that fail to increase ATP production in response to insulin infusion (Asmann et al. 2006). While a prominent role for mitochondrial function is more apparent in skeletal muscle, little is known about the physiological relevance of mitochondria in adipose tissue. Mitochondria in adipose tissue are necessary for adiponectin synthesis and positively correlated with lipogenic potential in human subcutaneous WAT (Kaaman et al. 2007; Koh et al. 2007). In addition, reductions in adipose tissue mitochondrial content and function recently have been observed in the epididymal fat of type 2 diabetic mice (Choo et al. 2006), suggesting a potential role for the disruption of adipose tissue mitochondrial content and function in type 2 diabetes.
The Otsuka Long–Evans Tokushima Fatty (OLETF) rats are a commonly studied model of obesity and type 2 diabetes (Kawano et al. 1992). Selectively bred for null expression of the cholecystokinin-1 receptor, OLETF rats exhibit a within-meal feedback defect for satiety, resulting in hyperphagia and obesity, and the spontaneous development of insulin resistance and type 2 diabetes (Moran & Bi, 2006). Recent findings also indicate that epididymal adipocyte mitochondrial content is reduced in 33-week-old OLETF animals (Choo et al. 2006), but these reductions in the context of progression from insulin resistance to type 2 diabetes was not examined. When allowed to exercise daily on voluntary running wheels, the accumulation of WAT is reduced and type 2 diabetes is prevented in OLETF animals (Shima et al. 1993; Rector et al. 2008a,b;). In addition, while not directly examined in OLETF animals, there is limited evidence that swimming exercise increases adipose tissue mitochondrial content (Stallknecht et al. 1991; Sutherland et al. 2009) and voluntary wheel running increases adipocyte fatty acid oxidation (Laye et al. 2009) in healthy animals. Furthermore, subcutaneous adipose tissue mitochondrial biogenesis is increased by peroxisome proliferator-activated receptor-γ (PPARγ) agonist treatment in type 2 diabetics and is associated with increased whole body insulin sensitivity and fatty acid oxidation (Boden et al. 2005). These findings suggest that therapies may be available for modulating adipose tissue mitochondrial content and function.
To our knowledge, no previous reports have prospectively examined the changes in WAT mitochondria content in a rodent model throughout the development and progression of insulin resistance and type 2 diabetes. Therefore, the purpose of the study was to examine changes in omental WAT mitochondrial content in a hyperphagic, obese animal model of type 2 diabetes and gain mechanistic insight into the potential role these changes may have on the initiation and progression of the disease. In addition, as anti-diabetic therapies have shown promise in altering adipose tissue mitochondrial concentration in type 2 diabetic mice (Choo et al. 2006), we sought to determine the impact of daily physical activity on WAT mitochondrial content in a diabetic model.
Methods
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. OLETF and LETO male rats at 4 weeks of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The OLETF-runners (OLETF-EX) were immediately housed (at the age of 28 days) in cages equipped with voluntary running wheels outfitted with a Sigma Sport BC 606 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA, USA) for measuring daily OLETF running activity. Voluntary running was selected to approximate the more natural activity state of the animal. Cages were in temperature-controlled animal quarters (21°C) with a 06.00–18.00 h light–18.00–06.00 h dark cycle that was maintained throughout the experimental period. All animals were provided standard rodent chow (Formulab 5008, Purina Mills, St Louis, MO, USA) in new cages at the beginning of each week when cages were changed and body weights obtained between 08.00 and 10.00 h. Body mass and food intake were measured weekly throughout the investigation. Running activity was obtained daily between 08.00 and 10.00 h, and rats in the running groups had daily access to wheels and food and water ad libitum until the designated time of killing (13, 20 or 40 weeks of age). Age-matched, sedentary OLETF (OLETF-SED) and LETO (LETO-SED) rats did not have access to running wheels throughout the duration of the study. Rat chow was removed on the day of killing at 0600 h. At 13, 20 and 40 weeks of age, rats were anaesthetized with sodium pentobarbital (100 mg kg−1) and killed by exsanguination 53 h after locking of wheels; the sedentary rats (OLETF-SED and LETO-SED) were killed at the same time. The timing of the last exercise bout was designed to mirror our previous study (Rector et al. 2008b) and also to examine the effects of chronic exercise, not the acute effects, of the last bout of exercise. All animals were fasted for 5 h prior to killing.
Dual-energy X-ray absorptiometry (DEXA)
Whole-body composition was measured using a Hologic QDR-1000/w DEXA machine calibrated for rats.
Western blotting
Western blot analyses were performed for the determination of the protein content of cytochrome oxidase IV-subunit I (COX anti-complex IV-subunit I; Molecular Probes, Eugene, OR, USA), total cytochrome c (Cell Signaling, Beverly, MA, USA), fatty acid synthase (FAS H-300, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mitochondrial fission protein 1 (FIS1) (Alexis Biochemical, San Diego, CA, USA), and sterol response element binding protein 1c (SREBP1c) (Santa Cruz Biotechnology), as previously described (Rector et al. 2008a,b;). In order to control for equal protein loading and transfer, the membranes were stained with 0.1% amido black (Sigma) or visualized with Ponceau S as previously described (Kump & Booth, 2005; Morris et al. 2008; Rector et al. 2008a,b;). All data are expressed in arbitrary units.
Fat pad collection
Omental adipose tissue fat pads were removed from exsanguinated animals and weighed. A portion was fixed in formalin, paraffin embedded, stained with haematoxylin and eosin, and analysed for cell diameter and cell number as previously described (Laye et al. 2007). At least three fields of view were used to size >300 adipocytes per animal using Image Pro (MediaCybernetics Inc., Bethesda, MD, USA) imaging software. An additional portion was frozen at −80°C for enzyme activity analyses and Western blotting.
Serum assays
Serum glucose (Sigma, St Louis, MO, USA), triglycerides (TG) (Sigma), free fatty acids (FFA; Wako Chemicals, Richmond, VA, USA), insulin (Linco Research, St Charles, MO, USA), leptin (Linco Research), and adiponectin (Linco Research) were measured using commercially available kits according to the manufacturer's instructions. Haemoglobin A1c (HbA1c) concentrations were determined in EDTA-whole blood by the Diabetes Diagnostics Lab at the University of Missouri using a boronate-affinity HPLC method (Primus Diagnostics, Kansas City, MO, USA).
Citrate synthase, β-hydroxyacyl-CoA dehydrogenase (β-HAD) and cytochrome c oxidase activities
Citrate synthase and β-HAD activities were determined in omental fat homogenate using the methods of Srere (1969) and Bass et al. (1969), respectively, and cytochrome c oxidase activity by a commercially available kit (Sigma) as previously described by our group (Rector et al. 2008a,b; Laye et al. 2009). Both β-HAD and cytochrome c oxidase activity was undetectable in omental adipose tissue homogenates.
Statistics
Each outcome measure was examined in five to eight animals per group. For each outcome measure, a one-way analysis of variance was performed (SPSS/15.0, SPSS, Chicago, IL, USA). A significant main effect (P < 0.05) was followed-up with Student–Newman–Keuls post hoc comparisons. Values are reported as means ± standard error of the mean (s.e.m.), and a P value less than 0.05 denotes a statistically significant difference.
Results
Animal characteristics
Average daily running distance and running time for the OLETF-EX groups are shown in Fig. 1A and B, respectively. As we have previously reported (Morris et al. 2008), initial running distance at 4 weeks of age averaged ∼4 km day−1 (∼150 min day−1). Peak distances of ∼12 km day−1 (∼275 min day−1) were achieved between 8 and 11 weeks of age, declining to ∼7 km day−1 (∼200 min day−1) at 20 weeks of age, and ∼4 km day−1 (∼150 min day−1) by 40 weeks of age. It remains unknown as to why the OLETF animals exhibited decreased voluntary wheel running as they age, but perhaps the OLETFs appropriately mirror the human condition in which aging is often associated with reduced physical activity.
Figure 1. Daily OLETF running distance (A) and time (B) and body weight gain (C).
Values are means ±s.e.m.;N= 6–8/group. *OLETF-SED is significantly different from other animal groups at respective ages (P < 0.001). In panel C, the values for OLETF-EX and LETO are essentially overlapping.
Consistent with our previous reports (Rector et al. 2008a,b;), body mass and body fat percent, determined by small animal DEXA, were significantly greater in the OLETF-SED animals compared with LETO-SED and OLETF-EX at all ages studied (P < 0.001, Fig. 1C and Table 1). A marker of exercise training, higher heart weight to body weight ratio, was found in all OLETF-EX animals compared with OLETF-SED and LETO-SED (Table 1).
Table 1.
Animal characteristics
| Groups | Age (weeks) | % body fat (g) | Food consumption (g week−1) | Food consumption (g week−1 (g BW)−1) | HW/BW (mg g−1) |
|---|---|---|---|---|---|
| LETO-SED | 13 | 10.0 ± 0.5a | 155.2 ± 2.6a | 0.43 ± 0.01a | 3.37 ± 0.13 a |
| 20 | 15.4 ± 1.1b | 159.1 ± 1.5b | 0.33 ± 0.01b | 2.92 ± 0.06 b | |
| 40 | 21.9 ± 1.3c | 164.7 ± 5.6 b | 0.30 ± 0.01b | 2.58 ± 0.03 c | |
| OLETF-EX | 13 | 7.2 ± 0.7a | 247.1 ± 6.61* | 0.67 ± 0.02a* | 4.00 ± 0.05a* |
| 20 | 11.7 ± 2.0b | 242.1 ± 4.5* | 0.56 ± 0.02b* | 3.57 ± 0.10b* | |
| 40 | 18.0 ± 1.8c | 231.5 ± 8.8* | 0.43 ± 0.01c* | 3.05 ± 0.06c* | |
| OLETF-SED | 13 | 21.4 ± 1.8a* | 214.2 ± 10.2a* | 0.41 ± 0.01a | 3.11 ± 0.05a |
| 20 | 30.2 ± 0.9b* | 219.8 ± 10.9a* | 0.36 ± 0.01b | 2.67 ± 0.07b | |
| 40 | 30.6 ± 4.5b* | 306.2 ± 21.4b*† | 0.45 ± 0.05a* | 2.71 ± 0.14b |
Values are means ±s.e.m. (n= 6–8). Values with different letter superscripts within each animal group are significantly different (P < 0.05).
Significantly different than other animal groups without symbol at respective age (P < 0.01).
Significantly different than OLETF-EX at respective age (P < 0.01). HW/BW = heart weight to body weight ratio.
Absolute weekly food consumption was significantly greater (P < 0.05) in OLETF-SED and OLETF-EX rats at 13, 20, and 40 weeks compared with non-hyperphagic, LETO-SED (Table 1). While absolute weekly food consumption only differed at 40 weeks between OLETF-EX and OLETF-SED, food consumption relative to body weight was significantly greater in the OLETF-EX animals at 13 and 20 weeks of age (Table 1), suggesting that observations in OLETF-EX animals are not due to consumption of less calories.
Serum characteristics
OLETF-SED animals exhibited age-associated increases in serum TG and FFAs and these concentrations were significantly higher than OLETF-EX and LETO-SED at all ages studied (Table 2). OLETF-SED animals displayed insulin resistance by 13 and 20 weeks, demonstrated by elevated levels of insulin and glucose compared with OLETF-EX and LETO-SED, and developed overt diabetes mellitus by 40 weeks with a ∼50% drop in serum insulin and 2-fold increase in HbA1c (Table 2), whereas OLETF-EX animals remarkably maintained glycaemic control to the level of the healthy LETO-SED animals at all ages studied (Table 2). Serum adiponectin showed an age-associated decrease in both of the OLETF groups, with the OLETF-EX dropping ∼30% between 13 and 20 weeks of age (P < 0.05) and the OLETF-SED dropping ∼45% between 20 and 40 weeks of age (Table 2). Conversely, adiponectin in the LETO-SED was ∼50% less than both OLETF-EX and OLETF-SED at 13 weeks, but significantly increased by 20 weeks (P < 0.05). By 40 weeks of age, adiponectin concentrations did not differ among groups.
Table 2.
Serum characteristics
| Groups | Age (weeks) | Serum TG (mg dl−1) | Serum FFAs (μmol l−1) | Serum glucose (mg dl−1) | Serum insulin (ng ml−1) | HbA1c (%) | Serum adiponectin (mg l−1) |
|---|---|---|---|---|---|---|---|
| LETO-SED | 13 | 38.5 ± 5.1 | 175.2 ± 47.0 | 253.8 ± 25.7 | 7.7 ± 0.9 | 4.7 ± 0.1 | 3.5 ± 0.3a |
| 20 | 45.2 ± 4.0 | 172.9 ± 27.1 | 348.1 ± 39.2 | 9.2 ± 0.8 | 4.6 ± 0.1 | 5.2 ± 0.3b | |
| 40 | 41.5 ± 3.6 | 179.6 ± 25.1 | 276.0 ± 16.8 | 11.1 ± 0.7 | 4.6 ± 0.1 | 5.0 ± 0.4b | |
| OLETF-EX | 13 | 53.7 ± 6.6 | 98.9 ± 5.0 | 260.4 ± 34.6 | 8.6 ± 1.5 | 4.8 ± 0.1 | 7.4 ± 1.0a* |
| 20 | 71.2 ± 12.5 | 127.5 ± 30.3 | 350.5 ± 52.4 | 9.9 ± 1.2 | 4.7 ± 0.1 | 5.1 ± 0.6b | |
| 40 | 81.2 ± 11.2 | 153.1 ± 20.4 | 302.1 ± 19.2 | 11.4 ± 0.8 | 4.8 ± 0.1 | 4.0 ± 0.4b | |
| OLETF-SED | 13 | 128.2 ± 14.0a* | 222.4 ± 10.7a* | 414.3 ± 32.1a* | 10.0 ± 1.3a | 5.4 ± 0.1a | 7.2 ± 1.0a* |
| 20 | 172.4 ± 28.8b* | 306.1 ± 54.0b* | 548.5 ± 67.8b* | 12.2 ± 0.7a* | 5.3 ± 0.1a | 7.3 ± 0.7a* | |
| 40 | 265.0 ± 35.4c* | 325.6 ± 62.8b* | 683.7 ± 43.6c* | 5.0 ± 1.3b* | 8.8 ± 0.8b* | 4.0 ± 0.8b |
Values are means ±s.e.m. (n= 5–8). Values with different letter superscripts within each animal group are significantly different (P < 0.05).
Significantly different from other animal groups without symbol at respective ages (P < 0.01).
Omental fat pad characteristics
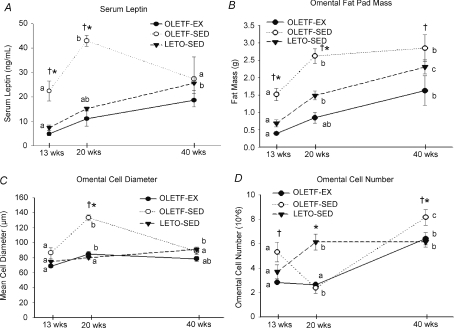
Omental fat pad mass significantly increased from 13 through 40 weeks in all groups (P < 0.05) (Fig. 2). However, omental fat pad mass increased significantly less in the OLETF-EX and LETO-SED animals at each age compared with OLETF-SED (Fig. 2). Mean fat cell diameter increased dramatically by 54% in the OLETF-SED from 13 to 20 weeks, corresponding with a drop in cell number. However, by 40 weeks of age, fat cell diameter had returned to 13 week levels in the OLETF-SED group, while cell number had increased dramatically (Figs 2 and 3). Serum leptin, a marker of fat mass, followed a similar pattern as fat cell diameter, with a dramatic rise from 13 to 20 weeks followed by a precipitous fall from 20 to 40 weeks (Fig. 2). The increase in cell size followed by a subsequent increase in cell number is similar to the progression of obesity and diabetes previously reported in high-fat fed mice (Strissel et al. 2007). In addition, while the LETO-SED showed an increase in adipocyte cell number at 20 weeks of age, the OLETF-EX did not have a similar increase until 40 weeks of age.
Figure 2. Changes in serum leptin (A), omental fat mass (B), omental fat cell diameter (adipocyte hypertrophy; C), and omental fat cell number (adipocyte hyperplasia; D).
Values (means ±s.e.m., n= 5–8) within each animal group with different letter superscripts are significantly different (P < 0.05). Significant differences (P < 0.05) between groups at a particular age are denoted by *LETO-SED vs. OLETF-SED and †OLETF-EX vs. OLETF-SED.
Figure 3. Changes in omental fat cell size and diameter.
Representative haematoxylin and eosin images for the calculation of omental fat cell size and cell number. Note the increase in fat cell diameter and a decrease in fat cell number from 13 to 20 weeks in the OLETF-SED animals. By 40 weeks of age, these changes had returned to 13 week levels in the OLETF-SED group. Histograms of adipocyte diameter at 13, 20, and 40 weeks are shown below.
Omental WAT mitochondrial content
Total content of cytochrome c, a nuclear DNA encoded mitochondrial protein, was significantly increased by 40 weeks in the LETO-SED and OLETF-EX animals but not in the OLETF-SED rats (Fig. 4A). Similarly, protein expression for the mitochondrial DNA encoded protein COXIV-subunit I was significantly increased at 40 weeks in the LETO-SED and OLETF-EX groups, but not the OLETF-SED animals (Fig. 4B). FIS1 protein, a marker of mitochondrial fission, did not differ among groups, but did display a dramatic age-associated reduction in all groups (Fig. 4C). Citrate synthase activity in omental WAT tended to be elevated in the OLETF-EX animals compared with OLETF-SED at 13 and 20 weeks of age (P= 0.07; Fig. 4D). LETO-SED and OLETF-SED groups showed an age-associated increase from 20 to 40 weeks, with LETO values significantly higher than OLETF-EX or OLETF-SED by 40 weeks. Mitofusion 2, a measure of mitochondrial fusion, also was assessed, but blots resulted in extensive non-specific binding combined with low signal (data not shown). In addition, cytochrome c oxidase and β-HAD activities were below detectable levels, highlighting the relatively low levels of mitochondria in omental WAT (data not shown).
Figure 4. Changes in markers of mitochondrial content: cytochrome c protein (A), COXIV-subunit I protein (B), Fis 1 protein (C), and citrate synthase activity (D) in omental fat.
Values (means ±s.e.m., n= 5–8) within each animal group with different letter superscripts are significantly different (P < 0.05). Significant differences (P < 0.05) between groups at a particular age are denoted by *LETO-SED vs. OLETF-SED, †OLETF-EX vs. OLETF-SED, and ‡OLETF-EX vs. LETO-SED.
Lipogenic proteins in omental WAT
The OLETF-EX rats exhibited significantly elevated FAS protein content compared with LETO-SED and OLETF-SED rats at 13 weeks of age (Fig. 5). Although there was an age-associated drop in FAS content in all groups, the elevated levels in the OLETF-EX group persisted at 20 and 40 weeks of age. SREBP1c did not differ among groups regardless of age (representative Western blot in Fig. 5).
Figure 5. Changes in protein expression of FAS and SREBP1c in omental fat.
Representative blots are shown. Values are means ±s.e.m.; n= 5–8. Within each animal group, different letter superscripts are significantly different (P < 0.05). Significant differences (P < 0.05) between groups at a particular age are denoted by *LETO-SED vs. OLETF-SED, †OLETF-EX vs. OLETF-SED, and ‡OLETF-EX vs. LETO-SED.
Discussion
Adipose tissue is becoming widely recognized as an active endocrine organ and an important regulator in whole body glucose metabolism. Limited data suggest that type 2 diabetes and obesity may be associated with lower epididymal/subcutaneous mitochondrial content and an increased accumulation of visceral WAT stores (Choo et al. 2006; Sutherland et al. 2008). Here we provide novel mechanistic insight into WAT mitochondrial content changes during the transition from insulin resistance (13 weeks) to type 2 diabetes (40 weeks) in a well characterized rodent model of hyperphagia-induced obesity and type 2 diabetes. We also examined the changes in WAT mitochondria in animals protected from type 2 diabetes by daily exercise. During the progression of type 2 diabetes, WAT mitochondrial content failed to increase in the OLETF-SED animals to the same magnitude as in lean, healthy LETO controls. In addition, daily exercise normalized changes in mitochondrial content to the level of the healthy controls. We also demonstrate that FAS protein content decreased from 13 to 40 weeks of age, but was sustained at a higher level with daily exercise in the absence of a concurrent increase in fat mass. Lastly, in the OLETF-SED rats there was a pattern of omental WAT expansion with adipocyte hypertrophy and a decrease in cell number which was followed by a return of adipocyte diameter and increase in cell number, a response that was similar to that previously reported in high-fat fed mice (Strissel et al. 2007).
It recently has been reported that mitochondrial dysfunction exists in epididymal adipocytes in type 2 diabetic mice (Choo et al. 2006). Here we report novel findings of suppressed mitochondrial protein content in omental WAT in the type 2 diabetic OLETF rat, but these differences were not apparent until overt diabetes was present. In addition, these differences appear to be unrelated to alterations in mitochondrial fission and fusion, as FIS1 protein expression decreased with age in all animal groups studied. Our findings support previous work demonstrating reduced epididymal adipocyte mitochondrial content in 33-week-old OLETF animals (Choo et al. 2006), and are in agreement with observations that adipose tissue mitochondrial content is reduced subsequent to impaired glucose homeostasis following high-fat feeding (Sutherland et al. 2008). It appears that reduced WAT mitochondrial content may not play a causal role in initiation of insulin resistance, but may be involved in a later progression to type 2 diabetes.
The progression of adipocyte hypertrophy is associated with an increase in macrophage infiltration and subsequent reduction in both adipocyte number and size (Strissel et al. 2007). Consistent with these findings, adipocyte size in the OLETF-SED group peaked at 20 weeks, corresponding with reduced cell number. By 40 weeks of age, adipocyte size was smaller, but omental fat mass was maintained due to adipocyte hyperplasia exceeding the cell number at 13 weeks of age, all of which was prevented by daily exercise. Circulating serum leptin in OLETF-SED rats followed a similar pattern as fat cell diameter, with a dramatic rise from 13 to 20 weeks followed by a precipitous fall from 20 to 40 weeks. Serum adiponectin did not follow a similar pattern, as adiponectin did not decrease until between weeks 20 and 40 in the OLETF-SED animals. In the 40-week-old OLETF-SED animals, serum leptin and adiponectin were negatively correlated with HbA1c and positively correlated with percentage body fat, suggesting that the inability to maintain elevated levels of leptin and adiponectin during hyperphagic and sedentary conditions may negatively impact glycaemic control. Additionally, mature adipocytes are known to have higher levels of mitochondrial content compared with preadipocytes (Wilson-Fritch et al. 2003), and thus a shift in cell population to a greater adipocyte : preadipocyte ratio may partially explain the differences in mitochondrial content observed in the current report. These findings warrant future investigation.
To our knowledge, we are the first to demonstrate that increased daily physical activity allows for the upregulation of WAT mitochondrial content in the hyperphagic OLETF rat, findings not seen in the sedentary OLETF animals. PPARγ agonists have been reported to reverse reductions in WAT mitochondria content and function in obese rodents in a manner similar to the effects of physical activity reported here in (Choo et al. 2006; Laplante et al. 2006). Although we did not observe a change in total PPARγ protein in WAT of young wild-type rats undergoing wheel running (Kump & Booth, 2005), others have shown an increase in PPARγ DNA binding activity with wheel running (Petridou et al. 2007). We also observed an age-associated decrease in FAS, another PPARγ-responsive protein, suggesting that increased PPARγ activity is unlikely in the current study. Interestingly, Sutherland et al. (2009) recently demonstrated that 2 h of swimming a day in rats increases mitochondrial protein content in both epididymal and retroperitoneal adipose depots. The authors attributed their findings to increases in circulating catecholamines (Sutherland et al. 2009). However, voluntary wheel running likely does not increase catecholamines as much as swimming (Higashida et al. 2008), and we did not observe an increase in mitochondrial content relative to healthy sedentary controls as did Sutherland et al. (2009). This may have also been related to the specific adipose depots examined, and future examination is needed to discern mitochondrial changes in both visceral and non-visceral adipose tissue depots. Collectively, these findings emphasize the need for future studies addressing the mechanism responsible for omental WAT mitochondrial biogenesis and the role physical activity may play in its regulation.
Because insulin-stimulated lipogenesis is positively correlated to human subcutaneous adipose mtDNA copy number (Kaaman et al. 2007), we also measured FAS and SREBP1c, two markers of lipogenesis. Unexpectedly, SREBP1c protein content did not differ among groups; however, in agreement with other studies examining epididymal WAT (Nogalska et al. 2003; Zhu et al. 2007), we observed an age-associated decrease in FAS expression in omental WAT. Although leptin is known to directly downregulate both FAS and SREBP1c (Nogalska & Swierczynski, 2004), alternative pathways may be involved since circulating leptin in our animals did not change in the same direction as FAS or SREBP1c protein content. Furthermore, unlike in human subcutaneous adipose tissue (Kaaman et al. 2007), FAS protein content was not associated with markers of mitochondrial content in omental WAT. Loss of mitochondrial content paired with the reduced FAS protein content may reduce the ability of adipose tissue to utilize glucose for lipogenesis (Choo et al. 2006), thus reducing glucose uptake into adipocytes and contributing to the elevated blood glucose levels and the diabetic condition in the OLETF-SED animals. Dysregulated glucose homeostasis and insulin resistance have been observed in adipose-specific GLUT4 knockout mice (Abel et al. 2001), indicating the importance of adipose tissue in systemic glucose homeostasis. Interestingly, daily exercise resulted in significantly higher WAT FAS protein expression at all ages studied compared with sedentary animals. The ability of exercise to increase FAS in WAT may prevent the accumulation of malonyl-CoA in a hyperphagic environment, reduce inhibition of carnitine palmitoyltransferase-1, and increase fatty acid oxidation (Hulver et al. 2005). It also is possible that increased WAT mitochondria in the LETO-SED and OLETF-EX rats may contribute to increased glyceroneogenesis and allow for better re-esterification of fatty acids, as it is known that adipose tissue mitochondria are associated with glyceroneogenesis (Wilson-Fritch et al. 2004).
Lastly, although WAT mitochondria have been shown to be essential for adiponectin synthesis (Koh et al. 2007), we failed to see an association between serum adiponectin and mitochondrial content in the omental WAT. However, the observed lack of difference in adiponectin between voluntary wheel running OLETF and sedentary OLETF rats 38–40 weeks of age is consistent with a previous report by Kimura et al. (2006). Interestingly, they reported increased serum adiponectin in pair-fed OLETF animals, which may be due to differences in adipose tissue distribution rather than the metabolic status, as others have shown (Phillips et al. 2008).
In conclusion, we demonstrate for the first time that increases in omental adipose tissue (an index of upper visceral WAT) mitochondrial content are attenuated in the hyperphagic, obese OLETF-SED rat through 40 weeks of age. Remarkably, these changes were prevented in hyperphagic OLETF-EX animals, implying that the changes were independent of dietary energy intake. The mechanism responsible for the increase in mitochondrial content appears to be unrelated to changes in FIS1 and lipogenic proteins, and likely does not play a causal role in the development of insulin resistance. However, the failure of WAT mitochondrial content to increase may play a key role in the worsening of the type 2 diabetic condition.
Acknowledgments
The OLETF and LETO-SED rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Suzie Ridenhour for excellent technical assistance and Whitney Collins and Aaron Bunker for help with animal husbandry. The authors also would like to thank the Diabetes Diagnostics Lab at the University of Missouri for help with the hemoglobin A1c measurements. This work was partially supported by the College of Veterinary Medicine at University of Missouri-Columbia, NIH grant HL-36088, and the American Heart Association (M.J.L.).
Acknowledgments
The OLETF and LETO-SED rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Suzie Ridenhour for excellent technical assistance and Whitney Collins and Aaron Bunker for help with animal husbandry. The authors also would like to thank the Diabetes Diagnostics Lab at the University of Missouri for help with the hemoglobin A1c measurements. This work was partially supported by the College of Veterinary Medicine at University of Missouri-Columbia, NIH grant HL-36088, and the American Heart Association (M.J.L.).
Glossary
Abbreviations
- COXIV-subunit I
cytochrome oxidase IV-subunit I
- FAS
fatty acid synthase
- FFA
free fatty acids
- FIS1
fission-1
- LETO
Long–Evans Tokushima Otsuka rats
- OLETF
Otsuka Long–Evans Tokushima Fatty rats
- SREBP1c
sterol response element binding protein 1c
- WAT
white adipose tissue.
Author contributions
All experiments in this investigation were performed at the University of Missouri, Columbia, MO, USA. M.J.L. – conception and design, collection of data, analysis and interpretation, drafting and revision, and final approval for publication. R.S.R. – conception and design, collection of data, analysis and interpretation, drafting and revision, and final approval for publication. S.O.W. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. S.P.N. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. A.L.P. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. G.M.U. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. M.H.L. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. J.P.T. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. F.W.B. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. J.A.I. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication.
Author contributions
All experiments in this investigation were performed at the University of Missouri, Columbia, MO, USA. M.J.L. – conception and design, collection of data, analysis and interpretation, drafting and revision, and final approval for publication. R.S.R. – conception and design, collection of data, analysis and interpretation, drafting and revision, and final approval for publication. S.O.W. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. S.P.N. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. A.L.P. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. G.M.U. – collection of data, analysis and interpretation, drafting and revision, and final approval for publication. M.H.L. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. J.P.T. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. F.W.B. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication. J.A.I. – conception and design, analysis and interpretation, drafting and revision, and final approval for publication.
References
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, Cheung P. Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes. 2005;54:880–885. doi: 10.2337/diabetes.54.3.880. [DOI] [PubMed] [Google Scholar]
- Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- Higashida K, Higuchi M, Terada S. Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem Biophys Res Commun. 2008;374:587–591. doi: 10.1016/j.bbrc.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50:2526–2533. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kimura M, Shinozaki T, Tateishi N, Yoda E, Yamauchi H, Suzuki M, Hosoyamada M, Shibasaki T. Adiponectin is regulated differently by chronic exercise than by weight-matched food restriction in hyperphagic and obese OLETF rats. Life Sci. 2006;79:2105–2111. doi: 10.1016/j.lfs.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56:2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol. 2005;565:911–925. doi: 10.1113/jphysiol.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Berger JP, Deshaies Y. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes. 2006;55:2771–2778. doi: 10.2337/db06-0551. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol. 2009;106:161–168. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol. 2007;102:1341–1347. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, Rosato FE, Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- Nogalska A, Pankiewicz A, Goyke E, Swierczynski J. The age-related inverse relationship between ob and lipogenic enzymes genes expression in rat white adipose tissue. Exp Gerontol. 2003;38:415–422. doi: 10.1016/s0531-5565(02)00210-3. [DOI] [PubMed] [Google Scholar]
- Nogalska A, Swierczynski J. Potential role of high serum leptin concentration in age-related decrease of fatty acid synthase gene expression in rat white adipose tissue. Exp Gerontol. 2004;39:147–150. doi: 10.1016/j.exger.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Petridou A, Tsalouhidou S, Tsalis G, Schulz T, Michna H, Mougios V. Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor gamma in rat adipose tissue. Metabolism. 2007;56:1029–1036. doi: 10.1016/j.metabol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab. 2008;295:E842–850. doi: 10.1152/ajpendo.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008a;586:4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008b;294:G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Turnbull D. Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci. 2008;1147:21–29. doi: 10.1196/annals.1427.016. [DOI] [PubMed] [Google Scholar]
- Shima K, Shi K, Sano T, Iwami T, Mizuno A, Noma Y. Is exercise training effective in preventing diabetes mellitus in the Otsuka-Long-Evans-Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Metabolism. 1993;42:971–977. doi: 10.1016/0026-0495(93)90009-d. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol Endocrinol Metab. 1991;261:E410–414. doi: 10.1152/ajpendo.1991.261.3.E410. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and epinephrine increase PGC-1α mRNA expression in rat adipose tissue. J Physiol. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008;295:E1076–1083. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Rohrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Lee GD, Ding L, Hu J, Qiu G, de Cabo R, Bernier M, Ingram DK, Zou S. Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp Gerontol. 2007;42:733–744. doi: 10.1016/j.exger.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]