Abstract
Endocrine disruptive compounds (EDC) alter hormone-stimulated, nuclear receptor-dependent physiological and developmental processes by a variety of mechanisms. One recently identified mode of endocrine disruption is through hormone sensitization, where the EDC modulates intracellular signaling pathways that control nuclear receptor function, thereby regulating receptor transcriptional activity indirectly. Methoxyacetic acid (MAA), the primary, active metabolite of the industrial solvent ethylene glycol monomethyl ether and a testicular toxicant, belongs to this EDC class. Modulation of nuclear receptor activity by MAA could contribute to the testicular toxicity associated with MAA exposure. In the present study, we evaluated the impact of MAA on the transcriptional activity of several nuclear receptors including the androgen receptor (AR), which plays a pivotal role in the development and maturation of spermatocytes. AR transcriptional activity is shown to be increased by MAA through a tyrosine kinase signaling pathway that involves PI3-kinase. In a combinatorial setting with AR antagonists, MAA potentiated the AR response without significantly altering the EC50 for androgen responsiveness, partially alleviating the antagonistic effect of the anti-androgens. Finally, MAA treatment of TM3 mouse testicular Leydig cells markedly increased the expression of Cyp17a1 and Shbg while suppressing Igfbp3 expression by ∼90%. De-regulation of these genes may alter androgen synthesis and action in a manner that contributes to MAA-induced testicular toxicity.
Introduction
Endocrine disruptive compounds (EDC) modulate hormonal signaling causing adverse physiological responses. Direct binding to steroid hormone receptors (nuclear receptors) leading to receptor activation, or to receptor antagonism, is a well-studied mode of endocrine disruption. However, recent studies have established that EDCs can interfere with hormone signaling through other, indirect mechanisms with effects on nuclear receptors that mediate steroid hormone action (Tabb and Blumberg, 2006). These indirect mechanisms include modulation of coactivator expression (Inoshita et al., 2003; Lonard et al., 2004), alteration of the rate of proteasome-dependent nuclear receptor degradation (Masuyama et al., 2002), changes in DNA methylation (Anway et al., 2005) and hormone sensitization (Jansen et al., 2004). Hormone sensitizing EDCs affect nuclear receptor activity via non-genomic intracellular signaling pathways, and may lead to an increase in the intrinsic transcriptional activity of the receptor without direct interactions between the EDC and the hormone or its receptor.
MAA, the toxic metabolite of the widely used industrial solvent ethylene glycol monomethyl ether (EGME) (Welsch, 2005; Bagchi and Waxman, 2007), is a hormone sensitizer that enhances the transcriptional activity of several nuclear receptors without itself being a hormone mimetic (Jansen et al., 2004). The most noticeable outcome of MAA exposure in males is testicular degradation (Li et al., 1996; Krishnamurthy et al., 1998) due to apoptosis of pachytene spermatocytes (Ku et al., 1994). The precise testicular cell target(s) of MAA and the mechanism whereby it induces germ cell apoptosis are not known. Spermatocyte apoptosis could result from direct actions of MAA on germ cells and/or could be due to indirect effects mediated through somatic cells, including Sertoli cells and/or Leydig cells, in the testis. Nuclear receptors are expressed in both germ cells and somatic cells of the testis and play an important role in spermatocyte survival and maturation. Thus, androgen receptor (AR) is required for androgen biosynthesis and spermatogenesis (Roberts and Zirkin, 1991; De Gendt et al., 2004) and estrogen receptor (ER) α is an essential mediator of the effects of estrogen on Leydig cell development (Abney, 1999). ERβ is widely expressed in testicular germ cells and somatic cells and likely contributes to germ cell maturation (O'Donnell et al., 2001). Retinoic acid receptors (RARs) also play a critical role in testes development and spermatogenesis (Vernet et al., 2006). Effects of MAA on these receptors could thus disrupt the critical physiological balance that governs nuclear receptor activity in the testes, leading to the observed germ cell toxicity.
In the present study, we investigate the effects of MAA on the transcriptional activity of AR and several other nuclear receptors. MAA is shown to enhance AR-dependent transcription without alteration of the receptor's intrinsic androgen responsiveness. This potentiation of AR activity is shown to require tyrosine kinase signaling that is independent of the Ras → MEK→ ERK signaling pathway but requires PI3 kinase activity. Furthermore, in a combinatorial setting with AR antagonists, MAA is shown to partially alleviate the antagonistic effect of anti-androgens, particularly at androgen concentrations that induce a low, suboptimal response. Finally, MAA is shown to alter the expression of several genes associated with androgen action in studies carried out in a cultured mouse Leydig cell model.
Materials and Methods
Chemicals
Dimethyl sulfoxide, testosterone, 17β-estradiol, tri-iodothyronine, all trans-retinoic acid, MAA, horse serum, linuron and vinclozolin were purchased from Sigma Chemical Co. (St. Louis, MO). The synthetic progesterone R5020 (promegestone) was purchased from Perkin Elmer Inc. (Waltham, MA). U0126 and antibodies against ERK (cat. #9102), and phosphorylated ERK (cat. #9101), were purchased from Cell Signaling Technology, Inc. (Danvers, MA). SB203580, SP600125, bisindolylmaleimide, LY294002, and PP2 were obtained from Calbiochem (San Diego, CA) and PD98059 from BioSource International (Camarillo, CA). DMEM and DMEM-F12 culture medium, fetal bovine serum (FBS) and TRIzol reagent were purchased from Invitrogen Corp. (Carlsbad, CA). Bicalutamide was a gift from AstraZeneca Pharmaceuticals (Waltham, MA).
Plasmids
The human AR expression plasmid pSG5-hAR and the androgen-responsive reporter plasmid pProbasin-Luc, which contains two naturally occurring androgen response elements (AREs), were obtained from Dr. Ollie Janne (Institute of Biomedicine, Univ. of Helsinki, Finland). The human ERα and ERβ expression plasmids, pcDNA3.1-hERα and pcDNA3.1-hERβ, were obtained from Dr. Myles Brown (Dana-Farber Cancer Institute, Boston, MA). The ER-C3-luciferase reporter plasmid was obtained from Dr. Kevin Gaido (CIIT Centers for Health Research, Research Triangle Park, NC). Thyroid hormone receptor (TR)-β expression plasmid, pCDM8-TR, a luciferase reporter vector containing three copies of a thyroid hormone-responsive DR4 element, pTK-TR(DR4)3, and the retinoic acid reporter plasmid pGL3-β-RARE-luc were obtained from Dr. David Moore (Baylor College of Medicine, Houston Texas). The retinoic acid receptor (RAR) expression vectors pSG-mRARα, pTL-mRARβ and pTL-mRARγ were provided by Dr. Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France). Expression plasmids for the coactivators GRIP1, p300 and CARMI were obtained from Dr. Michael R. Stallcup (Univ. of Southern California, Los Angeles, CA). Human progesterone receptor expression plasmid (hPR-B) and its reporter plasmid were gifts from Dr. Donald P. McDonnell (Duke University Medical Center, Durham, NC). pSV-βgal vector, containing the SV40 early promoter and enhancer upstream of bacterial lacZ gene, encoding β-galactosidase, was purchased from Promega Corp. (Madison, WI).
Cell culture and transient transfection
tsA201 cells, a derivative of the cell line HEK293, were obtained from Dr. J. Larry Jameson (Northwestern Univ. Medical School, Chicago, IL) and grown in DMEM containing 10% FBS. TM3 mouse Leydig cells and TM4 mouse Sertoli cells (American Type Culture Collection, Manassas, VA) were grown in DMEM-F12 containing 5% horse serum and 2.5% FBS. HepG2 human hepatocellular carcinoma cells (American Type Culture Collection) were grown in Eagle's Minimum Essential Medium containing 10% FBS. For transfection studies, ∼ 3 × 104 cells/well (tsA201, HepG2 and TM3 cells) were plated in 48-well tissue culture plates. 24 h later, the cells in individual wells were transfected with a total of 250 ng DNA/well using 0.3 μl of TransIT-LT1 transfection reagent (Mirus Bio, Madison, WI) and salmon sperm DNA as a carrier. TransIT-LT1 (0.3 μl/well) was pre-incubated with DMEM without serum, following which DNA was added to the TransIT-DMEM mix and incubated for 15 min at room temperature. This cocktail was then aliquoted into each well to be transfected. To improve the transfection efficiency of TM3 cells, Hoechst dye 33258 (Molecular Probes, Eugene, OR) was included in the transfection mix at 100 μM final concentration. DNA to be transfected was added to the serum free medium followed by addition of Hoechst 33258 and incubated for 30 min at room temperature. TransIT-LT1 was preincubated with serum free medium for 5 min and added to the Hoechst dye-DNA mix and further incubated for 15 min. The mix was then added equally to each well to be transfected (Shipley and Waxman, 2006). Transfections were performed using the following amounts of plasmid DNA per well of a 48-well tissue culture plate: 10 ng of receptor plasmid (AR, ERα, ERβ, TRβ, PR-B, RARα, RARβ or RARγ), 90 ng of reporter plasmid (pPB-luc, ERC3-luciferase, pTK-TR(DR4)3, progesterone receptor reporter plasmid or pGL3-β-RARE-luc), 20 ng of pSV-βgal (for normalization of luciferase activity) and 130 ng salmon sperm DNA. For coactivator experiments, transfections were performed using 1 ng or 15 ng AR, 90 ng of pProbasin-luc reporter plasmid, 20 ng of pSV-βgal, 50 ng of coactivator plasmids (10 ng GRIP1, 20 ng p300 and 20 ng CARM1) and 80 ng salmon sperm DNA.
Reporter assay
Transfected cells were stimulated for 24 h (or 6 h for the inhibitor studies) with the nuclear receptor ligands testosterone, 17β-estradiol (E2), tri-iodothyronine (T3), R5020 or all-trans retinoic acid dissolved in DMSO (10 nM each, except for the dose-response curves shown in Fig. 5) in the presence or absence of 5 mM MAA, as specified in each experiment. Each treatment (with vehicle, hormone, etc.) was administered to transfected cells in three separate wells. MAA stock solution was prepared by diluting MAA in culture medium (without serum), followed by adjustment of the pH to 7.4 with 10 N NaOH, to yield a final MAA concentration of 5 mM. Cells were treated with 0.1% DMSO as a vehicle control. For inhibitor experiments, transfected cells were pretreated with the inhibitor for 30 min before addition of testosterone and/or MAA to the culture medium. Following treatment, the cultured medium was removed, and 100 μl passive lysis buffer (Promega) was added to each well for 15 min to lyse the cells. Firefly luciferase and β-galactosidase activities were respectively assayed using Luciferase assay reagent from Promega Corp. (Madison, WI) and Galacto-Light Plus™ beta-Galactosidase Reporter Gene Assay System from Applied Biosystems (Foster City, CA) and a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). The resultant firefly luciferase activity was divided by the measured β-galactosidase activity to normalize for transfection efficiency. For each transfection experiment, triplicate sets of analyses were carried out for each data point on every bar graph and line graph. The n=3 reported for each transfection experiment thus indicates normalized reporter activities obtained from three separate platings of cells from the same passage, each of which was transfected and treated individually and processed for reporter assays, in parallel. Reporter gene assays (luciferase and β-galactosidase) were carried out on each of the triplicate cell samples and normalized reporter activities were calculated. The normalized activity obtained from cells stimulated with ligand alone was set = 1.0 and activities corresponding to all other treatments were expressed in relation to this value. Data shown are mean ± SD values, based on the triplicate analyses. Furthermore, in all cases, the experimental results were verified using at least two or three other passages of the cell line, each of which was carried out as a set of three independent transfections (i.e., n=3), as outlined above.
Fig. 5. Impact of MAA in combination with AR antagonists on AR dose-response curve.
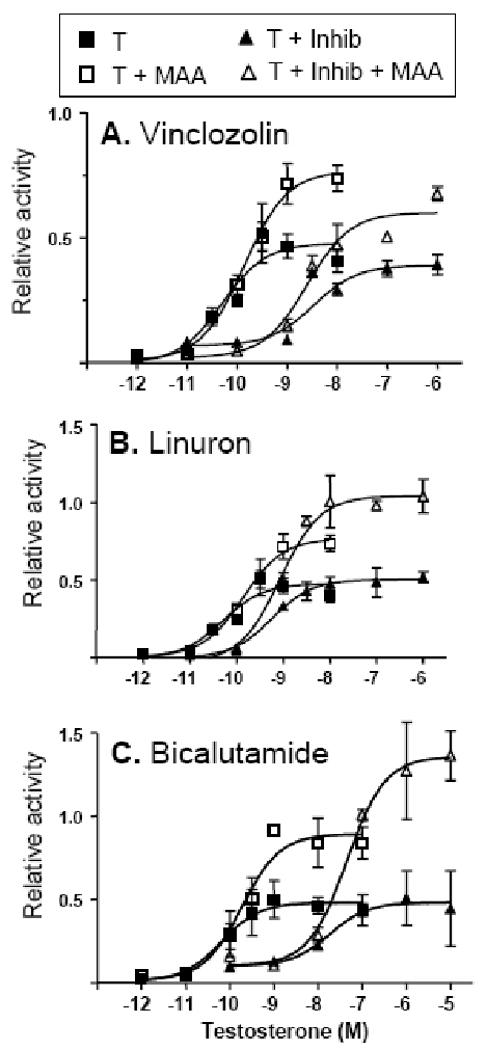
tsA201 cells were transfected as described in Fig. 3B and stimulated for 24 h with increasing concentrations of testosterone (T), either alone or in combination with MAA and/or each of the indicated AR antagonists at 10 μM: vinclozolin (A), linuron (B), and bicalutamide (C). Determination of normalized luciferase reporter activity was as described in Fig. 1. Non-linear regression analysis was performed using GraphPad Prism software based on n= 3 separate transfections from the same passage of cells for each data point. EC50 values for testosterone stimulation were as specified under Results. Comparison of the four curves within each panel by F-test confirmed that the dose-response curves were significantly different from each other, p < 0.0001 in the case of panels A, B and C.
Determination of EC50 values
tsA201 cells transfected with AR expression plasmid and pPB luciferase reporter plasmid were treated for 24 h with six or more concentrations of testosterone alone or in combination with MAA, and in the presence or absence of an AR antagonist. Controls included vehicle alone (DMSO, 0.1%), MAA alone (5 mM), AR antagonist alone (10 μM), and MAA + antagonist combined. Normalized reporter activities determined as described above were expressed relative to the DMSO control. EC50 values were determined by non-linear regression analysis using GraphPad Prism software, version 4.0 (GraphPad, San Diego, CA).
Western blotting
tsA201 cells were seeded in 6-well plates at ∼ 1.5 × 105 cells/well and allowed to grow overnight, following which the standard culture medium was replaced by starvation medium (DMEM without phenol red + 0.5% charcoal stripped serum). Twenty four hour later, the cells were treated for time periods ranging from 2 to 15 min with 5 mM MAA, with culture medium alone (negative control) or with culture medium + 10% FBS (positive control). Where indicated, 10 μM U0126 was added to the cells 30 min prior to the addition of MAA or stimulation of the cells with FBS. Cells lysates (20 μg protein/lane) were analyzed on Western blots probed with anti-phospho-ERK and anti-ERK antibodies according to the manufacturers' protocol. Membranes were incubated in 5% non-fat milk blocking buffer for 1 h at room temperature, washed 3× with 10 mM Tris, 150 mM NaCl, and 0.1% v/v Tween 20 and then incubated with primary antibody (1:1000 dilution) overnight at 4°C. The membrane was subsequently washed and incubated with HRP-conjugated secondary antibody and developed using Amersham ECL™ detection reagent (GE Healthcare, Piscataway, NJ).
qPCR analysis
TM3 cells were seeded in 6-well plates, cultured overnight to ∼ 60% confluence and then treated with 5 mM MAA or with culture medium alone for 3 h or 24 h. Total RNA was prepared using TRIzol reagent following the manufacturer's protocol. RNA samples were treated with RQ1 RNAse-free DNAse for 1 h at 37°C followed by heating at 75°C for 5 min. cDNA synthesis and real time qPCR analysis using SYBR Green I-based chemistry was as described (Holloway et al., 2006). Dissociation curves were examined after each qPCR run to ensure amplification of a single, specific product at the correct melting temperature. qPCR primers were designed using Primer express software (Applied Biosystems) (Table 1). Relative RNA levels were calculated after normalization to the 18S rRNA content of each sample and are based on duplicate RNA samples for each of three independent sets of experiments (mean ± SE for n = 3).
Table 1.
Mouse qPCR Primer sets and Genbank Accession Numbers
| Gene | Oligo No. | Accession | Amplicon(nt) | Forward primer | Reverse primer |
|---|---|---|---|---|---|
| AR | 1762/1763 | NM_013476 | 51 | AACTCGATCGCATCATTGCA | TTGAGCAGGATGTGGGATTCT |
| ABP | 1742/1743 | NM_013476 | 65 | CTGGCCAGCTTGAAATCCA | CCGAGGACCAAAGCCTACTGT |
| Igfbp3 | 1764/1765 | NM_008343 | 54 | CATATGCCTGAGGCTCATGGT | GCCAGGCCCTTATTCAGAGAT |
| StAR | 2028/2029 | NM_011485 | 53 | TTCATCCGCAGTGCCATTT | CCCACACGATAAGGGACAGAA |
| Cyp19a1 | 2030/2031 | NM_007810 | 51 | CCGAGCCTTTGGAGAACAATT | GCCCGTCAGAGCTTTCATAAA |
| Cyp17a1 | 1408/1409 | NM_007809 | 51 | TGCCCCTGGTGGGTAGTCTA | CATGCATATGACCACGTCTGG |
Statistical analysis
Statistical analyses were implemented using GraphPad Prism, v4.0 software. In Figures 1-4 and 6, unpaired student's t test was used to compare treatment groups for differences in responsiveness to hormone or MAA treatments. Non-linear regression analysis followed by F test was used to determine whether nuclear receptor dose-response curves presented in Fig. 5 were significantly different from each other. A value of p <0.05 was used as the limit of significance.
Fig. 1. MAA enhances ligand-induced transcriptional activity of select nuclear receptors.
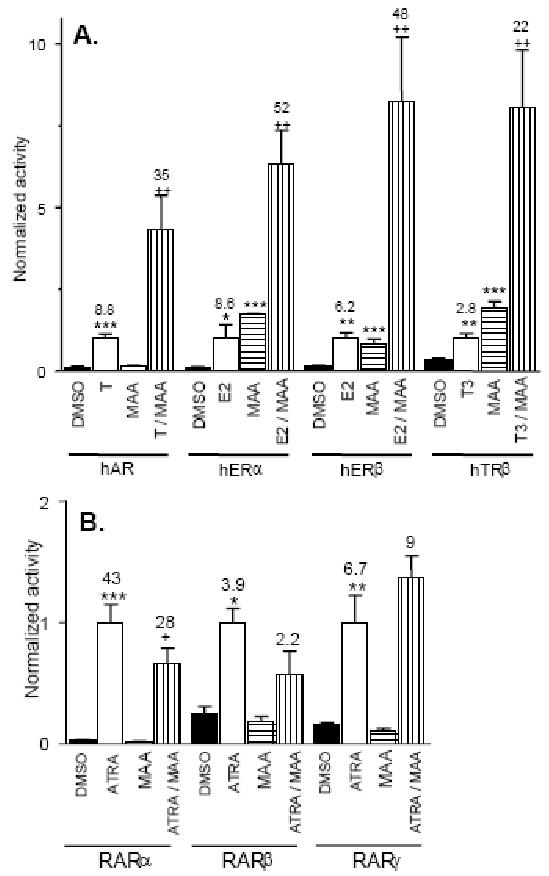
tsA201 cells were co-transfected with human (h) AR, ERα, ERβ or TRβ (panel A) or RARα, β or γ (panel B), as indicated, and their corresponding response element-directed firefly luciferase reporters. Cells were transfected with pSV-βgal plasmid as an internal control. ∼20 h after transfection, the cells were treated for 24 hr with 10 nM each ligand, testosterone (T), estradiol (E2) or triiodothyronine (T3) (panel A) or all-trans retinoic acid (ATRA, panel B), alone or in combination with 5 mM MAA. Cells treated with 0.1 % DMSO (vehicle) or with MAA alone served as controls. Firefly luciferase and β-galactosidase activity were measured and normalized reporter activity was determined, with the activity obtained from cells stimulated with ligand alone set = 1.0. Data shown are mean ± SD values for n= 3 independent transfections from a single passage of cells. The fold-change in hormone-stimulated activity compared to DMSO controls were 8.8, 8.6, 6.2 and 2.8 respectively for AR, ERα, ERβ and TRβ, as noted above each bar. Receptor activity increased to 35, 52, 48 and 22 (increased 4.3, 6.3, 8.2 and 8-fold, above treatment with hormone alone), in cells treated with hormone + MAA (panel A). Fold change in activity in the case of RAR isoforms α, β and γ, were 43, 3.9 and 6.7-fold, respectively, compared to the vehicle control. However, there was no increase in activity when the cells were treated with ATRA + MAA (panel B). Statistical analysis was carried out by T test: *, ** and *** indicate p < 0.05, p < 0.01 and p <0.001, respectively, for receptor reporter activity obtained in cells treated with hormone versus DMSO control, and for cells treated with MAA versus DMSO control. +, ++ and +++, indicate p < 0.05, p < 0.01 and p <0.001, respectively, for differences in activity between cells treated with hormone + MAA compared to hormone treatment alone.
Fig. 4. Tyrosine kinase and PI3 kinase signaling is required for MAA to increase AR transcriptional activity.
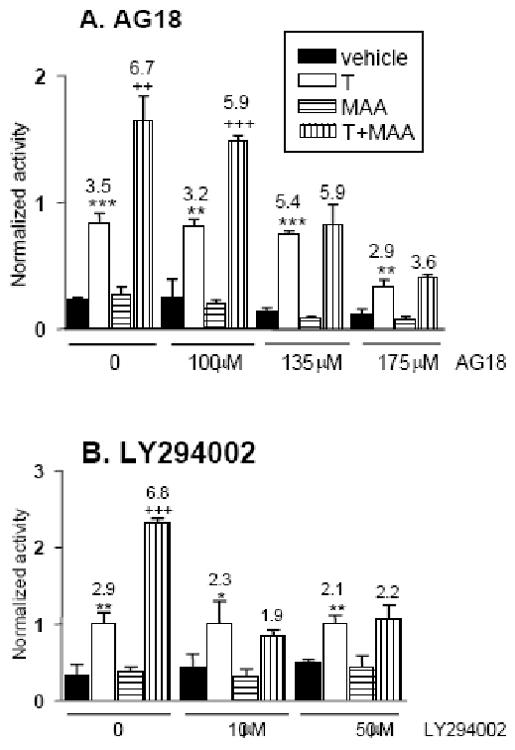
A. tsA201 cells were transfected and treated as described in Fig. 3B, except that the cells were treated for 6 h with testosterone, MAA and/or the tyrosine kinase inhibitor AG18 at the indicated concentrations. MAA potentiation of AR activity (increase in activity from 3.5-fold to 6.7-fold) was abolished in presence of 135 μM AG18. B. Experiment was as described in panel A, except that the cells were pre-treated with LY294002 at the indicated concentrations for 30 min, and then treated with LY294002 plus testosterone and MAA for the duration of the experiment, as indicated. Normalized reporter activity and data presentation were as described in Fig. 1. LY294002 abolished the MAA-dependent increase in AR transcriptional activity (increased from 2.9-fold to 6.8-fold) at both 10 μM and 50 μM.
Fig. 6. Gene expression changes induced by MAA treatment of TM3 cells.
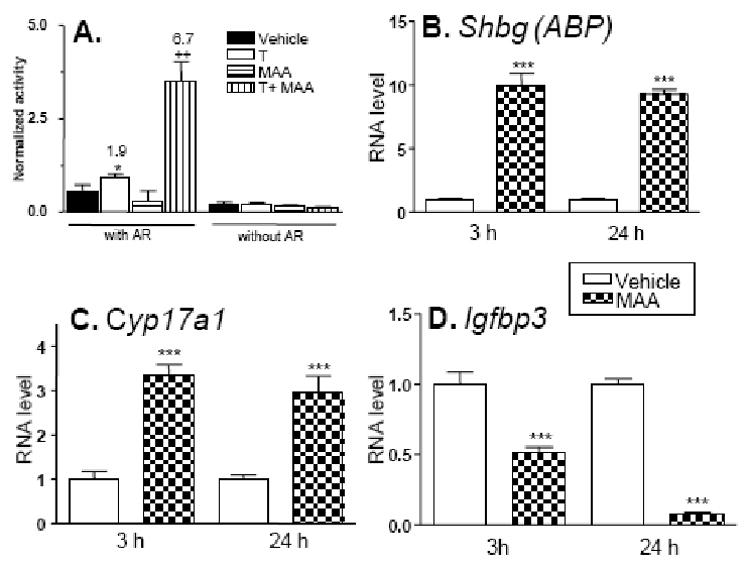
A. TM3 cells were assayed for 24 h testosterone-stimulated AR transcriptional activity using pProbasin-luc reporter, with or without transfection of exogenous AR expression plasmid. Normalized reporter activity and data presentation were as described in Fig. 1. Panels B, C and D: TM3 cells were treated for 3 h or 24 h with culture medium in the absence or presence of 5 mM MAA. RNA was isolated and analyzed by qPCR for expression of Shbg (ABP), Cyp17a1, or Igfbp3 as described in Materials and Methods. Data are graphed as relative RNA levels (mean ± SE), normalized to the 18S rRNA content of each sample, for duplicate samples obtained for each of three different batches of RNA prepared from separate passages of TM3 cells. Statistical analysis was carried out by T-test using GraphPad Prism version 4.0, *** indicates p<0.001, for culture medium alone vs. MAA treatment.
Results
MAA enhances transcriptional activity of steroid hormone-activated nuclear receptors
The effect of MAA on the transcriptional activities of AR and several other nuclear receptors was evaluated in a cell-based reporter gene assay. These studies were carried out in tsA201 cells, which are readily transfected (Margolskee et al., 1993) and express negligible levels of several nuclear receptors (unpublished results). Cells were transfected with plasmids encoding AR, ERα, ERβ, TRβ or RARα, β, γ together with their cognate reporter plasmids. Cells were stimulated for 24 h with ligands specific for each receptor in the presence or absence of MAA; MAA was used at a concentration (5 mM) similar to that found in EGME-treated rats and in human urine after occupational exposure to EGME (Terry et al., 1994; Shih et al., 2001). Each ligand activated the transcriptional activity of its respective receptor as shown in Fig. 1A and Fig. 1B. MAA enhanced the hormone-stimulated transcriptional activity of AR 4.3-fold (increase in normalized activity from 8.8 to 35-fold in the presence of MAA; Fig. 1A). Similarly, MAA increased the activity of ERα, ERβ and TRβ by 6.3, 8.2 and 8-fold, respectively, when compared with ligand alone (Fig. 1A). No potentiation of transcriptional activity was observed in case of the RARs (Fig. 1B), demonstrating the selectivity of MAA for a subset of nuclear receptors.
MAA potentiates coactivator-mediated increases in AR activity
Nuclear receptors recruit coactivator proteins to remodel chromatin structure and activate the transcription initiation complex. Binding of an agonist ligand modifies the receptor's conformation in a way that promotes coactivator binding (Glass and Rosenfeld, 2000). Coactivators of the p160 family, such as GRIP1, facilitate the binding of other coactivators, such as p300, which mediate histone acetylation and formation of stable complexes with basal transcription factors. GRIP1 also facilitates the binding of protein methyl transferases, such as CARM1, which methylates specific arginine residues in histones and other proteins in the transcription initiation complex and enhances transcription. Nuclear receptor transcriptional activity can be markedly increased upon co-expression of these coactivators, in particular, when low receptor amounts are used in transfection assays (∼1-4 ng receptor/well of a 48-well plate) (Lee et al., 2002). The impact of MAA on coactivator-mediated increases in AR transcriptional activity was investigated in tsA201 cells transfected with AR at both low and high plasmid levels (1 and 15 ng/well, respectively) in the absence (AR) and presence (AR + CoAc) of the coactivators GRIP1, p300 and CARM1. Fig. 2 shows that these coactivators enhanced AR activity > 2 fold at both low and high AR levels, evidencing the functional expression of these coactivators. MAA further enhanced this activity >2 fold, indicating that potentiation by MAA proceeds through a mechanism distinct from that used by these coactivators.
Fig. 2. MAA enhances coactivator-mediated increases in AR transcriptional activity.
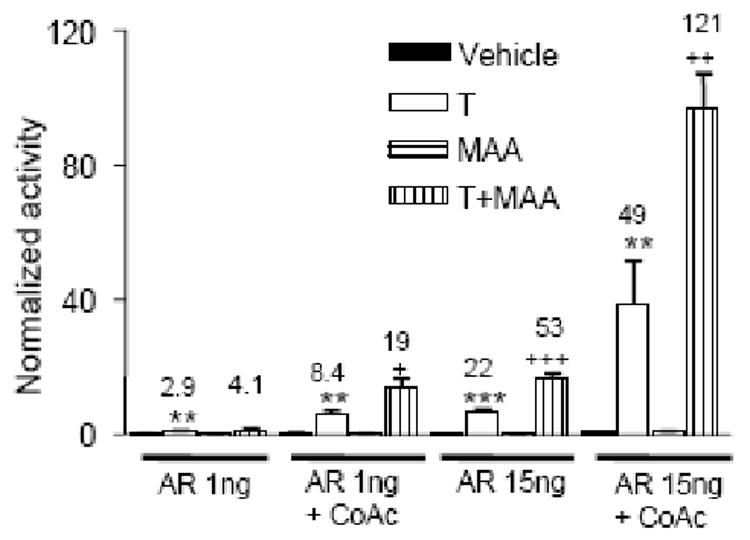
tsA201 cells seeded in 48-well plates were transfected with either 1ng or 15 ng of AR expression plasmid, as indicated, in the presence or absence of expression plasmids for a combination of the coactivators (CoAc), GRIP1 (10 ng), p300 (20 ng) and CARM1 (20 ng). All cells were also transfected with 90 ng of AR reporter plasmid pProbasin-luc and pSV-βgal as internal control. Transfected cells were stimulated for 24 h with testosterone (T), alone or in combination with MAA. Cells treated with vehicle or with MAA alone served as controls. Normalized reporter activity, data presentation and statistical analysis were as described in Fig. 1. In cells co-transfected with the coactivators, testosterone-stimulated AR reporter activity increased from 2.9-fold to 8.4-fold in the presence of 1 ng AR expression plasmid, and from 22-fold to 49-fold in cells transfected with 15 ng AR. MAA enhanced this coactivator mediated increase in T response from 8.4 to 19-fold and from 49 to 121-fold, as shown.
MAP kinase activation
MAA is reported to increase kinase activity in spermatocytes, and kinase inhibitors can block MAA-induced germ cell toxicity (Jindo et al., 2001). In other studies, MAA induced MAP kinase activity in HeLa cells, resulting in phosphorylation of ERK, while U0126, a selective inhibitor of ERK phosphorylation, decreased the potentiation of progesterone receptor transcriptional activity by MAA (Jansen et al., 2004). We therefore investigated 1) whether MAP kinase signaling was activated by MAA in tsA201 cells, and 2) if the inhibition of this activity would interfere with MAA-induced potentiation of AR signaling. MAA treatment led to a striking increase in ERK phosphorylation, which peaked at 5 min (Fig. 3A). MAA-induced ERK phosphorylation was completely blocked by the ERK MAP kinase pathway inhibitor U0126 (Fig. 3A). However, U0126 did not inhibit the potentiation of AR signaling by MAA (Fig. 3B). A second inhibitor of ERK phosphorylation, PD98059, was also without effect (Table 2). To determine if the inhibitory effect of U0126 on MAA potentiation of progesterone receptor activity in HepG2 cells reported earlier (Jansen et al., 2004) was specific to progesterone receptor and/or the HepG2 cell model, we investigated the effect of U0126 on MAA-induced potentiation of AR and progesterone receptor activity in HepG2 cells. U0126 did not block MAA-induced potentiation of progesterone receptor activity (Fig. 3C) or AR activity (data not shown) in HepG2 cells. Inhibitors of the p38 and JNK MAP kinase pathways were also without effect on MAA potentiation (Table 2).
Fig. 3. MAA-activated MAPK signaling is not required for potentiation of AR activity.
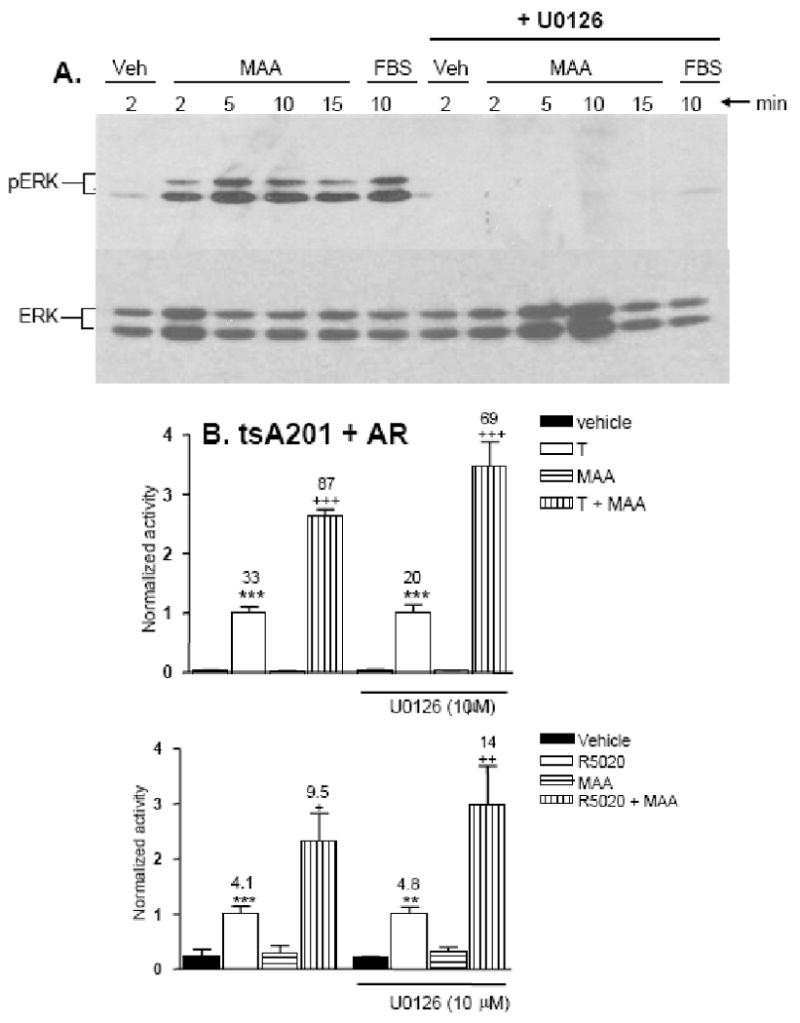
A. tsA201 cells were treated with culture medium alone (Veh), medium + 10 % FBS, or medium + 5 mM MAA, for 2′, 5′, 10′ or 15′, as indicated, in the presence or absence of the MAPK pathway inhibitor U0126 (10 μM). Whole cell lysates were analyzed on a Western blot probed with anti-phospho-ERK (top panel) and anti-ERK antibody (bottom panel). Phosphorylation of the pair of ERK MAPK proteins was maximal after 5 min of MAA treatment and was completely blocked by U0126. FBS treatment served as a positive control for MAPK pathway activation and for the inhibitory effect of U0126. B. tsA201 cells co-transfected with AR, pProbasin-luc and pSV-βgal were treated for 24 h with vehicle, testosterone (T), MAA or testosterone + MAA in the presence or absence of 10 μM U0126. Normalized reporter activity and data presentation were as described in Fig. 1. Co-treatment with MAA increased the T-induced activity by 2.6-fold and 3.4-fold, in the absence and presence of U0126, respectively, indicating that U0126 does not block MAA potentiation of AR transcriptional activity. C. HepG2 cells were co-transfected with progesterone receptor expression and reporter plasmids and pSV-βgal as an internal control. ∼20 h post-transfection, cells were treated for 24 h with vehicle, the synthetic progestin R5020 (10 nM), MAA, or with R5020 (10 nM) + MAA, in presence or absence of 10 μM U1026, as indicated. Normalized reporter activity and data presentation were as described in Fig. 1. MAA-potentiation of progesterone receptor activity (increase from 4.1-fold to 9.5-fold) was not inhibited by U0126 in HepG2 cells (increase from 4.8-fold to 14-fold).
Table 2. Effects of signaling pathway inhibitors on MAA-induced potentiation of AR activity.
Data shown are the fold-increase in testosterone-stimulated AR activity observed in the presence of MAA alone, or in the presence of MAA + inhibitor, as compared to testosterone treatment alone (set = 1.0). The concentration(s) of each inhibitor tested and the enzyme or pathway that is inhibited were as indicated. Each value shown in the table is based on triplicate determinations using the same methods and experiment design described for U0126, AG18 and LY294002 in Fig. 3 and Fig. 4. Data are presented for two independent sets of such experiments for four of the inhibitors, as listed. None of the inhibitors blocked MAA potentiation of AR activity, since no reduction in the MAA-induced “fold-increase” in AR activity was observed (compare column 4 to column 3). MEK, ERK1 and ERK2 kinase; JNK, c-Jun N-terminal kinase; PKC, protein kinase C.
| Inhibitor (concentration) | Enzyme/Pathway inhibited | MAA potentiation (fold-increase) |
MAA + Inhibitor (fold-increase) |
|---|---|---|---|
| PD98059 (20 μM) | MEK | 1.8 | 1.9 |
| SB203580 (10 μM) | p38 | 1.8, 1.7 | 2.2, 2.3 |
| SP600125 (10 μM) | JNK | 1.8 | 1.9 |
| PP2 (1 and 10 μM) | Src kinase | 1.9, 1.9 | 2.5, 2.4 |
| Bisindolyl- maleimide (1 μM) | PKC | 1.7, 2.0 | 1.9, 2.0 |
| N-acetyl cysteine (10 mM) | Glutathione oxidation | 1.7, 2.4 | 1.9, 2.4 |
Tyrosine kinase and phosphatidyl inositol (PI)-3 kinase inhibitors block MAA-induced AR potentiation
ERK phosphorylation is typically a downstream response to receptor tyrosine kinase activation, suggesting that MAA may induce receptor tyrosine kinase activity in tsA201 cells. Tyrosine kinases such as Her2/Neu stimulate androgen signaling in some cells (Craft et al., 1999; Yeh et al., 1999). We therefore investigated the effect of tyrosine kinase inhibitors on the potentiation of AR activity by MAA. AG18, a broad range inhibitor of protein tyrosine kinases (Jaleel et al., 2004), fully blocked MAA-induced potentiation of AR activity at 135 μM (Fig. 4A). Thus, tyrosine kinase activity is required for the AR stimulatory effect of MAA.
The testis is rich in non-receptor protein tyrosine kinases including Src family proteins, which mediate both physiological and pathological events in this organ (Elberg et al., 1995). Strong Src immunostaining is observed in rat spermatocytes that are undergoing apoptosis following MAA treatment (Jindo et al., 2001). Experiments using the Src inhibitor PP2 indicated, however, that Src kinase activity is not required for the enhancement of AR activity by MAA (Table 2). Similarly, the MAA response was not blocked by bisindolyl maleimide, an inhibitor of protein kinase C, which is activated downstream of several tyrosine kinase signaling pathways (Table 2).
PI3 kinase signaling, downstream of tyrosine kinase signaling, has been shown to enhance AR transcriptional activity (Sharma et al., 2002). We therefore examined the effects of LY294002, a PI3 kinase-selective inhibitor. LY294002 (10 μM) fully blocked the potentiation of AR transcriptional activity by MAA (Fig. 4B). We conclude that the MAA-dependent increase in AR activity proceeds via tyrosine kinase activation leading to PI3 kinase activation, which is typically associated with the activation of the serine/threonine kinase Akt and other downstream signaling pathways (Cantley, 2002).
MAA decreases the ratio of reduced glutathione to oxidized glutathione in germ cells, triggering apoptosis; restoration of glutathione levels by treatment with N-acetyl cysteine prevents germ cell death (Rao and Shaha, 2002). Several intracellular signaling pathways, including tyrosine kinase signaling, are modulated by the intracellular redox state (Kamata and Hirata, 1999), suggesting that the effects of MAA on tyrosine kinase signaling and AR potentiation could involve changes in redox state. We investigated this possibility by testing whether N-acetyl cysteine could block the potentiation of AR by MAA, however, no such effect was observed (Table 2), suggesting this mechanism does not contribute to AR potentiation.
Combination of MAA with AR antagonists
Several EDCs have been characterized as anti-androgens that can induce toxicity both in vivo and in vitro by antagonizing AR action (Kelce et al., 1998). This prompted us to investigate whether the stimulatory effects of MAA on AR activity lead to a moderation of the anti-androgenic activity of these agents, or alternatively, whether MAA potentiates the inhibitory activity of these EDCs in a combinatorial setting. AR reporter gene assays were used to investigate the effects of three AR antagonists: linuron, a herbicide; vinclozolin, a pesticide; and bicalutamide, a pharmaceutical agent used for prostate cancer treatment (10 μM each). First, we determined that testosterone activated AR with an EC50 of ∼ 0.18 nM, both in the presence and in the absence of MAA (Fig. 5). This indicates that MAA enhances the maximal transcriptional activity of AR without altering the receptor's intrinsic sensitivity to androgen. Next, the EC50 for testosterone stimulation of AR activity was determined in the presence of each AR antagonist. Linuron increased the EC50 (testosterone) from 0.18 nM to 0.58 nM (3.2-fold increase). The EC50 for testosterone stimulation was increased 19-fold (EC50 = 3.4 nM) and 100-fold (EC50 = 18 nM) in the presence of vinclozolin and bicalutamide, respectively. These findings are consistent with earlier reports that these anti-androgens are competitive inhibitors of androgen binding (Blackledge, 1996; McIntyre et al., 2000; Molina-Molina et al., 2006). EC50 values of 0.86 nM, 2.5 nM and 45 nM were respectively determined for the three anti-androgens, when combined with MAA (Fig. 5). Thus, all three anti-androgens retain their AR inhibitory activity in the presence of MAA. However, as seen in Fig. 5, MAA can potentiate AR signaling even in the presence of these AR antagonists. Of note, at some testosterone concentrations, MAA increased AR activity measured in the presence of antagonist to the activity level obtained with testosterone alone. For example, AR transcriptional activity determined at 1 nM testosterone in the presence of linuron + MAA is similar to AR activity determined in cells treated with 1 nM testosterone alone (Fig. 5B). Thus, MAA can partially alleviate the inhibitory effects of these anti-androgens.
MAA induces gene expression changes in mouse Leydig cells
MAA elicits changes in gene expression in primary spermatocytes and in Sertoli cells (reviewed by (Bagchi and Waxman, 2007)), however, the effects of MAA on other testicular cells are not well characterized. As germ cell development and viability requires Leydig cells, as well as Sertoli cells, MAA-induced gene expression changes in these cell types could contribute to MAA-induced toxicity. Two mouse testicular cell lines, TM3 Leydig cells and TM4 Sertoli cells, were investigated for their ability to support MAA potentiation of AR activity. MAA induced potentiation of AR activity was observed in TM3 cells (Fig. 6A, left) but not in TM4 cells (data not shown). This potentiation was dependent on transfection of exogenous AR, as the endogenous level of AR was too low to support reporter gene activity (Fig. 6A, right). Next we investigated whether MAA induces changes in the expression of androgen responsive genes or genes associated with AR signaling in TM3 cells. Genes that were studied included steroid 17α-hydroxylase/17,20-lyase (Cyp17a1), aromatase (Cyp19a1), steroidogenic acute regulatory protein (Star), androgen binding protein (Shbg) and insulin-like growth factor binding protein-3 (Igfbp3). These genes are all expressed in Leydig cells except Shbg, which encodes androgen-binding protein (ABP), and reportedly is expressed in Sertoli cells (Bardin et al., 1981). Gene expression changes were monitored by qPCR analysis of RNA isolated from TM3 cells treated with MAA for 3 h and 24 h. Significant increases in expression were observed for Cyp17a1 (3-fold increase) and Shbg (10-fold increase) while Igfbp3 expression was markedly decreased (by ∼90% at 24 h) following MAA treatment (Fig. 6B-6D). Negligible changes in expression were observed for Star (data not shown). Cyp19a1 (aromatase) expression was undetectable with or without MAA treatment (data not shown). Thus, MAA induces significant changes in the expression of Leydig cell genes involved in AR signaling.
Discussion
Nuclear receptors play a critical role in the survival and proper functioning of testicular cells. The present studies show that MAA can potentiate the transcriptional activity of several testis expressed nuclear receptors, including AR and ER, but not RARs (α, β or γ). The selectivity of MAA for certain nuclear receptors could reflect differences in the requirements of each receptor for coactivators, which are potential targets of MAA. However, our finding that MAA potentiates AR transcriptional activity even in the presence of potent coactivators of AR suggests that this potentiation proceeds by a mechanism that is independent of these coactivators. Since the balance between androgens and estrogens is critical for normal testicular development (Sharpe et al., 1998) and adult male reproductive behavior (Williams et al., 2001), any disturbance of this balance caused by MAA could potentially be harmful and may contribute to testicular toxicity. AR plays a critical role in male sexual development, and the expression of several genes in the testes is dependent upon appropriate levels of AR signaling (Zhou et al., 2005; Eacker et al., 2007). Increased AR signaling in response to MAA exposure could thus lead to deregulation of these genes and contribute to testicular toxicity.
AG18, a general tyrosine kinase inhibitor, blocked the AR potentiating activity of MAA, indicating a requirement for tyrosine kinase activity for MAA action. MAA was shown to induce the phosphorylation of the MAP kinases ERK1 and ERK2, however, inhibition of this phosphorylation using the MEK inhibitor U0126 did not decrease the extent to which MAA potentiated AR transcriptional activity. In contrast, the PI3 kinase inhibitor LY294002 blocked the potentiation of AR transcriptional activity by MAA, indicating a key role for PI3 kinase pathway in MAA action, as outlined in the model for MAA action shown in Fig. 7. A functional link between AR and PI3 kinase signaling pathways has been described (Craft et al., 1999; Yeh et al., 1999; Sharma et al., 2002; Taneja et al., 2005). Conceivably, PI3 kinase may augment the transcriptional activity of AR by inducing nuclear accumulation of β-catenin, which binds to the AF-2 domain of the agonist bound AR (Song et al., 2003) and increases AR transcriptional activity (Sharma et al., 2002; Song et al., 2003).
Fig. 7. Proposed pathways leading to potentiation of AR transcriptional activity by MAA.
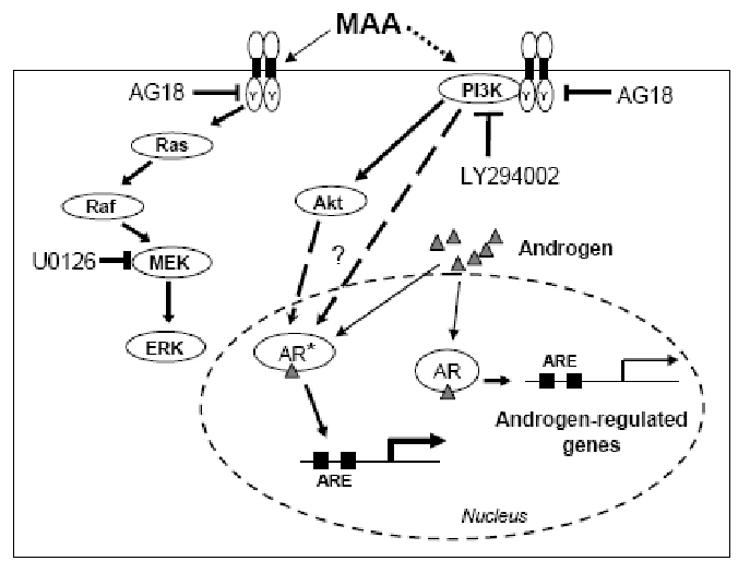
The broad range tyrosine kinase inhibitor AG18 blocks the potentiation of AR transcription by MAA. Activation of tyrosine kinase(s) at the cell surface, or elsewhere in the cell, leads to the activation of multiple intracellular signaling pathways, including the ERK MAP kinase pathway (left) and PI3 kinase signaling (right). MAA treatment activates phosphoryation of the MAP kinase ERK1 and ERK2, however, inhibition of this pathway using the MEK inhibitor U0126 does not block MAA-induced AR potentiation. In contrast, inhibition of PI3 kinase signaling using LY294002 blocks the enhancement of AR transcriptional activity by MAA. The detailed mechanism whereby PI3 kinase causes super-activation of AR (AR*) is unknown but could involve the serine/threonine protein kinase Akt (a common target of PI3 kinase), leading to downstream effects such as an increase in coactivator binding or chromatin modifications associated with an increase in AR transcriptional output.
In mammals, androgens are essential for development of the male reproductive phenotype. Exposure to anti-androgens de-masculanizes the male fetus resulting in a variety of morphological and physiological alterations (Hotchkiss et al., 2002). Sources of anti-androgens include many pesticides, industrial chemicals, phytochemicals and pharmaceuticals (Gray et al., 2001). Presently we investigated the impact of MAA on the activities of three anti-androgens, vinclozolin, linuron and bicalutamide, each of which competitively inhibits androgen binding to AR and can induce a variety of endocrine disruptive responses. These include altered sex differentiation in male rats, hypospadias, ectopic testes, vaginal pouch formation and nipple retention (Kelce and Wilson, 1997), as well as reduced testicular and epididymal weights, lower numbers of testicular spermatids and reduced fertility (Gray et al., 1999). Each of the AR antagonists investigated shifted the androgen dose-response curve to the right, consistent with the competitive inhibition mechanism reported for each of the agents. Furthermore, our studies of the combination of MAA with AR antagonists revealed that MAA did not alter the dose response curve for androgen activation, either in the presence or in the absence of an AR antagonist. Thus, MAA did not alter the affinity of AR towards androgen or towards anti-androgens. As such, MAA was able to stimulate the reduced AR activity measured in the presence of AR antagonists, thereby alleviating, at least in part, anti-androgenic activity. However, the combined effect of MAA and AR antagonists on gene expression and physiology in vivo may be more complex than suggested by these simplified model system studies because many anti-androgens interact with multiple signaling pathways and can affect reproduction and development by more than one mechanism. For example, linuron not only acts as an AR antagonist, it also reduces fetal testosterone production significantly in male rats (Wilson et al., 2004).
Investigation of the effects of MAA on TM3 mouse Leydig cells revealed changes in the expression of several genes involved in androgen synthesis and action. This is the first report of MAA-induced changes in Leydig cell gene expression. Substantial increases in expression were observed for Cyp17a1 (∼3-fold), which is required for Leydig cell testosterone biosynthesis (Payne, 1990), and for Shbg, which codes for androgen-binding protein, ABP (10-fold increase), whereas Igfbp3 expression was markedly reduced (90% decrease by 24 h). Cyp17a1 is negatively regulated by testosterone (Burgos-Trinidad et al., 1997) and positively regulated by FSH and cAMP (Youngblood and Payne, 1992). ABP is expressed in testes (Selva et al., 2000), where it binds sex steroids with high affinity and transports them to the epididymis. ABP synthesis and secretion is dependent on FSH and androgens (Selva et al., 2000). Our finding that ABP is markedly induced in MAA-treated mouse Leydig cells is of interest, insofar as the overexpression of ABP in transgenic mice partially blocks spermatogenesis at the first meiotic division leading to apoptotic death of growth arrested germ cells (Selva et al., 2000). Furthermore, immunohistochemical analysis of testis tissue evidenced the accumulation of pachytene and metaphase spermatocytes in ABP transgenic mice, suggesting that the elevated ABP levels may interrupt spermatogenesis and induce germ cell degeneration (Selva et al., 2000), perhaps by lowering the free androgen threshold level. However, even though MAA induced ABP expression has been observed in whole testes (Bartlett et al., 1988; Tirado et al., 2003), no change in androgen levels was observed (Bartlett et al., 1988). Thus the impact of MAA-induced increase in ABP expression and its potential contributions to testicular toxicity are uncertain.
IGFBP-3 is an abundant carrier protein that binds to and transports IGF1 in serum (Hwa et al., 1999). IGFBP-3 can inhibit cell growth and induce apoptosis through both IGF1-dependent and IGF1-independent mechanisms (Peng et al., 2006). Cell growth-inhibitory concentrations of androgens induce significant increases in Igfbp3 expression at both the mRNA and protein level through an AR-dependent mechanism (Peng et al., 2006). Moreover, Igfbp3 expression is increased ∼1.5-fold in male rat testes 6 h after treatment with EGME (Fukushima et al., 2005). However, in our studies a decrease in Igfbp3 expression was observed in MAA-treated TM3 Leydig cells. Whether this decrease also occurs in Leydig cells following EGME or MAA treatment in vivo is not known.
All three MAA-responsive Leydig cell gene products described here play critical roles in androgen action, and modulation of their expression could have negative impact on testicular cell survival and signaling. The mechanisms through which MAA induces these effects on TM3 Leydig cell gene expression are not known. Androgens cause feedback inhibition of Cyp17a1 expression (Burgos-Trinidad et al., 1997) and induce Igfbp3 expression (Peng et al., 2006) via an AR-dependent mechanism, as noted above. However, AR levels in TM3 cells are very low and were unchanged upon MAA treatment, as determined by qPCR analysis (data not shown). Also, others have reported that there is no significant change in testosterone levels in the testes following MAA treatment (Bartlett et al., 1988). These observations suggest that these responses to MAA might not be AR-dependent. However, since all three genes can also be regulated by cyclic AMP, as noted above, with a decrease in expression in the case of Igfbp3 (Wang et al., 2001), the observed changes in TM3 cell gene expression could result from an increase in cyclic AMP levels. Indeed, cAMP signaling is readily induced upon MAA treatment of tsA201 cells, as determined using Clontech, Inc. pathway profiling vectors (G.B. and D.J.W., unpublished experiments). Star and Cyp19a1 are also positively regulated by cAMP, however, we did not see any MAA-dependent changes in their expression.
In conclusion, the present study demonstrates that MAA can regulate multiple intracellular pathways related to androgen synthesis and action. In addition to modifying the activity of nuclear receptors, MAA has the potential to induce changes in expression of other genes that may indirectly affect signaling by AR and other nuclear receptors. The full significance of the potentiation of nuclear receptor activity by MAA is uncertain. The proximal cause of MAA-induced spermatocyte apoptosis appears to be a fall in the level of reduced glutathione as compared to oxidized glutathione, insofar as spermatocyte apoptosis is substantially reduced when cellular glutathione levels are restored by treatment with N-acetyl cysteine, an antioxidant (Rao and Shaha, 2002). In our study, treatment of cells with N-acetyl cysteine had no impact on MAA-induced potentiation of AR activity, indicating that the enhancement of AR transcriptional activity is not a consequence of changes in cellular glutathione levels. Alterations in AR signaling in cells exposed to MAA could contribute to testicular toxicity in a variety of ways. Although the most prominent outcome of MAA exposure is apoptosis of primary spermatocytes and testicular degradation, which can be indicative of androgen deprivation, testosterone levels are, in fact, not decreased following MAA treatment (Bartlett et al., 1988). Thus, an alternative hypothesis to be considered is that MAA-induced testicular toxicity involves, at least in part, altered AR signaling leading to a change in the balance between androgenic and estrogenic activity that is critical for normal testicular development (Sharpe et al., 1998). Several genes in the testes require appropriate levels of AR signaling for expression (Zhou et al., 2005; Eacker et al., 2007) and could serve as targets for this effect of MAA. Further studies are required to identify the downstream targets of MAA-modulated AR signaling, and to elucidate their role in MAA-induced testicular toxicity in vivo.
Acknowledgments
We thank Dr. John Tullai, Boston University, for many useful discussions.
Supported in part by National Institutes of Health grant 5 P42 ES07381, Superfund Basic Research Program at Boston University (to D.J.W.).
Abbreviations
- ABP
androgen-binding protein, encoded by the Shbg gene
- AR
androgen receptor
- EDC
endocrine disruptive compound
- DMEM
Dulbecco's modified Eagle medium
- EC50
effective concentration for half-maximal response
- EGME
ethyleneglycol monomethyl ether
- ER
estrogen receptor
- FBS
fetal bovine serum
- IGF1
insulin-like growth factor 1
- MAA
methoxyacetic acid
- MAPK
mitogen-activated protein kinase
- PI
phosphatidyl inositol
- qPCR
quantitative, real-time PCR
- RAR
retinoic acid receptor
- TR
thyroid hormone receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abney TO. The potential roles of estrogens in regulating Leydig cell development and function: a review. Steroids. 1999;64:610–617. doi: 10.1016/s0039-128x(99)00041-0. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi G, Waxman DJ. Toxicity of ethylene glycol monomethyl ether: impact on testicular gene expression. Int J Androl. 2007 doi: 10.1111/j.1365-2605.2007.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin CW, Musto N, Gunsalus G, Kotite N, Cheng SL, Larrea F, Becker R. Extracellular androgen binding proteins. Annu Rev Physiol. 1981;43:189–198. doi: 10.1146/annurev.ph.43.030181.001201. [DOI] [PubMed] [Google Scholar]
- Bartlett JM, Kerr JB, Sharpe RM. The selective removal of pachytene spermatocytes using methoxy acetic acid as an approach to the study in vivo of paracrine interactions in the testis. J Androl. 1988;9:31–40. doi: 10.1002/j.1939-4640.1988.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Blackledge GR. Clinical progress with a new antiandrogen, Casodex (bicalutamide) Eur Urol. 1996;29 2:96–104. doi: 10.1159/000473847. [DOI] [PubMed] [Google Scholar]
- Burgos-Trinidad M, Youngblood GL, Maroto MR, Scheller A, Robins DM, Payne AH. Repression of cAMP-induced expression of the mouse P450 17 alpha-hydroxylase/C17-20 lyase gene (Cyp17) by androgens. Mol Endocrinol. 1997;11:87–96. doi: 10.1210/mend.11.1.9871. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, Braun RE. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21:895–907. doi: 10.1210/me.2006-0113. [DOI] [PubMed] [Google Scholar]
- Elberg G, Li J, Leibovitch A, Shechter Y. Non-receptor cytosolic protein tyrosine kinases from various rat tissues. Biochim Biophys Acta. 1995;1269:299–306. doi: 10.1016/0167-4889(95)00124-8. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Yamamoto T, Kikkawa R, Hamada Y, Komiyama M, Mori C, Horii I. Effects of male reproductive toxicants on gene expression in rat testes. The Journal of toxicological sciences. 2005;30:195–206. doi: 10.2131/jts.30.195. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- Gray LE, Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Holloway MG, Laz EV, Waxman DJ. Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4alpha. Mol Endocrinol. 2006;20:647–660. doi: 10.1210/me.2005-0328. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG, Gray LE., Jr Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ Health Perspect. 2002;110 3:435–439. doi: 10.1289/ehp.02110s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- Inoshita H, Masuyama H, Hiramatsu Y. The different effects of endocrine-disrupting chemicals on estrogen receptor-mediated transcription through interaction with coactivator TRAP220 in uterine tissue. J Mol Endocrinol. 2003;31:551–561. doi: 10.1677/jme.0.0310551. [DOI] [PubMed] [Google Scholar]
- Jaleel M, Shenoy AR, Visweswariah SS. Tyrphostins are inhibitors of guanylyl and adenylyl cyclases. Biochemistry. 2004;43:8247–8255. doi: 10.1021/bi036234n. [DOI] [PubMed] [Google Scholar]
- Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, McDonnell DP. Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2004;101:7199–7204. doi: 10.1073/pnas.0402014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindo T, Wine RN, Li LH, Chapin RE. Protein kinase activity is central to rat germ cell apoptosis induced by methoxyacetic acid. Toxicol Pathol. 2001;29:607–616. doi: 10.1080/019262301753385933. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Gray LE, Wilson EM. Antiandrogens as environmental endocrine disruptors. Reprod Fertil Dev. 1998;10:105–111. doi: 10.1071/r98051. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Wilson EM. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med. 1997;75:198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Weinbauer GF, Aslam H, Yeung CH, Nieschlag E. Quantification of apoptotic testicular germ cells in normal and methoxyacetic acid-treated mice as determined by flow cytometry. J Androl. 1998;19:710–717. [PubMed] [Google Scholar]
- Ku WW, Ghanayem BI, Chapin RE, Wine RN. Comparison of the testicular effects of 2-methoxyethanol (ME) in rats and guinea pigs. Exp Mol Pathol. 1994;61:119–133. doi: 10.1006/exmp.1994.1031. [DOI] [PubMed] [Google Scholar]
- Lee YH, Koh SS, Zhang X, Cheng X, Stallcup MR. Synergy among nuclear receptor coactivators: selective requirement for protein methyltransferase and acetyltransferase activities. Mol Cell Biol. 2002;22:3621–3632. doi: 10.1128/MCB.22.11.3621-3632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Wine RN, Chapin RE. 2-Methoxyacetic acid (MAA)-induced spermatocyte apoptosis in human and rat testes: an in vitro comparison. J Androl. 1996;17:538–549. [PubMed] [Google Scholar]
- Lonard DM, Tsai SY, O'Malley BW. Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol. 2004;24:14–24. doi: 10.1128/MCB.24.1.14-24.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, McHendry-Rinde B, Horn R. Panning transfected cells for electrophysiological studies. Biotechniques. 1993;15:906–911. [PubMed] [Google Scholar]
- Masuyama H, Inoshita H, Hiramatsu Y, Kudo T. Ligands have various potential effects on the degradation of pregnane X receptor by proteasome. Endocrinology. 2002;143:55–61. doi: 10.1210/endo.143.1.8578. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW, Foster PM. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicol Appl Pharmacol. 2000;167:87–99. doi: 10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Molina-Molina JM, Hillenweck A, Jouanin I, Zalko D, Cravedi JP, Fernandez MF, Pillon A, Nicolas JC, Olea N, Balaguer P. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol Appl Pharmacol. 2006;216:44–54. doi: 10.1016/j.taap.2006.04.005. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Payne AH. Hormonal regulation of cytochrome P450 enzymes, cholesterol side-chain cleavage and 17 alpha-hydroxylase/C17-20 lyase in Leydig cells. Biol Reprod. 1990;42:399–404. doi: 10.1095/biolreprod42.3.399. [DOI] [PubMed] [Google Scholar]
- Peng L, Malloy PJ, Wang J, Feldman D. Growth inhibitory concentrations of androgens up-regulate insulin-like growth factor binding protein-3 expression via an androgen response element in LNCaP human prostate cancer cells. Endocrinology. 2006;147:4599–4607. doi: 10.1210/en.2006-0560. [DOI] [PubMed] [Google Scholar]
- Rao AV, Shaha C. N-acetylcysteine prevents MAA induced male germ cell apoptosis: role of glutathione and cytochrome c. FEBS Lett. 2002;527:133–137. doi: 10.1016/s0014-5793(02)03196-4. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Zirkin BR. Androgen regulation of spermatogenesis in the rat. Ann N Y Acad Sci. 1991;637:90–106. doi: 10.1111/j.1749-6632.1991.tb27303.x. [DOI] [PubMed] [Google Scholar]
- Selva DM, Tirado OM, Toran N, Suarez-Quian CA, Reventos J, Munell F. Meiotic arrest and germ cell apoptosis in androgen-binding protein transgenic mice. Endocrinology. 2000;141:1168–1177. doi: 10.1210/endo.141.3.7383. [DOI] [PubMed] [Google Scholar]
- Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Turner KJ, Sumpter JP. Endocrine disruptors and testis development. Environ Health Perspect. 1998;106:A220–221. doi: 10.1289/ehp.106-1533085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TS, Liou SH, Chen CY, Smith TJ. Urinary 2-methoxy acetic acid accumulation in response to 2-methoxy ethanol exposure. Arch Environ Health. 2001;56:20–25. doi: 10.1080/00039890109604050. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methylcholanthrene. Toxicol Appl Pharmacol. 2006;213:87–97. doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol Endocrinol. 2006;20:475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916–40924. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- Terry KK, Elswick BA, Stedman DB, Welsch F. Developmental phase alters dosimetry-teratogenicity relationship for 2-methoxyethanol in CD-1 mice. Teratology. 1994;49:218–227. doi: 10.1002/tera.1420490318. [DOI] [PubMed] [Google Scholar]
- Tirado OM, Martinez ED, Rodriguez OC, Danielsen M, Selva DM, Reventos J, Munell F, Suarez-Quian CA. Methoxyacetic acid disregulation of androgen receptor and androgen-binding protein expression in adult rat testis. Biol Reprod. 2003;68:1437–1446. doi: 10.1095/biolreprod.102.004937. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- Wang L, Ma X, Yeh LC, Adamo ML. Differential regulation of IGF-binding protein gene expression by cAMP in rat C6 glioma cells. Endocrinology. 2001;142:3917–3925. doi: 10.1210/endo.142.9.8357. [DOI] [PubMed] [Google Scholar]
- Welsch F. The mechanism of ethylene glycol ether reproductive and developmental toxicity and evidence for adverse effects in humans. Toxicol Lett. 2005;156:13–28. doi: 10.1016/j.toxlet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update. 2001;7:236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, Gray LE., Jr Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood GL, Payne AH. Isolation and characterization of the mouse P450 17 alpha-hydroxylase/C17-20-lyase gene (Cyp17): transcriptional regulation of the gene by cyclic adenosine 3′,5′-monophosphate in MA-10 Leydig cells. Mol Endocrinol. 1992;6:927–934. doi: 10.1210/mend.6.6.1323057. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod. 2005;72:1010–1019. doi: 10.1095/biolreprod.104.035915. [DOI] [PubMed] [Google Scholar]


