Abstract
Recent studies have suggested that a natural function of the immune system is to respond and destroy aberrant, dysfunctional cells by a process called immunosurveillance. These studies also suggest that the tumors that arise despite immunosurveillance have been immunosculpted by the immune system. The purported abilities of tumors to induce immune tolerance and suppression, the increased pathogenic behavior of the tumor cells following exposure to immune effectors and the loss of immunogenicity (i.e. immunoediting) often observed in advanced stage tumors could be the result of immunosculpting. In some cases, these immunosculpting features may be permanent and irreversible. However, in other cases, reversible epigenetic mechanisms may underlie the immune resistant tumor phenotype. Regardless, these immune-induced alterations could contribute to cancer pathogenesis. Understanding the mechanisms by which tumors evade immunity will be important for disease prevention and therapeutics.
Keywords: immunosurveillance, tumor immunity, vaccines, adoptive T cell therapy
A. Introduction
It has been proposed by several investigators that a natural function of the immune system is to seek out and eradicate aberrant (dysplastic and neoplastic) cells and tissues so that tumors will not form. If true, then in cases where tumors have emerged, the immune system has apparently failed due to immune tolerance or escape. As some studies suggest, interactions between the tumor and the immune system following immunosurveillance may result in sculpting of the tumor for increasingly aggressive growth and further resistance to immune destruction. The heterogeneous immune escape strategies and immune effector content of human tumors, as well as the unpredictable responses of tumors to immunotherapies, suggests that there may be considerable diversity in tumor:immune interactions. Understanding these interactions could lead to improved design of immunotherapies and more appropriate clinical testing. In this review, the focus is on the idea that tumor development and phenotype are a reflection of past interactions of the tumor and the immune system.
B. The immune System and Immunosurveillance
The concept of immunosurveillance was proposed several decades ago, but it is only recently that the theory has been rigorously examined. With the advent of knockout technologies, immunosurveillance has been directly demonstrated in mice. In humans, sophisticated assays and improved population sciences have provided compelling indirect evidence.
B.1. Immunosurveillance in mouse models
Murine models of spontaneously arising or chemically induced tumors have been useful in demonstrating that the immune system naturally surveys for aberrant cells and has an important role in preventing tumor formation. The landmark study that invigorated interest in immunosurveillance by demonstrating important anti-tumor roles of IFN-γ and lymphocytes was reported by Shankaran and colleagues [1]. In that study, mice were used that were insensitive to IFN-γ either by knocking out the IFN-γ receptor α chain, or the IFN-γ-inducible downstream transcription factor, STAT1. The key observation was an increase in 3-methylcholanthrene (MC)-induced sarcoma development in the IFN-γ insensitive mice relative to wild type mice. Mice that lack both B and T lymphocytes, (i.e. RAG2-/-), were also highly susceptible to chemically-induced tumor development, at similar rates compared to that of IFNγR-/- and STAT1-/- mice. Crossing the RAG2-/- and the STAT1-/- mice (RkSk mice) further enhanced tumor incidence, suggesting that multiple, yet non-overlapping, mechanisms contribute to immunosurveillance. Aged RkSk mice also demonstrated a high incidence of spontaneous tumor development compared to age-matched control. Studies from other groups using perforin knockout mice suggest, as expected, that cytotoxic cells (NK, NKT and cytotoxic CD8 T lymphocytes, CTL) are among the major lymphocyte contributors to immunosurveillance. Perforin deficient mice have reduced ability to control development of transplanted tumor; increased susceptibility to sarcoma development following chemical exposure [2], and increased spontaneous lymphoma development [3]. Another molecule expressed by cytotoxic T cells that has recently shown to be involved in immunosurveillance is TRAIL, a transmembrane protein of the TNF family [4-10]. TRAIL preferentially induces apoptotic cell death in transformed cells but not in normal cells in vitro, a finding that further supports the notion that the immune system has evolved mechanisms to specifically deal with neoplastic cells [11]. Several other immune effector cells and molecules have been directly knocked to demonstrate their respective roles immunosurveillance in mice, including natural killer (NK) cells, NK-T cells [12, 13], γδ T cells [14, 15], IL-12 [16], granulocyte-macrophage colony stimulating factor (GM-CSF) [17], and α-galactosylceramide (α-GalCer) [18].
B.2. Circumstantial evidence in humans supports immunosurveillance
Natural immunosurveillance is difficult to examine in human cancers. Despite this, there have been studies over the past decade that provide compelling, albeit indirect, evidence. These studies can be roughly classified into three major areas of study; infiltration of tumors with immune effectors, the generation of endogenous immunity to tumor antigens, and the observations that patients with immune deficiencies demonstrate abnormal risks for cancer development.
B.2.1. Tumors attract in immune cells very early in the course of disease
Infiltration of immune effectors is a well-documented observation in most if not all cancers. Emerging studies now reveal that infiltration occurs very early in the course of disease. In benign proliferative disease of the breast, a pre-breast cancer lesion, 30-fold increases in T cell infiltration have been observed and this level remains fairly constant throughout the course of progression into invasive breast cancer [19]. In many cases, T cell infiltration correlates with disease outcomes. One of the cornerstone studies demonstrating a biologic role for tumor-infiltrating T cells came from Zhang and colleagues with their analysis of infiltrating T cells in 186 frozen ovarian cancer specimens [20]. Of the 186 patient samples, 102 (54.8%) lesions had CD3 T cells infiltrating the tumor parenchyma, and there were significant differences in the distributions of progression free and overall survivals according to the presence or absence of infiltrating T cells. The median overall survival rate for patients with intratumoral T cells was 38% compared while only 4.5% for patients without detectable infiltrating T cells. In those patients who had a complete clinical response to surgery and chemotherapy the differences in median survival was more pronounced, with a median survival of 74% in patients with intratumoral T cells and 11.9% in those without intratumoral T cells. In a follow up study by Sato and colleagues, it was found that those ovarian cancer patients that had high levels of CD8 T cells infiltrating into the tumors had an improved outcome relative to those with lower levels [21]. Similarly, infiltrating T cells were associated with improved survival in colorectal cancer patients [22, 23]. In colorectal cancer, immune effectors may protect against disease progression, since the lack of immune infiltration is associated with the development of metastases [22].
B.2.2. Patients generate immune responses to tumor antigens, which correlate with disease outcome
In addition to infiltration of immune effectors, cancer patients also have elevated tumor antigen-specific immune responses relative to the levels observed in normal healthy individuals. Despite this elevation, the magnitude of the antigen-specific immune response remains typically very weak, an example of which is the folate receptor-α (FRα) (also referred to as folate binding protein), which is a glycophosphatidyl-anchored membrane glycoprotein that mediates cellular uptake of folate [24]. This protein is overexpressed on greater than 90% of ovarian cancers at levels of up to 80-90-fold relative to the levels observed on normal tissues [25]. Knutson and colleagues, using a highly sensitive IFN-γ ELIspot assay with predicted FRα–derived CD4 T helper cell epitopes, discovered the presence of pre-existing FRα-specific T cell immunity in breast and ovarian cancer patients. The increased immunity, relative to healthy donors, was largely confined to the amino terminal half of the receptor suggesting that overexpression may lead to presentation of subdominant epitopes by the immune system. Elevated T cell immunity to several other tumor antigens, such as HER-2/neu, CEA, and NY-ESO-1, has been recently reported [26-28]. Tumor antigen-specific IgG responses have also been observed in cancer patients and in some cases are associated with improved survival. For example in one study, ovarian cancer patients (Stages III-IV) with p53-specific antibodies had a median survival of 51 months compared to 24 months for those without detectable antibody levels [29]. The findings of both humoral and T cell immunity to tumor antigens might indicate that the immune system is just as capable of eliciting a coordinated immune response to tumors as it is to a foreign antigen.
B.2.3. The immunosuppressed have an elevated cancer risk
It has long been known that transplant patients have elevated risks of developing a variety of cancers. Overall, the risk of cancer is 3- to 8-fold higher in transplant patients as compared with age-matched controls in the general population [30]. However, the preferential anatomic sites of disease are not aligned with the general population, suggesting that multiple factors contribute to the increased incidence. For example, in immunosuppressed patients risks are elevated for skin and lip cancer, lymphomas, and Kaposi's sarcoma but not always for more common malignancies such as breast and prostate cancers [31]. The risk of developing non-viral digestive tract cancers (i.e. colorectal cancer), common in industrialized nations, is however also elevated in kidney transplant patients [32]. There are at least two potential sources that may interact to augment risk of cancer development following transplant, namely viral oncogenesis and reduced tumor immunosurveillance. In a study by Vajdic and colleagues in renal transplant patients, 17 anatomic sites of increased cancer risk were identified in a cohort of nearly 29,000 patients, in which there was greater than 3-fold risk of cancer compared to the general population. Of the 17 cancers, eight were at sites in which there is some association with human papillomavirus infection, notably, tongue, mouth, vulva, vagina, penis, salivary gland, esophagus, and nasal cavity [32]. Two of the seventeen cancers, Hodgkin disease, and non-hodgkins lymphoma are known to be caused by Epstein Barr virus. Lastly, two others, liver cancer and Kaposi sarcoma are associated with hepatitis B (or C) virus and human herpes virus, respectively. Thus, it is likely that the increased frequency of these cancers was due to the inability to control viral infections appropriately. However, it seems likely that the body's natural antitumor defense mechanisms (i.e. immunosurveillance) may also have had a role because the remaining four cancers for which there was an elevated risk (>3-fold) are not typically associated with viral infection, namely lip, biliary, thyroid, and connective tissue. Risks of developing several other common non-viral cancers such as colon cancer were increased as well but lower than 3-fold threshold. There is also some suggestion that cancer risk increases with increasing potency of immunosuppression. For example, potent immunosuppression with OKT3, an antibody which eliminates T cells, have been associated with an increased risk of post-transplant lymphoproliferative disorders compared with other immunosuppressive regimens [30]. It is however unclear if the anatomic distribution differs with increasing potentcy. A complicating factor in assessing relative risk is the contribution of inflammation-induced disease progression which is likely also inhibited during immunosuppression and may balance out anti-tumor effects of the immune system, such as in breast cancer where risks often decline in immunosuppressed individuals (reviewed in [31]).
C. The Paradoxical Roles of the Immune System in Malignancy
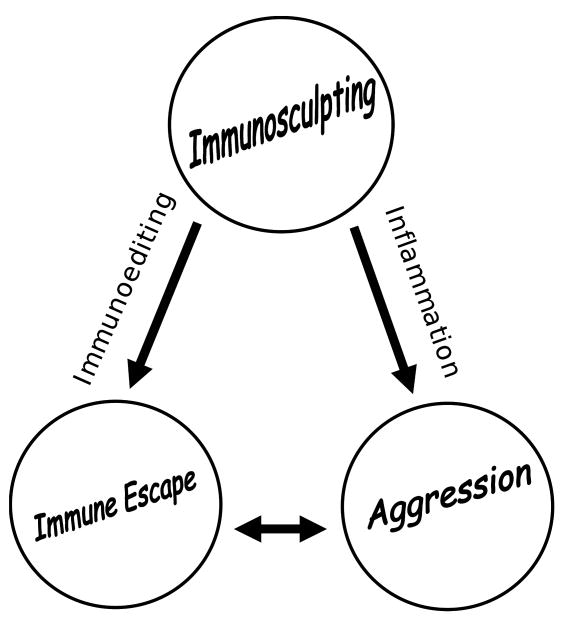
The recently coined term, immunoediting, remains difficult to define in light of what we know about the interactions of the immune system with tumors. As previously published, the term applies to those immune-induced tumor alterations that make the tumor acceptable to the immune system. In contrast, a broader definition might include any alteration, adaptation, or change that occurs in the tumor in response to the immune response. These alterations may include those that would not otherwise be associated with immune escape such as transformation and increased aggressiveness (i.e. inflammation-induced tumor promotion) [33]. Perhaps an appropriate term to encompass all immune-mediated changes in the tumor is immunosculpting, a process that includes the most minimal changes such as amino acid substitutions in key antigenic proteins (i.e. mutations) to major reprogramming strategies such as epithelial to mesenchymal transition (EMT). Further, it includes both permanent (e.g. mutations) and non-permanent (e.g. reversible ligand induced cytokine production) events. Immunoediting might be thought of as the component of immunosculpting, which imparts immune escape properties (See Figure).
Figure.
Immunosurveillance: Unless the immune system can eradicate the dysfunctional cells or tissue, chronic immunosurveillance results in immunosculpting of which there are two outcomes, immune escape and aggression, which are mediated by two mechanisms, immunoediting and inflammation-induced tumor promotion, respectively. These two mechanisms interact to promote disease progression in cancer.
C.1. The immune system is linked to tumor development and progression
Inflammation-induced tumor promotion is a rapidly growing area of study. Chronic inflammation and cancer have been linked since the 1850s and current thought has it as a major contributor to cancer pathogenesis. For example, inflammatory bowel diseases such as ulcerative colitis and Crohn's diseases are associated with an increased risk of colorectal cancer [34, 35]. Individuals with ulcerative colitis have a 10-fold greater risk of developing colorectal cancer compared to the risk defined for the general population [36-38]. Chronic exposure to irritants that cause inflammation in the bronchial track such as cigarette smoke, asbestos, and silica are associated with an elevated risk of lung cancers [39, 40]. Many cancers are linked with chronic pathogen exposure such as gastric cancers and Helicobacter pylori [41]. Molecular, cellular, and organism studies in recent years emphasize a broad role of NF-κB pathways in mediating development and progression of cancer [42-44]. In addition to its anti-apoptotic survival signals in dysplastic cells, activation of the NF-κB signaling pathway in immune effector cells leads to the production of cytokines, differentiation and survival signals, all of which further contribute to chronic inflammation and malignant progression [42]. A key example is work by Greten and colleagues who used a murine ulcerative colitis-associated colorectal cancer model to study the role of the NF-kB pathway in both intestinal epithelial cells and myeloid cells [45]. In this model, mice were treated with the carcinogenic regimen, azoxymethane and dextran sodium salt, which resulted in chronic inflammation and an increased incidence of colorectal cancers. The investigators observed that when the NF-kB pathway was blocked in the epithelial cells (but not in myeloid cells) the incidence of tumors was greatly reduced, but that the sizes of the tumors that did grow out remained similar to control animals. However, when the NF-kB pathway was blocked only in myeloid cells (and not the tumor cells), there was a significant decrease in both tumor incidence and size, indicating that the immune response contributed by macrophages (and potentially other myeloid cells) affected both tumor development and disease progression. In addition to myeloid cells, recent studies also suggest a role for the adaptive immune system in inflammation-induced disease progression. An example of this is the recent study by de Visser and colleagues in K14-HPV16 mice [46] which contain the HPV16 early region genes expressed as a transgene under control of the human keratin 14 gene. These mice develop squamous cell skin carcinomas that have a premalignant phase in which there is substantial infiltration of both innate and adaptive immune effectors. B and T lymphocyte deficiency in these mice limits the disease to benign hyperplasias, which unlike lymphocyte sufficient mice, do not have significant infiltration of mast cells, granulocytes and most of the other CD45+ leukocytes. As a result, the incidence of invasive cancer dropped from 47% to 6%.
Collectively, several recent studies now strongly support the concept that an immune response is generated early in the course of disease, likely at the benign dysplastic stage and prior to tumor formation. Although it is unclear if the thrust of this immune response is to eradicate tumor (i.e. immunosurveillance), the inflammatory mediators produced as a result drive further mutation and selection resulting in immunosculpting. In reiteration of several investigators, there is a complex and often paradoxical role of the immune system in cancer in which immune cells are cast in a protagonist versus antagonist role [31, 33, 47]. On one hand, immunity may protect against cancer and on the other, it appears to be pathologic.
C.1.2. Immunosculpting and Immunoediting
Recent studies have begun to define the types of immune cells, both adaptive and innate effectors, as well as their products (e.g. cytokines) that have a role in immunosculpting. Many, if not all, alterations induced by the immune effectors would favor immune escape, otherwise spontaneous regressions would be expected.
C.1.2.1. Immune effectors can simultaneously sculpt tumors for aggressiveness and immune subversion
Phagocytic cells are the best described of the immune effectors that are capable of sculpting the phenotype of dysplastic and neoplastic cells. Macrophages, one of several phagocytic cells, are among the first cells recruited into aberrant tissues. For example, relative to normal breast epithelium, the levels of macrophages are nearly 4-fold higher in early benign proliferative lesions of the breast and increase to approximately 20-fold in invasive lesions [19]. There are a multitude of chemokines and cytokines that are known to be released by neoplastic lesions that result in the influx and differentiation of macrophages, including GM-CSF, TGF-β1 and CCL3 [48, 49].
In most human cancers, macrophages are associated with a poor prognosis and modulate the tumor microenvironment, notably by producing survival/growth factors (e.g. vascular endothelial growth factor) and inducing an aggressive phenotype [48, 50-55]. One of the more prevalent immunosculpting outcomes of macrophages is increased aggressiveness (e.g. invasiveness, migration, growth, etc) of cancer cells. Several macrophage and tumor-derived molecules are implicated in the macrophage-induced augmented tumor aggression of which Wnt 5a is an example. Pukrop and colleagues showed that the invasiveness of the breast cancer cell line, MCF-7, was strongly enhanced by co-culture with macrophages, an effect, which was completely, eliminated using the Wnt antagonist, dickkopf-1. These studies demonstrated that the noncanonical Wnt signaling pathway was the predominant pathway activated by Wnt 5a [56]. The authors then went on to further demonstrate that Wnt 5a was upregulated in the macrophages during co-culture and that the direct application of purified Wnt 5a to MCF-7 tumor cells in the absence of macrophages resulted in a similar enhanced invasion which was also accompanied by increased matrix metalloproteinase activity, the latter of which likely contributed to the phenotype. Studies from Hageman and colleagues found that multiple intracellular signaling pathways are used by tumor cells in response to macrophage exposure, including both the NF-κB and JNK pathways [57]. Furthermore, the interactions between the tumor cells and macrophages may be reciprocal and self-perpetuating as was shown be Goswami and colleagues who recently discovered a paracrine loop involving colony-stimulating factor-1 (CSF-1) and epidermal growth factor (EGF) [58]. In this loop, macrophages express EGF that acts directly on breast tumors to increase both invasiveness and increased tumor cell production of CSF-1. The loop is completed when CSF-1 acts on the macrophages for enhanced EGF release. Characterization of the ligands and signaling pathways activated in tumors and macrophage could provide therapeutic options to inhibit or reverse aggressive tumor cell phenotypes. Perhaps a relevant example of this approach is the use of anti-inflammatory cyclooxygenase-2 (COX-2) inhibitors to prevent cancer progression and occurrence [59]. Based on these studies, it appears that immunosculpting of tumors by infiltrating immune effectors may be mutually beneficial to both the tumor and the immune cell. Such reciprocal interactions could potentially contribute to persistent inflammation and increased tumor pathogenesis.
Accumulating evidence suggests that neutrophils may also immunosculpt tumors for increased aggressiveness. Neutrophils are attracted into lesions by tumor IL-8 production and once in the tumor microenvironment, neutrophils produce many factors that sculpt the tumor [60]. Imai and colleagues observed that neutrophils augment the invasiveness of tumor cells during co-culture one effect that was completely blocked by the inclusion of a monoclonal anti-hepatocyte growth factor (HGF) antibody [61]. Subsequent experiments revealed that the tumor cells produced a factor that induced HGF production by the neutrophils, again demonstrating a mutually beneficial interaction between tumor and immune effect. HGF is well known for its abilities to induce scattering and migration of a variety of cell types, for example by upregulating chemokine receptors such as CXCR4 [62]. In addition to HGF, Queen and colleagues recently showed that tumor cells can also induce neutrophils to produce oncostatin M, an IL-6-related cytokine [63]. Like, HGF and other cytokines, oncostatin M also augments tumor cell invasiveness. Tumors therefore appear to interact with multiple phagocytic cells using several different pathways, a complex network that is likely to pose a significant challenge to target with exogenous agents aimed at preventing such interactions.
Recent data, in murine models, suggests that phagocytic cells are not the only effector able to sculpt tumors for increased aggressiveness, and that T cells may perform similar functions. For example, Liu and colleagues found that tumors that emerged in animals treated with adoptive CD8 T cell therapy demonstrated increased aggressiveness accompanied by increased resistance of the tumor to CD8 T cell-mediated lysis [64].
In addition to those already described above, a number of other effector molecules may also have a role in increasing cancer aggressiveness. One well-known example is TNF-α, a T cell and macrophage-produced pleiotropic cytokine that can result in one of at least two potential outcomes depending on its prevalence. The first outcome is the induction of inflammation and cell survival in which TNF-α causes the release of variety of growth factors and upregulates negative regulators of apoptosis such as c-FLIPL and Bcl-2 [65]. At higher doses, however, the effects are the opposite and include activation of apoptosis, and at higher doses yet, it can lead to hemorrhagic necrosis through its destruction of blood vessels, hence the derivation of its name [66]. TNF-α has widely been implicated in both early and late events in initiation, promotion, and progression of cancers. In one example, elimination of TNF-α signaling in the mouse epidermis protects against chemical induction of tumors demonstrating its role in progressing damaged skin cells to a neoplastic state [67]. In another study, Pilarsky and colleagues found in a liver cancer model, that the role of TNF-α at its earliest point is to promote the transition of a lesion from dysplasia to neoplasia [68]. In this model, TNF-α triggers activation of the NF-kB signaling pathway in dysplastic hepatocytes, resulting in the upregulation of pro-survival pathways. The role of hematopoietic cell-derived TNF-α may only be discernable at the earliest stages of cancer development since it has been observed that progression of cancer is associated with the acquisition of the tumor to produce its own TNF-α, which could then act in an autocrine and paracrine fashion. It is likely at the early stages of tumor development that immune effectors select for those tumor cells that have upregulated pro-survival signals and are capable of avoiding harsh inflammatory environments. TNF-α is also known to augment its own activity by promoting the release of other soluble mediators. Devoogdt and colleagues recently showed, using gene silencing, that Secretory Leukocyte Protease Inhibitor (SLPI), induced by TNF-α in lung carcinoma cells, was involved in the tumor-promoting activity of TNF-α by an as of yet unknown mechanism [69-71].
Although general mechanisms by which tumor cells acquire increased aggressiveness (e.g. invasiveness, migration) and resistance to immune destruction (e.g. resistance to apoptosis) are incompletely understood, one mechanism that may account for some phenotypic changes for epithelial tumors is epithelial to mesenchymal transition (EMT). Epithelial cells, form organized layers of cells that are adjoined by membranous structures such as adherens junctions. Further, epithelial cells display apical and basolateral polarization, a cellular architecture in which proteins are delivered to specific sites within the cellular membranes, which provide for tissue-specific vectorial functions. While motile, epithelial cells do not typically detach and move away from the epithelial layer, but rather move within the epithelial plane. Mesenchymal cells, on the other hand, neither form organized layers nor demonstrate basolateral/apical polarization. In culture, mesenchymal cells are generally highly motile and demonstrate a spindle-shaped morphology, analogous to the scattering morphology observed by exposure of tumor cells to a variety of factors or cells (e.g. macrophages). A number of key phenotypic changes occur when epithelial tumor cells undergo EMT. Tight, adherens and other membrane junctions are dissolved permitting greater cell mobility. Increased levels of matrix metalloproteinases (MMPs) permit increased capabilities of the cells to move through complex extracellular matrices. In health, EMT is an integral component of several morphogenetic and organogenetic processes such as gastrulation [72-75]. In vivo, EMT induction is mediated by complex interplay of extracellular signals, which include growth factors, cytokines and extracellular matrix molecules. Growth factors commonly associated with EMT include TGF-β, fibroblast growth factor (FGF), HGF, EGF and Wnt family members [76]. An important recent discovery is that cytokines derived from immune effectors can act as potentiators of EMT. For example, Bates and colleagues reported that TNF-α potentiated TGF-β induced EMT of human colon cancer cells in vitro [77]. Another recently discovered cytokine that may also induce EMT is interleukin-like EMT inducer (ILEI), which is encoded by the FAM3C gene [78]. ILEI is expressed abundantly in chronically inflamed tissues, often localizing to macrophages, mast cells, and lymphocytes [78]. When transfected into tumor cells, ILEI causes EMT, tumor growth, and metastasis. Some more recent results suggest that T cell immunity can cause EMT. In one by Knutson and colleagues, it was demonstrated in a CD4 T cell immune-mediated tumor rejection model, that the development of a strong anti-tumor immune response, while initially causing rejection, could result in a stable immune escape phenotype that had lost the target antigens (e.g. neu) [79]. Morphologic and microarray data of the antigen-loss variants revealed that the immunoedited tumor cells underwent EMT. The transition was accompanied by an upregulation of invasion factors and increased invasiveness characteristic of mesenchymal tumor cells. Further analysis of the tumors also revealed that the tumor cells that had undergone EMT were able to directly suppress T cell proliferation and had reduced expression of danger signals, and therefore demonstrated evidence of immunoediting [79, 80]. Although the T cell derived signals that induce EMT remain unclear, in vitro studies by Kmieciak and colleagues, using the same cell line, showed that IFN-γ could directly induce methylation of the epithelial MMTV promoter resulting in antigen-loss [81]. However, it is unclear if IFN-γ induces complete EMT. One remarkable characteristic was the greatly amplified expression of stromal-derived factor 1 (SDF-1) in the tumor cells that underwent EMT. It is probable that the enhanced secretion of SDF-1 into the tumor microenvironment resulted in an increased capability to evade immunity through immunosuppression. SDF-1 can enhance chemotaxis of immature plasmacytoid dendritic cells into the tumor microenvironment, the latter of which are known to induce high levels of IL-10 release from T cells [82]. SDF-1 may also directly induce release of the immunosuppressive cytokine, IL-10 from antigen-experienced T cells as was recently demonstrated by Kremer and colleagues [83]. Several other mechanisms of immune suppression and tolerization have been described in recent years including recruitment of regulatory cells (e.g. regulatory T cells and myeloid suppressor cells), tumor expression of immunosuppressive molecules (e.g. B7-H1), and induction of T cell anergy (e.g. CD3 zeta-chain reductions) [84-93]. Although definitive mechanisms have yet to emerge, the results seem to suggest that the immune system increases the aggressiveness of the tumor cells while simultaneously imparting an ability to suppress antitumor immunity.
C.1.2.2. Editing for reduced MHC and antigen expression and loss of IFN-γ responsiveness
Aside from immunosuppression, tumors may also evade destruction by reducing immunogenicity. In initial studies that led to the development of the immunoediting hypothesis, Shanakaran and colleagues observed that MCA-induced sarcomas that grew in lymphocyte-deficient mice (deficient of T and B cells) were rejected when transplanted into wild-type mice [1]. In contrast, MCA-induced tumors that emerged in the wild-type mice were never rejected on transplantation suggesting that lymphocytes shape immunogenicity, and hence immunoedit the tumor. Although viral oncogenesis in the immunodeficient mice could have explained the rejection of the tumors transplanted into the wild-type mice, this was ruled out using a variety of assays. These same investigators have also shown that the immunoedited tumors that develop in the wild-type mice following induction with MCA lost responsiveness to IFN-γ [94], suggesting the loss of the capability to upregulate MHC antigen-presentation was responsible for the lack of immunogenicity. Indeed, this was confirmed in follow-up studies which showed that the reconstitution of MHC class I presentation resulted in tumor rejection [1]. Additionally, CD4 and CD8 antibody depletion studies revealed that T lymphocytes were responsible for tumor rejection of the MHC class I reconstituted tumor. These elegant studies demonstrated that the concept of immunoediting could be modeled in animal disease models, and provided the seed for many discoveries from several other groups over the last few years. More profoundly, the studies provided compelling evidence for the first time in vivo that the immune system could affect the phenotype of the tumor in a stable fashion and, hence, the emergence of the field of immunoediting. Whether or not there is a human biology connection to these studies has remained uncertain; however, the evidence of antigen processing and presentation defects in human tumors posits the theory.
Over the last decade, tens of studies have been published showing defective MHC class I expression in human tumor cells. While the loss of MHC class I expression in human tumors is not definitive evidence of immunoediting, this widely observed defect remains some of the best to date. Loss of MHC class I in human tumors is frequently observed in several cancers including B cell lymphomas, colorectal, ovarian, breast, melanoma, lung and renal cancers [95-113]. Ongoing studies continue to define the molecular mechanisms of downregulation of MHC molecules. Perhaps the best understood defects and mechanisms of MHC downregulation come from studies of colorectal cancers. Downregulation of MHC class I in colorectal cancers is associated with a poorer outcome, relative to MHC class I expressing tumors [114]. Studies suggest that several mechanisms are responsible for loss of MHC expression. Haplotype loss is observed in approximately 40% of colorectal tumors [99]. For tumors that retain heterozygosity, loss of expression could be explained by gene (e.g. β2-microglobulin) inactivation or downregulation (e.g. LMP2) [99, 101]. Maleno and colleagues reported that it is not uncommon to observe both loss of heterozygosity accompanied by complete MHC loss, suggesting multiple mechanisms interact for loss of MHC expression [99].
Although the reasons remain incompletely understood, total loss of MHC class I is more frequent in microsatellite unstable (MSI-H) colorectal cancers [97] This may reflect an increased propensity of this subset of colorectal cancers to undergo point mutations in key pathways [115]. Indeed, Kloor and colleagues reported a high frequency of mutations in the coding microsatellites of the β2-microglobulin and antigen processing machinery (APM) genes in MSI-H tumors, whereas these point mutations are not present in microsatellite stable tumors [97]. In contrast, in microsatellite-stable tumors, in addition to loss of heterozygosity, down-regulation of APM components such as LMP7 and TAP2 at the protein level explain MHC class I loss [101]. In addition to the loss of basal expression of MHC class I, recent human studies have began to confirm the findings of other prior murine studies that have shown loss of IFN-γ inducibility of MHC class I or its associated APM molecules, which could further render tumors incapable of immune destruction. For example, Caki-2 cells, a human-derived renal cancer cell line, were recently shown to have lost inducibility of TAP1 and LMP2 as a result of a defect in the proximal signaling pathway of IFN-γ [116]. Loss of responsiveness was not due to reduced expression of key proteins such as the IFN-γ receptor, Stat1, Jak1, and Jak2. However, the defect eliminated IFN-γ induced Jak kinase activation, and while the nature of the defect remains unclear, it could be the result of point mutations as has been found in uterine leiomyosarcomas [117]. In the study of leiomyosarcomas, it was observed that loss of TAP1 and LMP2 inducibility by IFN-γ was due to a non-conserving G-to-A point mutation in the ATP binding region of Jak1, which resulted in a change from glycine to glutamic acid.
Although the loss of MHC expression in humans is suggestive of immunoediting, what remains to be determined is whether immunosurveillance drives the phenotypic changes. A recent study by Riemersma and colleagues suggests that such correlations can be documented experimentally. They observed that those aggressive B cell lymphomas that show loss of MHC class I and class II are often infiltrated with cytotoxic T cell whereas few T cells are observed in lesions that retained expression MHC, suggesting that increased T cell responses curb the growth of tumors they directly recognize.
Loss of MHC class I expression in human tumors may actually be associated with a better outcome and less aggressive tumors as has been seen in human breast cancers [105, 110, 114]. About 47% of breast tumors are completely negative for MHC class I expression while another 34% show very weak staining suggesting that the majority of breast cancers (i.e. 81%) have impairments [110]. When outcomes were compared between those that were negative of MHC and those that were positive (i.e. weak, moderate and strong expression), it was observed that MHC loss conferred improved survival. This seemingly paradoxical outcome might be explained by two different immunoediting hypotheses. First, it is possible that immunoediting of tumors resulted in the selection of loss variants which are less aggressive tumors. However, this hypothesis seems inconsistent with other studies showing increased aggressiveness in immunoedited tumors. Alternatively, the improved survival may be due to the increased vulnerability of MHC-loss variants to NK cell-mediated killing. This latter hypothesis is supported by the recent findings of Watson and colleagues in colorectal cancers where it was observed that tumors with reduced but not complete loss of MHC demonstrated the worse outcome [114]. A natural function of NK cells is to kill cells that fail to express MHC class I [118]. In the Watson study, about 77% of tumors retained strong MHC class I heavy chain staining with 10% showing weak expression and the remaining tumors showing complete loss of expression [114]. Patients (all cases, early and late-stage) with high or absent expression of MHC had median survivals of 68 months and 60 months respectively, while patients with low level, but not absent, expression had significantly reduced survival of 41 months. These results suggest that those tumors that maintain low-level expression of MHC avoid killing by both cytotoxic T cells as well as NK cells. Although NK cells are found at high levels (i.e. ∼60%) in colorectal tumors, their direct association with clinical outcome and, particularly, the absence of MHC class I expression has yet to be described. While the studies described above tended to focus on one element involved in immune recognition, studies by Cicinnati and colleagues suggest that tumors can acquire multiple defects related to T cell recognition [119]. In their analysis of p53 epitope-specific T cells in lesions from patients with hepatocellular carcinoma, they observed that higher levels of antigen-specific T cells in the tumor bed are associated not only with loss of HLA-A2 (as opposed to a global downregulation of HLA class I heavy chain) but also co-stimulatory molecules such as CD80.
Recent murine studies show that the adaptive immune response can also edit tumor antigen expression. For example, with the use of haemaglutinin (HE)-overexpressing kidney cancer cells and CD4 T cells that are transgenic for an HE-derived, I-Ed peptide, Zhou and colleagues found that the reconstitution of antigen-specific T cells with tumors in vivo, directly down-modulated tumor antigen expression at both the protein and mRNA level [120]. An important finding from that study was that antigen expression was not completely eliminated but was retained at low levels, which the authors suggest likely led to the observed induction of T cell tolerance. In contrast, Knutson and colleagues demonstrated that mice that were injected with breast tumors overexpressing rat neu (a foreign antigen), generated immunoedited tumors that were completely devoid of rat neu antigen expression at both the protein and RNA levels [79]. The loss of antigen from the tumor cells depended entirely on CD4 T helper cells. That there can be both induction of T cell tolerance and immunoediting seems to reconcile an ongoing debate that these two phenomena are mutually exclusive [120].
Taken all together, these results provide compelling evidence that the adaptive immune system can immunoedit the phenotype of tumor and cause the emergence of tumors which have lost immune recognition molecules. These alterations reflect the true sense of immunoediting, namely, the evolution of tumor cells that can avoid immune destruction.
C.1.2.3. Immunosculpting may be either reversible or permanent
Tumor immunosculpting likely reflects both reversible (e.g. some epigenetic) and permanent (i.e. mutations and irreversible epigenetic) events that could influence its ability to evade immune eradication. A probable example of a reversible response is EMT. Studies have shown that the mesenchymal phenotype of tumor cells that were derived from epithelial tumor cells using EMT is stable in culture but that despite this stability there is the reverse process termed mesenchymal to epithelial transition (MET) [79]. Like EMT, MET is a fundamental process activated during embryogenesis [121]. Chaffer and colleagues demonstrated that tumor cells that had undergone MET were more efficient at colonization of permissive tissues, compared to their mesenchymal counterparts, following intracardiac injection, despite reduced capabilities of invasion and migration [123]. It may be that inflammation induced at the tumor site by macrophages or other immune effectors could lead to EMT resulting in physical evasion of tumor cells from the primary mass followed by reseeding tissues where immunity is not prevalent. This would be consistent with other studies that show that mesenchymal tumor cells that develop, under immune pressure, in immunocompetent animals can revert using MET when transplanted into immune tolerant mice (Reiman and Knutson, unpublished observations). In addition to EMT, there is likely a host of other epigenetic changes that could be reversible. An example is illustrated in a recent study by Abouzhar and colleagues who described the characteristics of a CD8 T cell-selected tumor cell that resisted killing by perforin and granzyme B [124]. The resistant tumor cells apparently retained expression of antigen and MHC class I since it was observed that they were still able to trigger activation of the cytotoxic T cells. Transcriptional profiling revealed that the actin-related genes, ephrin-A1 and scinderin, were overexpressed in the resistant tumor, relative to the parental tumor. Importantly, silencing of these genes reversed the resistance. Establishing whether this phenotype is reversible in vivo when T cells are removed or blocked may be an important objective and could establish a potential therapeutic strategy.
Immunosculpting mediated by permanent or other irreversible alterations is probably in many cases a reflection of Darwinian selection. Immune effectors can be directly mutagenic and lead, by way of chance, to generation of a new phenotype of cell that is able to evade immune destruction. For example, it has recently been observed by Yan and colleagues that TNF-α is a potent immunogen [125]. In that cell line study, TNF-α resulted in increased gene mutations, gene amplification, chromosomal instability and micronuclei formation. TNF-α could also lead to increased malignant transformation of otherwise ordinary mouse fibroblasts. Randomly acquired mutations may result in a phenotype that is ignored by the immune system or one that actively suppresses the immune response.
C.2. Immunoediting in immune-based therapeutics
Some of the most compelling evidence for immunoediting comes from a variety of mouse and human immunotherapy studies, which underlies the importance of monitoring not only the immune response during the course of immunotherapy, but also what changes occur in the tumor to compensate for the altered immunologic environment. In the clinic, immunoediting has been the most evident in early trials of adoptive T cell therapy. Dudley and colleagues over the past several years have reported results of a T cell therapy paradigm by which patients with refractory metastatic melanoma are treated with nonmyeloablative lymphodepletion followed by the infusion of ex vivo expanded autologous tumor-infiltrating lymphocytes [126]. In one study of this therapy, 51% (18 of 35) of patients demonstrated objective clinical responses to the treatment. Twenty-eight percent (5/18) of these showed evidence of immunoediting with either loss of antigen expression (e.g. MART-1) or MHC class I expression. Yee and colleagues conducted a similar study in which ten patients with advanced melanoma were treated with antigen-specific CD8 T cell clones [127]. Of the ten patients, five underwent pre- and post-treatment biopsy analysis to examine for the generation of antigen-loss variant and of those five, three showed evidence of the generation of antigen-loss variants demonstrating immunoediting. Again, two possible mechanisms may account for the loss of antigen. The first mechanism is simply Darwinian selection of an already existing antigen-negative population. Given the heterogeneous nature of tumors, it is likely to some extent that the infusions of antigen-specific T cells resulted in killing of those cells with both antigen and MHC expression, while permitting the survival of cells that lost antigen expression. However, as explained above, there may be epigenetic mechanisms that could account for the loss of antigen and MHC class I expression. In these key studies of adoptive T cell therapy, immunoediting was a frequent event demonstrating the importance of understanding the mechanisms by which tumor escape immune-based therapies. Active vaccinations may also result in the generation of immunoedited tumors as has been shown in both murine models and humans [128, 129]. In the neu-transgenic mouse model, for example, Singh and colleagues showed that a neu-based Listeria monocytogenes-based vaccine induced potent immunity which caused the outgrowth of tumors with mutations in unique T cell epitopes that were recognized by the vaccine-induced T cells [128].
Antibody-based therapies can also immunoedit tumors, again a finding in both mice and humans. Antibodies that target growth factors (e.g. HER-2/neu) can lead to the antigen-loss variants by inducing receptor internalization, epigenetic mechanisms, or immunoselection. In a recent murine study by Knutson and colleagues, anti-neu antibody treatment of animals bearing neu-positive tumors resulted in a rapid outgrowth of antigen-negative variants by an as of yet unknown mechanism, and the results showed that loss of antigen was stable, indicating that something other than receptor down-regulation was responsible for the immune escape. HER-2/neu loss has also been observed in patients treated with trastuzumab, a recombinant humanized anti-HER-2/neu antibody widely used for HER-2/neu-overexpressing breast cancers. In one study, loss of HER-2/neu expression was seen in breast cancer patients undergoing preoperative, neoadjuvant trastuzumab therapy. In that study, patients with HER-2/neu-overexpressing tumors were treated with trastuzumab and paclitaxel prior to conventional (i.e. surgery and chemotherapy) therapy and HER-2/neu status was determined before treatment and at the time of surgery (i.e. following trastuzumab/paclitaxel neoadjuvant therapy)[130]. Seventy-five percent of the patients had objective clinical responses to preoperative trastuzumab and paclitaxel therapy. Twenty-seven (68%) of the patients had assessable tumors following preoperative therapy, in which it was observed that seven had significantly reduced levels of HER-2/neu staining. While the authors suggest that down-modulation may have occurred, the analyses did not appear to be extensive enough to rule out the possibility that more stable antigen-negative variants were generated. In lymphoma patients treated with monoclonal anti-CD20 antibody (i.e. rituximab) it was observed that treatment resulted in reduced CD20 expression in the tumor cells localized to the bone marrow but not those localized to lymph nodes [131]. What remains to be determined is if loss of antigen is associated with treatment failure.
C.3. Preventing and overcoming immunosculpting and immunoediting during immunotherapy
As the mechanisms by which tumor immunoediting become elucidated, there is an increasing recognition that immune-based approaches should be designed to incorporate combination approaches that not only augment anti-tumor immune response but also simultaneously block or overcome immunosculpting. Several potential strategies are relevant to this problem.
C.3.1. Targeting multiple antigens
Most antigen-specific vaccines, antibody, or T cell therapy strategies involve targeting single antigens (e.g. MART-1 or HER-2/neu). Furthermore, in many cases, antigen loss appears to be confined to only to a single antigen [127]. Several different antigens have now been discovered making it possible to target multiple antigens and reduce selection for antigen-loss variants [132]. Alternatively, another approach is to create vaccine conditions more favorable for natural epitope spreading to several antigens. By increasing the epitope coverage of the immune response there is less likelihood of escape by selection or generation of epitope- or antigen-loss variants. Epitope spreading, was first described in autoimmune disease, and it represents the generation of an immune response to a particular portion of an immunogenic protein, and then the natural spread of that immunity to other areas of the protein, or even to other antigens present in the environment [133]. Theoretically, epitope spreading represents endogenous processing of antigen at sites of inflammation initiated by a specific T cell response, or “driver clone” [134]. Incorporating CD4 T cell epitopes into vaccines has been reported to induce epitope spreading. For example, immunization with HER-2/neu T helper peptide-based vaccines resulted in epitope spreading within the HER-2/neu protein (i.e. intramolecular epitope spreading) being observed in the majority of patients, and significantly correlated with the generation of HER-2/neu protein-specific T cell immunity [135]. In addition, evaluation of the pre- and post-immunization tumor antigen-specific antibody immunity generated during the trial suggested the development of epitope spreading to additional antigens (e.g. p53). [136]. In murine studies, Nava-Parada and colleagues have shown that depletion of Tregs with an anti-CD25 antibody prior to immunization with CD8 T cell epitopes strongly enhanced epitope spreading and led to a more efficient anti-tumor response [137]. Epitope spreading has been linked with survival benefit after immunotherapy in patients with melanoma [138].
C.3.2. Targeting MHC-defective tumors
In cases where MHC class I or APM expression are defective or lost or where the tumor cells are resistant to killing using the traditional CD8 T cell targeting approaches, alternative strategies have emerged. Tumors thrive in specific locations because the stroma is particularly effective at providing growth factors, essential nutrients, and an overall permissive microenvironment [139]. Fibroblasts synthesize a number of factors that directly support growth and progression of tumors [140, 141]. One example is fibroblast activation protein (FAP), a serine protease that is specifically overexpressed by >90% of fibroblasts in colon, lung and breast cancers. Loeffler and colleagues, recently showed that CD8 T cell killing of fibroblasts, induced by FAP-specific DNA vaccination, was able to suppress primary tumor growth and metastases [142]. Another study from the same team suggested that eradicating tumor-associated macrophages also using a DNA vaccine could suppress tumor growth [143]. In that study, the stress-related macrophage protein, legumain, was the target protein. Together, these results demonstrate that targeting the tumor itself may not always be a necessary approach, but that disrupting its niche may also be an effective strategy.
As described earlier, several human cancers show loss of APM components, which limits the presentation of MHC:peptide complexes at the surface of the tumor cells. These defective tumor cells may still be susceptible to a T cell response. For example, using a TAP-deficient animal model, van Hall and colleagues recently described the existence of a unique population of CTLs that target an alternative (i.e. neoantigen) repertoire of peptide epitopes that emerge in the MHC class I molecules in TAP-deficient cells [144].
C.3.3. Blocking epigenetic mechanisms
In cases where reversible epigenetic mechanisms result in immunosculpting, treatment with a variety of different drugs may reverse the phenotype. In murine models of melanoma, a potent anti-melanoma immune response can be elicited by targeting normal healthy melanocytes with plasmids encoding hsp70 and suicide genes [145]. With suboptimal vaccinations, amelanotic tumors arise that have lost several antigens such as tyrosinase and tyrosinase-related protein-2 (TRP-2). These edited tumor cells could be induced to re-express antigen when treated with 5-azacytidine (5-aza), increasing their susceptibility to further vaccination. There is some recent data to suggest that such an approach may work in the human clinical setting as well. Human renal and melanoma cell lines are highly resistant to the antiproliferative effects of Type 1 interferons through the epigenetic silencing of interferon-response genes. Treatment of the cell lines with 5-aza-2′-deoxycytidine restored responsive to interferons, resulting in both decreased proliferation and an increase in the numbers of apoptotic cells [146]. Changes in the differentiation states (e.g. EMT) may also be blocked during immunotherapies that could lead to improved anti-tumor outcomes. For example, the cysteine protease inhibitor, cystatin C was recently found to act as a novel TGF-β type II receptor antagonist, which blocked induction of EMT in murine mammary epithelial cells [147, 148]. Another antagonist of TGF-β is HTS 466284, which was recently shown to increase the efficacy of DC-based vaccinations by preventing vaccine-induced EMT [149].
D. Conclusions
With modern tools and technology, the concept of immunosurveillance has garnered much interest in recent years. A simplified view of immunosurveillance is that the chronic interaction selects for cells that are no longer immunogenic (i.e. immunoediting). However, emerging concepts indicate a far more complex interaction (See Figure) that includes a dynamic tumor:immune interaction that increases both immune evasion and pathogenesis. Understanding the underlying mechanisms will likely be necessary to improve the immune therapies for cancer prevention and treatment.
Acknowledgments
This work was supported by the Mayo Clinic Comprehensive Cancer Center and NCI grants K01-CA100764 and R01-CA113861
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 2.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–90. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, Smyth MJ. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–90. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–9. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol. 2001;214:194–200. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–70. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–13. [PubMed] [Google Scholar]
- 10.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–61. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 11.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 12.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi Y, Jungbluth A, Richards EC, Old LJ. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1996;93:11798–801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–9. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:9464–9. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 21.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 23.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 24.Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev. 2004;56:1099–109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Li PY, Del Vecchio S, Fonti R, Carriero MV, Potena MI, Botti G, Miotti S, Lastoria S, Menard S, Colnaghi MI, Salvatore M. Local concentration of folate binding protein GP38 in sections of human ovarian carcinoma by in vitro quantitative autoradiography. J Nucl Med. 1996;37:665–72. [PubMed] [Google Scholar]
- 26.Rentzsch C, Kayser S, Stumm S, Watermann I, Walter S, Stevanovic S, Wallwiener D, Guckel B. Evaluation of pre-existent immunity in patients with primary breast cancer: molecular and cellular assays to quantify antigen-specific T lymphocytes in peripheral blood mononuclear cells. Clin Cancer Res. 2003;9:4376–86. [PubMed] [Google Scholar]
- 27.Disis ML, Knutson KL, Schiffman K, Rinn K, McNeel DG. Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat. 2000;62:245–52. doi: 10.1023/a:1006438507898. [DOI] [PubMed] [Google Scholar]
- 28.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, McIntosh MW, Disis ML. Antibody Immunity to the p53 Oncogenic Protein Is a Prognostic Indicator in Ovarian Cancer. J Clin Oncol. 2006;24:762–8. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 30.Vial T, Descotes J. Immunosuppressive drugs and cancer. Toxicology. 2003;185:229–40. doi: 10.1016/s0300-483x(02)00612-1. [DOI] [PubMed] [Google Scholar]
- 31.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 32.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. Jama. 2006;296:2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 33.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Chambers WM, Warren BF, Jewell DP, Mortensen NJ. Cancer surveillance in ulcerative colitis. Br J Surg. 2005;92:928–36. doi: 10.1002/bjs.5106. [DOI] [PubMed] [Google Scholar]
- 35.Friedman S. Cancer in Crohn's disease. Gastroenterol Clin North Am. 2006;35:621–39. doi: 10.1016/j.gtc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–4. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 38.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 39.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 40.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–26. 29. discussion 30-2. [PubMed] [Google Scholar]
- 41.Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: shifting the global burden. World J Gastroenterol. 2006;12:5458–64. doi: 10.3748/wjg.v12.i34.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 43.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 44.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 45.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 46.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–45. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 48.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 49.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–7. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 51.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–31. [PubMed] [Google Scholar]
- 52.Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Symp. 2004;256:158–68. discussion 68-72, 259-69. [PubMed] [Google Scholar]
- 53.Valkovic T, Fuckar D, Stifter S, Matusan K, Hasan M, Dobrila F, Jonjic N. Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol. 2005;131:453–8. doi: 10.1007/s00432-004-0667-3. [DOI] [PubMed] [Google Scholar]
- 54.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–51. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 55.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 56.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 58.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 59.Meric JB, Rottey S, Olaussen K, Soria JC, Khayat D, Rixe O, Spano JP. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 61.Imai Y, Kubota Y, Yamamoto S, Tsuji K, Shimatani M, Shibatani N, Takamido S, Matsushita M, Okazaki K. Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: an in vitro study. J Gastroenterol Hepatol. 2005;20:287–93. doi: 10.1111/j.1440-1746.2004.03575.x. [DOI] [PubMed] [Google Scholar]
- 62.Matteucci E, Locati M, Desiderio MA. Hepatocyte growth factor enhances CXCR4 expression favoring breast cancer cell invasiveness. Exp Cell Res. 2005;310:176–85. doi: 10.1016/j.yexcr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 64.Liu K, Caldwell SA, Greeneltch KM, Yang D, Abrams SI. CTL adoptive immunotherapy concurrently mediates tumor regression and tumor escape. J Immunol. 2006;176:3374–82. doi: 10.4049/jimmunol.176.6.3374. [DOI] [PubMed] [Google Scholar]
- 65.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 66.Lejeune FJ. Clinical use of TNF revisited: improving penetration of anti-cancer agents by increasing vascular permeability. J Clin Invest. 2002;110:433–5. doi: 10.1172/JCI16493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23:1902–10. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 68.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 69.Devoogdt N, Revets H, Kindt A, Liu YQ, De Baetselier P, Ghassabeh GH. The tumor-promoting effect of TNF-alpha involves the induction of secretory leukocyte protease inhibitor. J Immunol. 2006;177:8046–52. doi: 10.4049/jimmunol.177.11.8046. [DOI] [PubMed] [Google Scholar]
- 70.Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci U S A. 2003;100:5778–82. doi: 10.1073/pnas.1037154100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann N Y Acad Sci. 2004;1028:380–9. doi: 10.1196/annals.1322.044. [DOI] [PubMed] [Google Scholar]
- 72.Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091–9. doi: 10.1016/s0006-2952(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 73.Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta Anat (Basel) 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- 74.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 75.Viebahn C. Epithelio-mesenchymal transformation during formation of the mesoderm in the mammalian embryo. Acta Anat (Basel) 1995;154:79–97. doi: 10.1159/000147753. [DOI] [PubMed] [Google Scholar]
- 76.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Bates RC, Mercurio AM. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14:1790–800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227–39. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 79.Knutson KL, Lu H, Stone B, Reiman J, Gad E, Smorlesi A, Disis ML. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 80.Manjili MH, Arnouk H, Knutson KL, Kmieciak M, Disis ML, Subjeck JR, Kazim AL. Emergence of immune escape variant of mammary tumors that has distinct proteomic profile and a reduced ability to induce “danger signals”. Breast Cancer Res Treat. 2005;96:233–41. doi: 10.1007/s10549-005-9044-4. [DOI] [PubMed] [Google Scholar]
- 81.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mouse mammary carcinoma are actively induced by T cell-mediated anti-tumor responses. Eur J Immunol. 2007;37:675–85. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 83.Kremer KN, Kumar A, Hedin KE. Haplotype-Independent Costimulation of IL-10 Secretion by SDF-1/CXCL12 Proceeds via AP-1 Binding to the Human IL-10 Promoter. J Immunol. 2007;178:1581–8. doi: 10.4049/jimmunol.178.3.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12:4794–803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 85.Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, Azeke E, Disis ML. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 86.Knutson KL, Disis ML, Salazar L. CD4 Regulatory T Cells in Human Cancer Pathogenesis. Cancer Immunol Immunother. 2006 doi: 10.1007/s00262-006-0194-y. Epub ahead of print: 10.1007/s00262-00262-0006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–78. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 90.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson RH, Dong H, Kwon ED. Implications of b7-h1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 92.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 94.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watanabe N, Hizuta A, Tanaka N, Orita K. Localization of T cell receptor (TCR)-gamma delta + T cells into human colorectal cancer: flow cytometric analysis of TCR-gamma delta expression in tumour-infiltrating lymphocytes. Clin Exp Immunol. 1995;102:167–73. doi: 10.1111/j.1365-2249.1995.tb06651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42:541–6. doi: 10.1016/j.molimm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 97.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–24. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 98.Cabrera CM, Jimenez P, Concha A, Garrido F, Ruiz-Cabello F. Promyelocytic leukemia (PML) nuclear bodies are disorganized in colorectal tumors with total loss of major histocompatibility complex class I expression and LMP7 downregulation. Tissue Antigens. 2004;63:446–52. doi: 10.1111/j.0001-2815.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 99.Maleno I, Cabrera CM, Cabrera T, Paco L, Lopez-Nevot MA, Collado A, Ferron A, Garrido F. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56:244–53. doi: 10.1007/s00251-004-0692-z. [DOI] [PubMed] [Google Scholar]
- 100.Dierssen JW, de Miranda NF, Mulder A, van Puijenbroek M, Verduyn W, Claas FH, van de Velde CJ, Jan Fleuren G, Cornelisse CJ, Corver WE, Morreau H. High-resolution analysis of HLA class I alterations in colorectal cancer. BMC Cancer. 2006;6:233. doi: 10.1186/1471-2407-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cabrera CM, Jimenez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211–9. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 102.Cabrera T, Collado A, Fernandez MA, Ferron A, Sancho J, Ruiz-Cabello F, Garrido F. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens. 1998;52:114–23. doi: 10.1111/j.1399-0039.1998.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 103.Kaklamanis L, Gatter KC, Hill AB, Mortensen N, Harris AL, Krausa P, McMichael A, Bodmer JG, Bodmer WF. Loss of HLA class-I alleles, heavy chains and beta 2-microglobulin in colorectal cancer. Int J Cancer. 1992;51:379–85. doi: 10.1002/ijc.2910510308. [DOI] [PubMed] [Google Scholar]
- 104.Romero JM, Aptsiauri N, Vazquez F, Cozar JM, Canton J, Cabrera T, Tallada M, Garrido F, Ruiz-Cabello F. Analysis of the expression of HLA class I, proinflammatory cytokines and chemokines in primary tumors from patients with localized and metastatic renal cell carcinoma. Tissue Antigens. 2006;68:303–10. doi: 10.1111/j.1399-0039.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 105.Ramnath N, Tan D, Li Q, Hylander BL, Bogner P, Ryes L, Ferrone S. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–9. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, Altermann W, Handke D, Atkins D, Seliger B, Kiessling R. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–94. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 107.So T, Takenoyama M, Mizukami M, Ichiki Y, Sugaya M, Hanagiri T, Sugio K, Yasumoto K. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005;65:5945–52. doi: 10.1158/0008-5472.CAN-04-3787. [DOI] [PubMed] [Google Scholar]
- 108.Riemersma SA, Oudejans JJ, Vonk MJ, Dreef EJ, Prins FA, Jansen PM, Vermeer MH, Blok P, Kibbelaar RE, Muris JJ, Schuuring EM, Kluin PM. High numbers of tumour-infiltrating activated cytotoxic T lymphocytes, and frequent loss of HLA class I and II expression, are features of aggressive B cell lymphomas of the brain and testis. J Pathol. 2005;206:328–36. doi: 10.1002/path.1783. [DOI] [PubMed] [Google Scholar]
- 109.Palmisano GL, Pistillo MP, Capanni P, Pera C, Nicolo G, Salvi S, Perdelli L, Pasciucco G, Ferrara GB. Investigation of HLA class I downregulation in breast cancer by RT-PCR. Hum Immunol. 2001;62:133–9. doi: 10.1016/s0198-8859(00)00241-x. [DOI] [PubMed] [Google Scholar]
- 110.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248–55. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
- 111.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–73. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 112.Baldi A, Battista T, De Luca A, Santini D, Rossiello L, Baldi F, Natali PG, Lombardi D, Picardo M, Felsani A, Paggi MG. Identification of genes down-regulated during melanoma progression: a cDNA array study. Exp Dermatol. 2003;12:213–8. doi: 10.1034/j.1600-0625.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 113.Vetter CS, Lieb W, Brocker EB, Becker JC. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br J Cancer. 2004;91:1495–9. doi: 10.1038/sj.bjc.6602123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 115.Michor F, Iwasa Y, Lengauer C, Nowak MA. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15:484–93. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Dovhey SE, Ghosh NS, Wright KL. Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res. 2000;60:5789–96. [PubMed] [Google Scholar]
- 117.Hayashi T, Kobayashi Y, Kohsaka S, Sano K. The mutation in the ATP-binding region of JAK1, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene. 2006;25:4016–26. doi: 10.1038/sj.onc.1209434. [DOI] [PubMed] [Google Scholar]
- 118.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–11. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 119.Cicinnati VR, Zhang X, Yu Z, Ferencik S, Schmitz KJ, Dworacki G, Kaczmarek E, Oldhafer K, Frilling A, Baba HA, Schmid KW, Grosse-Wilde H, Broelsch CE, DeLeo AB, Gerken G, Beckebaum S. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119:2851–60. doi: 10.1002/ijc.22251. [DOI] [PubMed] [Google Scholar]
- 120.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–92. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–43. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- 122.Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 123.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 124.Abouzahr S, Bismuth G, Gaudin C, Caroll O, Van Endert P, Jalil A, Dausset J, Vergnon I, Richon C, Kauffmann A, Galon J, Raposo G, Mami-Chouaib F, Chouaib S. Identification of target actin content and polymerization status as a mechanism of tumor resistance after cytolytic T lymphocyte pressure. Proc Natl Acad Sci U S A. 2006;103:1428–33. doi: 10.1073/pnas.0510454103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, Li F, Dewhirst MW, Li CY. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–70. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 126.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67:1887–92. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- 129.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR, Weinstein S, Orel SG, Vonderheide R, Coukos G, DeMichele A, Araujo L, Spitz FR, Rosen M, Levine BL, June C, Zhang PJ. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–52. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 130.Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP. Preoperative Therapy With Trastuzumab and Paclitaxel Followed by Sequential Adjuvant Doxorubicin/Cyclophosphamide for HER2 Overexpressing Stage II or III Breast Cancer: A Pilot Study. J Clin Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 131.Foran JM, Norton AJ, Micallef IN, Taussig DC, Amess JA, Rohatiner AZ, Lister TA. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): a retrospective cohort analysis. Br J Haematol. 2001;114:881–3. doi: 10.1046/j.1365-2141.2001.03019.x. [DOI] [PubMed] [Google Scholar]
- 132.Knutson KL. Cancer Vaccines: The Next Generation. Drug Discov Today. 2006;2:323–30. [Google Scholar]
- 133.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 134.Sercarz EE. Driver clones and determinant spreading. J Autoimmun. 2000;14:275–7. doi: 10.1006/jaut.2000.0380. [DOI] [PubMed] [Google Scholar]
- 135.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu Peptide-based vaccines. J Clin Oncol. 2002;20:2624–32. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 136.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol. 2004;24:571–78. doi: 10.1023/B:JOCI.0000040928.67495.52. [DOI] [PubMed] [Google Scholar]
- 137.Nava-Parada P, Forni G, Knutson KL, Pease LR. Peptide Vaccine administered with a toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2006;67:1326–34. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, Cochran AJ, Glaspy JA, Economou JS. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 139.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ganss R. Tumor stroma fosters neovascularization by recruitment of progenitor cells into the tumor bed. J Cell Mol Med. 2006;10:857–65. doi: 10.1111/j.1582-4934.2006.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 141.Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest. 2006;35:297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 142.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–62. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, de Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12:417–24. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 145.Daniels GA, Sanchez-Perez L, Diaz RM, Kottke T, Thompson J, Lai M, Gough M, Karim M, Bushell A, Chong H, Melcher A, Harrington K, Vile RG. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 2004;22:1125–32. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 146.Reu FJ, Bae SI, Cherkassky L, Leaman DW, Lindner D, Beaulieu N, MacLeod AR, Borden EC. Overcoming resistance to interferon-induced apoptosis of renal carcinoma and melanoma cells by DNA demethylation. J Clin Oncol. 2006;24:3771–9. doi: 10.1200/JCO.2005.03.4074. [DOI] [PubMed] [Google Scholar]
- 147.Sokol JP, Neil JR, Schiemann BJ, Schiemann WP. The use of cystatin C to inhibit epithelial-mesenchymal transition and morphological transformation stimulated by transforming growth factor-beta. Breast Cancer Res. 2005;7:R844–53. doi: 10.1186/bcr1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sokol JP, Schiemann WP. Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol Cancer Res. 2004;2:183–95. [PubMed] [Google Scholar]
- 149.Rausch MP, Akporiaye ET, Mahadevan D. Enhancement of DC-based immunotherapy using a small molecule TGF-{beta} inhibitor. AACR Meeting Abstracts. 2005;2005:1255–e56. [Google Scholar]