Abstract
MnTBAP is often referred to as an SOD mimic in numerous models of oxidative stress. We have recently reported that pure MnTBAP does not dismute superoxide, but commercial/ill-purified samples are able to perform O2•− dismutation with low-to-moderate efficacy via non-innocent Mn-containing impurities. Herein, we show that neither commercial nor pure MnTBAP could substitute for SOD enzyme in the SOD-deficient E. coli model, while MnTE-2-PyP-treated SOD-deficient E. coli grew as well as wild-type strain. This SOD-specific system indicates that MnTBAP does not act as an SOD mimic in vivo. In another model, carrageenan-induced pleurisy in mice, inflammation was evidenced by increased pleural fluid exudate, and neutrophil infiltration and activation: these events were blocked by 0.3 mg/kg of MnTE-2-PyP and to a slightly lesser extent with 10 mg/kg of MnTBAP. Also, 3-nitrotyrosine formation, an indication of the peroxynitrite existence in vivo, was blocked by both compounds; again MnTE-2-PyP was 33-fold more effective. Pleurisy model data indicate that MnTBAP exert some protective actions in common with MnTE-2-PyP, which are not O2•−-related, and can be fully rationalized if one considers that the common biological role shared by MnTBAP and MnTE-2-PyP is related to their reduction of peroxynitrite and carbonate radical, the latter arising from ONOO− adduct with CO2. The log kcat (O2•−) value for MnTBAP is estimated to be about 3.16, which is ~5 and ~7 orders of magnitude smaller than the SOD activity of the potent SOD mimic MnTE-2-PyP and Cu, Zn-SOD, respectively. This very low value indicates that MnTBAP is very inefficient in dismuting superoxide to be of any biological impact, which was confirmed in the SOD-deficient E. coli model. Peroxynitrite scavenging ability of MnTBAP, however, is only ~2.5 orders of magnitude smaller than that of MnTE-2-PyP and is not significantly affected by the presence of the SOD-active impurities in commercial MnTBAP sample (log kred(ONOO−) = 5.06 for pure and 4.97 for commercial sample). The reduction of carbonate radical is equally fast with MnTBAP and MnTE-2-PyP. The dose of MnTBAP required to yield oxidative stress protection and block nitrotyrosine formation in the pleurisy model is >1.5 orders of magnitude higher than that of MnTE-2-PyP, which could be related to the smaller ability of MnTBAP to scavenge peroxynitrite. The slightly better protection observed with the commercial MnTBAP sample (relative to the pure MnTBAP one) could arise from its impurities, which, by scavenging O2•−, reduce consequently the overall peroxynitrite, and secondary ROS/RNS levels. These observations have profound biological repercussions as they may suggest that the effect of MnTBAP observed in numerous studies may conceivably relate to peroxynitrite scavenging. Moreover, provided that pure MnTBAP is unable to dismute superoxide at any significant extent, but is able to partially scavenge peroxynitrite and carbonate radical, this compound may prove valuable to distinguish ONOO−/CO3•− from O2•− pathways.
Keywords: SOD mimic, peroxynitrite scavenger, carbonate radical scavenger, MnTBAP, MnTE-2-PyP, SOD-deficient E. coli, carrageenan-induced pleurisy in mouse
Introduction
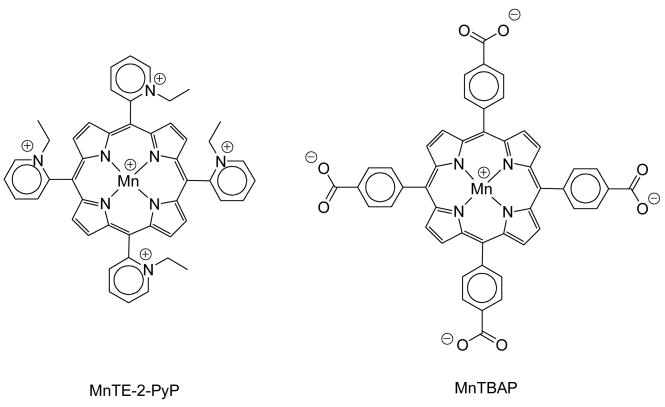
There has been a great deal of interest in developing superoxide dismutase (SOD) mimics and peroxynitrite scavengers as both mechanistic probes and therapeutic drugs for treating oxidative stress injuries [1–13]. The most potent porphyrin-based compounds developed based on structure-activity relationships (SAR), such as Mn (III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP; Fig. 1), its hexyl analogue MnTnHex-2-PyP and Mn (III) meso-tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin, bear positively charged ortho pyridyl or di-ortho imidazolyl moieties close to the metal site, which thus offer the thermodynamic and electrostatic facilitation [1–4,14–16] for the reaction with negatively charged superoxide and peroxynitrite, and are the most efficient in helping SOD-deficient E. coli to grow aerobically [4,14,17]. Along with SAR, a stringent SOD model based on the aerobic growth of SOD-deficient E. coli in restricted 5 amino acid medium has proven extremely useful to assess the potentialities of Mn porphyrins as candidate therapeutics for ameliorating diseases/conditions that have oxidative stress in common [4,14]. Negatively charged porphyrins, which lack thermodynamic and electrostatic facilitation for O2•− dismutation, are either poor SOD mimics (e.g., MnTSPP) or not at all able to dismute O2•− (e.g., MnTBAP; Fig. 1) [4,18,19], and in turn can poorly or not at all substitute for the SOD in SOD-deficient E. coli [19]. Although commercial preparations of MnTBAP have been widely used to test mechanistic concepts and/or for therapeutic purposes, the preparation of a pure MnTBAP sample showed unambiguously that MnTBAP per se has no SOD-like activity in aqueous systems [18], and that all common commercially available MnTBAP preparations contain varying amounts of highly SOD-active Mn oxo/hydroxo/acetato species as contaminants; such impurities are responsible for the observed in vitro SOD-activity. Moreover, all of these commercial preparations exhibit modest to high ability to inhibit xanthine oxidase [18], which is a common source of ROS in vivo [20].
Figure 1.
Structural diagram of MnTE-2-PyP and MnTBAP.
Due to a high number of studies indicating that MnTBAP is effective in ameliorating in vivo oxidative stress injuries, we kept trying to understand the origin of such effects. Our in vitro and in vivo experiments suggest that while MnTBAP is not an SOD mimic, the benefits often observed with MnTBAP in cell/animal models may conceivably be related, at least in part, to its capacity to scavenge peroxynitrite or other reactive species derived from it. Thus we analyzed herein the ability of pure and impure MnTBAP to reduce ONOO−.
Finally we devised in vivo experiments to investigate whether pure MnTBAP and commercial MnTBAP differ with respect to their in vivo behavior in mediating oxidative stress in two well-established models: an SOD-deficient E. coli model [21–23] and an inflammatory, mouse carrageenan-induced pleurisy model [24–26]. We compared the effects of MnTBAP to MnTE-2-PyP, which is both a potent SOD mimic and peroxynitrite scavenger [4,27].
Experimental
Materials
Pure MnTBAP was synthesized and characterized as previously reported [18]. A commercial sample of MnTBAP was bought from Alexis (catalogue # 430-069-M010, lot# L06883); analyses of this commercial sample showed 3% of Mn-free ligand. Another batch of commercial MnTBAP (lot# 19498) was found unsuitable for any cell/animal testing as it contained ~24% of the Mn-free ligand [28]. MnTE-2-PyP was prepared and thoroughly characterized as described in [4,28]. All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Milan, Italy). The compounds for animal work were obtained from Sigma-Aldrich Company Ltd. (Milan, Italy) and all other chemicals were of the highest commercial grade available.
Kinetics of peroxynitrite scavenging
Stopped-flow kinetic measurements were carried out as described before [27] using an SX-17MV Stopped-Flow from Applied Photophysics coupled with a 1-cm-long mixing cell. Briefly, MnTBAP samples (6 μM) in 100 mM phosphate buffer pH 7.0 were mixed in a 1:1 v/v ratio with 10- to 40-fold molar excess of ONOO− in 15 mM NaOH; the final pH was 7.3. The reaction was monitored by the change in the absorbance of the Soret band of MnTBAP (468 nm [18]), and the plots were fitted to a single exponential function. All experiments were carried out at 37 °C. The pH was measured at the outlet of the stopped-flow.
E. coli growth experiments
Escherichia coli strains used in this study were AB1157, wild type (F-thr-1; leuB6; proA2; his-4; thi-1; argE2; lacY1; galK2; rpsL; supE44; ara-14; xyl-15; mtl-1; tsx-33), and JI132, SOD-deficient, sodA−sodB− (same as AB1157 plus (sodA::mudPR13)25 (sodB-kan)1-Δ2). Both strains were obtained from J. A. Imlay [21]. The experiments were carried out as described in detail in [14]. Briefly, cultures were grown aerobically in either casamino acid (M9CA) medium or in a 5 amino acid restricted medium (L-leucine, L-threonine, L-proline, L-arginine, L-histidine) in flasks on a water bath shaker at 37°C and 200 rpm. The effect of Mn porphyrins on the growth of the SOD-deficient strains was followed at 10 (M9CA medium) and 20 hours (5 amino acids medium), turbidimetrically at 700 nm (to minimize the interference of compounds studied) and compared to the growth curves of both strains in the absence of Mn porphyrin (controls). Deionized water was used throughout the study.
Animals
Six to eight week old CD1 male mice (Charles River, Calco, Italy) were used for the study. The animals were housed in a controlled environment and provided with standard rodent chow and water. The study was approved by the University of Messina Review Board for the care of animals. All animal experiments complied with regulations in Italy (D.M. 116192), Europe (O.J. of E.C. L 358/1 12/18/1986) and USA (Animal Welfare Assurance No A5594-01, Department of Health and Human Services, USA).
Carrageenan-induced pleurisy
Pleurisy was induced by carrageenan as previously described [29]. We anesthetized the mice with isoflurane and made a skin incision at the level of the left sixth intercostal space. The underlying muscle was dissected and saline (0.2 ml) or saline containing 1% (w/v) λ-carrageenan (Sigma-Aldrich Ltd, 0.2 ml) was injected into the pleural cavity. The skin incision was closed with a suture and the animals were allowed to recover. At 4 h after the injection of carrageenan, the animals were killed. The chest was carefully opened and the pleural cavity rinsed with 2 ml of saline solution containing heparin (5 U/ml) and indomethacin (10 μg/ml). The exudate and washing solution were removed by aspiration and the total volume measured. Any exudate, which was contaminated with blood, was discarded. The amount of exudate was calculated by subtracting the volume injected (2 ml) from the total volume recovered. The leukocytes in the exudate were suspended in phosphate-buffer saline (PBS, 0.01M, pH7.4) and counted with an optical microscope in a Burker’s chamber after vital Trypan Blue staining.
Experimental groups
Mice were allocated into one of the following groups: (1) administration of carrageenan only (CAR group, N=10), (2a) commercial MnTBAP (10.0 mg/kg) given as an intraperitoneal (i.p.) bolus 30 minutes before carrageenan (CAR + commercial MnTBAP group, N=10), (2b) same as 2a except that pure MnTBAP (10.0 mg/kg) was used instead of commercial MnTBAP (CAR + pure MnTBAP group, N=10), (2c) same as 2a except that MnTE-2-PyP (0.3 mg/kg) was used instead of commercial MnTBAP (CAR + MnTE-2-PyP group, N=10), (3) a sham-operated group in which identical surgical procedures to the CAR group was performed, except that the saline (vehicle) was administered instead of carrageenan (Sham group, N=10). (4a) Sham + commercial MnTBAP group: Same as Sham group except for the administration of commercial MnTBAP (10.0 mg/kg) which was given 30 minutes before the saline injection (N=10), (4b) same as 4a except that pure MnTBAP (10.0 mg/kg) was used instead of commercial MnTBAP (Sham + pure MnTBAP group, N=10), (4c) same as 4a except that MnTE-2-PyP (0.3 mg/kg) was used instead of commercial MnTBAP (Sham + MnTE-2-PyP group, N=10).
Histological examination
Lungs were taken 4 h after carrageenan or vehicle injection. Tissues were fixed for 1 week in 10 % (w/v) PBS-buffered formaldehyde solution at room temperature, dehydrated using graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, USA). Lung sections were then deparaffinized with xylene, stained with hematoxylin and eosin. All sections were studied using light microscopy (Dialux 22 Leitz).
Myeloperoxidase activity
Myeloperoxidase (MPO) activity, an indicator of polymorphonuclear leukocyte (PMN) accumulation, was determined in lung tissues after carrageenan-injection as previously described [30]. Subplantar and lung tissues obtained from ten animals per group were homogenized in a solution containing 0.5% (w/v) hexadecyltrimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) using a Polytron homogenizer (3 cycles of 10 sec at maximum speed) and centrifuged for 30 min at 20,000 × g at 4° C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM hydrogen peroxide. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μM of peroxide/min at 37 °C and was expressed in units per gram of wet tissue.
Immunohistochemical localisation of 3-nitrotyrosine
At the end of the experiment, the tissues were fixed in 10% (w/v) PBS-buffered formaldehyde and 8 μm sections were prepared from paraffin embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min. The sections were permeabilized with 0.1% (w/v) Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with biotin and avidin (DBA, Milan, Italy), respectively. Sections were incubated overnight with 1) with anti-nitrotyrosine rabbit polyclonal antibody (1:500 in PBS, v/v). Sections were washed with PBS, and incubated with secondary antibody. Specific labeling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (DBA, Milan, Italy). In order to confirm that the immunoreaction for the nitrotyrosine was specific some sections were also incubated with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM) to verify the binding specificity.
Statistical evaluation
All values in the figures and text are expressed as mean ± standard error of the mean (s.e.m.) from 10 mice for each group. For the in vivo studies n represents the number of animals studied. In the experiments involving histology, the figures shown are representative of at least three experiments (histological coloration) performed on different experimental days on the tissues section collected from all the animals in each group. The results were analyzed by one-way ANOVA followed by a Bonferroni’s post-hoc test for multiple comparisons and the effect of on was assessed with 2-way ANOVA for repeated measures and followed by Student’s t test. A p-value of less than 0.05 was considered statistically significant.
Results
ONOO− scavenging and O2•− dismuting properties of the Mn porphyrins
The ability of the pure and the commercial MnTBAP samples to scavenge peroxynitrite was determined by stopped-flow measurements and is summarized in Table 1, which includes also the data on MnTE-2-PyP and Cu, Zn-SOD for comparison. Although the SOD activity of the pure and the commercial (impure) MnTBAP samples differ by 2 orders of magnitude [18], their ability to scavenge peroxynitrite is essentially the same (Table 1). This indicates that while the redox-active impurities in the commercial samples (Mn oxo/hydroxo/acetate clusters) possess SOD activity in their own right and alter the properties of the MnTBAP sample, these impurities do not play any significant role in peroxynitrite scavenging.
Table 1.
The SOD-like (kcat for O2•− dismutation),, peroxynitrite and carbonate radical scavenging (kred for the reduction of ONOO− and CO3•−) properties of Mn(III) porphyrins and Cu, Zn-SOD.
| Compound | log kcat (O2•−) | log kred (ONOO−) | log kred (CO3•−) |
|---|---|---|---|
| MnTE-2-PyP | 7.76a | 7.53f (> 7)g,h | 8.5f (9.3)f,g |
| Pure MnTBAP | <3.50b (3.16)c,d | 5.02d | ~9.1j |
| Commercial MnTBAP | 5.16b | 4.96d | 9.1f |
| Cu, Zn-SOD | ca 9e | 3.97i | – |
Ref. [4].
Ref. [18].
This work.
Calculated using the log kcat (O2•−) vs. log kred (ONOO−) relationship given in Fig. 2.
Ref. [27].
Value for the corresponding Mn(II) species.
Ref. [55].
Ref. [58].
The estimated value is based on the similar reactivity of pure and commercial MnTBAP with ONOO−.
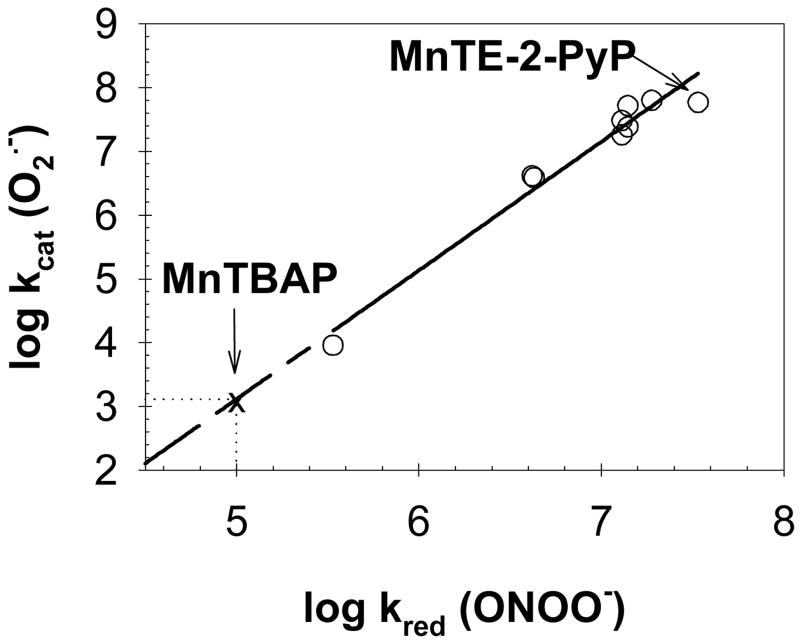
We have previously observed that the ability of Mn porphyrins to scavenge peroxynitrite and dismute superoxide is governed by the closely related SAR [27] as both reactions are predominantly affected by the electron-deficiency and redox-cycling of the Mn site. It follows, thus, that log kcat(O2•−) and log kred (ONOO−) are related by a linear relationship (Fig. 2), from which the SOD activity of pure MnTBAP may be estimated. Because of the low SOD activity of the pure MnTBAP (log kcat < 3.50), only an upper limit for the rate constant was accessible by the direct experimental measurement [18]. Conversely, the determination of the peroxynitrite scavenging ability of MnTBAP could be achieved accurately (Table 1). Given the linear relationship between the SOD and peroxynitrite scavenging properties of Mn porphyrins (Fig. 2), the log kcat (O2•−) value for MnTBAP is estimated to be about 3.16.
Figure 2.
Linear relationship between the SOD-like and peroxynitrite scavenging activities of Mn porphyrins (y = −6.98 + 2.02 x; R2 = 0.96). Data points from [27]. The MnTBAP point was not included in the linear regression as log kcat (O2•−) was too low to be measured accurately (< 3.50); this value was estimated to be ~3.16 based on log kred (ONOO−) of 5.02 determined for pure MnTBAP (see Table 1).
Protection of SOD-deficient E. coli
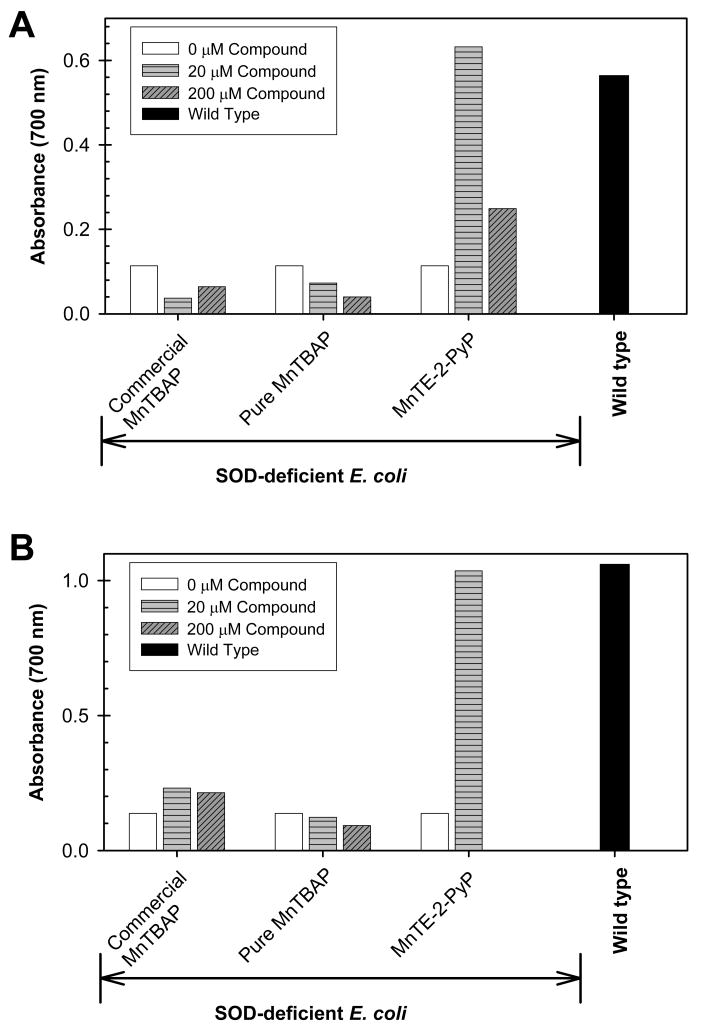
SOD-deficient E. coli can only grow under aerobic conditions in restricted five amino acid-medium if a compound is added to substitute for superoxide dismutase enzyme. In the presence of such a functional SOD mimic, E. coli can, then, synthesize other essential amino acids by utilizing enzymes that are superoxide sensitive [22,23]. Neither pure nor commercial 20 or 200 μM MnTBAP protects SOD-deficient E. coli against superoxide toxicity when grown aerobically in restricted medium (Fig. 3), while 20 μM MnTE-2-PyP was fully protective. In M9CA, which is a richer medium, only MnTE-2-PyP was fully protective at 20 μM, whereas only a slight growth of SOD-deficient E. coli was observed with the commercial MnTBAP preparation at a 10-fold higher concentration (200μM) (Fig. 3). Therefore, pure MnTBAP does not substitute for SOD in either rich (M9CA) or restricted (5 amino acid) medium (Figs. 3A and 3B). The growth of E. coli in M9CA and 5 amino acid restricted medium in the presence and absence of compounds is shown here at 10 and 20 hours, respectively. In both media wild type was in stationary phase, while SOD-deficient E. coli still did not grow. We however followed the growth slightly longer and into the stationary phase to able to notice the effect of MnTBAP, if any. We have previously reported the growth curves of SOD-deficient E. coli [4,17], which clearly showed that MnTE-2-PyP protected and accelerated the growth of SOD-deficient mutants to a rate that is comparable to that of the wild type cells.
Figure 3.
Aerobic growth of SOD-deficient (JI132) and wild type (SOD-proficient, AB1157) E. coli strains in the presence and absence of 20 or 200 μM of commercial MnTBAP, pure MnTBAP, and MnTE-2-PyP in (A) 5 amino acid-medium after 20 hours, and (B) in nutrient rich M9CA medium at 10 hours. Figures are representative of at least 3 experiments performed on different days.
Effect of MnTBAP and MnTE-2-PyP on carrageenan-induced pleurisy, lung MPO activity, and histological examination
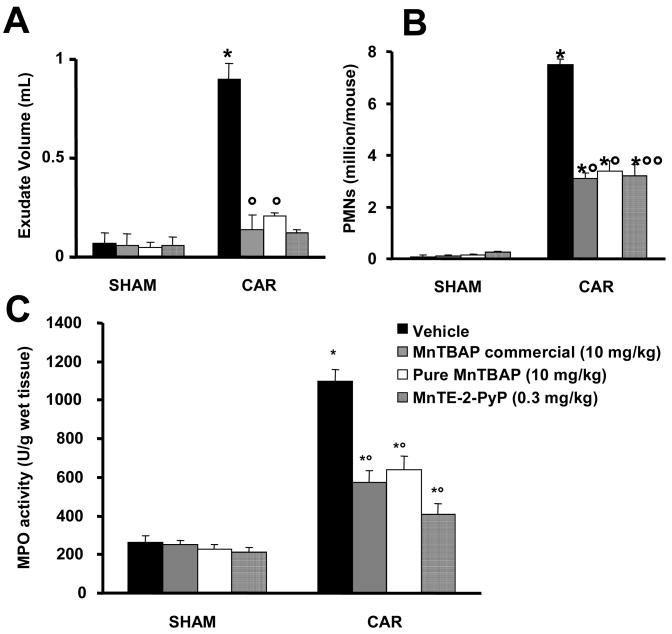
When compared to the sham-treated group, intrapleural injection of carrageenan led to the development of acute pleurisy producing turbid exudate containing a large amount of PMNs within 4 hours (Fig. 4A, B). The presence of pleural exudate and the number of inflammatory cells in the pleural cavity at 4 hours after carrageenan administration was significantly reduced by the treatment with commercial or pure MnTBAP (both at 10 mg/kg) or MnTE-2-PyP (0.3 mg/kg) (Fig. 4A, B). When tested at 0.3 mg/kg commercial or pure MnTBAP had no protective effect (n=6/group, not shown). The presence of inflammatory cells in the pleural cavity appeared to be related to the influx of leukocytes into the lung tissue. Indeed, myeloperoxidase activity was significantly elevated at 4 h after carrageenan injection in vehicle-treated mice (Fig. 4C, p < 0.01), and this was significantly inhibited by commercial or pure MnTBAP (both at 10 mg/kg) or MnTE-2-PyP (0.3 mg/kg) (Fig. 4C, p < 0.01).
Figure 4.
Effects of commercial MnTBAP, pure MnTBAP, and MnTE-2-PyP on carrageenan-induced pleural exudate production, accumulation of polymorphonuclear cells in pleural cavity and myeloperoxidase activity (MPO). A significant production in pleural exudate (A), polymorphonuclear and (B) cells infiltration was observed in pleural cavity from vehicle-treated mice at 4h after carrageenan administration. Furthermore, MPO activity in lung tissues was significantly elevated at 4 h after carrageenan administration in vehicle-treated mice (C). The treatment with commercial or pure MnTBAP, and MnTE-2-PyP significantly reduced the presence of pleural exudate (A) and the number of inflammatory cells (B) as well as lung tissue levels of MPO (C). Data are means ± SEM of 10 mice for each group. *p < 0.01 vs. SHAM; °p < 0.01 vs. carrageenan group.
No histological alterations were observed in lung tissues collected from vehicle-treated mice (Fig. 5). On the contrary, histological examination of lung sections collected at 4 h from all of carrageenan-treated mice showed tissue injury as well as inflammatory cells infiltration. Commercial or pure MnTBAP or MnTE-2-PyP significantly reduced lung injury at 4 h after carrageenan injection as evidenced by histological examination.
Figure 5.
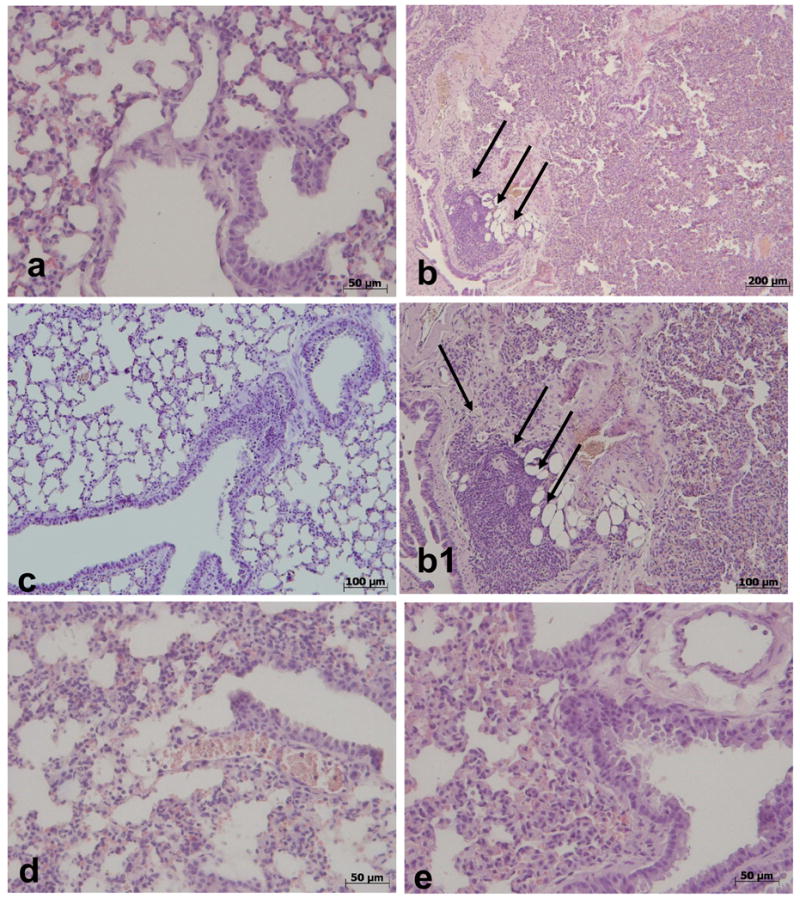
Effects of commercial MnTBAP, pure MnTBAP and MnTE-2-PyP on lung injury. Lung tissues collected at 4 h were stained with hematoxylin and eosin. No injury was observed in the lung tissues collected from sham (vehicle-treated) mice (a). Histological examination of lung sections collected at 4 h from all carrageenan-injected mice showed tissue injury as well as inflammatory cells infiltration (see arrows, b and b1). The treatment of mice with commercial MnTBAP (c), pure MnTBAP (d), and MnTE-2-PyP (e) significantly reduced the lung injury at 4 h after carrageenan injection. Figure is representative of at least 3 experiments performed on different days.
Effects of MnTE-2-PyP and MnTBAP administration on carrageenan-induced immunohistochemical localization of 3-nitrotyrosine in the lung tissues after pleurisy
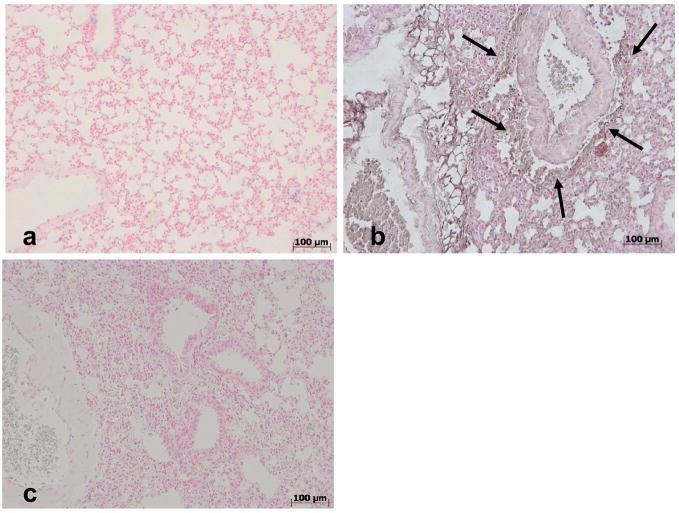
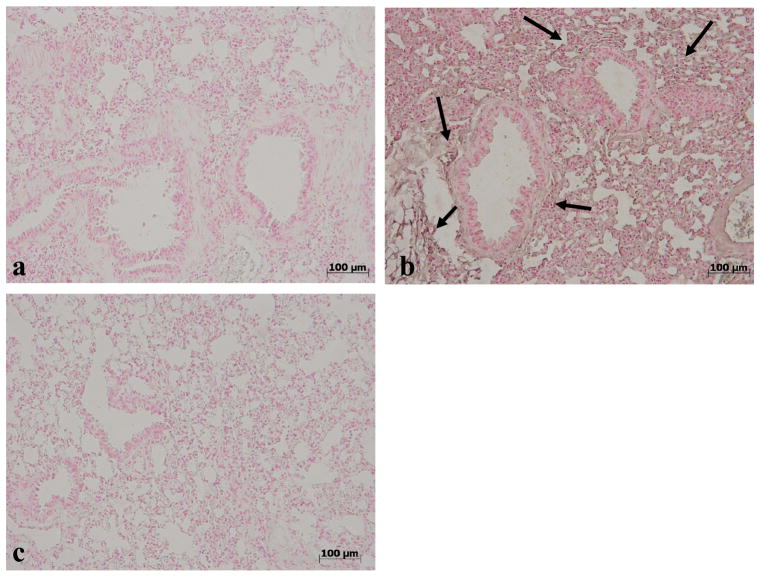
No positive staining for nitrotyrosine (Fig. 6a) was found in lung sections from vehicle-treated mice. Enhanced staining for nitrotyrosine was evident in lung sections obtained from mice at 4h after carrageenan administration (Fig. 6b) and this was reduced by MnTE-2-PyP (Fig. 6c). The same has been observed with 10 mg/kg of commercial MnTBAP (Figure 7, [26]).
Figure 6.
Effects of MnTE-2-PyP on nitrotyrosine formation in the lung. No positive staining for nitrotyrosine was observed in lung tissues obtained from sham mice (a). In contrast, tissue sections obtained from carrageenan-treated mice at 4h after carrageenan administration demonstrate positive staining for nitrotyrosine (see arrows b) and this was blocked by MnTE-2-PyP (c). Figure is representative of at least 3 experiments performed on different experimental days.
Figure 7.
Effects of commercial MnTBAP on nitrotyrosine formation in the lung. No positive staining for nitrotyrosine was observed in lung tissues obtained from sham mice (a). On the contrary, tissue sections obtained 4h after the administration of carrageenan demonstrate positive staining for nitrotyrosine (see arrows, b) and this was blocked by MnTBAP (10 mg/kg, c). Figure is representative of at least 3 experiments performed on different experimental days.
Discussion
Superoxide and its progeny peroxynitrite are among the major reactive oxygen and nitrogen species implicated in a series of physiological processes and pathological conditions [31,32]. The ability of Mn porphyrins to dismute superoxide or scavenge peroxynitrite is an intrinsic property that is quantified, by physicochemical means, via measurement of the rate constants associated with the processes.
MnTBAP has been widely used in in vivo animal models of oxidative stress injuries as an SOD mimic often showing protective effects. Based on kinetic and thermodynamic grounds there is no basis for such an SOD activity. Indeed we have prepared and characterized a pure MnTBAP [18] and showed that it has no SOD-like activity in aqueous system (log kcat < 3.5, [18]; of note Cu, Zn-SOD has log kcat ~9 [33–35]). We compared this pure sample to several common commercial preparations, among which the apparent log kcat varied by >350-fold [18]. Furthermore, we have shown that the SOD-like activity in the commercial samples arise from impurities (Mn oxo/hydroxo/acetato complexes), whose levels differed from one batch to another even within the same supplier [18]. In addition to scavenging O2•−, the commercial preparations also have significant ability to inhibit xanthine oxidase, which may affect superoxide levels in vivo not by scavenging it, but by decreasing its production [20]. Some researchers have also proposed other alternative role/s for MnTBAP, such as induction of hemeoxygenase-1 [36] or modulation of Ca2+ levels [37,38], which do not invoke a MnTBAP superoxide dismuting ability per se. The possibility of MnTBAP action via superoxide reductase (in aqueous media) or superoxide oxidase (in lipophilic environments, such as biological membranes) mechanisms was fully contemplated [18] and found unlikely on thermodynamic grounds [18].
Herein, as a continuation of our efforts to understand the in vivo effects reported on commercial MnTBAPs, we decided to determine the peroxynitrite scavenging ability of MnTBAP and to test a pure MnTBAP in vivo. We used two different samples to study the possible effects of MnTBAP: a pure preparation [18] and a commercial (impure) one. We utilized an SOD-specific model, which is based on the inability of a SOD-deficient E. coli strain (JI132) to grow under aerobic conditions in the absence of a suitable SOD mimic [21–23], and a mouse carrageenan-induced pleurisy model, where inflammation has been previously ascribed at least in part to the involvement of superoxide and peroxynitrite [26,39].
Because of the nature of its formation and decomposition, ONOO− cannot be directly measured in vivo [40] and investigators have been relying on markers of its production. To this end, detection of the formation of 3-nitrotyrosine has been widely used for over a decade as a “footprint marker” to verify its presence [40]. However, several other in vivo sources of nitrating oxidants exist (for example, hydrogen peroxide, nitrite and myeloperoxidase [40–42]. Therefore, detection of 3-nitrotyrosine in vitro or in vivo, can be reliably used as a surrogate “footoprint marker” for ONOO−, only, if it is blocked pharmacologically with agents that remove superoxide (i.e., superoxide dismutase mimetics), nitric oxide (i.e., nitric oxide synthase NOS inhibitors) and specifically by catalytic scavengers of ONOO− such as metalloporphyrins [40,43,44]. We have previously demonstrated that the expression of spinal 3-nitrotyrosine during the development of morphine antinociceptive tolerance is derived from in situ formation of ONOO− since it was blocked by each class of the above-mentioned pharmacological agents targeting ONOO− indirectly or directly [45].
SOD-deficient E. coli strain (JI132) exhibits several auxotrophies and grows slowly aerobically as it has little protection against superoxide; it grows, however, as well as wild type (AB1157) under anaerobic conditions or in the presence of a compound that is able to behave as an SOD mimic and, thus, substitute for the SOD enzyme [22,23]. Such a behavior has provided us with a very useful model to quickly screen the in vivo activity of SOD mimics [3,4,14,16,17,19]. E. coli experiment undoubtedly shows that neither commercial nor pure MnTBAP preparations at 20 or 200 μM levels can substitute for the SOD enzyme in both nutrient-restricted and rich medium (Fig. 3). The potent SOD mimic, MnTE-2-PyP (log kcat = 7.76 [4]), allows full growth of SOD-deficient E. coli at 20 μM (Fig. 3). The very low SOD-like activity of the commercial (Alexis) MnTBAP (kcat = 5.16, Table 1 [18]), which arises from its impurities (SOD-active Mn oxo/hydroxo/acetato complexes [18]), is clearly insufficient to protect SOD-deficient E. coli (Fig. 3). The same inefficacy of MnTBAP has been found with two other SOD-specific systems, MnSOD [46], and Cu, ZnSOD [47] knock-out yeast where Calbiochem MnTBAP was used.
Pleurisy model
Carrageenan-induced local inflammation is commonly used to evaluate non-steroidal anti-inflammatory drugs (NSAID). Therefore, carrageenan-induced local inflammation (pleurisy) is a useful model to asses the contribution of mediators involved in vascular changes associated with acute inflammation. In particular, the initial phase of acute inflammation (0–1h) which is not inhibited by NSAID such us indomethacin or aspirin, has been attributed to the release of histamine, 5-hydroxytryptamine and bradykinin, followed by a late phase (1–6h) mainly sustained by prostaglandin release and more recently has been attributed to the induction of inducible cyclooxygenase (COX-2) in the tissue [25]. The development of inflammation in response to carrageenan has also been associated with increased pleural exudate production, myeloperoxidase activity, accumulation of polymorphonuclear cells in pleural cavity and the production of several reactive species including superoxide, nitric oxide and peroxynitrite resulting in increased nitrotyrosine formation [13]. Indeed, carrageenan-induced inflammation is markedly reduced in neutropenic animals and in animals treated with either superoxide dismutase mimics such as M40403 or M40401, inhibitors of nitric oxide synthase or peroxynitrite scavengers [24,25,48–52]. The effect of such compounds on the decreased neutrophil infiltration may occur through various pathways including inhibition of the expression of adhesion molecules, activation of redox-sensitive transcription factors and generation of cytokines [13]. Such pathways would likely involve the positive loop of the oxidative injury: the decreased levels of ROS/RNS by porphyrins would modulate cellular transcription activity resulting in decreased inflammatory cell and cytokine recruitment which would in turn decrease levels of secondary ROS/RNS. The data obtained herein show that an efficient SOD mimic and peroxynitrite scavenger, MnTE-2-PyP (at dose of 0.3 mg/kg) decreased exudate volume, MPO activity, infiltration of PMNs and nitrotyrosine levels (Figures 4, 5, and 6). At the same dose MnTBAP was ineffective. Similar anti-inflammatory effects were observed with MnTBAP but at doses at least 33-fold higher (10 mg/kg) than those used in the MnTE-2-PyP group (Figures 4, 5, 6 and 7) [26]. The anti-inflammatory effects of the impure (commercial) MnTBAP sample were just slightly more pronounced than those obtained with pure MnTBAP (Figures 4, 5 and 6). Our results confirm the general notion [40] that whatever the origin of the beneficial effect of MnTBAP is, it is significantly lower when compared to MnTE-2-PyP. Most importantly, they show that while pure MnTBAP has no SOD-like activity in aqueous systems [18] and is not efficient in O2•−-specific systems (i.e., the SOD-deficient E. coli model), it is able to diminish oxidative stress to some extent. Such data point to other possible roles of MnTBAP in oxidative stress injuries. Furthermore, the data also indicate that MnTBAP exert some protective actions in common with MnTE-2-PyP, which are not O2•−-related.
Due to the complexity of the mouse pleurisy model when compared to a more straightforward SOD-deficient E. coli system, the actual roles of MnTBAP in modulating oxidative stress is less clear-cut. In aqueous systems, the commercial MnTBAP sample has a moderate SOD activity (associated with its impurities) and is able to inhibit considerably xanthine oxidase [18], which may reduce, therefore, in vivo levels of O2•− and H2O2 and indirectly the levels of peroxynitrite. Such inhibition of xanthine oxidase, however, is not observed in the case of MnTE-2-PyP [4] and is considerably smaller with pure MnTBAP [18]. The effect of pure MnTBAP on other enzymatic systems or the modulation of Ca2+ levels [37,38] have not been explored. We also suggested [18] the possibility that MnTBAP may localize (upon protonation) in lipid membranes, where due to the different oxidation potential of the O2•−/O2 couple [53], it may act [18,54] as a superoxide scavenger and protect against lipid peroxidation [18]. This, however, can occur only if MnTBAP localizes in relatively acidic cellular compartments and if such compartments are major sources/targets of ROS/RNS.
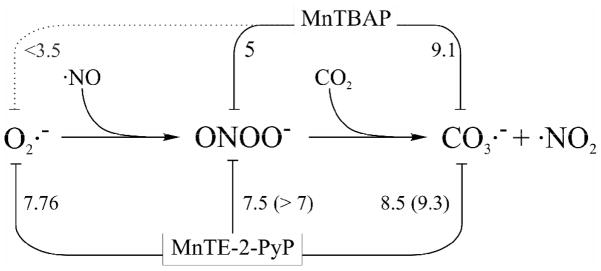
As an additional difference between MnTBAP and MnTE-2-PyP is that the latter is readily reduced in vivo by a number of flavoenzymes [55,56]. Such a facile reduction plays a major role in the antioxidant activity of this and similar Mn(III) ortho N-alkylpyridylporphyrins [56]; thus MnTE-2-PyP may remove O2− in vivo through flavoenzyme-superoxide oxido-reductase activity rather than superoxide dismutase activity. Further, such a reduction, allows Mn(II) porphyrins to reduce peroxynitrite by two electrons thus avoiding the formation of nitrogen dioxide, and to reduce carbonate radical 5 to 10 times faster than the Mn(III) compounds. So far we have not found any biological reductant capable of reducing Mn(III)TBAP to Mn(II)TBAP and this limits the redox activity of MnTBAP to being oxidized by peroxynitrite or carbonate radical. In Figure 8 we illustrate the differential reactivity between the two porphyrins discussed herein.
Figure 8.
Comparison of the reactivity of MnTBAP and MnTE-2-PyP. The numbers are the log k for the Mn(III) porphyrins and the reactants linked with each line (numbers in parenthesis correspond to the reactions with the corresponding Mn(II) species). Data are from [4,18,27,55] and this work.
At present, the in vivo data presented here can be fully rationalized if one considers that the common biological role shared by MnTBAP and MnTE-2-PyP is related to their peroxynitrite scavenging activities and the reduction of carbonate radical. The log kcat (O2•−) value for MnTBAP is estimated to be about 3.16 (based on the relationship given in Fig. 2), which is ~5 and ~7 orders of magnitude smaller than the SOD activity of the potent SOD mimic MnTE-2-PyP and Cu, Zn-SOD, respectively. This very low value indicates that MnTBAP is very inefficient at dismuting superoxide to be of any biological impact, which was confirmed in the SOD-deficient E. coli model. Worthwhile noting, however, is that peroxynitrite scavenging ability of MnTBAP is only ~2.5 orders of magnitude smaller than that of MnTE-2-PyP and is not significantly affected by the presence of the SOD-active impurities in commercial MnTBAP sample. Additionally, the reduction of carbonate radical by MnTBAP (log kred CO3•− = 9.1) and MnTE-2-PyP (log kred CO3•− = 8.5 for MnIIIP and 9.5 for MnIIP) is comparably fast [27]. These observations have profound biological repercussions as they may suggest that the effect of MnTBAP may conceivably relate to peroxynitrite and carbonate radical scavenging. The previous report on carrageenan induced pleurisy with same dose of commercial MnTBAP used in this study showed a significant decrease in levels of nitrotyrosine (Figure 7, [26]). The effect on nitrotyrosine levels was also reported in another model, carrageenan-induced-paw edema. MnTBAP further decreased nitrotyrosine levels in cultured H9C2 cardiomyocytes following a brief exposure to peroxynitrite and decreased nitrotyrosine formation in in vivo myocardial ischemia-reperfusion [57]. The dose of MnTBAP required to yield oxidative stress protection, and decrease damage from peroxynitrite, in the pleurisy model is >1.5 orders of magnitude higher than that of MnTE-2-PyP, which could be related to the smaller ability of MnTBAP to scavenge peroxynitrite and the lesser impact of carbonate radical which is formed only after peroxynitrite reacts with CO2. The slightly better protection observed with the commercial MnTBAP sample (relative to the pure MnTBAP one) could arise from its impurities, which by scavenging O2•− [18], reduce consequently the overall peroxynitrite, and secondary ROS/RNS levels. Of note is that a direct translation of the relative in vitro properties (rate constants) of MnTBAP and MnTE-2-PyP to their relative in vivo effects, particularly dosing requirements is most likely unattainable, as these porphyrins are by their own chemical nature, which governs uptake and biodistribution (e.g., overall charge, unrelated functional groups, size), considerably different. A direct comparison between the levels of these porphyrins in tissue was not easily achievable, as there is no reliable method for the determination of MnTBAP levels in biological samples and adaptation of the recently reported method for MnTE-2-PyP via reductive Mn to Zn transmetallation [1] to the MnTBAP case is far from straightforward (e.g., the ascorbate-reduction of MnTBAP to its Mn(II) analogue is precluded by its the very low MnIII/MnII reduction potential [18]). Because the development of anionic Mn porphyrin-based compounds (such as MnTBAP) as SOD mimic is severely hampered by unfavorable electrostatics [19], the positively-charged MnTE-2-PyP and related analogues [3,14] are still the most reliable positive controls as potent SOD mimics and ONOO2−/CO3•− scavengers.
In very practical terms, the parallel study of MnTE-2-PyP (as an indicator of both ROS and RNS) and pure MnTBAP (as an indicator of RNS) in an oxidative stress model may conceptually be useful in distinguishing the major contributions of ROS vs RNS pathways, as summarized in Table 2. ((Table 2))
Table 2.
Possible use of MnTE-2-PyP and pure MnTBAP as tools in oxidative stress mechanistic studies.
| RNS scavenger | Potent ROS and RNS scavenger (e.g., MnTE-2-PyP and congeners) |
||
|---|---|---|---|
| Effect Observed | Effect Not Observeda | ||
| Pure MnTBAP | Effect Observed | ONOO− may be a major player | MnTBAP probably regulates ROS/RNS via alternative pathways (e.g., enzyme inactivation; see text) |
| Effect Not Observeda | O2•− may be a major player | Likely not an oxidative stress event | |
given that cell uptake/biodistribution is granted.
Concluding remarks
In summary, physicochemical, in vitro, and in vivo data suggest that, whereas MnTBAP is not an SOD mimic, its overall in vivo efficacy may arise from its role as peroxynitrite scavenger. This may shed some light on the controversial effects reported for MnTBAP and justify further studies, particularly mechanistic ones, given that pure compounds are utilized or the levels and properties of the impurities in the samples are well established and characterized. Provided that pure MnTBAP is unable to dismute superoxide at any significant extent [18] but is able to partially scavenge peroxynitrite and carbonate radical, this compound may prove a valuable tool to distinguish the major contributions of ONOO−-dependent from O2•−-dependent pathways.
Acknowledgments
J.S.R. and I.B.H. acknowledge the support by the National Institutes of Health grant U19AI67798-01. I.S. thanks NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA014236-33). R.R. acknowledges grants from Howard Hughes Medical Institute and the International Centre of Genetic Engineering and Biotechnology. L.B. acknowledges grant MB07/04 from Kuwait University. S.C., E.M., and R.diP. acknowledge Associazione Italiana Ricerca sul Cancro (Milan, Italy) and a grant from Ministero dell’Università e della Ricerca. D.S. is thankful to the Saint Louis University Seed Funds. D.S. and I.B.H. thank for the support from NIH R01 DA024074.
Abbreviations Charges are omitted for clarity
- MnTBAP3−
Mn(III) meso-tetrakis(p-carboxylatophenyl)porphyrin
- MnTE-2-PyP5+
Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrins (AEOL10113)
- SOD-deficient
E. coli JI132
- SOD-proficient
wild type E. coli AB1157
- MnTSPP3−
Mn(III) meso-tetrakis(p-sulfonatophenyl)porphyrin
- MnBr8TSPP3−
Mn(III) β-octabromo-meso-tetrakis(p-sulfonatophenyl)porphyrin
- MnP
manganese porphyrin
- MPO
myeloperoxidase
- PMN
polymorphonuclear leukocyte
- CAR
carrageenan
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spasojević I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, StClair DK, Batinić-Haberle I. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants, and the ischemic Brain. J Exp Biology. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 3.Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso-tetrakis ortho N-alkylpyridylporphyrins. Synthesis, characterization and catalysis of O2•− dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 4.Batinić-Haberle I, Benov L, Spasojević I, Hambright P, Crumbliss AL, Fridovich I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of Manganese(III) and Iron(III) cationic and anionic porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 5.Soule BP, Hyodo F, Matsumoto K-I, Simone NL, Cook JA, Krishna MC, Mitchell JB. Therapeutic and clinical applications of nitroxide compounds. Antiox Redox Sign. 2007;9:1731–1743. doi: 10.1089/ars.2007.1722. [DOI] [PubMed] [Google Scholar]
- 6.Matthijssens F, Back P, Braeckman BP, Vanfleteren JR. Pro-oxidant activity of the SOD mimetic in proliferating and growth-arrested Escherichia coli cells. Free Radic Biol Med. doi: 10.1016/j. freeradicbiolmed.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;177:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z-Q, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 10.Cuzzocrea S, Thiemermann C, Salvemini D. Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem. 2004;11:1147–1162. doi: 10.2174/0929867043365396. [DOI] [PubMed] [Google Scholar]
- 11.Salvemini D, Cuzzocrea S. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med. 2003;31:S29–S38. doi: 10.1097/00003246-200301001-00005. [DOI] [PubMed] [Google Scholar]
- 12.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang Z-Q, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nature Rev Drug Discovery. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 14.Rebouças JS, Spasojević I, Tjahjono DH, Richaud A, Mendez F, Benov L, Batinić-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 15.Spasojević I, Batinić-Haberle I, Rebouças JS, Idemori YM, Fridovich I. Electrostatic contribution in the catalysis of O2•− dismutation by superoxide dismutase mimics. J Biol Chem. 2003;278:6831–6837. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 16.Batinić-Haberle I, Spasojević I, Stevens RD, Bondurant B, Okado-Matsumoto A, Fridovich I, Vujasković Ž, Dewhirst MW. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006:617–624. doi: 10.1039/b513761f. [DOI] [PubMed] [Google Scholar]
- 17.Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New class of potent catalysts of O2•− dismutation. Mn(III) methoxyethylpyridyl- and methoxyethylimidazolylporphyrins. J Chem Soc, Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 18.Rebouças JS, Spasojević I, Batinić-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: A case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Inorg Biol Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 19.Rebouças JS, DeFreitas-Silva G, Spasojević I, Idemori YM, Benov L, Batinić-Haberle I. Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: Protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier. Free Radic Biol Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imlay JA, Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987;169:2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benov L, Kredich NM, Fridovich I. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J Biol Chem. 1996;271:21037–21040. doi: 10.1074/jbc.271.35.21037. [DOI] [PubMed] [Google Scholar]
- 23.Benov L, Fridovich I. Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli. The transketolase connection. J Biol Chem. 1999;274:4202–4206. doi: 10.1074/jbc.274.7.4202. [DOI] [PubMed] [Google Scholar]
- 24.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 25.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuzzocrea S, Zingarelli B, Costantino G, Caputi AP. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy. Free Radic Biol Med. 1999;26:25–33. doi: 10.1016/s0891-5849(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer-Sueta G, Vitturi D, Batinić-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 28.Rebouças JS, Spasojević I, Batinić-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J Pharm Biomed Anal. 2008 doi: 10.1016/j.jpba.2008.08.005. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuzzocrea S, Caputi AP, Zingarelli B. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998;93:96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–487. [PMC free article] [PubMed] [Google Scholar]
- 31.Fridovich I. Oxygen toxicity: A radical explanation. J Exp Biol. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric Oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vance CK, Miller AF. A Simple Proposal That Can Explain the Inactivity of Metal-Substituted Superoxide Dismutases. J Am Chem Soc. 1998;120:461–467. [Google Scholar]
- 34.Ellerby RM, Cabelli DE, Graden JA, Valentine JS. Copper-Zinc Superoxide Dismutase: Why Not pH-Dependent? J Am Chem Soc. 1996;118:6556–6561. [Google Scholar]
- 35.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu, Zn-superoxide dismutase as a function of metal content – Order restored. Free Radic Biol Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Konorev EA, Kotamraju S, Zhao H, Shasi H, Kalivendi S, Joseph J, Kalyanaraman B. Paradoxical effects of metalloporphyrins on doxorubicin-induced apoptosis: scavenging of reactive species versus induction of heme oxygenase-1. Free Radic Biol Med. 2002;33:988–987. doi: 10.1016/s0891-5849(02)00989-9. [DOI] [PubMed] [Google Scholar]
- 37.Tauskela JS, Brunette E, Kiedrowski L, Lortie K, Hewitt M, Morley P. Unconventional neuroprotection against Ca2+-dependent insults by metalloporphyrin catalytic natioxidants. J Neurochem. 2006;98:1234–1342. doi: 10.1111/j.1471-4159.2006.03973.x. [DOI] [PubMed] [Google Scholar]
- 38.Tauskela JS, Brunette E, O’Reilly N, Mealing G, Comas T, Gendron TF, Monette R, Morley P. An alternative Ca2+-dependent mechanism of neuroprotection by metalloporphyrin class of superoxide dismutase mimetics. FASEB J. 2005;19:1734–1736. doi: 10.1096/fj.05-3795fje. [DOI] [PubMed] [Google Scholar]
- 39.Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 41.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 42.Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H, Kalyanaraman B, Radi R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32:501–515. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 43.Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, et al. Expression of inducible nitric-oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species. J Biol Chem. 2003;278:22901–22907. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- 44.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini M, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance. J Clin Invest. 2007;117:1–11. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles SS, Batinić-Haberle I, Perfect JR, Cox GM. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high-temperature growth. Eukariotic Cell. 2005;4:46–54. doi: 10.1128/EC.4.1.46-54.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munroe W, Kingsley C, Durazo A, Gralla EB, Imlay JA, Srinivasan C, Valentine JS. Only one of a wide assortment of manganese-containing SOD mimicking compounds rescues the slow aerobic growth phenotypes of both Escherichia coli and Saccharomyces cerevisiae strains lacking superoxide dismutase enzymes. J Inorg Biochem. 2007;101:1875–1882. doi: 10.1016/j.jinorgbio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci USA. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 50.Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De Loo FA, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162:1859–1866. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- 52.Cuzzocrea S, Costantino G, Mazzon E, Zingarelli B, De Sarro A, Caputi AP. Protective effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in paw oedema induced by carrageenan in the rat. Biochem Pharmacol. 1999;58:171–176. doi: 10.1016/s0006-2952(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 53.Sawyer DT, Valentine JS. How super is superoxide? Acc Chem Res. 1981;14:393–400. [Google Scholar]
- 54.Lahaye D, Muthukumaran K, Hung C-H, Gryko D, Rebouças JS, Spasojević I, Batinić-Haberle I, Lindsey JS. Design and Synthesis of Manganese Porphyrins With Tailored Lipophilicity: Investigation of Redox Properties and Superoxide Dismutase Activity. Bioorg Med Chem. 2007;15:7066–7086. doi: 10.1016/j.bmc.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrer-Sueta G, Hannibal L, Batinić-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 56.Kachadourian R, Johnson CA, Min E, Spasojević I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 57.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocytes in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez B, Demicheli V, Durán R, Trujillo M, Cerveñansky C, Freeman BA, Radi R. Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]