Abstract
Purpose
To compare the in vitro and in vivo efficacy of CAT-8015, a second-generation recombinant immunotoxin composed of disulfide linked affinity matured VH and VL chains of the mouse anti-CD22 monoclonal antibody RFB4 fused to PE38, to the parental compound CAT-3888.
Experimental Design
The biological activity of CAT-8015 was examined in vitro using B cell tumor lines and in vivo in a JD38-based subcutaneous tumor model in NCr athymic mice. Pharmacokinetics and interspecies scaling of CAT-8015 were evaluated in mice, rats, and Cynomologus monkeys. The potential toxicity of CAT-8015 was assessed in monkeys in a toxicological study and compared to CAT-3888.
Results
The IC50s of CAT-8015 in vitro using the EHEB, MEC1, Daudi, CA46, and JD38 cell lines ranged from 0.3 - 8.6 ng/mL. Pharmacokinetic studies with CAT-8015 were conducted in mouse, rat and Cynomolgus monkey. The T1/2 was calculated to be 0.42, 0.61, and 0.79 hr and the Vss was 1.37, 5.57, and 140.3 mL in mouse, rat, and monkey, respectively. In vivo, when JD38 tumor-bearing animals were treated with CAT-8015 at doses ≥ 75 μg/kg at 48 hr intervals for a total of 3 doses, a rapid reduction in tumor volume and in some cases complete remission in tumor growth was observed. The comparative toxicological study showed comparable clinical and anatomical pathology changes for CAT-8015 and CAT-3888.
Conclusions
CAT-8015 is a CD22-targeting immunotoxin that, in preclinical studies, has greatly improved efficacy as compared to CAT-3888.
Keywords: immunotoxin, CLL, NHL, PE-38, apoptosis
Introduction
In order to enhance the efficacy of immunotherapeutics, a number of strategies have been investigated involving the conjugation of the targeting antibody to radionuclides (1), protein or bacterial toxins (2), or chemotherapeutic drugs or prodrugs (3, 4). A series of Pseudomonas exotoxin A (ETA)-based immunotoxins have been developed over the last several years to a variety of cell surface targets and have evolved from a chemically conjugated immunotoxin to a fully recombinant form (5).
CD22 is a 135 KDa transmembrane sialoglycoprotein that is composed of an extracellular domain consisting of 7 Ig-like motifs, a transmembrane domain and a 141 amino acid cytoplasmic tail. CD22 was selected as a cell surface target for immunotoxin-based therapy for three reasons. First, it is a B-lymphocyte lineage restricted transmembrane protein expressed on the cell surface of mature B cells at a stage of differentiation when IgD expression is initiated (6). CD22 is strongly expressed in follicular (primary and secondary B cell zones), mantle, and marginal zone B cells. However, once B cells enter into the germinal centre and become activated, the level of CD22 expression decreases. Second, it is rapidly internalized. The basal half-life of CD22 is approximately 8 hr (6). Following ligand binding or antibody cross-linking the t½ of internalization for CD22 was less than 1 hr. There is no recycling of CD22 to the cell surface from the intracellular pool; since following internalization, CD22 is targeted to the lysosomal compartment where it is degraded. Third, CD22 has been reported to be present in 60 - 80% of the samples from patients with B cell malignancies (7, 8).
The initial immunotoxin developed for use in the treatment of B cell malignancies, designated CAT-3888 (BL22), consisted of disulfide linked VH and VL chains of the mouse anti-CD22 monoclonal antibody RFB4 fused to a truncated form of ETA, PE38 (5, 9). A second-generation CD22 targeted immunotoxin, CAT-8015 (HA22) has been under development for a number of years (10 - 12). While the PE38 portion of this construct is identical to that used for CAT-3888, the VH and VL chains have been affinity matured by phage display from a library targeting the CDR3 domain of the VH chain in a scFv format. A variant where CDR3 residues Ser-Ser-Tyr (SSY) were replaced by Thr-His-Trp (THW) possesses increased affinity (approximately 14-fold, Kd approximately 6 nM) towards the target, CD22, when compared to the parent protein (10). In dose ranging in vitro cytotoxicity assays, where primary cells from 4 CLL patients were treated for 72 hr with CAT-8015, the IC50 values ranged from 1.5 to 29 ng/mL (10).
In this study, we have conducted pre-clinical studies on CAT-8015. The biological activities of CAT-8015 and CAT-3888 were compared on human B cell lines in vitro. Pharmacokinetic studies were conducted with CAT-8015 in mice, rats, and monkeys. The antitumor activities of CAT-8015 and CAT-3888 were investigated in vivo in dose-ranging efficacy studies using a Burkitt's lymphoma subcutaneous (sc) xenograft tumor model.
Materials and Methods
Expression and purification of CAT-8015 and CAT-3888
For both of the immunotoxins the variable domain light chain (VL) and VH-PE38 were expressed separately in E. coli BL21 (λDE3). The proteins were purified from inclusion bodies as previously described (13).
Cell Culture and reagents
The EHEB, MEC-1, CA46 and Daudi cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD). The JD38 cells were a kind gift from Dr. Maryalice Stetler-Stevenson (Laboratory of Pathology, National Cancer Institute, Bethesda, MD). The medium composition and the culture conditions used for the various tumor cell lines were recommended by the suppliers.
Isolation of Human and Monkey PBMCs and FACS Analysis
Heparinized human or Cynomolgus monkey whole blood samples were maintained at room temperature until processed for FACS analysis. Human peripheral blood mononuclear cells (PBMCs) were prepared by Ficol centrifugation and resuspended in RPMI medium supplemented with 5% human AB serum, IL-4 and IL-10. The PBMCs were stained with biotinylated CAT-8015 and the cells were then washed and treated with streptavidin-FITC (10 ug/mL) followed by washing in PBS and analysis on a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) following standard procedures.
Cynomolgus Monkey Toxicity
A multi-dose two-cycle GLP toxicity study of CAT-8015 and CAT-3888 was conducted in cynomolgus monkeys. The dosing groups were composed of three male and three female in the main study animals, as well as two male and two female recovery animals. The animals were dosed on days 1, 3, 5, 15, 17, and 19 via iv bolus. The control group (study group 1) received the vehicle (phosphate buffered saline). Groups 2, 3, and 4 received CAT-8015 at the respective dose levels of 0.135, 0.405, and 1.35 mg/kg. Group 5 received CAT-3888 at a dose level of 1.35 mg/kg. The animals were sacrificed on Day 20, one day after the last dose. The recovery groups were sacrificed on Day 48. The study incorporated analyses of multiple clinical endpoints in addition to an extensive histopathology analysis.
Cytotoxicity Assays
Cell viability following various treatments was determined using the MTS assay (Promega, Madison, WI, USA). The cells were plated at a density of 2 × 105 cells/well and incubated overnight in a 5% CO2, 95% humidified atmosphere at 37°C. The test compounds were added and the cells were then incubated for an additional 44 hr. The data points represent the mean ± the standard deviation of triplicate determinations.
The effects of the various treatments on the level of protein synthesis were monitored by measuring the level of incorporation of [3H] leucine into protein. The various B cell tumor lines were seeded at a density of 2 × 105 cells/well and the cells were incubated overnight. The test compounds were then added and the cells were incubated for an additional 48 hr. Tritiated leucine was added to the culture medium for the last 2.5 hr of the incubation period.
ELISA Assays
Ninety-six-well plates were coated with 1 μg/mL CD22-Fc (R&D Systems, Minneapolis, MN), washed with PBS containing 0.1% Tween-20 (PBST) and blocked with casein blocker buffer (Pierce, Rockford, IL). Experimental and control plasma samples were diluted 1:20 in dilution buffer (PBST containing 10% casein blocker buffer). CAT-8015 and CAT-3888 containing plasma samples or negative controls were added to each well. After washing with PBST, biotinylated IP49 (1:1000 in dilution buffer) was added to the wells and incubated at room temperature for 30 minutes. After washing, Pierce ImmunoPure Streptavidin-HRP (1:40,000 in PBST) was added. After incubation, the plates were washed and Pierce TMB substrate was added. The endpoint optical density (OD) was read at 450 nm. The limit of detection under these conditions was 30 ng/mL. The calculated concentrations of CAT-8015 and CAT-3888 were determined with a four-parameter fitting of the standards using SOFTmax PRO (version 4.0, Molecular Devices, Sunnyvale, CA).
Pharmacokinetics
The athymic NCr mice or Sprague Dawley rats were injected intravenously (iv) with CAT-8015 diluted in PBS containing 0.5% human serum albumin. The blood samples were taken via cardiac puncture (mice) or tail vein bleeds (rats). For the monkey pharmacokinetic studies, blood samples were taken via a peripheral venous port. Plasma was prepared from the rodent or primate blood samples and then stored at -80°C until assayed. Plasma CAT-8015 concentration-time data were analyzed by non-compartmental methods. All of the pharmacokinetic analyses were made using WinNonlin 4.1 (Pharsight, Mountain View, CA).
In vivo tumor model
Female athymic NCr nude mice (Charles River, Wilminington, MA, approximately 21 g body weight, 4 - 6 weeks of age) were allowed to acclimate to the animal facility for at least 4 days prior to treatment. All of the animal related specific procedures are in compliance with the Animal Welfare Act Regulations (9CFR 3). The JD38 cells were maintained in RPMI 1640 (Mediatech, Herdon, VA) supplemented with 15% (v/v) fetal bovine serum (Hyclone, Logan, UT), 0.5% (v/v) penicillin (10,000 IU) and streptomycin sulfate (10,000 μg/mL) and incubated in a 5% CO2, 95% humidified atmosphere at 37°C. For tumor implantation, the JD38 cells were injected subcutaneously (sc) at a final concentration of 5 × 107 cells/mL in 100 μL of Ham's F12. The cells were allowed to grow until the tumors were 100 - 200 mm3 (5 - 7 days) prior to injecting the animals with test compounds. Every 3 to 4 days, the body weights were recorded and the tumors were measured.
Results
In vitro cytotoxicity and PBMC binding profiles
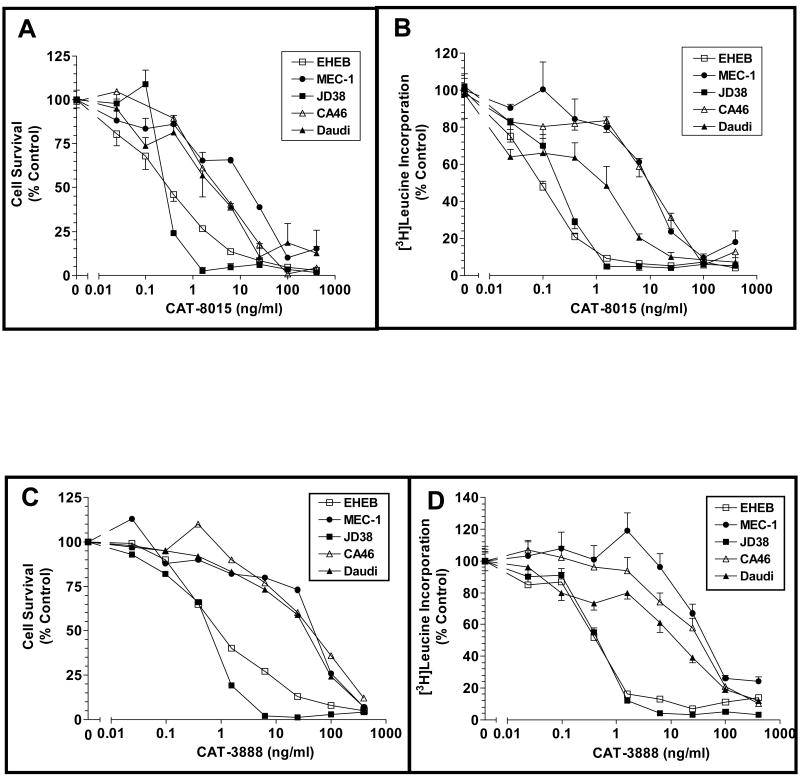
The cytotoxic activity of CAT-8015 occurs through the inhibition of protein synthesis that results from the ribosylation of EF2 on a phathmide residue (14). We characterized the in vitro efficacy of CAT-8015 using two different types of assays, cell survival and inhibition of protein synthesis. Five CD22 expressing cell lines were tested in both assays; three Burkitt's lymphoma lines, JD38, CA46 and Daudi, and two CLL lines, EHEB and MEC-1. The relative level of expression of CD22 in the B cell lines was low (874 RFI) in the EHEB cells, medium in the JD38 (2906), MEC-1 (5215) and CA46 (6189), and high (14196) in the Daudi cell line. The dose response curves relating the concentration of CAT-8015 to cell death or inhibition of protein synthesis responses are depicted in Figures 1A and 1B, respectively. The rank order of the sensitivities of the cell lines to CAT-8015 in the cell killing assay was JD38≈EHEB>Daudi>CA46>MEC1 while in the inhibition of protein synthesis assay the rank order was EHEB≈JD38>Daudi>MEC1>CA46. With three of the tumor cell lines, JD38, CA46 and EHEB, complete cell killing was achieved in this short-term assay at 1000 ng/mL. Following treatment with ≥ 50 ng/mL of CAT-8015 there was only 10% or less cell survival in all of the cell lines tested. CAT-8015 was more potent than CAT-3888 both in the cell survival and inhibition of protein synthesis assays (Figure 1C and 1D; Table 1). It is interesting to note that the extent of the response of the cells to immunotoxin was independent of the level of expression of CD22, suggesting that there are additional downstream regulatory steps in the immunotoxin induced cell death pathway.
Figure 1.
In vitro cytotoxicity of CAT-8015 and CAT-3888 on B cell tumor lines and effect of immunotoxin treatment on the level of [3H]leucine incorporation. (A and B) The B cell lines EHEB, MEC-1, JD38, CA46, and Daudi were incubated in the presence of the indicated concentrations of CAT-8015. (A) shows the dose dependent effect of CAT-8015 on lymphoma cell survival and (B) shows the inhibition of protein synthesis. (C and D) Effect of immunotoxin CAT-3888 treatment on lymphoma cell survival (C) and protein synthesis (D). The effect of the immunotoxin treatment on cell survival was determined using MTS after 48 hour incubation. Values represent the mean OD ± the standard error of the mean (SEM) of triplicate determinations. To assess the effect of CAT-8015 and CAT-3888 on protein synthesis the level of [3H]leucine incorporation by B cell tumor lines was measured. The tumor cell lines were incubated with immunotoxin for 48 hr and [3H]leucine was added to the culture medium for the final 2.5 hr. The incorporation of [3H]leucine into precipitable protein was then determined. Values represent the mean ± SEM of triplicate determinations.
Table 1.
Relative Level of CD22 Expression and Comparison of the In Vitro Efficacy of CAT-3888 versus CAT-8015.
| Cell Lines | Relative CD22 Expression1 | Cell Survival2 | Protein Synthesis Inhibition2 | ||
|---|---|---|---|---|---|
| CAT-8015 | CAT-3888 | CAT-8015 | CAT-3888 | ||
| JD38 | 2906 | 0.35 | 0.54 | 0.15 | 0.44 |
| EHEB | 874 | 0.3 | 1.18 | 0.08 | 0.42 |
| Daudi | 14196 | 2.46 | 25.79 | 0.4 | 9.6 |
| CA46 | 6189 | 3.46 | 40.52 | 7.15 | 28.16 |
| MEC1 | 5215 | 8.66 | 40.49 | 5.96 | 52.02 |
Relative mean fluorescence intensity
IC50 (ng/ml)
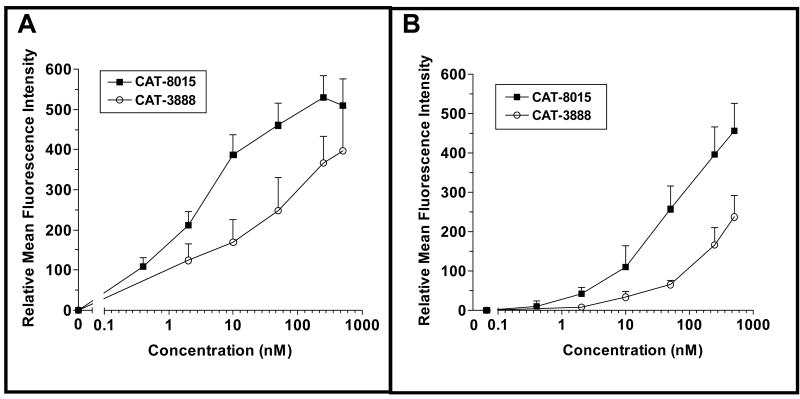
The binding profiles of the two immunotoxins were compared on freshly isolated human or Cynomologus monkey PBMCs. Consistent with its higher affinity for CD22, the binding curve for CAT-8015 was shifted to the left by approximately a log with both the human and monkey cells (Figure 2A and B, respectively).
Figure 2.
Binding of CAT-8015 or CAT-3888 to human or monkey PBMCs. Human (A) or monkey (B) purified PBMCs were incubated with biotinylated CAT-8015 at concentrations shown and then with streptavidin-FITC. Following washing in PBS, the samples were analyzed on a FACSCalibur flow cytometer (Becton-Dickinson).
Pharmacokinetics of CAT-8015 in mice, rats, and monkeys
Mice and rats were administered CAT-8015 at 0.5 or 0.25 mg/kg, respectively, and monkeys at 0.1, 0.5 or 2 mg/kg. Pharmacokinetic parameter estimates for CAT-8015 were derived from non-compartmental methods (Table 2). Following intravenous bolus injection, plasma CAT-8015 disposition was monophasic in mice and rats, and multiphasic in monkeys. The average peak concentration was 7.28 and 5.57 μg/mL in mice and rats, respectively. The volume of distribution in all three species was approximately equivalent to the plasma volume. Plasma clearance was estimated to be 0.04 mL/min in mice, 0.23 mL/min in rats, and ranged from approximately 2 to 4 mL/min in monkeys. In addition, the exposure in monkeys was dose-related but slightly under dose-proportional. Allometeric scaling of these data produced the equation CL= 1.063W0.86.
Table 2.
Pharmacokinetic Parameters for CAT-8015 in NCr Nude Mice, Sprague Dawley Rats and Cynomolgus Monkeys
| Parameter | Mouse (0.5 mg/kg) | Rat (0.25 mg/kg) | Monkey (mg/kg) | ||
|---|---|---|---|---|---|
| 0.1 | 0.5 | 2.0 | |||
| AUCinf (hr*μg/mL) | 4.37 | 4.12 | 2.03±0.31 | 8.13±0.84 | 32.20±4.9 |
| C0 (μg/mL) | 7.63 | 5.93 | 2.92±0.30 | 12.40±1.5 | 44.70±5.9 |
| CL (mL/min) | 0.04 | 0.23 | 2.39±0.29 | 2.83±0.52 | 3.26 ± 0.64 |
| Cmax (μg/mL) | 7.28 | 5.57 | 2.78±0.29 | 11.69±1.21 | 42.50±5.7 |
| t½ (hr) | 0.42 | 0.61 | 0.56±0.11 | 0.62±0.09 | 1.20±0.47 |
| MRTinf (hr) | 0.57 | 0.78 | 0.75±0.08 | 0.78±0.07 | 0.95±0.23 |
| Vss (mL) | 1.37 | 10.6 | 107.44±18.63 | 131.03±22.25 | 182.41±42.0 |
Human and Cynomologus Tissue Immunohistochemical Screen
The targeting scFv in CAT-8015 displays a 15-fold increase in affinity for CD22 as compared to the anti-scFv used in CAT-3888 (10). In order to confirm that the increase in affinity for CD22 did not also result in a shift in antigen specificity and thus a potential shift in the toxicity profile as compared to CAT-3888, an extensive immunohistochemical (IHC) study was conducted on a selected panel of twenty-three human tissues. The results from the study demonstrated that there was only specific positive staining of B cells in many of the tissues examined (data not shown). The corresponding monkey tissues were also studied and a similar B cell specific IHC staining pattern was observed.
Non-Human Primate Multi-dose Toxicological Study
The objective of the multi-dose two-cycle toxicological study was to assess the potential toxicity of CAT-8015 with CAT-3888 as a comparator. During the treatment phase, slightly reduced food consumption and body weight were noted for both drug candidates. However, there were no statistically significant differences in mean body weight between CAT-8015 and CAT-3888-treated and control animals.
Several hematology parameters were altered generally in a dose-dependent manner primarily on Day 6, one day following the third dose, in one or both sexes. These consisted of slight to moderate, transient reductions in one or more red blood cell parameters that were statistically significant in males and females receiving CAT-8015. Similar changes were observed in animals receiving CAT-3888. In addition, transient reductions were also noted in the absolute and/or relative lymphocyte numbers following treatment with CAT-8015 and CAT-3888. Administration of 1.35 mg/kg CAT-8015 or CAT-3888 was associated with 1.2- to 1.8-fold increases on average in total white blood cell counts on Day 6, relative to the Day -1 baseline, and a 1.3- to 2.5-fold increase relative in neutrophils. The effects upon these parameters were largely absent by Day 15 (prior to the fourth dose). Clinical pathology changes consisted of increases in white blood cell and neutrophil counts; decreases in red blood cell counts, hemoglobin concentration, and hematocrit, and decreases in serum total protein and albumin levels. The changes associated with the treatment were observed during or immediately following the dosing phase in animals that received intermittent injections of CAT-8015 or CAT-3888. The only histopathological alteration attributed to treatment was apoptosis of cells of the renal tubular epithelium in some animals. Overall, when used at the same dose, clinical and histopathological findings were very similar between the two drugs. The results suggest that the increased affinity of CAT-8015 for CD22 is not associated with additional or exacerbated side effects in cynomolgus monkeys.
Antitumor Activity
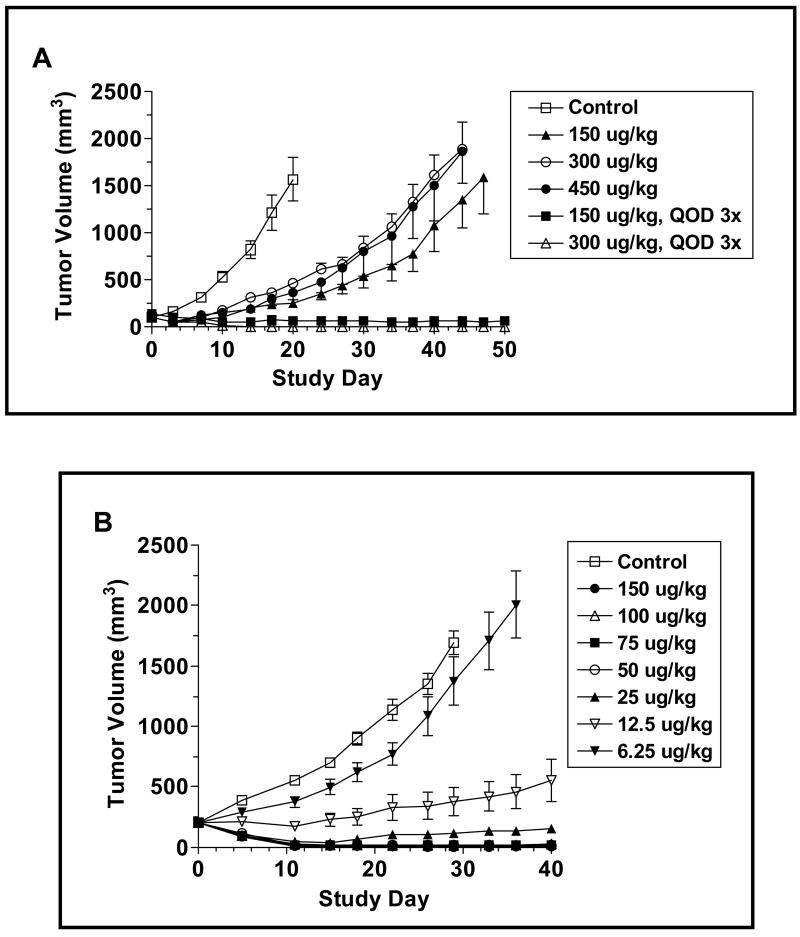
To evaluate the antitumor activity of CAT-8015 in vivo, we first investigated whether a single iv bolus of CAT-8015 was as efficacious as an every other day for 3 administrations (Q2D, x3) dosing schedule. Figure 3A depicts the change in the volume of the JD38 sc tumors over time following treatment with the indicated concentrations of CAT-8015 in each dosing paradigm. The JD38 tumors in the vehicle-treated group grew exponentially, so that by Study Day 7, 14, and 20 the tumors had increased in size by 3.2, 8.3 and 15.9-fold, respectively. On Study Day 20, when the average tumor volume reached 1565.5 ± 654.0 mm3, the animals had to be sacrificed due to tumor burden. The tumors in the groups that received a single iv bolus of CAT-8015 at 150, 300, or 450 μg/kg uniformly displayed slower growth profiles as compared to the vehicle-treated control group until approximately Study Day 36 when the growth rates increased. On Study Day 7, three days post-CAT-8015 administration, the mean volume of the tumors in the groups that received 150, 300 or 450 μg/kg were significantly different from the mean volumes of the tumors in the vehicle-treated control group (p < 0.0001 for all of the CAT-8015 treated groups versus the vehicle-treated control group). The inhibitory response to a single dose of immunotoxin appeared to saturate at 150 μg/kg. Furthermore, there was no apparent dose response relationship either during the initial growth period or subsequently during the later period when the relapse in tumor growth occurred.
Figure 3.
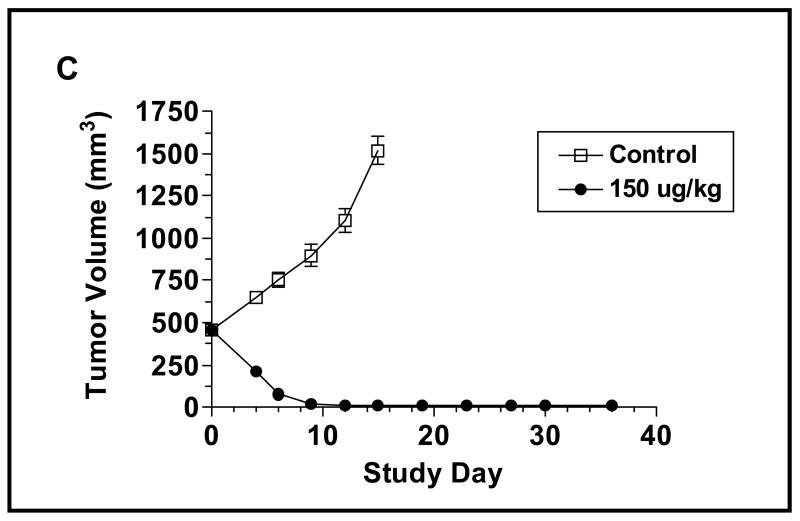
Effect of CAT-8015 on the growth of JD38 sc tumors in NCr athymic nude animals. Animals bearing JD38 sc tumors were randomized into groups based on tumor size on Study Day 0. (A) The animals were treated once (Study Day 0) or Q2D, x3 (Study Day 0, 2, and 4) with CAT-8015. The data points represent the mean ± SEM (n = 8). (B) JD38 tumor-bearing animals were treated Q2D, x3 on Study Day 0, 2, and 4 with an expanded range of doses of CAT-8015. (C) JD38 tumors with an average volume of 450 mm3 were treated Q2D, x3 on Study Day 0, 2, and 4 with vehicle or CAT-8015 at 150 μg/kg.
In contrast to the response observed following the single bolus administration of CAT-8015, when the immunotoxin was administered in the Q2D x3 regimen, a regression in the average volume of the tumors was noted in both the 150 and 300 μg/kg treatment groups. By Study Day 7, the average tumor volumes in the 150 and 300 μg/kg treatment groups were reduced by 40 and 51% (99.3 ± 7.7 to 59.5 ± 7.7 mm3, p = 0.0026 and 98.8 ± 7.3 to 48.5 ± 5.6 mm3, p<0.0001) respectively. The average tumor volumes in the groups receiving 150 or 300 μg/kg Q2D, x3 were significantly different from the average tumor volumes in the vehicle-treated group by Study Day 3 (p = 0.01, p < 0.0001, respectively) and remained so for the duration of the study. By Study Day 17, the tumor volumes had reached their nadir, representing a 62 and 100% decrease in size. The growth of the tumors in these groups was subsequently monitored for a period of 50 days and none of the tumors in either group relapsed.
Since the Q2D x3 dosing paradigm is more effective in suppressing the long-term growth of JD38 tumors, we expanded the range of doses tested using this paradigm (Figure 3B). Treatment with 6.25 μg/kg CAT-8015 proved ineffective in altering the growth of the JD38 tumors, whereas 12.5 μg/kg induced stable disease with a significant inhibition in tumor growth occurring by Study Day 11 (p = 0.005 compared to control group). The stable disease was long-lived in that there was no significant relapse in the growth of the tumors by Study Day 40 when the experiment was terminated. Doses of CAT-8015 >12.5 μg/kg induced a rapid regression in tumor volume that reached a nadir by Study Day 11 when the extent of the tumor shrinkage was ≥ 92% in comparison with the average volume of the tumors in the vehicle treated group. Furthermore, the tumors in the groups that received doses ≥ 50 μg/kg were still non-palpable on Study Day 40. Since the higher doses of CAT-8015 were effective in shrinking small-established tumor masses, we tested if larger tumors would respond in a similar manner. Starting with JD38 tumors in the range of 300 - 600 mm3, CAT-8015 was administered Q2D, x3 at a dose of 150 μg/kg (Figure 3C). As was the case with the smaller tumors, treatment with the immunotoxin induced a rapid shrinkage in the volumes of the larger tumors so that on Study Day 4, the day that the animals received the final dose of the immunotoxin, and Study Day 6, the average sizes of the tumors were reduced by 60 and 92%, respectively. This data suggests that the immunotoxin can effectively penetrate larger tumors.
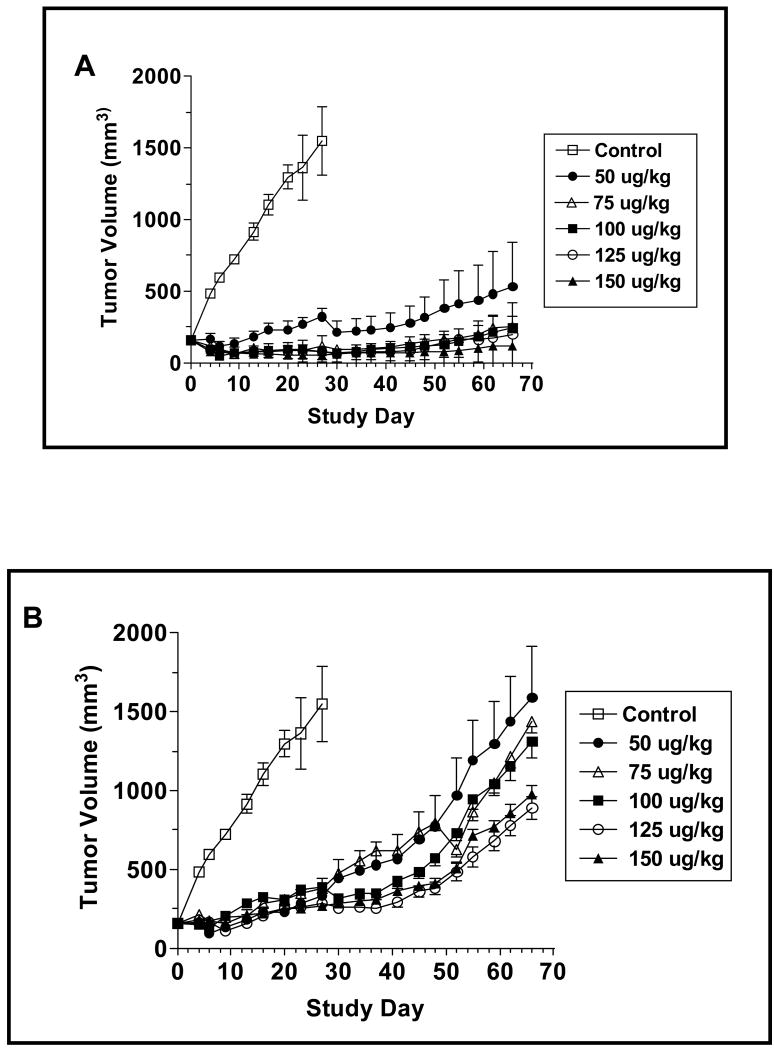
As CAT-8015 is a second-generation immunotoxin, we were interested in determining if, as was the case in the in vitro assays, its in vivo antitumor activity was improved in comparison to the parent compound, CAT-3888. Using the JD38 xenograft model, the animals were treated Q2D, x3 with the indicated concentrations of CAT-3888 (Figure 4A) or CAT-8015 (Figure 4B). CAT-3888 at 50 or 75 μg/kg suppressed the growth of the tumors for 30 days when a rebound in their growth rates was noted. A similar rebound in the growth rates of the tumors in the groups treated with 100, 125 or 150 μg/kg occurred between days 42 and 54. Similar to the previous results, when the tumor-bearing animals were treated with CAT-8015, there was an initial dose dependent decrease in the volume of the tumors in the groups that received doses ≥75 μg/kg. At the nadir, the range of the reduction in the volumes of the tumors was 46.9 - 66.0%. This initial period was followed by an extended interval during which the growth of tumors was inhibited. By the end of the experiment on Study Day 66, no significant relapse in the growth of the tumors had occurred.
Figure 4.
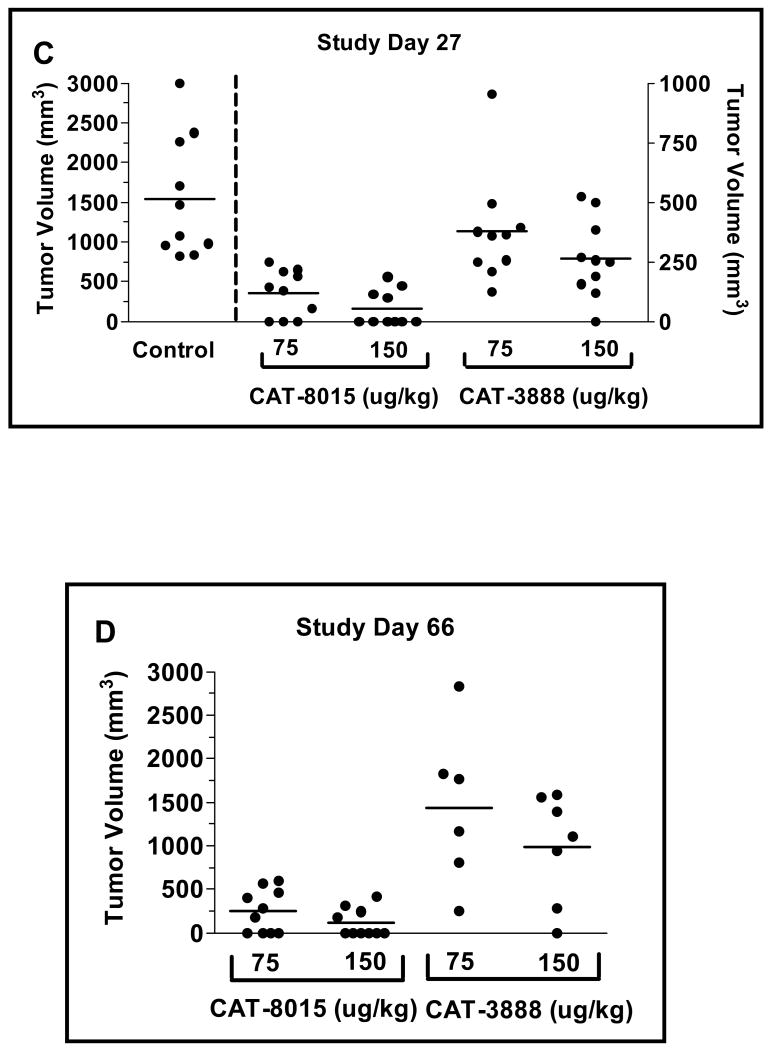
Comparison of the antitumor activity of CAT-3888 (A) and CAT-8015 (B). The immunotoxins were administered Q2D, x3 starting on Study Day 0 at 50, 75, 100, 125, 150 μg/kg or with the vehicle (control). The data points represent the average tumor volumes from groups with an n of 6 - 10. Scatter plots of the individual tumor volumes in the groups treated with 75 or 150 μg/kg CAT-8015 or CAT-3888 on Study Day 27 (C) or 66 (D). The solid bar represents the standard error of the mean. Comparing the mean tumor volumes in the groups receiving CAT-8015 at 75 or 150 μg/kg to the mean tumor volume of the control group the p value was >0.0001 in both cases. Comparing the mean tumor volumes in the groups dosed with CAT-8015 at 75 or 150 μg/kg to the respective groups dosed with CAT-3888 the p values were equal to 0.0013 and 0.0007, respectively.
A scatter plot depicting the individual tumor volumes for the control group versus the groups treated with CAT-8015 or CAT-3888 at 75 or 150 μg/kg on Study Day 27, the last day that the control group was intact, is shown in Figure 4C. In comparison to the control group or to the respective groups receiving CAT-3888, the groups that received CAT-8015 at either dose were significantly smaller. Figure 4D depicts a scatter plot of the individual tumor volumes on Study Day 66 in the groups receiving CAT-8015 or CAT-3888 at 75 or 150 μg/kg. There were 4/10 and 6/10 complete remissions of tumors in the groups that received CAT-8015 versus 0/10 and 1/10 in the groups that received CAT-3888, respectively. In comparison to the groups that received CAT-3888 at 75 or 150 μg/kg, the groups that received CAT-8015 were significantly smaller (p=0.0013 and p ≤ 0.0007, respectively).
Discussion
This study has focused on the in vitro and in vivo characterization of a second-generation CD22-targeted immunotoxin, CAT-8015. The results from the in vitro cytotoxicity assays demonstrated that CAT-8015 is highly effective in killing B cells from a variety of tumor cell lines. In addition, CAT-8015 was found to be up to approximately 12-fold more potent than CAT-3888 in cell survival based assays. In vitro binding studies with human or monkey PBMCs demonstrated that when used at an equal concentration, a higher relative mean fluorescence intensity signal was observed for CAT-8015 as compared to CAT-3888 suggesting that the increase in the affinity of the targeting scFv for CD22 results in an increase in receptor occupancy. The pharmacokinetic profiles of CAT-8015 were evaluated in single dose studies in mice, rats, and monkeys. These studies estimated the plasma clearance to be 0.04, 0.23, and approximately 2.0 - 4.0 mL/min in mice, rats, and monkeys, respectively. Although a single administration of CAT-8015 at a dose as low as 150 μg/mL was sufficient to induce a long-term inhibition in the growth of the Burkitt's lymphoma derived tumor cell line JD38, the immunotoxin was more effective when administered on a Q2D x3 dosing paradigm. When administered on this schedule, a dose as low as 12.5 μg/mL was sufficient to produce a long-lasting cytostatic response. Treatment with higher doses of the immunotoxin resulted in rapid tumor shrinkage, sustained inhibition of tumor growth, and in some cases, complete remissions.
The relative level of CD22 expression in the B cell lines tested ranged from a low of 874 (RFI) on EHEB cells to a high of 14196 (RFI) on Daudi cells. The IC50 values determined in cytotoxicity assays using CAT-8015 ranged from 0.3 to 8.6 ng/mL, approximately 30-fold. While both CLL (MEC-1 and EHEB) and Burkitt's lymphoma (JD38, CA46, and Daudi) derived cell lines were tested, representative lines from each tumor type, JD38 and EHEB, were determined to be very sensitive (IC50<0.5 ng/mL) to the immunotoxin. Furthermore, the sensitivity of the cell lines to killing with either immunotoxin did not directly correlate with the level of CD22 cell surface expression.
In preclinical models, CAT-8015 pharmacokinetics were consistent with other macromolecules in its molecular weight range. The volume of distribution was close to plasma volume, suggesting limited tissue distribution. Allometeric scaling of these data produced the equation CL=1.063W0.86. Based on this relationship, plasma clearance in a 70 kg human would be approximately 42 mL/min. With this plasma clearance, a CAT-8015 dose of 5 μg/kg (350 μg total) would produce an AUC of approximately 0.13 hr*μg/mL. In HCL patients treated with CAT-3888, the peak levels were dose-related and median half-lives were about 3 hours at the 30 to 50 μg/kg Q2D, x3 dose level (15). A median AUC of 123 min*μg/mL (∼2 hr*μg/mL) was reported for these patients.
The toxicology profile of CAT-8015 appears to be consistent with that of CAT-3888 and the two drugs showed a very similar profile of clinical and histopathological findings. Given that efficacy was noted in mice at doses ≥ 0.0125 mg/kg, the non-clinical data suggest that a therapeutic window for CAT-8015 in humans may be achievable. Aside from the kidney, no other microscopic alterations related to immunotoxin treatment were identified. As no specific binding was seen in the kidney (in non-B-cells) in the in vitro tissue cross reactivity studies in monkeys and humans, the nephrotoxicity observed is not suspected to be a CD22 specific effect of CAT-8015 but rather a toxic result of non-specific localization of the immunotoxin. In clinical studies conducted with CAT-3888 in HCL patients, the predominant dose limiting toxicity has been hemolytic uremic syndrome (15).
A number of clinical trials are ongoing testing immunotoxins targeting a variety of cell surface antigens. IL13-PE38 (also known as cintredekin besudotox) is a recombinant chimeric toxin composed of human IL-13 fused to PE38. A phase I/II study examining the activity of IL-13-PE38 in glioblastoma multiforme (GBM) patients with the objective to determine the toxicity, maximum tolerated dose, and the response profile when the immunotoxin is delivered via convection enhanced delivery is ongoing (16). In addition, a phase III trial is ongoing to determine in patients following surgical tumor removal in the treatment of first recurrence of GBM after initial surgery and external beam radiation therapy if the overall survival duration, safety, and quality of life are improved with IL13-PE38 treatment as compared to Gliadel wafers (16). A phase I study is ongoing in mesothelioma with SS1(dsFv)-PE38 that targets mesothelin in combination with pemetrexed and cisplatin. Several phase II studies with LMB2, a CD25 targeting immunotoxin, are ongoing in hairy cell leukemia, chronic lymphocytic leukemia, and cutaneous T-cell lymphoma. Furthermore, a phase II study is ongoing with VB4-845 that targets EpCAM in bladder cancer (17).
CD22 targeting immunotherapeutics have recently been tested in several clinical trials in the form of a naked antibody, epratuzumab (18), as a radiolabelled antibody (19) or as a conjugate with calicheamicin, CMC-544, inotuzumab ozogamicin (20 - 22). Considering FL patients treated with epratuzumab, 24% responded with a 43% response in the 360 mg/m2 dose group and a 27% response in the 430 mg/m2. Epratuzumab was well tolerated with no dose-limiting toxicities reported (18). A dose-fractionated radioimmunotherapy based clinical trial has been conducted using 90Y-radiolabeled epratuzumab (19). In this study, 16 patients received treatment and the over-all objective response rate was 62% in both indolent (75%) and aggressive disease (50%). Complete responses were noted in 25% of patients and were durable from 14 - 41 months. In addition, a multi-center phase II clinical trial has been conducted with epratuzumab, in combination with rituximab in refractory or recurrent non-Hodgkin's lymphoma. Sixty-five patients were enrolled; 34 patients with FL, 15 patients with diffuse large B cell lymphoma (DLBCL) and 16 patients with other lymphomas. The objective response rate was 30 out of 64 patients (47%) in assessable patients with the highest response rates being in FL (64%; 21/33) and DLBCL (47%; 7/15) (23). Results from this open label phase II study suggest that the combination of targeting CD20 and CD22 is more efficacious than single agent CD20 therapy without an increase in toxicity in both FL and DLBCL patients. Thus based on these studies, there appears to be clinical utility in the use of immunotherapy-targeting CD22 at least in FL and DLBCL. Clinical trials are also underway investigating the efficacy of inotuzumab ozogamicin in combination with rituximab in FL and DLBCL.
The results of pre-clinical studies reported in this paper demonstrate that the second generation immunotoxin CAT-8015, which contains disulfide linked affinity matured VH and VL chains having a higher-affinity for CD22 than those used in the parental immunotoxin CAT-3888, has an increased cytotoxic activity in both in vitro and in vivo assays. The increase efficacy of CAT-8015 in pre-clinical models may translate to an increase in anti-tumor activity in B cell malignancies expressing relative low levels of CD22 on their cell surface.
Acknowledgments
The authors wish to thank Dr. E. Escandon and Dr. S. Sherwood for help in establishing the pharmacokinetic and FACS related assays, respectively.
Footnotes
Statement of Clinical Relevance: CAT-8015 (HA22) is a second generation recombinant Immunotoxin, targeting the B cell-specific surface antigen CD22. It is an affinity optimized version of CAT-3888 (BL22), which has produced promising results in early clinical trials. In a phase one trial, 46 B leukemia/lymphoma patients who had failed prior therapy were given CAT-3888. In HCL, which was represented by 31 patients, a high response rate was observed (61 % CRs, 19 % PRs). HCL typically shows high levels of CD22 expression, but the drug also showed signs of activity in other indications, such as CLL.
The results presented here demonstrate that CAT-8015 has significantly improved activity, due to the increased affinity for its target CD22. CAT-8015 is superior to its predecessor both in in vitro cytotoxicity assays as well as in in vivo models of B cell leukemia/lymphoma. CAT-8015 has already entered the clinic and is being evaluated in several indications, including HCL. The improved activity of this new compound may well translate into the clinical setting. Due to the increased affinity towards CD22, CAT-3015 may show improvements over CAT-3888 in particular in indications with lower CD22 cell surface expression.
References
- 1.Carter P. Improving the efficacy of antibody-based cancer therapies. Nature Rev Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 2.Schiavo G, Gisou van der Goot F. The bacterial toxin tool kit. Nature Rev. 2001;2:530–537. doi: 10.1038/35080089. [DOI] [PubMed] [Google Scholar]
- 3.Law CL, Cerveny CG, Gordon KA, et al. Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. Clin Cancer Res. 2004;10:7842–7851. doi: 10.1158/1078-0432.CCR-04-1028. [DOI] [PubMed] [Google Scholar]
- 4.Senter PD, Springer CJ. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv Drug Delivery Reviews. 2001;53:247–264. doi: 10.1016/s0169-409x(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 5.Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin therapy of Cancer. Nature Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 6.Shan D, Press OW. Constitutive endocytosis and degradation of CD22 by human B cells. J Immunol. 1995;154:4466–4475. [PubMed] [Google Scholar]
- 7.Vitetta ES, Stone M, Amlot P. Phase I immunotoxin trial in patients with B cell lymphoma. Cancer Res. 1991;51:4052–4058. [PubMed] [Google Scholar]
- 8.Clark EA. CD22, a B cell-specific receptor, mediates adhesion and signal transduction. J Immunol. 1993;150:4715–4718. [PubMed] [Google Scholar]
- 9.Tedder TF, Tuscano J, Sato S, et al. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore G, Beers R, Margulies I, et al. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 11.Bang SH, Nagata S, Onda M, et al. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11:1545–1550. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 12.Alderson RF, Escandon E, Chen T, et al. Characterization of CAT-8015: A Pseudomonas exotoxin based immunotoxin for the treatment of CD22-related hematological malignancies. Proc Amer Assoc Cancer Res. 2006;47:3728. [Google Scholar]
- 13.Mansfield E, Chiron MF, AmLot P, et al. Recombinant RFB4 single-chain immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 14.Wick MJ, Frank DW, Storey DG, et al. Structure, function, and regulation of Pseudomonas aeruginosa exotoxin A. Annu Rev Microbiol. 1990;44:335–363. doi: 10.1146/annurev.mi.44.100190.002003. [DOI] [PubMed] [Google Scholar]
- 15.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 16.Rainov NG, Söling A. Clinical studies with targeted toxins in malignant glioma. Rev Rec Clin Trials. 2006;1:119–131. doi: 10.2174/157488706776876454. [DOI] [PubMed] [Google Scholar]
- 17.Biggers K, Scheinfeld N. VB4-845, a conjugated recombinant antibody and immunotoxin for head and neck cancer and bladder cancer. Curr Opin in Mol Ther. 2008;10:176–186. [PubMed] [Google Scholar]
- 18.Leonard JP, Coleman M, Ketas JC, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 19.Linden O, Hindorf C, Cavallin-Stahl E, et al. Dose-fractionated radioimmunotherapy in non-Hodgkin's lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody epratuzumab. Clin Cancer Res. 2005;11:5215–5222. doi: 10.1158/1078-0432.CCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 20.DiJoseph JF, Goad ME, Dougher MM, et al. Potent and specific antitumor efficacy of CMC-544, a CD22-targeted immunoconjugate of calicheamicin, against systemically disseminated B-cell lymphoma. Clin Cancer Res. 2004;10:8620–8629. doi: 10.1158/1078-0432.CCR-04-1134. [DOI] [PubMed] [Google Scholar]
- 21.Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008;36:755–768. doi: 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 23.Strauss SJ, Morschhauser F, Rech J, et al. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:3880–3886. doi: 10.1200/JCO.2006.05.6291. [DOI] [PubMed] [Google Scholar]