Summary
Objective
We analyzed the CT and clinical findings of pulmonary alveolar proteinosis secondary to hematological malignancy.
Methods
Seven patients with hematological malignancy and pathologically proven secondary pulmonary alveolar proteinosis were identified from 2000−2007. Six had chest CT scans, which were analyzed retrospectively; medical records were also reviewed.
Results
Patient age ranged from 30−54 years. Four had chronic myelogenous leukemia, 1 had myelodysplastic syndrome, and 1 had cutaneous T cell lymphoma. As in idiopathic pulmonary alveolar proteinosis, geographic ground-glass opacities with or without septal thickening were most common (5/6). No axial or zonal predominance was present. Two patients died from respiratory failure.
Conclusion
It is important to consider secondary pulmonary alveolar proteinosis as a cause of geographic ground-glass opacities and septal thickening in a patient with hematological malignancy. Whereas idiopathic pulmonary alveolar proteinosis has a low mortality rate, the death of 2 of our 6 patients implies that secondary pulmonary alveolar proteinosis may have a worse prognosis. Our case of secondary pulmonary alveolar proteinosis associated with cutaneous T-cell lymphoma is the first described in the literature.
Keywords: Secondary Pulmonary Alveolar Proteinosis, Hematological Malignancy, Leukemia, Myelodysplastic Syndrome, Cutaneous T-Cell Lymphoma
Introduction
In pulmonary alveolar proteinosis (PAP), PAS-positive lipoproteinaceous material accumulates in the alveoli [1]. The underlying cause is failure of macrophages to clear degradation products of surfactant. Most cases of PAP are idiopathic (IPAP). Secondary PAP (SPAP) arises in association with hematological disorders, medications, certain infections, acute silicosis, and immunodeficiency [2, 3]. SPAP may be misinterpreted as infection in immunocompromised patients, given its nonspecific radiographic findings. In addition, there is new interest in PAP with the recent introduction of therapy with granulocyte-macrophage colony stimulating factor (GM-CSF). We analyzed the CT and clinical findings in 6 patients with SPAP associated with hematological malignancies.
Material and Methods
Retrospective review of patient data and images was approved by our institutional review board. No patient consent was required for our HIPAA-compliant study. Between 2000 and 2007, 7 patients with pathologically-proven PAP in the setting of hematological malignancy were identified at our institution. Six of these patients had chest CT scans performed to evaluate their respiratory symptoms and were included in our study.
Three studies from 2004 to 2007 were performed on a 64-slice LightSpeed VCT GE CT scanner (GE Medical Systems, Waukesha, WI). The 3 earlier studies were performed on either a 4-slice or an 8-slice multidetector GE CT scanner. The CT parameters were 120 kVp, 180−400 mA, and 1.25−5 mm slice thickness. Two cases were scanned without IV contrast. The other 4 scans were performed with IV contrast. Images were reviewed in lung, soft tissue, and bone windows.
CT scans were reviewed retrospectively by an experienced chest radiologist and separately by a resident. Disagreements were then resolved by consensus. CT abnormalities were characterized as ground-glass opacities, septal thickening (interlobular and intralobular), and consolidation. The axial and zonal distribution of disease was also noted. Other findings were also recorded, including nodules and pleural effusion. Medical records were reviewed to ascertain the temporal relationship between onset of lung disease and drug treatment, induction chemotherapy, or stem cell transplantation. Treatment of SPAP and outcome were also logged. Because the number of patients was small, no statistical analysis was warranted.
Results
Patient ages ranged from 30−54 years (average 42 years). Four men and two women were included. The underlying malignancies were chronic myelogenous leukemia (n=4), myelodysplastic syndrome (n=1), and cutaneous T-cell lymphoma (n=1). Three patients had concomitant infection: Mycobacterium avium complex (patient 2), Acinetobacter baumanii and Aspergillus fumigatus (patient 3), and Cladosporium (patient 4).
In the 6 cases, the predominant pattern was ground-glass opacity in 3, ground-glass opacity plus septal thickening in 2, and peripheral consolidation in 1 (Figure 1). No clear axial pattern was demonstrated. (In 2 patients the ground-glass opacities were predominately peripheral, in 1 central, in 1 posterior, and in 2 random or diffuse). Other than the diffuse pattern demonstrated in patient 6, all CT scans demonstrated patchy, relatively well-demarcated (geographic) lung abnormalities (Figure 2). An upper zonal predominance was noted in 2, and a lower zonal predominance in 3. A uniform distribution across all zonal patterns was noted in 1 (Table 1).
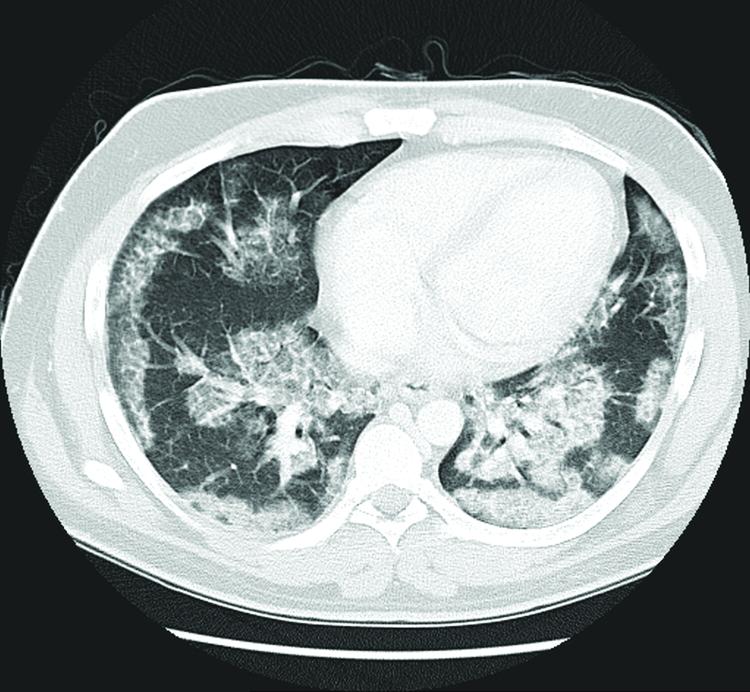
Figure 1. Patient 3.
“Crazy-paving” CT pattern with sharply marginated (geographic) ground-glass opacities and superimposed interlobular and intralobular septal thickening.
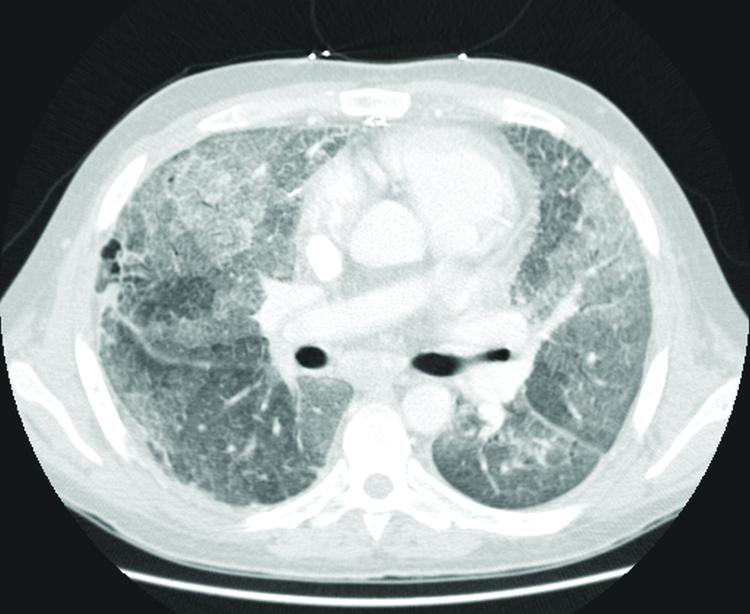
Figure 2. Patient 6.
Diffuse ground-glass opacities and interlobular/intralobular septal thickening predominantly in the right middle lobe and lingula.
Table 1.
--Summary of CT findings in 6 patients with pathologically proven secondary PAP. As in primary PAP, the most common pattern was ground-glass opacities with or without septal thickening.
| Patient | GGO | Septal thickening | Consolidation | Predominant pattern | Axial | Other |
|---|---|---|---|---|---|---|
| 1 | Lower greater than middle greater than upper lung zone involvement | Minimal | Patchy lower greater than middle greater than upper lung zone involvement | Consolidation | Peripheral except lower lung zone where diffuse | Small left pleural effusion, small ill-defined pulmonary nodules |
| 2 | Bilateral upper greater than middle greater than lower lung zone involvement | Same as GGO | Minimal | GGO | Central | Left pleural effusion |
| 3 | Lower greater than middle greater than upper lung zone involvement | Same as GGO | none | GGO and septal thickening | Peripheral | |
| 4 | Left greater than right; lower zones greater that middle zonal involvement | Mild left>right | Nodular patchy lower lung consolidation | GGO | Posterior greater than central | Upper lobe pulmonary nodules |
| 5 | Bilateral upper and middle with less involvement of lower lungs | Same as GGO | Minimal patchy in all lobes | GGO and septal thickening | No clear distribution | Bilateral pleural effusions |
| 6 | Uniform | Same as GGO | Lower lungs | GGO>>>septal thick and consolidation | Diffuse |
GGO: Ground-glass opacities
The etiology of SPAP was most often considered to be hematological malignancy; however, other etiologies were also considered in 3 patients, including infection (Mycobacterium avium complex and Cladosporium) and drug therapy (with dasatinib or FLAN [fludarabine, cytosine-arabinoside, mitoxantrone]) (Table 2). Although 3 patients were eventually treated with stem cell transplantation, transplantation did not coincide with clinical or CT evidence of PAP. Patient 4 was treated with antifungal agents. Patients 2 and 3 were treated with GM-CSF with no response to therapy. Patients 5 and 6 did not receive therapy—patient 5 had mild disease while patient 6 succumbed to respiratory failure before treatment for SPAP could begin.
Table 2.
Patient data. Two of the six patients died from respiratory failure. Two patients were treated with GM-CSF with no diminution in signs, symptoms, or CT abnormalities.
| Patient | Age | Major comorbidity | Means of diagnosis | Probable cause | Outcome | Treatment |
|---|---|---|---|---|---|---|
| 1 | 40 M | MDS | Surgical biopsy | MDS | Resolution | Stem cell transplantation though there was improvement even before treatment |
| 2 | 45 M | CML | BAL | CML or MAC | Death from respiratory compromise | Stem cell transplantation and GM-CSF |
| 3 | 30 M | CTCL | Surgical biopsy | CTCL | Discharged without complete resolution of PAP; Secondarily infected with Acinetobacter baumanii and Aspergillus fumigatus, later in disease course | GM-CSF discontinued after one week due to no response |
| 4 | 52 F | CML | BAL and surgical biopsy | CML or Cladosporium or FLAN induction therapy | Resolution with treatment for Cladosporium with amphotericin and caspofungin | Amphotericin and caspofungin |
| 5 | 54 F | CML | Surgical biopsy | CML or drug (dasatinib) | Stable; minimal initial symptoms; stable radiological abnormalities. | None acutely, eventual bone marrow transplant |
| 6 | 35 M | CML | BAL and autopsy | CML | Death from respiratory compromise | None |
MDS: Myelodysplastic syndrome, CML: Chronic myelogenous leukemia, CTCL: Cutaneous T-cell lymphoma, MAC: Mycobacterium avium complex, SCT: Stem cell transplant, BAL: Bronchoalveolar lavage FLAN: Fludarabine, cytosine arabinoside, mitoxantrone, GM-CSF: Granulocyte-macrophage colony stimulating factor
Outcome was mixed (Table 2). In patient 3, treatment with GM-CSF was not effective, and he died from sepsis caused by cutaneous infection about 15 months later. Patients 1 and 4 improved with stem cell transplantation and antifungal treatment, respectively. Patient 5 had mild symptoms and did well with no intervention. Patients 2 and 6 died from respiratory failure. Autopsy revealed that SPAP was the sole cause of death in patient 6, with no concomitant infection identified (Figure 3). Patient 2 perished from respiratory failure from combined Mycobacterium avium complex infection and SPAP.
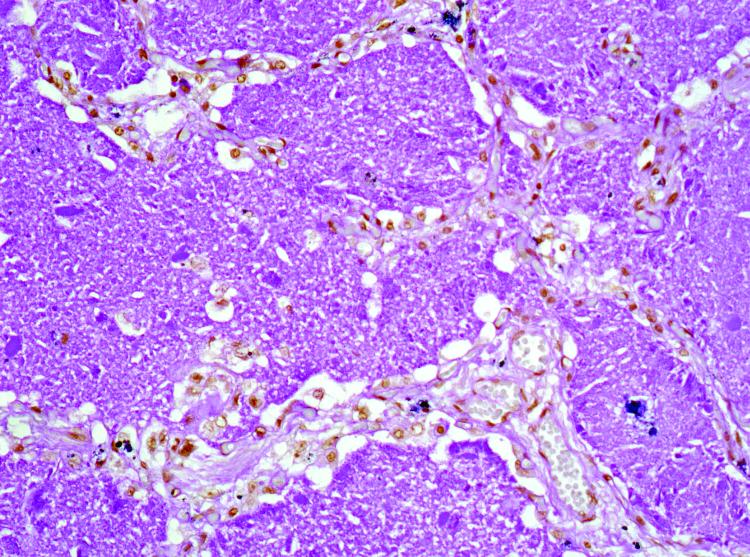
Figure 3. Patient 6.
Homogeneous PAS-positive eosinophilic material stains with a granular pattern highly suggestive of PAP.
Discussion
As opposed to IPAP, SPAP has received little attention in the radiology literature. The hematological malignancies most often related to SPAP are acute and chronic myelogenous leukemias; also linked are myelodysplastic syndrome and lymphoblastic leukemia [4-7]. CML was the most common cause of SPAP in our series (4/6 patients). Three of these patients had accompanying drug therapy (with FLAN induction treatment or dasatinib) or infection (Mycobacterium avium complex or Cladosporium), which may have contributed to SPAP. To our knowledge, case 3 is the first case of SPAP associated with cutaneous T-cell lymphoma. Three of our patients had stem cell transplantation. SPAP may arise as a late complication of stem cell transplantation, but in our patients SPAP occurred before transplantation [8].
Infection often coexists with SPAP, as in our series [9], although a causal relationship between PAP and infection is not clearly defined. Organisms associated with SPAP include Aspergillus, Nocardia, viruses, and Mycobacterial species. Superimposed infection accounts for a significant degree of morbidity and mortality in patients with SPAP [2]. PAP may promote infection because of impaired macrophage function, dysfunction of surfactant, or retention of proteinaceous materials [9, 10].
Macrophage dysfunction underlies all forms of PAP. This may arise anywhere along the pathway for GM-CSF-related macrophage activation. IPAP is likely autoimmune, and autoantibodies to GM-CSF may be detected, especially in adults [2], but autoantibodies are not found in SPAP [2, 11]. In SPAP arising in hematological malignancy, the alveolar macrophage may be defective because it derives from the malignant clone and has a defective GM-CSF transduction pathway [6, 12]. Treatment of IPAP with aerosolized and subcutaneous GM-CSF has produced mixed results [13, 14]. Treatment of SPAP is directed at the underlying malignancy. Whole lung lavage has also been used; however, the efficacy of GM-CSF therapy in SPAP has yet be determined [4, 9, 15]. Two patients in our study (patients 2 and 3) did not respond to therapy with GM-CSF.
Whole-lung lavage has boosted survival rates for IPAP from 70% to as high as 100% [3, 16]. Mortality rates in SPAP are harder to define because cases are rare. However, there are many reported deaths, including respiratory demise solely from SPAP, implying that SPAP may have a worse prognosis than IPAP [17-20]. SPAP played a significant role in two of our patients’ deaths. Mortality in patient 6 was attributed solely to SPAP as there was no evidence of infection or concomitant lung disease on autopsy. SPAP also was the primary cause of death in patient 2, though this patient was recently treated for Mycobacterium avium complex infection.
The classic CT findings in IPAP have been termed “crazy paving,” representing ground-glass opacity combined with septal thickening [21, 22]. Crazy paving is not pathognomonic for PAP, since it also occurs with Pneumocystis and viral pneumonia, lipoid pneumonia, pulmonary edema, diffuse alveolar damage, bronchioloalveolar cell carcinoma, and pulmonary hemorrhage [21]. Holbert demonstrated that the most common pattern in IPAP was a geographic pattern of ground-glass opacities without a zonal distribution [23]. A similar pattern of disease was also demonstrated in an earlier study in which 8 of 9 patients demonstrated ground-glass opacities or consolidation [22].
To our knowledge, there has been no study on the CT findings in SPAP. The most common pattern in our series was ground-glass opacities with or without septal thickening. No definite zonal distribution could be established, although a lower zonal predominance was present in half of patients. Overall, the pattern in our cases was similar to that described in IPAP. Consolidation was absent or minimal except in patient 1, who had pathological findings of cryptogenic organizing pneumonia in addition to SPAP. The pattern of peripheral consolidation is more suggestive of cryptogenic organizing pneumonia rather than PAP [24]. Given the rarity of SPAP and the nonspecificity of ground-glass opacities and septal thickening in patients with hematological malignancy, SPAP can be easily misdiagnosed as infection, particularly Pneumocystis carinii or viral pneumonia [25].
Our study was limited by small sample size and the relative inexperience of the resident reader. In addition, in patients with concomitant infection, some of the CT abnormalities might have reflected infection rather than SPAP alone.
In conclusion, SPAP is an uncommon form of PAP with the same CT finding as in IPAP, namely a crazy-paving pattern of ground-glass opacity. In cancer patients, this finding is easily misdiagnosed as infection, particularly Pneumocystis or viral pneumonia. Our cases demonstrate the importance of recognizing this entity in the setting of hematological malignancy in order to prevent a delay in diagnosis. Although our small sample size precludes any definitive statement about mortality in SPAP, the death of 2 of our 6 patients suggests that SPAP may have a worse prognosis than IPAP.
Acknowledgments
Grant from the NIH, CA18029.
Contributor Information
Jonathan H. Chung, Department of Radiology UW Medical Center Box # 357115 Seattle, WA 98195 jchung2@u.washington.edu.
Sudhakar J. Pipavath, Department of Radiology UW Medical Center Box 357115 Seattle, WA 98195.
David H. Myerson, 1100 Fairview Avenue N., D2−190 P.O. Box 19024 Seattle, Washington 98109−1024.
J. David Godwin, Department of Radiology UW Medical Center Box 357115 Seattle, WA 98195.
References
- 1.Rosen S, Castleman B, Liebow A. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell B, Whitsett J, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 3.Ioachimescu OC KM. Pulmonary alveolar proteinosis. Chron Respir Dis. 2006;3:149–159. doi: 10.1191/1479972306cd101rs. [DOI] [PubMed] [Google Scholar]
- 4.Cordonnier C, Fleury-Feith J, Escudier E, Atassi K, Bernaudin J. Secondary alveolar proteinosis is a reversible cause of respiratory failure in leukemic patients. Am J Respir Crit Care Med. 1994;149:788–794. doi: 10.1164/ajrccm.149.3.8118651. [DOI] [PubMed] [Google Scholar]
- 5.Gacouin A, Le Tulzo Y, Suprin E, et al. Acute respiratory failure caused by secondary alveolar proteinosis in a patient with acute myeloid leukemia: a case report. Intensive Care Med. 1998;24:265–267. doi: 10.1007/s001340050563. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi T YG, Shijubo N, Takagi-Takahashi Y, Itoh T, Takahashi H, Satoh M, Koba H, Nakata K, Abe S. Secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome. Intern Med. 2003;42:187–190. doi: 10.2169/internalmedicine.42.187. [DOI] [PubMed] [Google Scholar]
- 7.Pamuk G, Turgut B, Vural O, et al. Pulmonary alveolar proteinosis in a patient with acute lymphoid leukemia regression after G-CSF therapy. Leuk Lymphoma. 2003;44:871–874. doi: 10.1080/1042819021000055093. [DOI] [PubMed] [Google Scholar]
- 8.Coy D, Ormazabal A, Godwin J, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005;25:305–317. doi: 10.1148/rg.252045037. discussion 318. [DOI] [PubMed] [Google Scholar]
- 9.Birsak CA vRR, Nijhuis-Heddes JM, Maartense E. Pulmonary alveolar proteinosis: a complication in patients with hematologic malignancy. Neth J Med. 2000;56:193–197. doi: 10.1016/s0300-2977(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 10.Prakash U, Barham S, Carpenter H, Dines D, Marsh H. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987;62:499–518. doi: 10.1016/s0025-6196(12)65477-9. [DOI] [PubMed] [Google Scholar]
- 11.Trapnell BC WJ. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 12.Dirksen U HU, Schneider P, Schroten H, Gobel U, Bocking A, Muller KM, Murray R, Burdach S. Defective expression of granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 receptor common beta chain in children with acute myeloid leukemia associated with respiratory failure. Blood. 1998;92:1097–1103. [PubMed] [Google Scholar]
- 13.Wylam ME TR, Prakash UB, Nadrous HF, Clawson ML, Anderson PM. Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur Respir J. 2006;27:585–593. doi: 10.1183/09031936.06.00058305. [DOI] [PubMed] [Google Scholar]
- 14.Venkateshiah SB YT, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006;130:227–237. doi: 10.1378/chest.130.1.227. [DOI] [PubMed] [Google Scholar]
- 15.Ceruti M RG, Stella GM, Adami A, Bolongaro A, Baritussio A, Pozzi E, Luisetti M. Successful whole lung lavage in pulmonary alveolar proteinosis secondary to lysinuric protein intolerance: a case report. Orphanet J Rare Dis. 2007;2:14. doi: 10.1186/1750-1172-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour JF PJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 17.Kita H MS, Nakano Y, Hattori N, Mizutani T, Kagioka H, Fujita M. [An autopsy case of acute lymphocytic leukemia associated with secondary pulmonary alveolar proteinosis and systemic aspergillosis]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:374–378. [PubMed] [Google Scholar]
- 18.Ruben FL TT. Secondary pulmonary alveolar proteinosis occurring in two patients with acquired immune deficiency syndrome. Am J Med. 1986;80:1187–1190. doi: 10.1016/0002-9343(86)90683-2. [DOI] [PubMed] [Google Scholar]
- 19.Shoji N IY, Kimura Y, Nishimaki J, Kuriyama Y, Tauchi T, Yaguchi M, Payzulla D, Ebihara Y, Ohyashiki K. Pulmonary alveolar proteinosis as a terminal complication in myelodysplastic syndromes: a report of four cases detected on autopsy. Leuk Res. 2002;26:591–595. doi: 10.1016/s0145-2126(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 20.Doki N HT, Irisawa H, Sakura T, Miyawaki S. [Acute myeloid leukemia complicated with pulmonary alveolar proteinosis at presentation]. Rinsho Ketsueki. 2005;46:522–526. [PubMed] [Google Scholar]
- 21.Rossi SE EJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509–1519.. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 22.Godwin JDMN, Takasugi JE. Pulmonary alveolar proteinosis: CT findings. Radiology. 1988;169:609–613. doi: 10.1148/radiology.169.3.3186983. [DOI] [PubMed] [Google Scholar]
- 23.Holbert JMCP, Li W, Hoffman RM, Rogers RM. CT features of pulmonary alveolar proteinosis. AJR Am J Roentgenol. 2001;176:1287–1294. doi: 10.2214/ajr.176.5.1761287. [DOI] [PubMed] [Google Scholar]
- 24.Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier A. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27:595–615. doi: 10.1148/rg.273065130. [DOI] [PubMed] [Google Scholar]
- 25.Kanne J, Godwin J, Franquet T, Escuissato D, Müller N. Viral pneumonia after hematopoietic stem cell transplantation: high-resolution CT findings. J Thorac Imaging. 2007;22:292–299. doi: 10.1097/RTI.0b013e31805467f4. [DOI] [PubMed] [Google Scholar]