Abstract
Background & Aims
Hepatocyte transplantation-induced liver inflammation impairs cell engraftment. We defined whether proinflammatory cytokines and chemokines played roles in regulation of hepatocyte engraftment in the liver.
Methods
We performed studies over up to three weeks in rat hepatocyte transplantation systems. Expression of 84 cytokine-chemokine genes was studied by quantitative real-time polymerase chain reactions. Expression of selected upregulated genes was verified by immunohistochemistry. Hepatic recruitment of neutrophils was demonstrated by myeloperoxidase activity assays and Kupffer cell activation was established by carbon phagocytosis assays. The role of neutrophils and Kupffer cells in regulating expression of cytokine-chemokine genes as well as cell engraftment was determined by cell depletion studies.
Results
Within six hours after syngeneic cell transplantation, expression of 25 cytokine-chemokine genes increased by 2- to 123-fold, p<0.05. These genes were largely associated with activated neutrophils and macrophages, including chemokine ligands, CXCL1, CXCL2, CCL3, CCL4, chemokine receptors, CXCR1 or CXCR2, CCR1, CCR2, and regulatory cytokines TNF-α and IL6. Inflammatory cells in the liver immunostained for CCR1, CCR2, CXCR1 and CXCR2, which indicated that upregulated mRNAs were appropriately translated. When neutrophils and Kupffer cells were depleted with neutrophil anti-serum and gadolinium chloride, respectively, before transplanting cells, cell transplantation-induced cytokine-chemokine responses were attenuated. Virtually all abnormalities subsided in animals treated with neutrophil anti-serum plus gadolinium chloride. Moreover, depletion of neutrophils or Kupffer cells improved engraftment of transplanted cells.
Conclusions
Cell transplantation-induced liver inflammation involves proinflammatory cytokine-chemokine systems capable of modulation by neutrophils and Kupffer cells. This offers new directions for optimizing cell therapy strategies.
Keywords: Cell transplantation, Chemokine, Cytokine, Kupffer cell, Liver, Neutrophil
Introduction
Repopulation of the liver with hepatocyte transplantation has substantial therapeutic potential. However, multiple barriers restrict engraftment and proliferation of transplanted hepatocytes.1,2 For instance, 70–80% of transplanted cells are cleared from the liver within one or two days after cell transplantation. Subsequently, disruption of the sinusoidal endothelial barrier is required for transplanted cells to enter and integrate in the liver parenchyma.2 During that period, multiple types of cell-cell interactions take place, including with liver sinusoidal endothelial cells (LSEC), hepatic stellate cells (HSC) and Kupffer cells.3–5 Some interactions are beneficial, e.g., activation of HSC promotes cell engraftment,4 whereas others are deleterious, e.g., Kupffer cells phagocytose transplanted cells.5
Recently, cytokines and chemokines have been more specifically incriminated in tissue damage in inflammation, sepsis, immune disorders, cancer, and other conditions.6 Chemokines are small proteins of 8–10 kilodalton size, and are grouped into four categories, depending on the distribution of cysteine residues in conserved locations – CC chemokines contain two adjacent cysteines near amino terminus and constitute over 25 ligands (CCL1 to CCL28) with ability to attract monocytes, NK, dendritic, and T and B cells, as well as eosinophils and basophils; CXC chemokines, with separation of two amino-terminal cysteines by an amino acid (X), constitute 17 ligands and attract neutrophils and lymphocytes; C chemokines contain two cysteine residues and constitute two ligands, XCL1 and XCL2, with ability to attract T cell precursors to the thymus; and CX3C chemokines contain three amino acids between two cysteine residues, with CX3CL1 being the prototype member, which additionally serves as an adhesion molecule. Chemokine ligands recognize widely distributed cellular receptors, characterized by seven G protein-coupled transmembrane domains, including ten CC chemokine receptors (CCR1-CCR10) and seven CXC chemokine receptors (CXCR1-CXCR7). Each chemokine receptor may bind several ligands, which produces much complexity in chemokine responses. Nonetheless, chemokine ligands and receptors are attractive therapeutic targets. Similarly, much therapeutic focus has centered on pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, which serves major roles in coordinating or amplifying inflammation in diseases.7
Here, to define the nature of cytokine-chemokine-dependent liver inflammation after hepatocyte transplantation, we studied dipeptidyl peptidase IV-deficient (DPPIV-) rats, where transplanted cells can be conveniently identified.2–5 We studied inflammatory changes over up to three weeks after cell transplantation, determined what cell types contributed to inflammation, characterized expression of cytokines-chemokines under defined experimental conditions, and demonstrated whether prevention of inflammatory cell activation improved cell engraftment.
Materials and Methods
Animals
DPPIV- F344 rats, 8–10 weeks old and weighing 120–200 g, and Long Evans Agouti (LEA) rats weighing 200–250 g, were from Special Animal Core of Marion Bessin Liver Research Center. F344 rats were from National Cancer Institute (Bethesda, MD). Animal Care and Use Committee at Albert Einstein College of Medicine approved experimental protocols.
Hepatocyte transplantation
Hepatocytes were isolated by collagenase perfusion of liver from F344 or LEA rats and 2×107 freshly isolated cells were injected within seconds into the spleen as described previously.2 Control rats were not subjected to any manipulations.
Macroaggregated albumin particles (MAA)
These were commercially available (TechneScan®, Mallinckrodt Medical Inc., St. Louis, MO) and used as described previously. 8 Each TechneScan® vial contains 8±4×106 MAA and 90% particles are in 10 to 40 μm size range. Approximately 2×107 MAA were injected intrasplenically into DPPIV- rats.
Analysis of mRNA expression by real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA was isolated from liver with TRIzol Reagent (Invitrogen, Carlsbad, CA). After DNAse treatment, 1 μg RNAs were converted to cDNA with RT2 PCR Array First Strand Kit (SABiosciences, Frederick, MD), followed by RT-PCR with rat cytokine-chemokine arrays (APRN 011C; SABiosciences), as recommended by the manufacturer. The array included 84 chemokine, cytokine and receptor genes, 5 housekeeping genes, 1 control for genomic DNA contamination, 3 reverse-transcription controls to assess cDNA conversion, and 3 positive controls with pre-dispensed DNA and oligonucleotide primers. PCR amplification was with Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Data were analyzed with an Excel-based template from the manufacturer (SABiosciences). Fold-changes in gene expression were determined with the Ct method by comparing experimental and control samples after data were normalized with β-actin, which was expressed invariantly. Changes in gene expression of 2-fold or greater with p-values <0.05 were considered significant. In some instances, end-point PCR products were analyzed in 2% agarose gels with ethidium bromide to verify single PCR products.
Identification of transplanted cells
Liver samples were frozen in cold methylbutane and stored at −80°C. Cryostat sections of 5 μm thickness were fixed in chloroform-acetone (1:1 vol/vol) for 10 min, air-dried and stained histochemically for DPPIV with hematoxylin counterstain. 1–5 Transplanted cell numbers were measured in 100 areas centered on portal radicles under ×100 magnification 3 d after cell transplantation (n=3 rats per condition).
Hepatic γ-glutamyltranspeptidase (GGT) expression
Cryostat sections of 5 μm thickness were fixed in chloroform-acetone (1:1 vol/vol) for 10 min, air-dried and stained histochemically for GGT with hematoxylin counterstain, as described previously. 9 Liver areas with GGT were measured with Adobe Photoshop CS3 software (Adobe Systems Inc., San Jose, CA).
Neutrophil activation
Hepatic myeloperoxidase (MPO) was measured with tetramethylbenzidine substrate (Sigma Chemical Co., St. Louis, MO) as described previously. 10 For MPO histochemistry, 5 μm thick cryosections were fixed in 4% paraformaldehyde and 50% ethanol followed by incubation with Peroxidase Indicator Reagent, as recommended (procedure 390A, Sigma). Presence of neutrophils was verified with immunostaining by fixing sections in cold acetone for 10 min and blocking with 3% goat serum in phosphate buffered saline, pH 7.4 (PBS), 0.1% bovine serum albumin (BSA) and 0.1% Triton (Sigma) followed by incubation with rabbit anti-rat neutrophil serum, 1:100 in PBS (51140 AD; Inter-cell Technologies, Jupiter, FL). After washing with PBS, sections were incubated with goat anti-rabbit IgG (1:500; Sigma) for 30 min at 37°C and developed with DAB+ (K3467, DakoCytomation, Dako North America Inc., Carpinteria, CA). Neutrophils were counted in 25 consecutive areas centered on portal radicles under ×200 magnification (n=3 animals per condition).
Kupffer cell and other inflammatory cell activations
Animals were given 1 h pulse of 0.1 mL India ink injected intrasplenically before sacrifice, as previously described.3 Liver samples were frozen and 5 μm cryostat sections were stained for DPPIV activity to identify transplanted cells followed by hematoxylin counterstaining to visualize Kupffer cells with carbon. Kupffer cell activity was graded in 25 areas centered on portal radicles under ×200 magnification (n=3 rats per condition): grade 1, minimal carbon; grade 2, intermediate carbon; and grade 3, maximal carbon incorporation. Sections were stained with hematoxylin and eosin to determine accumulation of other inflammatory cell types, e.g., lymphocytes, eosinophils and basophils.
Verification of cytokine expression
Cryosections of 5 μm thickness were fixed in 4% paraformaldehyde in PBS for 10 min and blocked with 3% serum in PBS, 0.1% BSA, 0.1% Triton. This was followed by incubation with 1:50 to 1:100 goat or rabbit antibodies against CCR1 (sc-6125, Santa Cruz Biotechnology Inc., Santa Cruz, CA), CCR2 (sc-31564, Santa Cruz), CCR3 (18–511–244683, GenWay Biotech, Inc., San Diego, CA), CCR5 (ab65850, Abcam Inc., Cambridge, MA), CXCR1 (NB300–697, Novus Biologicals, Littleton, CO), and CXCR2 (ab14935, Abcam) for 30 min at room temperature and overnight at 4°C. Secondary antibodies were anti-goat or anti-rabbit peroxidase-conjugated IgG (1:200, Sigma) and color was developed with DAB+ (K3467, DakoCytomation).
Manipulation of animals to decrease cytokine activation
To deplete neutrophils, 1 ml rabbit anti-neutrophil rat antiserum (anti-PMN) (Inter-cell Technologies) was given intraperitoneally 16–18 h before cell transplantation, as described previously. 11 To deplete Kupffer cells, gadolinium chloride (GdCl3) was dissolved in normal saline and 10 mg/kg was given intrasplenically 16–18 h before cell transplantation, as described previously. 3 To deplete both neutrophils and Kupffer cells, GdCl3 was given initially followed by neutrophil antiserum.
Study design
First, we examined onset of inflammation 6 h, 1 d, 3 d, and 1, 2 and 3 weeks after cell transplantation (n=3 each), plus untreated control rats (n=3; total, 21 rats). Tissues were analyzed for cell engraftment, liver ischemia and hepatic inflammation, including cytokine-chemokine profiles. In additional animals, nonparenchymal liver cells were isolated by collagenase perfusion with untreated control rats (n=3), and rats 6 h or 1d after cell transplantation (n=3 each), to demonstrate inflammatory cells by flow cytometry (total, 9 rats). Rats were given MAA to demonstrate changes in hepatic ischemia, inflammation and cytokine-chemokine expression after 1 d and 3 d (n= 3 each; total 6 rats). Additional rats were given allogeneic LEA rat hepatocytes followed by analysis of cytokine-chemokine responses after 6 h, 1 d and 3 d (n=3 each; total 9 rats). Next, we examined whether depletion of neutrophils and/or Kupffer cells with anti-PMN and GdCl3, respectively, altered cell transplantation-induced changes (n=3 each; total 12 rats). Finally, we addressed whether depletion of neutrophils and Kupffer cells improved cell engraftment after 3 days and after 2 weeks compared with untreated control rats (n=3 each; total 18 rats). The experiments were repeated 2–3 times to reproduce major findings.
Statistical Analysis
Data are shown as means±SD. Significances were analyzed by t-test, Chi-square test or ANOVA and Holm-Sidak method for pairwise comparisons of mean responses with SigmaStat 3 (Systat Software Inc., Point Richmond, CA). P <0.05 was considered significant.
Results
Cell transplantation activated hepatic inflammation
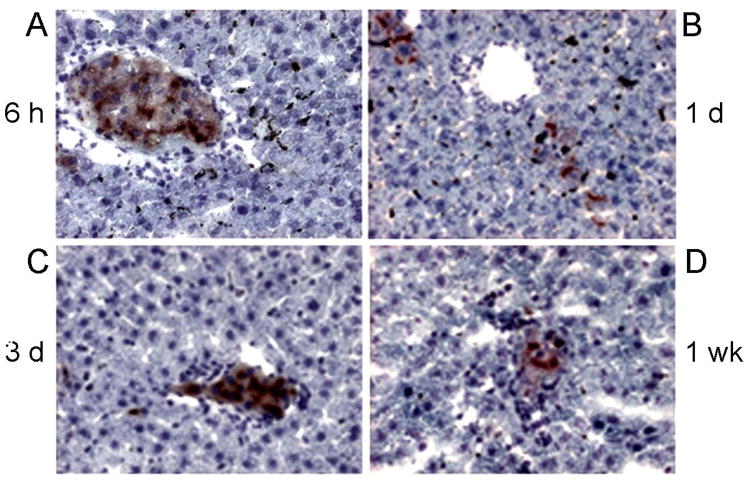
Transplanted syngeneic hepatocytes were in portal vein radicles and liver sinusoids 6 h after intrasplenic injection of cells (Fig. 1A), and were present 1 d, 3 d and 1, 2 and 3 weeks after syngeneic cell transplantation, although transplanted cells had largely been cleared from vascular spaces within 3 d, whereas cells surviving beyond 3 d showed engraftment throughout the 3 week period (Fig. 1B–D). After cell transplantation, GGT was widely expressed in hepatocytes, hepatic MPO activity increased and neutrophils-expressing MPO and reacting with anti-PMN serum accumulated, maximally 6 h and 1 d after cell transplantation (see below), followed by return of MPO-positive neutrophils to normal levels 3 d after cell transplantation. Kupffer cells incorporated more carbon throughout after cell transplantation, although this was greatest 6 h after cell transplantation.
Figure 1. Transplanted hepatocytes identified by DPPIV histochemistry in DPPIV- rats.
Shows transplanted cells (red color) in the liver 6 h (A), 1 d (B), 3 d (C) and 1 week (D) after transplantation. Note transplanted cells in sinusoids and portal radicles after 6 h (A). Fewer transplanted cells were present later (B–D), although transplanted cells were present throughout the 3-week duration of studies. Orig. mag. ×400; hematoxylin counterstain.
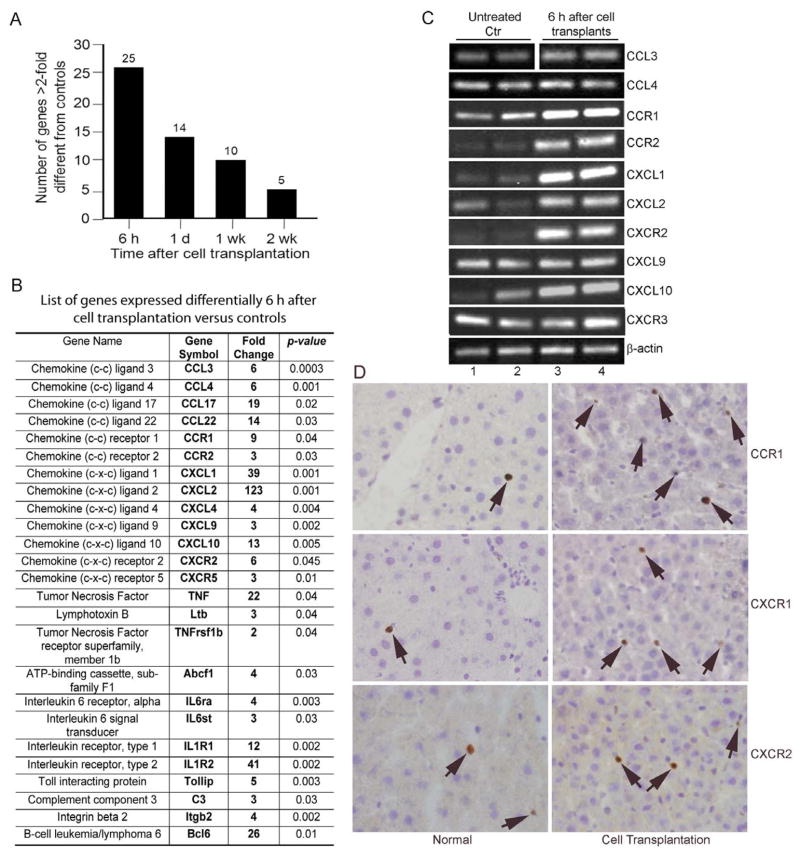
Studies with qRT-PCR arrays indicated significant changes in cytokine-chemokine genes, such that 6 h after cell transplantation 25 of 84 genes were upregulated by >2-fold before declining subsequently (Fig. 2A). This was similar to the kinetics of changes in neutrophils and Kupffer cells after cell transplantation. Gene expression increased from 2- to over 120-fold, including genes typically associated with neutrophils (e.g., CXCL1, CXCL2, and the receptor, CXCR2), macrophages (e.g., CCL3, CCL4, and the receptors, CCR1 and CCR2, along with CCL9, CCL10), and other cells, such as lymphocytes (e.g., CCL17, IL-1 and IL-6 groups) (Fig. 2B). TNF-α and associated genes, e.g., Ltb, TNFrSF1b and ABCF1, were also of interest. Analysis of PCR products verified presence of single bands (Fig. 2C). Immunostaining for several chemokine receptors further verified that changes observed at the mRNA level were representative of changes at the protein level (Fig. 2D). In normal livers, only occasional sinusoidal cells were positive for CXCR1, CXCR2 and CCR1, whereas after cell transplantation, increased numbers of infiltrating cells with these receptors, 3–6 cells per high power field, p<0.05, were present in the liver. These receptors were not expressed in parenchymal liver cells.
Figure 2. Expression of Chemokine and cytokine genes.
(A) Shows cumulative changes in gene expression. (B) List of 25 genes expressed more after cell transplantation. (C) Shows endpoint PCR products of selected genes. (D) Immunohistochemical staining of CCR1, CXCR1 and CXCR2 in liver from normal animal and animals 6 h after cell transplantation. Note that proteins were expressed in only infiltrating cells (arrows). Orig. mag. ×400; hematoxylin counterstain.
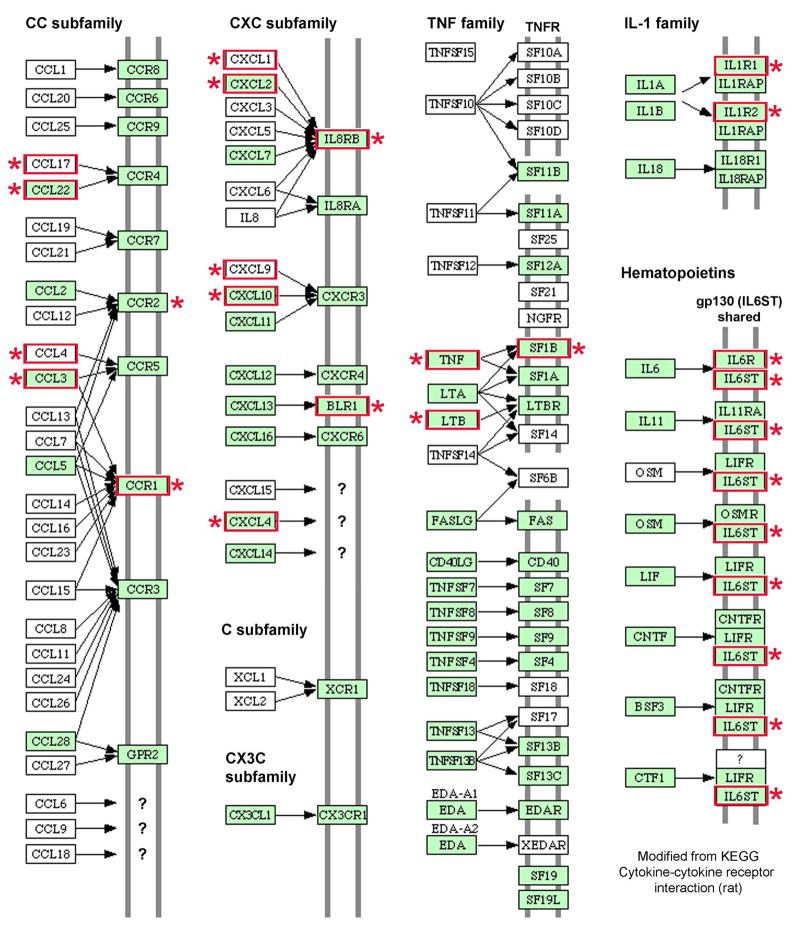
The choice of these receptors for immunostaining was determined by the connections between upregulated chemokine ligands and receptors, which are complex, as indicated by mapping of cell transplantation-induced changes (Fig. 3). Some inflammatory effectors were more activated after cell transplantation, e.g., neutrophil and macrophage-related genes were represented much more, rather than lymphocyte-related chemokines, which was to be expected after transplantation of syngeneic cells, where acquired immune responses should not have been induced.
Figure 3. Mapping of chemokine-cytokine and receptor interactions.
Genes with increased expression after cell transplantation are shown (asterisks, red boxes) in map modified from Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.ad.jp/dbget-bin/get_pathway?org_name=rno&mapno=04060). Expression of other genes not included in the array might also have been altered. Note, IL8RB = CXCR2; BLR1 = CXCR5.
To determine whether hepatic changes following cell transplantation were different from those induced by inanimate particles, we studied animals after injection of MAA, which reproduced the distribution of hepatocytes in liver sinusoids in previous studies. 8 After MAA administration, we observed greater GGT expression (12±2% liver area after MAA versus 9±1% after hepatocyte transplantation, p<0.05, ANOVA with Holm-Sidak method), indicating onset of hepatic ischemia, 9 as well as evidence for hepatic inflammation with activation of neutrophils and Kupffer cells, although these cells were less pronounced compared with after cell transplantation, 11±1 versus 21±1, and 18±1 versus 32±1 cells per lobule, respectively) (Fig. 4). Analysis of cytokine-chemokine expression with arrays was instructive since changes after MAA were restricted to 40-fold increase in TNF expression only after 6 h and increased expression of the receptor, TNFrsf1a, to 421-fold and 114-fold above normal after 6 h and 3 d, respectively. This contrasted markedly with extensive changes in cytokine-chemokines after transplantation of syngeneic hepatocytes.
Figure 4. Hepatic changes after sinusoidal deposition of MAA or syngeneic hepatocytes.
Shown is hepatic GGT expression (panels on left), MPO-positive neutrophils (middle panels) and activated Kupffer cells containing carbon in tissues from normal untreated animals (A), animals after transplantation of syngeneic cells (B) and animals given MAA (C). Arrows indicate representative GGT-stained area, MPO-positive neutrophils and carbon-containing Kupffer cells. Note that cell transplantation and MAA resulted in activation of GGT expression, neutrophil infiltration and carbon containing Kupffer cells. Charts in (D) provide cumulative analysis of these changes. Asterisks in charts indicate p<0.05 versus untreated controls. Orig. mag., panels showing GGT, ×40, other panels, ×400; hematoxylin counterstain.
In limited studies, we also examined changes in cytokine-chemokine expression following transplantation of allogeneic LEA rats into DPPIV- rats. As allogeneic cells are rejected through adaptive immune responses subsequent to the completion of cell engraftment, we restricted our studies to this cell engraftment phase. Remarkably, the number of cytokine-chemokines upregulated after allogeneic cells was similar to syngeneic cells, 23 versus 25 after 6 h and 17 versus 14 after 1 d. These responses were similar 83% and 88% cases and dissimilar in 17% and 22% cases after 6 h and 1 d, respectively. Additional expression of chemokines after allogeneic cell transplants pertained to recruitment or trafficking of lymphocytes, dendritic cells, NK cells, and eosinophils, including CCL11, CCL12, CCL19, CCL25, as well as CCR3, CCR6 and CCR7.
Consistent with the differences in the cellular responses following syngeneic and allogeneic transplants, we did not observe accumulation of lymphocytes, eosinophils or basophils in tissues after transplantation of syngeneic cells. Similarly, isolation of nonparenchymal cells by collagenase perfusion of livers followed by flow cytometry in recipients of syngeneic cells did not indicate increases in CD3-positive lymphocytes or CD161-positive NK cells (not shown). Therefore, we next approached the issue of what innate cell types were major contributors of tissue inflammation by cell depletion studies in syngeneic animals.
Neutrophils and Kupffer cells largely contributed to hepatic inflammation
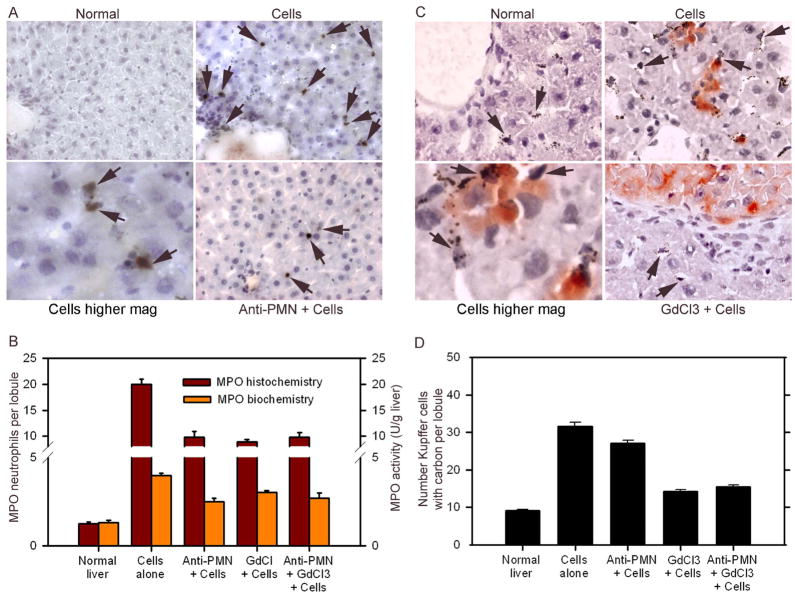
We transplanted syngeneic hepatocytes in rats after anti-neutrophil serum or GdCl3. As before, transplantation of cells without these manipulations activated neutrophils and Kupffer cells (Fig. 5). However, accumulation of neutrophils and of Kupffer cell activation was markedly attenuated after cell transplantation in animals treated with anti-PMN, GdCl3, or both.
Figure 5. Depletion of neutrophils and Kupffer cells after anti-PMN and GdCl3.
(A) MPO-stained neutrophils in the liver from normal rat, rat 6 h after cell transplantation alone, or rat treated with anti-PMN before cell transplantation. Note accumulation of neutrophils after cell transplantation (arrows), including their proximity to hepatocytes as shown in the panel at higher magnification, and their depletion after anti-PMN. Panel B shows cumulative decreases in MPO-positive cells and hepatic MPO activity after various treatments. (C) Shows carbon-containing Kupffer cells in normal rat, and rats 6 h after cell transplantation (arrows), where DPPIV-positive transplanted cells (red color) are present, whereas in animal treated with GdCl3, carbon uptake decreased. (D) Shows cumulative analysis of changes in Kupffer cell activation with fewer Kupffer cells containing carbon after GdCl3.
Depletion of neutrophils, as well as Kupffer cells, resulted in complete normalization of 17 and 13 of the 25 upregulated genes, 68% and 52%, respectively, whereas depletion of neutrophils plus Kupffer cells normalized 19 of 25 genes (76%) (Fig. 6). Moreover, many genes were expressed at lower levels. Taken together, cell transplantation in animals treated with anti-PMN plus GdCl3 either normalized or decreased expression in 24 out of 25 genes (96%). It was noteworthy that neutrophil depletion was especially effective in preventing upregulation of TNF-α-related genes, including TNF-α itself, TNFrSF1b, Ltb, and ABCF1. These genes were still expressed in GdCl3-treated rats. On the other hand, GdCl3 was more effective than anti-PMN in preventing upregulation of CCL3, CCL4, CXCL9, and IL1R1.
Figure 6. Changes in cytokine-chemokine gene expression after depletion of neutrophils and Kupffer cells.
(A) Shows gene expression profiles in rats 6 h after cell transplantation alone versus cell transplantation after anti-PMN, GdCl3 or both. (B) Chart indicates cumulative differences in gene expression after various treatments. The data indicated that anti-PMN plus GdCl3 largely normalized changes in gene expression.
To verify these qRT-PCR findings, we performed immunohistochemical stainings. This demonstrated marked increases 6 h after cell transplantation in infiltrating inflammatory cells expressing CCR1, CCR2 and CXCR1 (Fig. 7). By contrast, expression of these chemokine receptors in animals treated with anti-PMN, GdCl3, or both, diminished.
Figure 7. Immunostaining verified expression of CCR1, CCR2 and CXCR1 decreased after neutrophils and Kupffer cells were depleted.
Shown are livers from normal animals (A), animals after cell transplantation alone (B) and animals treated with anti-PMN (C), GdCl3 (D) and anti-PMN plus GdCl3 (E) before cell transplantation. Immunostaining is indicated for CCR1 (left), CCR2 (middle), and CXCR1 (right). Cell transplantation increased receptor expression (arrows, B), whereas depletion of neutrophils or Kupffer cells decreased their expression. Orig. mag. ×400; hematoxylin counterstain.
Regulation of hepatic inflammatory cells improved cell engraftment
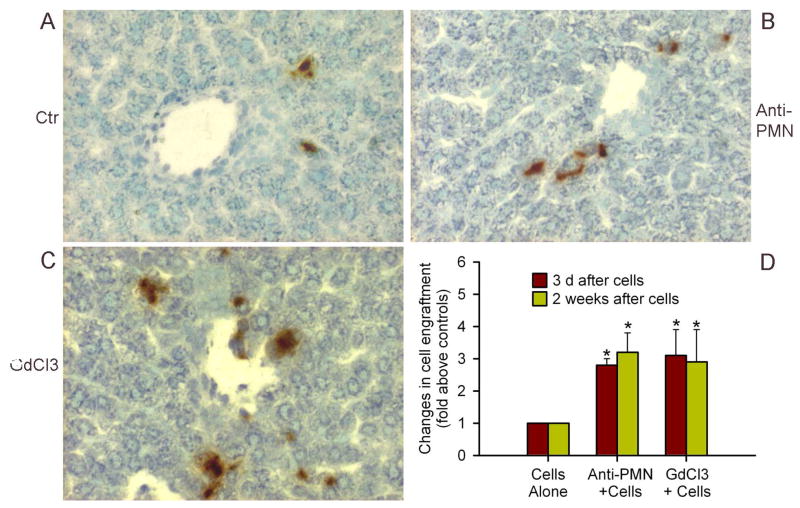
If chemokines and cytokines released from neutrophils and Kupffer cells affected transplanted cells, then depletion of these inflammatory cells should have improved cell engraftment. Therefore, we studied rats treated with anti-PMN, GdCl3, or both, followed by cell transplantation and analyzed cell engraftment after 3 d, as well as 2 weeks (Fig. 8). We found significant improvements in cell engraftment after either anti-PMN or GdCl3, which increased after 3 d on average by 2.8 and 3.2-fold above controls, respectively, p<0.05, and remained similarly increased after 2 weeks on average by 3.1 and 2.9-fold above controls, respectively, p<0.05. However, the combination of anti-PMN and GdCl3 produced mortality in animals 2–3 d after cell transplantation, leading to survival of only 1 out of 6 rats in two separate studies, although in the one surviving animal, transplanted cell numbers were greater than after either anti-PMN or GdCl3 alone.
Figure 8. Depletion of neutrophils and Kupffer cells improved cell engraftment in liver.
Shows DPPIV-positive transplanted cells (red color) in control rat (A), and rats treated with either anti-PMN (B) or GdCl3 (C). (D) Morphometric counts of transplanted cells indicating that anti-PMN and GdCl3 improved cell engraftment. Orig. mag. ×400; methylgreen counterstain.
Discussion
These studies advanced insights into innate responses following cell transplantation in the liver. First, profiling of chemokines and cytokines offered clues to the nature of inflammatory cells recruited by cell transplantation, i.e., neutrophils and Kupffer cells. Second, depletion of neutrophils and Kupffer cells permitted dissection of inflammatory mechanisms induced by cell transplantation. Third, suppression of chemokine and cytokine expression after depletion of neutrophils and Kupffer cells improved engraftment of hepatocytes in the liver.
Here, we limited studies of allogeneic cell transplants to the short-term and avoided xenogeneic cell transplants, which additionally activate a variety of adaptive immune responses.12,13 By contrast, transplantation of syngeneic cells permitted analysis of the innate immune system, which proved important in the initial clearance and engraftment of transplanted cells. Transplantation of autologous hepatocytes will be appropriate in some conditions for cell therapy with genetically-modified hepatocytes, as was examined in familial hypercholesterolemia.14 Also, suitable autologous cells, e.g., those derived from person-specific induced stem cells, will eventually be of interest for cell therapy. Of course, allogeneic and xenogeneic donor cells are of considerable interest for cell therapy, although analysis of relevant cytokine-chemokine-dependent pathways in such cell transplants will require detailed further work, where our studies here should provide an excellent comparison. Our targeted array-based approach to elucidate cytokine-chemokine changes was effective. High-density microarrays to study genome-wide transcriptional changes offer an alternative approach,15 although this approach generates far larger datasets, requiring more complex analyses, and our conclusions regarding inflammatory mechanisms would likely not have changed.
Neutrophils and Kupffer cells emerged in our studies as predominant effectors of cell transplantation-induced early inflammation. Increased expression of CCL3, CCL4, CXCL1 and CXCL2, which are released from macrophages, neutrophils or epithelial cells, 16,17 was in agreement with recruitment of neutrophils and Kupffer cells. CCL3 binds CCR1 and CCR5, CCL4 binds CCR5, and CXCL1 and CXCL2 bind CXCR2, as well as other receptors (Fig. 3).6 The potency of CXCL1 and CXCL2 in attracting neutrophils to sites of inflammation is well established,18 whereas CCL3 is chemotactic for monocytes, eosinophils and basophils.19 CXCL4 is expressed in activated platelets with additional roles in attracting neutrophils, monocytes or lymphocytes, although its receptor, CXCR3, was not overexpressed after syngeneic cell transplantation.20,21 Our results suggested potential roles for additional chemokines. For instance, CCL17, CCL22, CXCL9 or CXCL10 are released by macrophages, as well as eosinophils.22 CXCL10 may additionally be released from endothelial cells,23 which should be relevant since LSEC are damaged by cell transplantation.2 Similarly, Bcl6 (B-cell leukemia/lymphoma 6 protein) and its receptor CXCR5, were expressed after cell transplantation. Bcl6 is required for the function of germinal B cells though not for differentiation of memory B cells.15,24 Our histological studies did not demonstrate accumulation of eosinophils or basophils and we did not identify accumulation of lymphocytes or NK cells in the liver, which was in agreement with their playing little or no roles in this setting of hepatic inflammation after syngeneic cell transplants.
Our studies with anti-PMN and GdCl3 substantiated the role of neutrophils and Kupffer cells in the inflammatory response after cell transplantation. Simultaneous depletion of neutrophils and Kupffer cells virtually abolished the early inflammatory response after cell transplantation. Crosstalk between neutrophils and Kupffer cells likely accounted for these changes in chemokine expression. Depletion of neutrophils with anti-PMN before cell transplantation decreased CXCL1 expresssion, although CXCL2 expression increased, suggesting that the latter was additionally expressed elsewhere. In GdCl3-treated animals, expression of CXCL1, as well as CXCL2 increased by 60- and 457-fold, respectively, likely due to expression from neutrophils. By contrast, neutrophil depletion had smaller effects on CCL3 and CCL4 expression compared with normalization of their expression after Kupffer cell depletion, consistent with their production in the latter. Expression of CCR1, CCR2, CXCR1, CXCR2 and CXCR5 was normal under both conditions.
This activation of neutrophils and Kupffer cells after cell transplantation was in agreement with their roles in other forms of liver injury, e.g., ischemia-reperfusion injury, an inevitable additional accompaniment of hepatocyte transplantation.25 This type of liver injury occurs in phases, where Kupffer cells mediate the initial response through proinflammatory cytokines, e.g., TNF-α or IL-6, in association with neutrophils.26 Our data showing greater expression of TNF-α and its receptor, TNFrSF1b, as well as the associated genes Ltb and ABCF1, which participate in TNF-α-induced inflammation,27,28 along with normalization of this TNF-α group only after anti-PMN, indicated that these changes were due to neutrophil activation. Moreover, neutrophils may activate release of TNF-α and IL6 from macrophages.29 Also, TNF-α may induce expression of multiple chemokines by itself from parenchymal cells, e.g., CCL3, CCL4, as well as CCR1 and CCR2, 30 or in cooperation with other cytokines, e.g., TNF-α induced expression of CCL9, CCL10, CCL17, and CCL22 in the presence of IL4 or interferon-γ. 22,23 Therefore, TNF-α is likely to have played a major role in liver inflammation after cell transplantation. Similarly, upregulation of an IL6 receptor along with IL6 signal transducer, and of IL1 receptor along with tollip, 31 should indicate roles for these cytokines. Increased availability of C3 complement component and integrin beta2 was probably helpful in directing chemokines to suitable sites.6,32 Proteolytic cleavages of chemokines by matrix metalloproteinases, which are expressed after cell transplantation,4 may promote their chemokine activity.6
These mechanisms of inflammation should be highly significant for defining organ-specific perturbations after cell transplantation and for guiding strategies in cell and gene therapy. The role of chemokine receptors, such as CCR1, CCR2, CCR5 and CXCR3, in allografts has been studied in knockout mice for tissue (pancreatic islets), epithelium (cornea) or solid organ (cardiac) transplantation.33–35 The precise mechanisms by which the inflammatory environment of the liver following cell transplantation may impair cell engraftment are unknown at present. However, cell isolation with collagenase digestion produces significant perturbations in hepatocytes. While exposure of such hepatocytes to discrete pro-inflammatory cytokines, e.g., TNF-α, by itself, is insufficient for cell necrosis or apoptosis, 36 the combination of multiple cytokine-chemokines in the presence of additional perturbations related to ischemia or other hepatic changes may cause cytotoxicity. Our studies offer several chemokine and cytokine targets that can be directly blocked with drugs to demonstrate changes in cell engraftment and for improving outcomes in cell therapy. Among potential drug targets should include receptors, such as CXCR2 to block neutrophil-specific activities, and CCR1, CCR2 or CCR5 to block Kupffer cell or monocyte-specific activities. Similarly, blockade of TNF-α should offer suitable means to overcome additional inflammatory perturbations. Targeting of specific molecules should produce lesser general toxicity, as observed with the combination of anti-PMN plus GdCl3 in our studies. As chemokines and cytokines are naturally involved in allograft responses through NK, dendritic, antigen-presenting, and T and B cells, transplantation of allogeneic or xenogeneic cells will likely benefit from these insights, although further work will be necessary to establish the significance of additional cytokine-chemokine changes following such cell transplants.
Acknowledgments
Ms. Chaoying Zhang provided technical assistance.
Supported in part by NIH grants R01 DK46952 and P30-DK41296
Footnotes
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu YM, Joseph B, Berishvili E, Kumaran V, Gupta S. Hepatocyte transplantation and drug-induced perturbations in liver cell compartments. Hepatology. 2008;47:279–87. doi: 10.1002/hep.21937. [DOI] [PubMed] [Google Scholar]
- 2.Joseph B, Kumaran V, Berishvili E, Bhargava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44:1411–20. doi: 10.1002/hep.21416. [DOI] [PubMed] [Google Scholar]
- 3.Joseph B, Malhi H, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology. 2002;123:1677–1685. doi: 10.1053/gast.2002.36592. [DOI] [PubMed] [Google Scholar]
- 4.Benten D, Kumaran V, Joseph B, Gupta S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of engraftment. Hepatology. 2005;42:1072–1081. doi: 10.1002/hep.20889. [DOI] [PubMed] [Google Scholar]
- 5.Kumaran V, Joseph B, Benten D, Gupta S. Integrin and extracellular matrix interactions regulate engraftment of transplanted hepatocytes in the rat liver. Gastroenterology. 2005;129:1643–53. doi: 10.1053/j.gastro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 7.Melmed GY, Targan SR, Yasothan U, Hanicq D, Kirkpatrick P. Certolizumab pegol. Nat Rev Drug Discov. 2008;7:641–2. doi: 10.1038/nrd2654. [DOI] [PubMed] [Google Scholar]
- 8.Rajvanshi P, Fabrega A, Bhargava KK, Kerr A, Pollack G, Blanchard J, Palestro CJ, Gupta S. Rapid clearance of hepatocytes from pulmonary capillaries in rats allows development of surrogates for testing safety of liver repopulation. J Hepatol. 1999;30:299–310. [Google Scholar]
- 9.Gupta S, Rajvanshi P, Malhi H, Sokhi RP, Slehria S, Vasa SRG, Dabeva M, Shafritz DA, Kerr A. Cell transplantation causes loss of gap junctions and activates GGT expression permanently in host liver. Am J Physiol Gastroint Liver Physiol. 2000;279:G815–G826. doi: 10.1152/ajpgi.2000.279.4.G815. [DOI] [PubMed] [Google Scholar]
- 10.Koga HF, Aoyagi K, Matsumoto T, Iida M, Fujishima M. Experimental enteropathy in athymic and euthymic rats: synergistic role of lipopolysaccharide and indomethacin. Am J Physiol Gastrointest Liver Physiol. 1999;276:576–582. doi: 10.1152/ajpgi.1999.276.3.G576. [DOI] [PubMed] [Google Scholar]
- 11.Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, Roth RA. Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci. 2003;72:43–56. doi: 10.1093/toxsci/kfg019. [DOI] [PubMed] [Google Scholar]
- 12.Wu YM, Joseph B, Gupta S. Immunosuppression using the mTOR inhibition mechanism affects replacement of the rat liver with transplanted cells. Hepatology. 2006;44:410–419. doi: 10.1002/hep.21277. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S. Hog heaven on the road to liver cell therapy. Gastroenterology. 2007;32:450–453. doi: 10.1053/j.gastro.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Raper SE, Grossman M, Rader DJ, Thoene JG, Clark BJ, 3rd, Kolansky DM, Muller DW, Wilson JM. Safety and feasibility of liver-directed ex vivo gene therapy for homozygous familial hypercholesterolemia. Ann Surg. 1996;223:116–26. doi: 10.1097/00000658-199602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 16.Moreno C, Gustot T, Nicaise C, Quertinmont E, Nagy N, Parmentier M, Le Moine O, Devière J, Louis H. CCR5 deficiency exacerbates T-cell-mediated hepatitis in mice. Hepatology. 2005;42:854–62. doi: 10.1002/hep.20865. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Klintman D, Liu Q, Sato T, Jeppsson B, Thorlacius H. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;75:443–52. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- 18.Rittner HL, Mousa SA, Labuz D, Beschmann K, Schäfer M, Stein C, Brack A. Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain. J Leukoc Biol. 2006;79:1022–32. doi: 10.1189/jlb.0805452. [DOI] [PubMed] [Google Scholar]
- 19.Gaga M, Ong YE, Benyahia F, Aizen M, Barkans J, Kay AB. Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1alpha. Allergy. 2008;63:703–11. doi: 10.1111/j.1398-9995.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Z, Visentin GP, Dayananda KM, Neelamegham S. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 2008;112:1091–100. doi: 10.1182/blood-2008-04-153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, Williams TJ, Pease JE. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol. 2008;83:875–82. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- 22.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–8. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi A, Cantini G, Piscitelli E, Gelmini S, Francalanci M, Mello T, Ceni E, Varano G, Forti G, Rotondi M, Galli A, Serio M, Luconi M. A new mechanism involving ERK contributes to rosiglitazone inhibition of tumor necrosis factor-alpha and interferon-gamma inflammatory effects in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:718–24. doi: 10.1161/ATVBAHA.107.160713. [DOI] [PubMed] [Google Scholar]
- 24.Kuo TC, Shaffer AL, Haddad J, Jr, Choi YS, Staudt LM, Calame K. Repression of BCL-6 is required for the formation of human memory B cells in vitro. J Exp Med. 2007;204:819–30. doi: 10.1084/jem.20062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slehria S, Rajvanshi P, Ito Y, Sokhi R, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology. 2002;35:1320–1328. doi: 10.1053/jhep.2002.33201. [DOI] [PubMed] [Google Scholar]
- 26.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology. 2008 Jul 7; doi: 10.1002/hep.22471. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takasaki J, Kawauchi Y, Masuho Y. Synergistic effect of type II phospholipase A2 and platelet-activating factor on Mac-1 surface expression and exocytosis of gelatinase granules in human neutrophils: evidence for the 5-lipoxygenase-dependent mechanism. J Immunol. 1998;160:5066–72. [PubMed] [Google Scholar]
- 28.Richard M, Drouin R, Beaulieu AD. ABC50, a novel human ATP-binding cassette protein found in tumor necrosis factor-alpha-stimulated synoviocytes. Genomics. 1998;53:137–45. doi: 10.1006/geno.1998.5480. [DOI] [PubMed] [Google Scholar]
- 29.Wilsson A, Lind S, Ohman L, Nilsdotter-Augustinsson A, Lundqvist-Setterud H. Apoptotic neutrophils containing Staphylococcus epidermidis stimulate macrophages to release the proinflammatory cytokines tumor necrosis factor-alpha and interleukin-6. FEMS Immunol Med Microbiol. 2008;53:126–35. doi: 10.1111/j.1574-695X.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 30.Glabinski AR, Bielecki B, Kolodziejski P, Han Y, Selmaj K, Ransohoff RM. TNF-alpha microinjection upregulates chemokines and chemokine receptors in the central nervous system without inducing leukocyte infiltration. J Interferon Cytokine Res. 2003;23:457–66. doi: 10.1089/107999003322277874. [DOI] [PubMed] [Google Scholar]
- 31.Lu HL, Yang CY, Chen HC, Hung CS, Chiang YC, Ting LP. A novel alternatively spliced interleukin-1 receptor accessory protein mIL-1RAcP687. Mol Immunol. 2008;45:1374–84. doi: 10.1016/j.molimm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Vorup-Jensen T, Chi L, Gjelstrup LC, Jensen UB, Jewett CA, Xie C, Shimaoka M, Linhardt RJ, Springer TA. Binding between the integrin alphaXbeta2 (CD11c/CD18) and heparin. J Biol Chem. 2007;282:30869–77. doi: 10.1074/jbc.M706114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdi R, Means TK, Ito T, Smith RN, Najafian N, Jurewicz M, Tchipachvili V, Charo I, Auchincloss H, Jr, Sayegh MH, Luster AD. Differential role of CCR2 in islet and heart allograft rejection: tissue specificity of chemokine/chemokine receptor function in vivo. J Immunol. 2004;172:767–75. doi: 10.4049/jimmunol.172.2.767. [DOI] [PubMed] [Google Scholar]
- 34.Hamrah P, Yamagami S, Liu Y, Zhang Q, Vora SS, Lu B, Gerard CJ, Dana MR. Deletion of the chemokine receptor CCR1 prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2007;48:1228–36. doi: 10.1167/iovs.05-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnickel GT, Bastani S, Hsieh GR, Shefizadeh A, Bhatia R, Fishbein MC, Belperio J, Ardehali A. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180:4714–21. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Lo CR, Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–8. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]