Abstract
OBJECTIVE
To investigate whether L-isoleucine was effective in the treatment of hot flushes and whether L-isoleucine, L-valine, or the combination of both amino acids reduced fasting serum homocysteine.
METHODS
After a 1-week baseline period, 100 postmenopausal women experiencing at least five moderate-severe hot flushes per day were randomized with equal probability to one of four groups (phase 1/phase 2): placebo/L-valine, placebo/L-valine and L-isoleucine, L-isoleucine/L-valine, and L-isoleucine/L-valine and L-isoleucine. Phase 1 was 12 weeks long, and phase 2 was 10 weeks long. Patients took five capsules by mouth, twice a day throughout the study, with each capsule containing 500 mg of compound. Data were obtained from daily hot flush diaries, fasting blood work, and several questionnaires. The primary outcome variable was the percent change in hot flush composite score from baseline to week 12.
RESULTS
In phase 1 of the study, there were no significant differences between the L-isoleucine and placebo groups for any of the outcome measures. At week 12, there was a mean 13.9% decrease in hot flush composite score compared with baseline in the L-isoleucine group and a mean 25% decrease in the placebo group (P=.28). In phase 2 of the study, there was no significant change in fasting serum homocysteine levels associated with any of the amino acid therapies.
CONCLUSION
L-isoleucine therapy appears to be ineffective in the treatment of hot flushes in postmenopausal women. L-isoleucine and L-valine, either alone or in combination, appear to have no effect on fasting serum homocysteine levels.
Level of Evidence: I
Hot flushes affect about 75% of menopausal women1 and are associated with significantly reduced quality of life.2 Hormone replacement therapy had been the treatment of choice for hot flushes until increased risks of breast cancer, heart disease, stroke, and pulmonary embolism were shown to be linked to hormone replacement therapy use.3 Although hormone replacement therapy use in younger postmenopausal women may not affect their risk for heart disease, hormone replacement therapy is associated with increased risk of stroke in any age group of menopause.4 Safe and effective nonhormonal hot flush therapies are needed.
The nonhormonal prescription hot flush therapies that have been shown to be effective for at least 12 weeks based on participants’ daily hot flush records (ie, hot flush diaries) in randomized controlled trials (RCTs) are gabapentin,6,7 desvenlafaxine,8 and extended-release oxybutynin. Gabapentin appears to be as effective as hormone replacement therapy,7 whereas desvenlafaxine appears to be less effective than hormonal therapy as shown in two separate RCTs. Extended-release oxybutynin has not been directly compared with hormone replacement therapy. It has been theorized that gabapentin’s mechanism of action in treating hot flushes involves its affinity to its central nervous system (CNS)CNS correct as written out? binding site, the alpha-2-delta subunit of voltage-gated calcium channels.6 Several large neutral amino acids also have an affinity for this binding site, which is comparable to that of gabapentin. Of the amino acids that are classified as dietary supplements, L-methionine and L-isoleucine have the first and second-highest affinities in mouse cerebral cortex, respectively.9 At the time our study was initiated, elevated serum homocysteine was felt to be a risk factor for cardiovascular disease based on observational studies.10 Because L-methionine therapy was known to elevate serum homocysteine,11,12 L-isoleucine was chosen for further study.
We first performed an uncontrolled, unblinded pilot study involving 15 postmenopausal women experiencing at least five moderate-severe hot flushes a day. Each participant took L-isoleucine 2.5g by mouth, twice a day for 10 weeks. There was an average 62% reduction in hot flush composite score (combination of hot flush frequency and severity) during week 10 as compared with baseline (unpublished data). One participant withdrew from the study after 4 weeks because of hand edema, which is also a known side effect of gabapentin.
There also was an unexpected 11% reduction in fasting serum homocysteine among the 14 participants at week 10 compared with baseline and a 22% reduction among the six participants who had been taking either a multivitamin or a B-complex vitamin throughout the study (unpublished data). We theorized that this effect, if real, may have been mediated by L-isoleucine’s ability to induce the expression of the enzyme methylmalonyl-CoA mutase (MM),13 promoting the metabolism of homocysteine into propionyl-CoA and L-methylmalonyl-CoA, MM’s substrate. The amino acid L-valine also induces the expression of MM13 and is more effective than L-isoleucine in promoting the excretion of methylmalonic acid in human patients with vitamin B12-deficiency.14
We conducted a two-phase RCT in postmenopausal women to investigate whether L-isoleucine was effective in the treatment of hot flushes (phase 1) and whether L-isoleucine, L-valine, or the combination of both amino acids reduced fasting serum homocysteine (phase 2).
MATERIALS AND METHODS
Per journal style, only level-one heads are used in Original Research articles. All other headings have been deleted.
Participants were recruited from a TV segment on a local news channel and from newspaper advertisements. In this two-phase RCT, we enrolled 100 postmenopausal women who were not on hormone replacement therapy for at least 2 months, had baseline serum follicle-stimulating hormone levels higher than 40 mInternational Units/mL, and were experiencing at least five moderate-severe hot flushes per day. The study was approved by the Institutional Review Board at the University at Buffalo, and all participants provided written informed consent to participate.
This was a single site study conducted at the University at Buffalo’s Student Health Center. One hundred women were enrolled between February 2005 and September 2005. After a 1-week baseline period, participants were randomized with equal probability to one of four groups (phase 1/phase 2): placebo/L-valine (n=26), placebo/L-valine and L-isoleucine (n=24), L-isoleucine/L-valine (n=24), and L-isoleucine/L-valine and L-isoleucine (n=26). The computer-generated randomization plan was available only to the programmer at the University of Rochester who generated the plan and the pharmacist at the University at Buffalo who packaged the drug; no other study personnel had access to this plan. Phase 1 was 12 weeks long, and phase 2 was 10 weeks long. All capsules appeared identical and contained 500 mg of compound (Montiff, Inc., Santa Monica, CA). The dosage was five capsules orally twice daily throughout the study. In phase 2, there was an equal mix of L-valine and L-isoleucine in the capsules for the two groups assigned to combination amino acid therapy. Participants were informed during the consent process that there was a 50% chance for placebo during phase 1 of the study and a 0% chance for placebo during phase 2. A placebo group was not included in phase 2 because the main outcome variable for that phase was fasting serum homocysteine, a variable that we felt to be not susceptible to a placebo effect. The exact identities of the amino acids were not revealed to the participants during the study. This was done to help prevent participants from purchasing the amino acids over the counter and taking them during the study. All participants also were provided with, and instructed to take, one Equate Multivitamin (Wal-Mart, Bentonville, AR) and one B-complex vitamin (0.5 mg methylcobalamin, 0.6 mg folic acid, 0.6 mg biotin, 7 mg pyridoxine hydrochloride; Montiff, Inc., Santa Monica, CA) every day. These vitamins and dosages were chosen based on our pilot study, in which they appeared to maximize the potential serum homocysteine-lowering effects of L-isoleucine therapy.
Participants completed daily hot flush diaries for a 1-week period before the baseline visit, during weeks 1–4 and 8–12 during phase 1 of the trial, and again during weeks 16–17 and 21–22 during phase 2 of the trial. Participants were instructed to keep the diary with them and record each hot flush after it occurred as mild, moderate, or severe.15 Night sweats also were recorded. Participants mailed each completed diary directly to the General Clinical Research Center at the University of Rochester, Rochester, NY. The following questionnaires also were completed at baseline, week 12, and week 22: the Pittsburgh Sleep Quality Index,16 the Patient Perceived Hot Flash Score (PPHFS),17 and the Greene Climacteric Scale.18 Global Satisfaction with Treatment was assessed at weeks 12 and 22 using a numerical scale ranging from 1 (“extremely dissatisfied”) to 10 (“extremely satisfied”). Laboratory tests were assayed in the fasting state, and weight, blood pressure, and heart rate were assessed at enrollment, week 12, and week 22. Blood samples to be assayed for homocysteine were immediately placed in a 4°C refrigerator and centrifuged within 1 hour of the draw. The serum was then immediately poured into a separate tube, and homocysteine was assayed the same day. Laboratory testing was performed at Kaleida Health Laboratories, Buffalo, NY, and at Strong Memorial Hospital, Rochester, NY. Adverse events were assessed by the PIWhat does PI stand for? at each participant visit by inquiry.
The primary outcome measure was percent change in hot flush composite score from baseline to week 12 as determined by participant s’ daily hot flush diaries. The hot flush composite score combines frequency and severity into one score by assigning the numbers 1, 2, and 3 to mild, moderate, and severe hot flushes, respectively, and then summing across all hot flushes. Secondary outcome variables included percent change in hot flush frequency (number of hot flushes per day) from baseline to week 12, changes from baseline to week 12 in the PPHFS, Pittsburgh Sleep Quality Index Global Score, Greene Climacteric Scale Total Score and subscale scores, homocysteine level, weight, vital signs, and laboratory test results, and Global Satisfaction with Treatment at week 12. Changes from baseline to week 22 and from week 12 to week 22 also were considered in the statistical analyses.
For phase 1, the statistical analyses were performed according to the intention-to-treat principle and included data from all randomized participants. The hot flush diary outcomes were analyzed using a repeated measures analysis of variance model that included terms for treatment group (L-isoleucine, placebo), week (categorical), and the interaction between treatment group and week. This allowed estimation of the treatment effects separately for each week (including the primary time point at week 12) along with associated 95% confidence intervals (CIs). All available data from all randomized participants were included in these analyses, with maximum likelihood used to estimate the model parameters of interest in the setting of missing data.19 Changes from baseline to week 12 in the other outcome variables were compared between the L-isoleucine and placebo groups using Wilcoxon rank-sum tests, with missing data at week 12 imputed with the last available observation. Treatment effects were estimated using the Hodges-Lehmann estimates of the group differences in population medians and their associated 95% CIs.20
For phase 2, the significance of within-group changes in homocysteine level from week 12 to week 22 was examined using Wilcoxon signed rank tests. Pairwise comparisons among the treatment groups were performed using Wilcoxon rank-sum tests. Similar analyses were performed for the other outcome variables in phase 2. The statistical analyses for phase 2 were exploratory and did not use imputation for missing data.
The sample size of 100 participants was chosen to provide 80% power to detect a group difference (L-isoleucine–placebo) in mean response of 22% for the primary outcome variable of percent change from baseline to week 12 in hot flush composite score, using a t test and a two-tailed significance level of 5%. The chosen effect size of 22% was based on that observed in our earlier trial of gabapentin6 and the hypothesis that L-isoleucine and gabapentin have similar mechanisms of action. The calculation also assumed a standard deviation of 39% for the primary outcome variable based on results from the gabapentin trial.6
All statistical analyses were performed through the General Clinical Research Center at the University of Rochester.
RESULTS
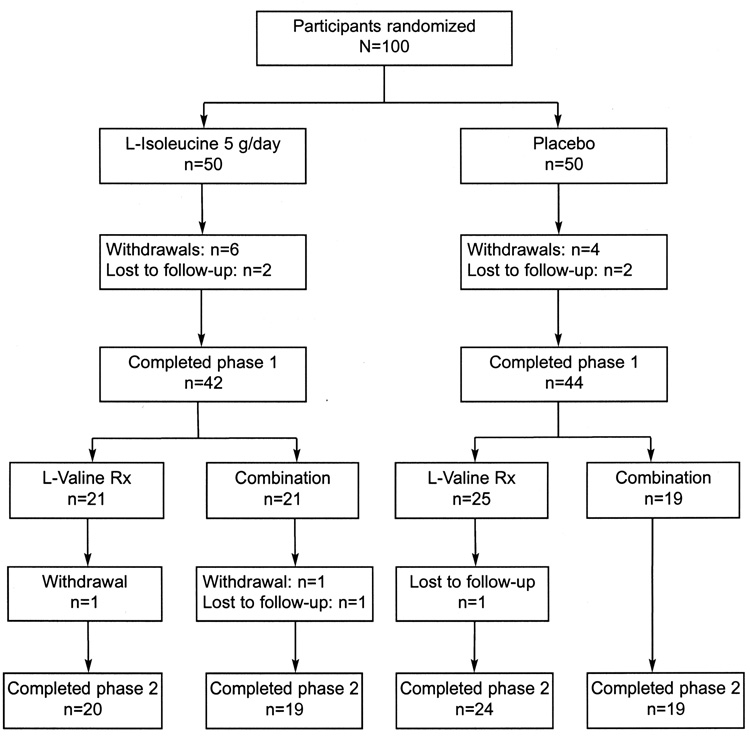
The baseline characteristics are summarized in Table 1 by treatment received in phase 1. The L-isoleucine and placebo groups were generally comparable, with the placebo group having a slightly higher hot flush frequency at baseline. Fourteen participants withdrew from phase 1 of the study. Five participants assigned to L-isoleucine treatment withdrew because of adverse events (herpes zoster and edema, arthralgias, stroke, infection, and nausea), one withdrew due to lack of efficacy (hot flushes), and two were lost to follow-up (Fig. 1). Six participants assigned to the placebo group withdrew from the study, one because of an adverse event (hypertension), one due to lack of efficacy (hot flushes), two for no reported reason, and two were lost to follow-up (Fig. 1). The most common phase 1 adverse events were nausea, which occurred in six participants (four on L-isoleucine and two on placebo), and edema, which occurred in five participants (three on L-isoleucine and two on placebo). The patient who experienced a stroke had a history of hypertension. Four participants withdrew from phase 2 of the study, two for myalgias and edema (one on L-valine and one on combination treatment) and two who were lost to follow-up (one on L-valine and one on combination treatment) (Fig. 1).
Table 1.
Participant Characteristics at Baseline by Treatment Assigned for Phase 1
| Characteristic | L-Isoleucine (n=50) | Placebo (n=50) |
|---|---|---|
| Age (y) | 56.1 (6.4) | 55.7 (6.7) |
| Daily HF frequency | 9.4 (3.1) | 10.9 (4.0) |
| Daily moderate and severe HF frequency | 7.5 (3.3) | 8.1 (4.0) |
| Daily HF composite score | 18.7 (7.0) | 21.6 (8.9) |
| Duration of amenorrhea (mo)* | 78.0 (24.0–124.0) | 66.0 (24.0–144.0) |
| Duration experiencing hot flushes (mo)* | 60.0 (30.0–120.0) | 48.0 (17.0–120.0) |
| Surgical menopause, n (%) | 15 (30%) | 18 (36%) |
| Non-Hispanic white, n (%) | 46 (92%) | 48 (96%) |
| Non-Hispanic black, n (%) | 4 (8%) | 2 (4%) |
| PPHFS | 6.1 (2.4) | 6.3 (2.1) |
| PSQI Global Score | 9.0 (3.3) | 9.9 (3.8) |
| GCS Total Score | 19.7 (8.2) | 19.4 (8.0) |
| Fasting serum homocysteine (µmol/L) | 9.7 (2.9) | 9.6 (3.3) |
| FSH (mInternational Units/mL) | 88.6 (33.2) | 96.7 (35.8) |
| LH (mInternational Units/mL) | 39.1 (14.6) | 39.6 (14.4) |
| Estradiol (pg/mL) | 16.3 (7.2) | 15.8 (6.1) |
HF, hot flush; PPHFS, Patient Perceived Hot Flash Score; PSQI, Pittsburgh Sleep Quality Index; GCS, Greene Climacteric Scale; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Values are mean (standard deviation) unless otherwise indicated.
Reported as median (25th percentile–75th percentile).
Fig. 1.
Flow diagram of participants. Combination, combination L-isoleucine and L-valine treatment.
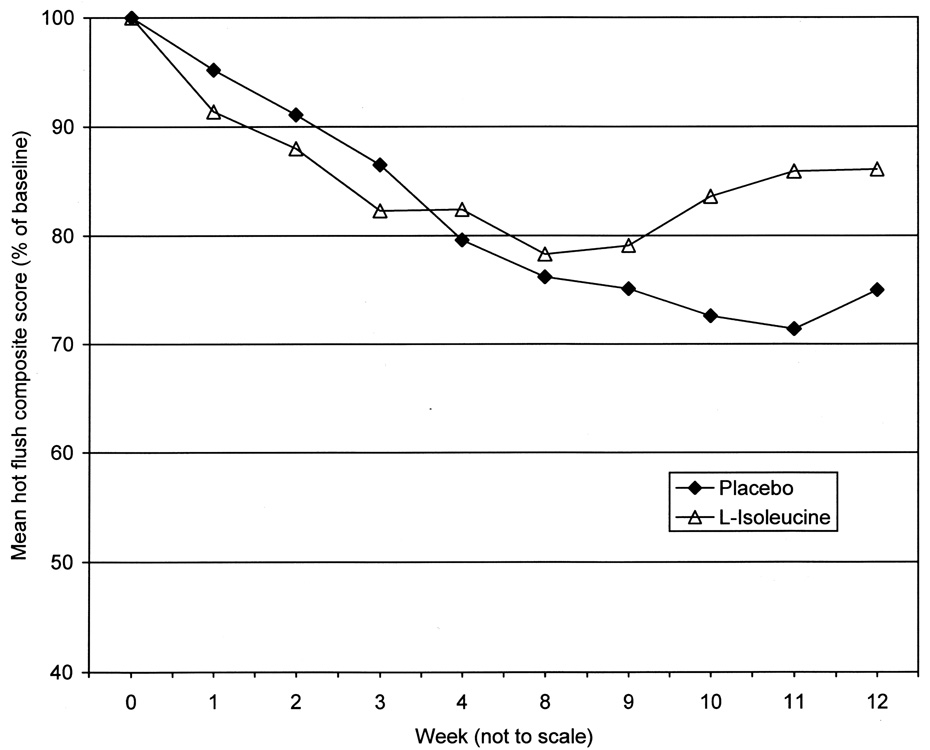
In phase 1 of the study, there were no significant differences between the L-isoleucine and placebo groups for any of the outcome variables (Fig. 2 and Table 2). For the hot flush composite score, the mean percent changes from baseline to week 12 (estimated from the repeated measures analysis of variance model) were −25.0% in the placebo group and −13.9% in the L-isoleucine group (treatment effect=11.0%, 95% CI −9.0 to 31.1%, P=.28). For hot flush frequency, the mean percent changes from baseline to week 12 (estimated from the repeated measures analysis of variance model) were −25.4% in the placebo group and −16.3% in the L-isoleucine group (treatment effect=9.1%, 95% CI −7.4 to 25.6%, P=.28).
Fig. 2.
Hot flush composite scores by week from study phase 1.
Table 2.
Treatment Effects on Primary and Secondary Outcome Variables from Study Phase 1
| Variable | L-Isoleucine (n=50) | Placebo (n=50) | Treatment Effect* | 95% CI | P |
|---|---|---|---|---|---|
| Hot flush frequency (% change) | −14.1 (44.6) | −26.4 (34.1) | 9.1 | (−7.4–25.6) | .28 |
| Hot flush composite score (% change) | −11.3 (57.1) | −27.4 (37.5) | 11.0 | (−9.0–31.1) | .28 |
| PPHFS | −0.74 (2.19) | −0.82 (2.65) | 0.00 | (−1.00–1.00) | .77 |
| PSQI Global Score | −0.26 (2.93) | −0.40 (2.78) | 0.00 | (−1.00–1.00) | .81 |
| GCS Total Score | −1.28 (5.65) | −0.88 (3.73) | 0.00 | (−2.00–0.00) | .52 |
| Global Satisfaction with Treatment | 4.37 (3.03) | 5.05 (2.91) | 0.00 | (−2.00–0.00) | .29 |
| Homocysteine (µmol/L) | −1.61 (2.78) | −1.89 (3.08) | 0.00 | (−1.20–0.80) | .90 |
| Weight (kg) | −0.36 (1.66) | −0.24 (1.29) | 0.00 | (0.00–1.00) | .87 |
CI, confidence interval; PPHFS, Patient Perceived Hot Flash Score; PSQI, Pittsburgh Sleep Quality Index; GCS, Greene Climacteric Scale.
Values are presented as mean (standard deviation) change from baseline except for Global Satisfaction with Treatment, for which the mean score at week 12 is presented.
Treatment effect is estimated from a repeated measures analysis of variance model for the hot flush diary outcomes and is the Hodges-Lehmann estimate of the group difference (L-isoleucine–placebo) in population medians for the other outcome variables; see text for details.
In phase 2 of the study, there were no significant changes from week 12 to week 22 in fasting serum homocysteine in any of the groups (Table 3), nor were there any significant between-group differences in these changes. Although within-group changes from week 12 to week 22 were apparent for the hot flush outcomes (Table 3), no differences between the groups were noted. Also, there was no placebo group in phase 2.
Table 3.
Changes from Week 12 to Week 22 in Outcome Variables (Study Phase 2)
| Variable | Placebo/L-Valine (n=24) | Placebo/Combination (n=19) | L-Isoleucine/L-Valine (n=20) | L-Isoleucine/Combination (n=19) |
|---|---|---|---|---|
| Homocysteine (µmol/L) | −0.41 (1.51) (P=.21) | −0.54 (3.03) (P=.31) | −0.37 (1.99) (P=.71) | −0.60 (2.41) (P=.42) |
| Hot flush frequency (% change) | −31.5 (39.8) (P=.0007) | −32.1 (39.3) (P=.004) | −23.3 (48.4) (P=.03) | −20.7 (50.0) (P=.03) |
| Hot flush composite score (% change) | −34.0 (41.1) (P=.0006) | −31.5 (46.5) (P=.02) | −29.1 (52.1) (P=.008) | −25.3 (44.4) (P=.02) |
| PPHFS | −1.25 (2.31) (P=.02) | −1.95 (2.76) (P=.004) | −1.20 (2.86) (P=.08) | −1.58 (2.52) (P=.02) |
| PSQI Global Score | −0.18 (3.39) (P=.99) | −1.27 (5.09) (P=.43) | −0.82 (4.14) (P=.43) | −0.56 (2.19) (P=.28) |
| GCS Total Score | −1.65 (5.06) (P=.09) | −4.08 (6.40) (P=.04) | −2.79 (6.83) (P=.07) | 0.00 (3.76) (P=.86) |
| Global Satisfaction with Treatment | 1.79 (3.60) (P=.03) | 1.11 (3.91) (P=.35) | 1.84 (4.00) (P=.08) | 1.89 (3.30) (P=.03) |
Combination, combination L-isoleucine and L-valine treatment; PPHFS, Patient Perceived Hot Flash Score; PSQI, Pittsburgh Sleep Quality Index; GCS, Greene Climacteric Scale.
Values are presented as mean (standard deviation) change from baseline. The P value in each group is for a test of significance of the change from week 12 to week 22 within that group (Wilcoxon signed rank test). No significant differences between groups were found (Wilcoxon rank sum tests).Per journal style, P values should be expressed to no more than the third decimal. Please restate the second and third P values in column 2.
DISCUSSION
This study demonstrated that L-isoleucine monotherapy is ineffective in the treatment of hot flushes in postmenopausal women. Although L-isoleucine, like gabapentin, binds to the theoretical therapeutic CNS target known as the alpha-2-delta subunit of voltage-gated calcium channels, it does so with about 40% less affinity than gabapentin.9 The L-isoleucine dosage used in this study was much higher than the gabapentin dosage used in previous hot flush studies; however, there is saturable transport of the large neutral amino acids across both the gut and the blood-brain-barrier, limiting the concentration of L-isoleucine reaching the CNS target regardless of the dosage.21,22 Also, L-isoleucine may have different physiological effects at this binding site than does gabapentin. The promising results of our open-label study highlight the necessity of evaluating potential hot flush therapies in a randomized, controlled trial design.
The significant reductions in hot flushes observed in all groups during phase 2 of the study may all be due to a placebo effect; however, this cannot be verified because there was no placebo group during phase 2. The magnitude of these reductions was within the placebo effect range shown in previous hot flush trials.6,7 The placebo effect also could explain the apparent changes in PPHFS and Global Satisfaction with Treatment from week 12 to week 22 (Table 3).
A possible explanation for the lack of effects of the amino acids on serum homocysteine in this study, in contrast to the pilot study, may be the more rigorous processing protocol of the blood samples in this study. Serum homocysteine can be falsely elevated if the blood sample is not immediately refrigerated and the serum separated from the cellular component within 1 hour of the draw. It is possible that the baseline homocysteine levels from the pilot study were falsely elevated from improper sample processing, whereas the on-treatment samples were correctly processed. This would have given a spurious association in the pilot study of decreased homocysteine levels with L-isoleucine therapy.
After the initiation of this RCT, two large RCTs were published23,24 and one was presented (WAFACS) at the American Heart Association 2006 Scientific Sessions, all showing that lowering serum homocysteine by 18–27% through the use of B-vitamin therapy leads to no cardiovascular benefit. These three RCTs, in addition to one previously published RCT,25 cumulatively enrolled more than 18,000 participants. The consistent negative findings among all four RCTs have cast significant doubt on a causative role of homocysteine in cardiovascular disease. This has encouraged us to revisit L-methionine therapy in the treatment of hot flushes. L-methionine has the same affinity as gabapentin for the proposed CNS therapeutic target,9 which may translate into improved efficacy.
In conclusion, L-isoleucine therapy appears to be ineffective in the treatment of hot flushes in postmenopausal women; L-isoleucine and L-valine, either alone or in combination, appear to have no effects on fasting serum homocysteine levels.
Acknowledgments
Supported by grant number 1K23-AT-1709-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and by grant number 5 MO1 RR00044 from the National Center for Research Resources (NCRR). Both centers are components of the National Institutes of Health (NIH). This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM, NCRR, or NIH.
Financial Disclosure
Dr. Guttuso is the inventor on US Patent 6,310,098, which is owned by the University of Rochester, covering the use of gabapentin and related compounds for treating hot flushes.
Footnotes
This patent has been licensed to three companies. The other authors have no potential conflicts of interest to disclose.
CLINCIAL TRIAL REGISTRATION:
Clinicaltrials.gov, www.clinicaltrials.gov, NCT00081952
References
- 1.Hammar M, Berg G, Fåhraeus L, Larsson-Cohn U. Climacteric symptoms in an unselected sample of Swedish women. Maturitas. 1984;6:345–350. doi: 10.1016/0378-5122(84)90007-0. [DOI] [PubMed] [Google Scholar]
- 2.Blumel JE, Castelo-Branco C, Binfa L, Gramegna G, Tacla X, Aracena B. Quality of life after the menopause: a population study. Maturitas. 2000;34:17–23. doi: 10.1016/s0378-5122(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause [published erratum appears in JAMA 2008;288:1426] JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 5.Ockene JK, Barad DH, Cochrane BB, Larson JC, Gass M, Wassertheil-Smoller S. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. Please cite reference 5 in order in the text or delete it from the list‥. [DOI] [PubMed] [Google Scholar]
- 6.Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 7.Reddy SY, Warner H, Guttuso T, Jr, Messing S, DiGrazio W, Thornburg L. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41–48. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- 8.Speroff L, Gass M, Constantine G, Olivier S. Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol. 2008;111:77–87. doi: 10.1097/01.AOG.0000297371.89129.b3. [DOI] [PubMed] [Google Scholar]
- 9.Thurlow RJ, Brown JP, Gee NS, Hill DR, Woodruff GN. [3H]gabapentin may label a system-L-like neutral amino acid carrier in brain. Eur J Pharmacol. 1993;247:341–345. doi: 10.1016/0922-4106(93)90204-m. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 11.van der Griend R, Biesma DH, Banga JD. Postmethionine-load homocysteine determination for the diagnosis hyperhomocysteinaemia and efficacy of homocysteine lowering treatment regimens. Vasc Med. 2002;7:29–33. doi: 10.1191/1358863x02vm407ra. [DOI] [PubMed] [Google Scholar]
- 12.Ditscheid B, Fünfstück R, Busch M, Schubert R, Gerth J, Jahreis G. Effect of L-methionine supplementation on plasma homocysteine and other free amino acids: a placebo-controlled double-blind cross-over study. Eur J Clin Nutr. 2005;59:768–775. doi: 10.1038/sj.ejcn.1602138. [DOI] [PubMed] [Google Scholar]
- 13.Narasimhan P, Sklar R, Murrell M, Swanson RA, Sharp FR. Methylmalonyl-CoA mutase induction by cerebral ischemia and neurotoxicity of the mitochondrial toxin methylmalonic acid. J Neurosci. 1996;16:7336–7346. doi: 10.1523/JNEUROSCI.16-22-07336.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gompertz D, Jones JH, Knowles JP. Metabolic precursors of methylmalonic acid in vitamin B12 deficiency. Clin Chim Acta. 1967;18:197–204. doi: 10.1016/0009-8981(67)90157-x. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. [Accessed July 6, 2007];Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms- recommendations for clinical evaluation. Available at: http://www.fda.gov/cder/guidance/5412dft.pdf.
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Evans ML, Pritts E, Vittinghoff E, McClish K, Morgan KS, Jaffe RB. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105:161–166. doi: 10.1097/01.AOG.0000147840.06947.46. [DOI] [PubMed] [Google Scholar]
- 18.Barentsen R, van de Weijer PH, van Gend S, Foekema H. Climacteric symptoms in a representative Dutch population sample as measured with the Greene Climacteric Scale. Maturitas. 2001;38:123–128. doi: 10.1016/s0378-5122(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken (NJ): John Wiley and Sons; 2002. [Google Scholar]
- 20.Hettmansperger T. Statistical inference based on ranks. New York (NY): John Wiley and Sons; 1984. [Google Scholar]
- 21.Lerner J, Larimore DL. Comparative aspects of the apparent Michaelis constant for neutral amino acid transport in several animal tissues. Comp Biochem Physiol B. 1986;84:235–248. doi: 10.1016/0305-0491(86)90071-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 23.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 24.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M. Homocysteine lowering with folic acid and B vitamins in vascular disease [published erratum appears in N Engl J Med 2006;355:746] N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 25.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]