Abstract
In a previous study using male rats, a correlation was found between the development of “at-level” allodynia in T6-7 dermatomes following severe T8 spinal contusion injury and the sparing of some myelinated axons within the core of the lesion epicenter. To further test our hypothesis that this sparing is important for the expression of allodynia and the supraspinal plasticity that ensues, an injury that severs all axons (i.e., a complete spinal cord transection) was made in 15 male rats. Behavioral assessments were done at level throughout the 30-day recovery period followed by terminal electrophysiological recordings (urethane anesthesia) from single medullary reticular formation (MRF) neurons receiving convergent nociceptive inputs from receptive fields above, at, and below the lesion level. None of the rats developed signs of at-level allodynia (versus 18 of 26 male rats following severe contusion). However, the terminal recording (206 MRF neurons) data resembled those obtained previously post-contusion. That is, there was evidence of neuronal hyper-excitability (relative to previous data from intact controls) to high- and low-threshold mechanical stimulation for “at-level” (dorsal trunk) and “above-level” (eyelids and face) cutaneous territories. These results, when combined with prior data on intact controls and severe/moderate contusions, indicate that (1) an anatomically incomplete injury (some lesion epicenter axonal sparing) following severe contusion is likely important for the development of allodynia and (2) the neuronal hyper-excitability at the level of the medulla is likely involved in nociceptive processes that are not directly related to the conscious expression of pain-like avoidance behaviors that are being used as evidence of allodynia.
Key words: medullary reticular formation, pain, spinal cord injury
Pain is considered by individuals with spinal cord injury (SCI) as one of the areas of highest priority (Anderson, 2004). The prevalence of pain among SCI patients varies across different studies, ranging from more than 25% (Johnson et al., 1998) up to 81% (Siddall et al., 2003), with an average estimated at 69% (Bonica, 1991). Classification of SCI pain by the International Association for the Study of Pain includes nociceptive (musculoskeletal and visceral) and neuropathic (above, at, and below lesion level) types of pain (Siddall et al., 1997). About 40% of the patients have neuropathic pain (Siddall et al., 2003; Werhagen et al., 2004), which could be spontaneous or mechanically evoked by normally innocuous mechanical stimulation (allodynia) (Hubscher and Johnson, 1999a; Drew et al., 2001).
A number of studies have shown a positive correlation between the presence/absence of “at-level” allodynia post-contusion injury and neuronal hyper-excitability to innocuous and noxious mechanical stimulation in at-level dermatomes both within the dorsal horn immediately rostral to the injury (wide dynamic range/spinothalamic neurons [Drew et al., 2001; Crown et al., 2005]) and supraspinally in the medullary reticular formation (MRF) (Hubscher and Johnson, 1999a) and thalamus (Gerke et al., 2003; Hubscher and Johnson, 2006). In a recent study using male rats, we also found a positive correlation between both the behavioral and electrophysiological (thalamic recordings 30 days post-injury) evidence of allodynia and the sparing of some myelinated axons within the core of the lesion epicenter (Hubscher and Johnson, 2006).
In the present study, an injury that severs all axons (i.e., a complete spinal cord transection) was made to further test our hypothesis that this core epicenter sparing is important for the expression of allodynia and the supraspinal plasticity that ensues. Fifteen male Wistar rats, 90 days of age, were completely spinalized at the T8 spinal level 30 days before terminal electrophysiological experiments. Animals were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg), injected intraperitonealy. Our standard SCI surgery procedures included administration of 0.5 mL of dual penicillin (Ambi Pen®; The Butler Company, Columbus, OH) prior to surgery, 5 mg/kg gentamicin (Abbott Laboratories, North Chicago, IL) once per day for 5 days post-surgery (to control for possible bladder infection) and 2.5 mg/kg of ketoprofen (Ketofen®; Fort Dodge Laboratories, Fort Dodge, IA) once per day for 2 days to alleviate post-surgical pain.
All surgeries were done under aseptic conditions, and the body temperature was controlled throughout the surgery and recovery period. A T7 laminectomy was performed to expose the underlying T8 spinal cord, and the dura was incised and reflected laterally. The spinal cord was cut using a pair of surgical microdissecting scissors. Gentle suction was used as needed to elevate the cut stump to verify the completion of the lesion (Kaddumi and Hubscher, 2007). Gelfoam soaked in thrombin was placed in the lesion cavity. The incision was closed using 4-0 nylon suture for the muscle layers and fascia, and surgical clips for the skin. The urinary bladder was expressed every 8 h.
Beginning 2 weeks post-injury (the period during which SCI pain is known to develop), the dorsolateral trunk was examined for sensitivity to mechanical stimulation in the at-level (T5-T8) dermatomes (Hubscher and Johnson, 2006) by stroking with a no. 5 paint brush (1.5 × 0.5 cm bristles, with an average pressure of 17 g). Avoidance behaviors, including efforts to escape or grab/push the stimulus probe away with the forelimbs (with or without vocalization), were taken as evidence of an allodynia-like response. An animal was considered sensitive to touch if it responded to at least 60% of the stimuli in a given session (minimum of five stimuli) for at least two sessions beyond the initial 2 weeks (Hubscher and Johnson, 2006). All avoidance responses to brush, if present, were assessed further for threshold values using a set of Semmes-Weinstein monofilaments (20 filament set, 15 of which are in the range of 0.008–15 g; obtained from Stoelting Co., Wood Dale, IL).
At 30 days post-transection, each animal was anesthetized with 50% urethane (1.2 g/kg), and the jugular vein, carotid artery, and trachea were exposed and intubated for anesthetic supplement (5% urethane, as needed), blood pressure monitoring, and respiratory rate/end expired pCO2 level monitoring, respectively. Body temperature was monitored throughout the experiment by an esophageal probe connected to a thermometer and maintained at around 37°C using a circulating water-heating pad. After mounting the animal onto a stereotaxic device, a dorsal incision was made to gain access to the brainstem. The dorsal surface of rostral medulla was exposed by removing part of the occipital bone and suctioning the caudal midline portion of the overlying cerebellum (Hubscher and Johnson, 1996, 1999b).
A tungsten microelectrode with ≈7 ± 1 MOhms impedance (Fredrich Haer and Co., Bowdoinham, ME) was lowered from the dorsal surface of the brainstem with a motorized drive (Fredrich Haer and Co.) into the MRF. Stereotaxic coordinates were 3400 μm rostral to obex, and 400 and 800 μm lateral to midline on both sides of the brainstem (two tracks per animal collectively). The search area for each dorso-ventral track was 2800–3000 μm in length, which covered the rostral part of the nucleus reticularis gigantocellularis (Gi), Gi pars alpha (GiA), and the medial part of the lateral paragigantocellular nucleus (LPGi) (Hubscher and Johnson, 1999a).
To insure, as in our previous studies, that the same population of somatovisceral convergent neurons were being sampled in our current experiments, pinching the ear was used as our search stimulus (many of these neurons respond to stimulation of the entire body surface, and 100% respond to ear pinch, as previously described [Hubscher and Johnson, 1999a, 2006]). Recording of a single neuron was established by monitoring the action potential on an oscilloscope with a spike-triggered analog delay module for discrimination of somato-dendritic neuron profiles from nerve fiber spikes as described previously (Hubscher and Johnson, 1996, 1999a, 2006). Once a single neuron responsive to ear pinching was found, responses to mechanical (stroke and pinch) stimuli of the dorsal trunk and to other above level somatic areas (eyelids and face) were tested. MRF neuronal responses were recorded to videotape and analyzed offline using Data Wave software (www.dwavetech.com). Onset and offset of the mechanical stimuli were recorded to tape on a second channel with an external manual trigger device which generated an electrical timing pulse.
At the end of the experiment each animal was perfused transcardially using 0.9% normal saline followed by 4.0% paraformaldehyde. Brainstems were removed and sectioned at 100 μm thickness on the vibratome and stained with cresyl violet. The electrophysiological tracks were identified in these sections under the light microscope and the location of each track was confirmed (Hubscher and Johnson, 1999a, 2006) and reconstructed (Paxinos and Watson, 1998). The spinal cord containing the lesion area was extracted from the transected animals and sectioned sagittally at 18 μm thickness in the cryostat and stained with both luxol fast blue and cresyl violet (Kluver-Barrera Method). The sections were viewed under the light microscope to confirm the completion of the spinal cord transection. Data was analyzed for significance using chi-square test with p < 0.05 level of significance. All animal procedures were reviewed and approved by the Institutional Animal Use and Care Committee, University of Louisville School of Medicine.
None of the 15 rats met our avoidance criteria that would indicate sensitivity to touch (allodynia) “at level,” as tested up to 30 days following transection. One of the 15 rats, however, vocalized to an 8- and 60-g monofilament (left and right side at level, respectively), but did not show any avoidance responses. Four of the 15 rats showed evidence of excessive grooming below level beginning 2–3 weeks post-injury. Three had very small spotty patches of sores, one rat on just the abdomen (bilateral), one rat on the abdomen and inner thigh (left side only), and the other rat on just the lower abdomen and inner thigh (bilateral). The fourth rat had two large sores angled from midline outward on the ventral side of the body on the left side only; one close to but below the lesion level transitional zone (T8-9 region), which measured 3.0 × 0.5 cm, and the other caudal to that (T11-12 region; 2.5 × 0.5 cm).
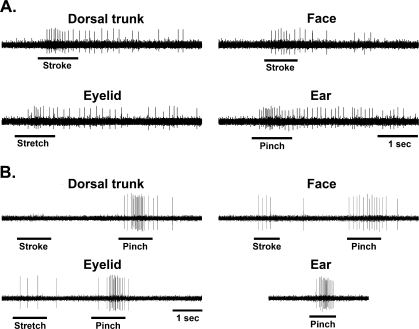
A total of 206 MRF neurons were found responsive to the search stimulus, pinching the ear. The neuronal characteristics of these neurons did not differ significantly (χ2, p > 0.05) from intact controls (Kaddumi and Hubscher, 2006). Most neurons were excitatory (73.2%) and many had spontaneous discharges at rest (36%), with a mean activity rate of 17.5 ± 1.3 spikes/sec. There was a significant (χ2, p < 0.05) increase in the percentage of MRF neurons responding to mechanical stimulation of different somatic areas (face, eyelid, and dorsal trunk above the level of spinal cord transection) compared to our previous findings using intact rats. In addition, there was a significant increase in the percentage of MRF neurons responding to just low-threshold levels of stimulation (touch/gentle pressure) for all above level areas tested. A summary of the MRF neuronal responses to stimulation of the different somatic regions in normal versus spinalized animals is presented in Table 1. Examples of responses recorded in two MRF neurons to high- and low-threshold stimuli are shown in Figure 1.
Table 1.
Summary of Medullary Reticular Formation (MRF) Responses
| Trunk | Face | Eyelid | |
|---|---|---|---|
| (A) Percentage of responsive MRF neurons: | |||
| Normala | 68.1% | 40.4% | 85.1% |
| Tx | 93.7%* | 76.7%* | 97.1%* |
| (B) Percentage of MRF neurons in A responsive to low threshold stimuli: | |||
| Normala | 17.2% | 7.9% | 27.5% |
| Tx | 42.0%* | 26.0%** | 57.0%* |
Kaddumi and Hubscher, 2006.
Significantly different (χ2, p < 0.001) than normal.
Significantly different (χ2, p < 0.025) than normal.
FIG. 1.
Typical examples of stimulus-response characteristics in medullary reticular formation (MRF) neurons from spinally transected animals. (A) Example of excitatory responses of a single MRF neuron located in the left nucleus reticularis gigantocellularis pars alpha to low-threshold stimulation of dorsal trunk, face and eyelid. As shown, this neuron responded to gentle pinching of the ear (search stimulus). (B) Example of excitatory responses of another single MRF neuron located in the left nucleus reticularis gigantocellularis to high-threshold stimulation of dorsal trunk, face, and eyelid. This neuron did not respond to low-threshold stimulation of trunk but responded to low-threshold stimulation of the face and eyelid. Horizontal bars indicate the onset and duration of the mechanical stimulus.
The 24.8% increase in the proportion of dorsal trunk touch-responsive MRF neurons (indicating hyper-excitability) after spinal cord transection is similar to the 29% increase seen previously in the MRF of spinally contused rats showing signs of allodynia (Hubscher and Johnson, 1999a). Our spinally transected rats, however, did not show any behavioral signs of allodynia, while 69% of the contused animals developed allodynia after injury (Hubscher and Johnson, 1999a, 2006). These results support the idea that at least some intact spinal axons traversing the site of injury are a requisite for the development and maintenance of at-level allodynia (Johnson and Hubscher, 2002; Hubscher and Johnson, 2006). Although chronic transitional zone pain is more frequent in patients with “clinically complete” injuries (Beric, 1999), as is the case for rats with “severe” contusions (Hubscher and Johnson, 1999a; Lindsey et al., 2000), studies show (Finnerup et al., 2004) that many of these patients labeled as “clinically complete” are really anatomically incomplete (i.e., discomplete) (Sherwood et al., 1992), raising the potential of sub-clinical functions for residual ascending and descending spinal tracts. This notion is consistent with our demonstration of residual myelinated axons at the lesion epicenter (Hubscher and Johnson, 2006). Those rats all had “at-level” allodynia, but no electrophysiological evidence (based on thalamic recordings) of any functional ascending sensory pathways from “below-level” territories. An alternate explanation is that “at-level” allodynia in rats with severe contusion may result from local ischemia. Although we did not directly measure the degree of ischemia, this possibility is not likely since ischemia will occur in all rats with severe contusion injuries (anatomically discomplete), and we have shown in several studies that allodynia develops in only about 70% (Hubscher and Johnson, 1999a; 2006). Other groups have also found that not all rats develop allodynia, and likewise, not all SCI humans experience neuropathic pain.
The disconnection between the behavioral and electro-physiological data suggests that neuronal changes in MRF (i.e., Gi, GiA, and LPGi) may not be directly associated with the circuitries that are important for the expression of avoidance to gentle mechanical touch. A similar disconnection was obtained previously for neurons in the intralaminar nuclei of the thalamus, which receives input from the MRF (Peschanski and Besson, 1984; Jones and Yang, 1985; Peschanski and Ralston, 1985). As with MRF neurons, those in the intralaminar regions had a 23.3% increase in the number of neurons responding to touch “at-level” post-contusion regardless of the presence/absence of behavioral signs of allodynia (Hubscher and Johnson, 2006). Similar increases in sensitivity found in neurons residing in other thalamic nuclei (within ventral and posterior subregions) were, however, positively correlated with the presence of allodynia. Since the thalamic intralaminar region (like the MRF) has been implicated in contributing to arousal and vigilance (Kinomura et al., 1996; Bester et al., 1999), it is possible that the plasticity within these and other CNS areas are related to other aspects of sensation that we are not testing for. Alternatively, since these two nuclear regions project into limbic regions including the anterior cingulate cortex (ACC), a region associated with the motor avoidance response to a painful stimulus (Johansen et al., 2001), enhanced neuronal firing patterns may provide input into the motivational-affective dimension of pain but the ACC requires input from the sensory-discriminitive component pathways or some other input from the spared spinal axons in order to give rise to a stimulus-oriented motor response.
In terms of surviving circuitries contributing to neuropathic pain “at level” following contusion injury, behavioral evidence of allodynia was correlated to novel responses to touch/gentle pressure of “at-level” dermatomes for ventral and posterior thalamic neurons which normally receive widespread nociceptive-specific somatovisceral convergent inputs (Hubscher and Johnson, 2006) and are integral in pain perception pathways. The partial loss of many below-level inputs to individual groups of neurons that normally receive widespread (often whole body) inputs involved in nociceptive processing may produce an imbalance that precipitates a cascade of cellular events leading to altered responsiveness to spared inputs (i.e., dorsal trunk) and subsequent to that the eventual behavioral output (avoidance to touch). In a recent study (Hubscher et al., 2008), the degree of allodynia was found to be significantly correlated to the amount of ventrolateral funiculus damage/sparing at or immediately above the lesion epicenter. Thus, some of the surviving axons traversing the anatomically incomplete contusion site as shown in our previous study (Hubscher and Johnson, 2006) may be some remaining spinothalamic projection neurons that contribute to altered thalamic neuronal responsiveness. In addition, since incoming segmental inputs ascend and descend several segments before terminating in the spinal dorsal horn, partial loss of these inputs following SCI at any given level may likewise be responsible for some of the cellular changes noted for spared spinothalamic dorsal horn neurons “at level” (Mills and Hulsebosch, 2002; Crown et al., 2005), which likely also contributes to the altered neuronal responsiveness within the thalamus. Note, however, that like the MRF, the hyper-excitability of “at-level” dorsal horn neurons may also not be directly associated with the circuitries that are important for the expression of avoidance to gentle mechanical touch.
In a study using a combined contusion/transection lesion (Hoheisel et al., 2003), allodynia was found in 13 of 16 female rats. The differences between our results may relate to the procedures used to define the presence of allodynia. The response criteria demonstrating the presence of allodynia in the Hoheisel et al. (2003) study not only included defense reactions but also withdrawal movements or vocalization, two behaviors that are not adequate for assessing cortically mediated pain perception, as they can, for example, be evoked in decerebrate rats (Woolf, 1984). Other labs also use reflex withdrawals as all or part of their response criteria for at-level and below-level allodynia (Eaton et al., 1997; Hutchinson et al., 2004; Oatway et al., 2004; Zhang et al., 2005; Hains and Waxman, 2006). Simple reflexes do not distinguish motor versus sensory effects. Moreover, unconscious nociceptive reactions and consciously perceived pain, however, may be confounded (Vierck et al., 1989, 2005), so care must be taken when a SCI-induced change is related to specific (or a group of) behaviors.
Acknowledgments
We wish to thank James Armstrong for excellent technical assistance. This study was supported by NS40919 from the NIH and grant number RR015576 from the National Center for Research Resources (NCRR), a component of the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Beric A. Spinal cord damage: injury. In: Wall P.D., editor; Melzack R., editor. Textbook of Pain. Churchill Livingstone; New York: 1999. pp. 915–927. [Google Scholar]
- Bester H. Bourgeais L. Villanueva L. Besson J.M. Bernard J.F. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J. Comp. Neurol. 1999;405:421–449. [PubMed] [Google Scholar]
- Bonica J.J. Introduction: semantic, epidemiology, and educational issues. In: Casey K.L., editor. Pain and Central Nervous System Disease: The Central Pain Syndromes. Raven Press; New York: 1991. pp. 13–29. [Google Scholar]
- Crown E.D. Ye Z. Johnson K.M. Xu G.Y. McAdoo D.J. Westlund K.N. Hulsebosch C.E. Upregulation of the phosphorylated form of CREB in spinothalamic tract cells following spinal cord injury: relation to central neuropathic pain. Neurosci. Lett. 2005;384:139–144. doi: 10.1016/j.neulet.2005.04.066. [DOI] [PubMed] [Google Scholar]
- Drew G.M. Siddall P.J. Duggan A.W. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 2001;893:59–69. doi: 10.1016/s0006-8993(00)03288-1. [DOI] [PubMed] [Google Scholar]
- Eaton M.J. Santiago D.I. Dancausse H.A. Whittemore S.R. Lumbar transplants of immortalized serotonergic neurons alleviate chronic neuropathic pain. Pain. 1997;72:59–69. doi: 10.1016/s0304-3959(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B. Gyldensted C. Fuglsang-Frederiksen A. Bach F.W. Jensen T.S. Sensory perception in complete spinal cord injury. Acta Neurol. Scand. 2004;109:194–199. doi: 10.1034/j.1600-0404.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- Gerke M.B. Duggan A.W. Xu L. Siddall P.J. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–722. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- Hains B.C. Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel U. Scheifer C. Trudrung P. Unger T. Mense S. Pathophysiological activity in rat dorsal horn neurones in segments rostral to a chronic spinal cord injury. Brain Res. 2003;974:134–145. doi: 10.1016/s0006-8993(03)02571-x. [DOI] [PubMed] [Google Scholar]
- Hubscher C.H. Hall B.J. Lally J.E. Vukmanic E.V. Armstrong J.E. Gupta D.S. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Asymmetrical lesion effects on central neuropathic pain following spinal cord injury. Program No. 570.2. Online. [Google Scholar]
- Hubscher C.H. Johnson R.D. Responses of medullary reticular formation neurons to input from the male genitalia. J. Neurophysiol. 1996;76:2474–2482. doi: 10.1152/jn.1996.76.4.2474. [DOI] [PubMed] [Google Scholar]
- Hubscher C.H. Johnson R.D. Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. J. Neurotrauma. 1999a;16:533–541. doi: 10.1089/neu.1999.16.533. [DOI] [PubMed] [Google Scholar]
- Hubscher C.H. Johnson R.D. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. I. Ascending pathways. J. Neurophysiol. 1999b;82:1381–1389. doi: 10.1152/jn.1999.82.3.1381. [DOI] [PubMed] [Google Scholar]
- Hubscher C.H. Johnson R.D. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp. Neurol. 2006;197:177–188. doi: 10.1016/j.expneurol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Hutchinson K.J. Gomez-Pinilla F. Crowe M.J. Ying Z. Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Johnson R.D. Hubscher C.H. Plasticity in supraspinal viscerosomatic convergent neurons following chronic spinal cord injury. In: Burchiel K., editor; Yezierski R.P., editor. Spinal Cord Injury Pain: Assessment, Mechanisms, Treatment. IASP Press; Seattle: 2002. [Google Scholar]
- Johnson R.L. Gerhart K.A. McCray J. Menconi J.C. Whiteneck G.G. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36:45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- Jones B.E. Yang T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kaddumi E.G. Hubscher C.H. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J. Physiol. 2006;572:393–405. doi: 10.1113/jphysiol.2005.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddumi E.G. Hubscher C.H. Urinary bladder irritation alters efficacy of vagal stimulation on rostral medullary neurons in chronic T8 spinalized rats. J. Neurotrauma. 2007;24:1219–1228. doi: 10.1089/neu.2007.0276. [DOI] [PubMed] [Google Scholar]
- Kinomura S. Larsson J. Gulyas B. Roland P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Lindsey A.E. LoVerso R.L. Tovar C.A. Hill C.E. Beattie M.S. Bresnahan J.C. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Hulsebosch C.E. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci. Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- Oatway M.A. Chen Y. Weaver L.C. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110:259–268. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peschanski M. Besson J.M. A spino-reticulo-thalamic pathway in the rat: an anatomical study with reference to pain transmission. Neuroscience. 1984;12:165–178. doi: 10.1016/0306-4522(84)90145-3. [DOI] [PubMed] [Google Scholar]
- Peschanski M. Ralston H.J.D. Light and electron microscopic evidence of transneuronal labeling with WGA-HRP to trace somatosensory pathways to the thalamus. J. Comp. Neurol. 1985;236:29–41. doi: 10.1002/cne.902360104. [DOI] [PubMed] [Google Scholar]
- Sherwood A.M. Dimitrijevic M.R. McKay W.B. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J. Neurol. Sci. 1992;110:90–98. doi: 10.1016/0022-510x(92)90014-c. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. McClelland J.M. Rutkowski S.B. Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. Taylor D.A. Cousins M.J. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Jr. Acosta-Rua A.J. Johnson R.D. Bilateral chronic constriction of the sciatic nerve: a model of long-term cold hyperalgesia. J. Pain. 2005;6:507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Jr. Cooper B.J. Ritz L.A. Greenspan J.D. Inference of pain sensitivity from complex behaviors of laboratory animals. In: Chapman C.R., editor; Loeser J.D., editor. Issues in Pain Management. Raven Press; New York: 1989. pp. 93–115. [Google Scholar]
- Werhagen L. Budh C.N. Hultling C. Molander C. Neuropathic pain after traumatic spinal cord injury–relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665–673. doi: 10.1038/sj.sc.3101641. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. Long-term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Zhang H. Xie W. Xie Y. Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons in segments rostral to the injury. Brain Res. 2005;1055:103–110. doi: 10.1016/j.brainres.2005.06.072. [DOI] [PubMed] [Google Scholar]