Abstract
Sle3 is an NZM2410/NZW-derived lupus susceptibility interval on murine chromosome 7, that is associated with spontaneous lupus nephritis, and also anti-GBM induced glomerulonephritis. The tissue kallikrein gene cluster is located within the Sle3 interval and constitutes potential candidate genes for this locus. We have recently reported that renal kallikrein expression was up-regulated by anti-GBM antibody challenge in a strain-specific manner and that it was significantly under-expressed in the anti-GBM sensitive strains, including B6.Sle3. Further sequencing and functional studies reported previously provided evidence that kallikreins could constitute disease genes in lupus. In the present report, we have used an adenoviral vector to deliver the klk1 gene to B6.Sle3 congenics to directly test if kallikreins might have a protective effect against anti-GBM induced nephritis. Our data shows that klk1 gene delivery ameliorated anti-GBM induced nephritis in B6.Sle3 congenics. Taken together with previous studies, these findings indicate that kallikreins play an important protective role in autoantibody-initiated glomerulonephritis, and could constitute potential candidate genes for anti-GBM induced and spontaneous lupus nephritis.
Keywords: Kallikrein, anti-GBM, glomerulonephritis, adenovirus, lupus
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production and immune complex (ICs) formation (1-7). Immune-mediated nephritis caused by deposition of pathogenic autoantibodies and ICs in the glumeruli is the leading cause of mortality and morbidity in this disease. The studies of spontaneous lupus nephritis (SLN) in mouse models and experimental anti-GBM disease have provided valuable insights into the underlying mechanisms of human lupus nephritis (8-14). The experimentally induced anti-GBM model has proven to be a particularly useful approach for studying end-organ susceptibility to immune-mediated damage (9-12). Our previous studies have revealed that NZW mice exhibit increased susceptibility to experimental anti-GBM induced glomerulonephritis (EAG), compared to C57BL/6 (B6) (10). Instead of performing a traditional mapping study to identify the responsible genetic loci, we took advantage of the fact that several NZW/NZM2410-derived “Sle” susceptibility loci had already been bred onto the normal B6 background as congenic intervals (13-25). One such lupus susceptibility interval is Sle3z, on chromosome 7 (19, 22, 26). Unlike the other B6.Sle congenic strains tested, B6.Sle3z mice (bearing the NZM2410/NZW-derived “z” allele of Sle3 on the relatively normal B6 background) exhibit increased susceptibility to EAG compare with B6 (27). Further fine mapping using recombinant sub-congenics bearing progressively narrower subintervals of Sle3 revealed the susceptibility genes to be located between D7mit157 and D7mit158 on chromosome 7 (27), an interval harboring the kallikrein cluster of genes. Microarray and real-time PCR studies indicated that several EAG susceptible strains (such as 129/svJ, NZW and DBA/1) as well as the B6.Sle3 congenic strain had significantly reduced renal expression of kallikreins, compared to B6 and BALB/c controls, following anti-GBM challenge. Furthermore, sequence comparison of several klk genes indicated that nephritis-prone mouse strains and patients with lupus nephritis possessed different klk alleles, compared to controls (27).
The above studies suggested that kallikreins may be renoprotective in immune-mediated nephritis. Indirect evidence for this was provided by demonstrating that bradykinins (which are generated by kallikreins) can be renoprotective, while bradykinin receptor blockade aggravated anti-GBM induced nephritis (27). The previous studies did not address if kallikreins themselves could modulate disease when deliberately administered to nephritis-susceptible mice. In this communication, we directly test if systemic delivery of kallikreins is renoprotective against autoantibody-induced nephritis, using B6.Sle3z congenic mice as the disease model.
MATERIAL AND METHODS
Construction and preparation of recombinant adeno-klk1
The recombinant adenoviral Ad-GFP vector (AdEasy™ vector system, Stratagene, USA) was used for making the Ad-mklk1 construct, following the vendor’s instructions. Briefly, the mouse klk1 gene coding region (786 bp) was PCR amplified from the B6 strain using the following primers: forward 5′-AGC GTC GAC ACC ATG AGG TTC CTG ATC-3′, reverse 5′-AGC CTC GAG GTC ATT TTC AGC CAT AG-3′, and inserted into the multi-cloning site of pShuttle-IRES-hrGFP vector. Once constructed, the shuttle vector was linearized with Pme I and co-transformed into BJ5183 competent cells together with pAdEasy-1, a supercoiled viral DNA plasmid. Transformants were selected for kanamycin resistance, and recombinants containing mKlk1 insert were subsequently identified by restriction digestion. Once a recombinant was identified, it was produced in bulk using the recombination-deficient XL10-Gold strain. Purified recombinant Ad-mklk1 plasmid DNA was digested with Pac I to expose its inverted terminal repeats (ITR), and then used to transfect AD-293 cells, in which deleted viral assembly genes are complemented in vivo. Ad-mklk1 was amplified and purified from these cells, and the titer of recombinant virus was measured by plaque assays. The Ad-GFP vector was used as a control.
Animal studies
C57BL/6 (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.Sle3z is a congenic stain bearing the lupus susceptibility interval, Sle3z (15, 19, 26). All mice were maintained in a specific pathogen-free colony. 2-3 month old females were used for all studies. To induce EAG, 10 mice from each strain were sensitized on day 0 with rabbit IgG (250 μg/mouse, i.p.), in adjuvant. On day 3, mice of each strain were randomly divided into two groups of 5 mice each. One group received recombinant Ad-mklk1 virus via tail vain injection (1 × 107 plaque-forming units per mouse) and another group receive the same dose of Ad-GFP vector as control. On day 5, all mice were challenged (i.v.) with rabbit anti-GBM IgG (200 μg per 25 g of body weight). Twenty-four-hour urine and serum samples were collected from all mice on days 0, 7, 14 and 21, for measuring proteinuria, serum BUN and kallikrein activity. All animals were sacrificed on day 21, and the kidneys were processed for histo-pathological examination by light microscopy. Five mice were included in each experimental group.
Detection of Klk1 expression in serum by Western blotting
Serum samples were collected from each experimental mouse at day 0, 7, 14 and 21. Sera were diluted 1:10 with PBS and protein concentration was measured using the BCA protein assay kit (Pierce, Rockford, IL). 10 ug of serum protein from each sample was subjected to SDS-PAGE and transferred to nylon membrane for western blot analysis using a rabbit anti-mouse kallikrein-1 antibody (1:1000), as described (27). Immunoreactivity was detected by chemiluminescence (Pierce).
Detection of urine kallikrein excretion by enzymatic activity assay
24-hour urine samples were collected from each mouse using metabolic cages on days 0, 7, 14, and 21. Total urinary kallikrein enzymatic activity was measured using the synthetic chromogenic substrate HD-Val-Leu-Arg-pNA (S-2266), as described by Moodely et al (28). Briefly, 50 ul of mouse urine sample was added to 50 ul of assay buffer (0.2M Tris-HCl, pH 8.2, containing 300ug/l SBTI and 375ug/l EDTA) and incubated at 37°C for 30 min. Then, 50 ul of S-2266 was added and incubated for 3 h at 37°C. The plate was read at 405 nm. A standard curve was constructed using purified human urinary kallikrein (HUK, Calbiochem). The amount of total protein in the urine was measured using the BCA Protein assay kit (Pierce).
Assessing renal disease
Twenty-four-hour urine samples were collected from experimental mice on days 0, 7, 14 and 21, using metabolic cages, with free access to drinking water. Urinary protein concentration was determined using the Coomassie Plus protein assay kit (Pierce). Serum was collected on day 14, for measuring blood urea nitrogen (BUN), using a urea nitrogen kit (Sigma-Aldrich, St. Louis, MO). Three-micrometer sections of formalin-fixed, paraffin-embedded kidney tissues were cut and stained with H&E and periodic acid-Schiff. All sections were examined in a blinded fashion, for any evidence of pathology in the glomeruli, tubules, or interstitial areas, as described before (12). The severity of glomerulonephritis (GN) was graded on a 0-4 scale as follows: 0, normal; 1, mild increase in mesangial cellularity and matrix; 2, moderate increase in mesangial cellularity and matrix, with thickening of the GBM; 3, focal endocapillary hypercellularity with obliteration of capillary lumina and a substantial increase in the thickness and irregularity of the GBM; and 4, diffuse endocapillary hypercellularity, segmental necrosis, crescents, and hyalinized end-stage glomeruli. The severity of tubulointerstitial nephritis (TIN) was graded on a 0-4 scale, based on the extent of tubular atrophy, inflammatory infiltrates, and interstitial fibrosis, as detailed previously (29).
Statistics
Results are expressed as mean ± sem. Data were analyzed using the unpaired Student’s t test or ANOVA, with the use of GraphPad Prism 5.0 software (San Diego, CA). Values were considered significantly different if P < 0.05.
RESULTS
Expression of kallikrein in B6 and B6.Sle3z mice after klk1 gene delivery
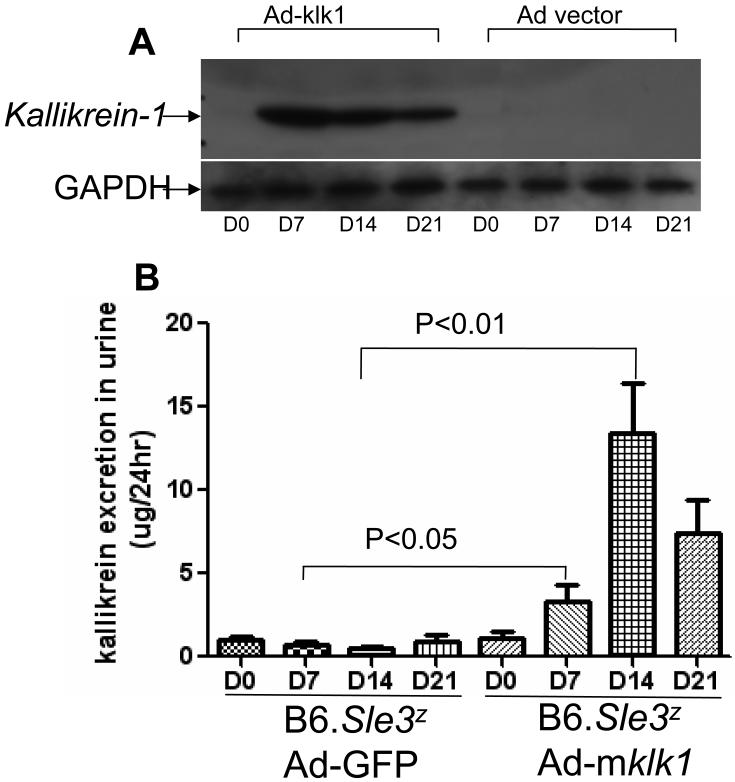
Following intravenous injection of an adenovirus encoding the mouse klk1 gene, recombinant kallikrein levels in mouse sera and tissues were assayed by Western blot using a rabbit anti-mKlk1 antibody. All B6 and B6.Sle3z mice receiving Ad-mklk1 expressed significantly elevated kallikrein levels in their blood and liver, but not in the kidneys or spleen (Fig. 1 and data not shown), compared to the control mice. Figure 1A shows a representative Western blot. Klk1 expression reached its peak on day 7 (i.e., 5 days after Ad-mklk1 injection) and then started to decrease. The expression of Klk1 in mouse serum was still detectable on the day of sacrifice (day 21). Mice receiving Ad-GFP vector control did not have detectable kallikrein expression in their serum. Kallikrein excretion was also detected in the urine of B6.Sle3z mice receiving Ad-mklk1 injection on days 7, 14 and 21 (3.35±2.23, 13.38±6.86 and 7.44±4.42 ug/24hr, respectively), compared to the vector control group (0.69±0.43, 0.49±0.3, and 0.95±0.66 ug/24hr, respectively) (P values: 0.03, 0.007, 0.05, respectively), as summarized in Figure 1B.
Figure 1. The expression and excretion of recombinant mouse klk1 protein after Ad-mklk1 gene delivery.
(A)Anti-GBM disease was induced in B6.Sle3z mice with or without klk1 gene delivery. Serum was collected on days 0, 7, 14 and 21 from each mouse and klk1 protein expression was gauged using Western blot. Five biological replicates were included in each group. The left panel shows that high klk1 expression was detected from the mice with klk1 gene transfer (Ad-mklk1) on day 7 after anti-GBM challenge, and the levels persisted until day 21. The right panel depicted the serum Klk levels from mice receiving Ad-GFP vector as control.
(B)Kallikrein level in urine was determined using a kallikrein enzymatic activity assay. Klk1 gene transferred mice (Ad-mklk1) showed increased kallikrein excretion from day 7 (right panel). No kallikrein activity was detected in the control group of mice (Ad-GFP).
Effect of kallikrein gene delivery on proteinuria and serum BUN
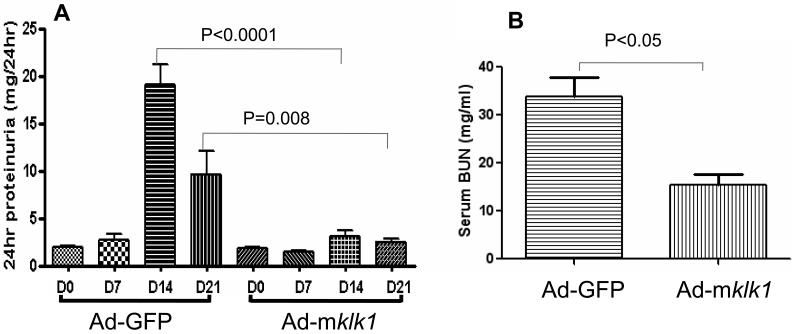
As expected, the B6.Sle3z mice in the control group (receiving Ad-GFP vector injection) developed high levels of proteinuria on day 14 (19.2±2.1 mg/24hr) and day 21 (9.7±2.5 mg/24hr), after anti-GBM Ab challenge. In contrast, B6.Sle3z mice in the klk1 gene delivery group (receiving Ad-mklk1 injection) showed significantly reduced proteinuria on day 14 and day 21 (3.2±0.6 mg/24 hr and 2.6±0.3 mg/24hr, respectively) compared to the vector control group (P<0.001, n=5), as diagramed in Figure 2A. There was no significant increase in proteinuria in un-challenged B6 mice receiving the adenoviral vector (data not shown). Likewise, on day 14 after anti-GBM challenge, the serum BUN levels in B6.Sle3z mice injected with Ad-GFP control vector reached 33.9±4.0 mg/ml. However, B6.Sle3z mice treated with recombinant Ad-mklk1 showed significantly lower serum BUN (18.5±4.6 mg/ml) compared to the vector control mice (P=0.004), as shown in Figure 2B. Unchallenged B6 mice treated with Ad-GFP or Ad-mklk1 showed low levels of serum BUN, which was similar to the levels in klk1-treated B6.Sle3z mice (data not shown).
Fig 2. Urinary protein and blood urea nitrogen levels in B6.Sle3z mice after kallikrein gene delivery.
(A)Total urinary protein levels in Ad-mklk1 and Ad-GFP vector treated mice were assayed. Urine samples were collected on days 0, 7, 14 and 21 and urine protein was determined by ELISA. Kallikrein gene delivery attenuated anti-GBM induced proteinuria. Values are expressed as mean ± SEM (N = 5 per group).
(B)Blood urea nitrogen (BUN) levels were gauged after adenoviral gene delivery. Blood was collected on day 14 after anti-GBM challenge (i.e., 12 days after klk1 gene transfer). Kallikrein administration reduces anti-GBM induced BUN. Values are expressed as mean ± SEM (N = 5 per group).
Effect of kallikrein gene delivery on renal histology
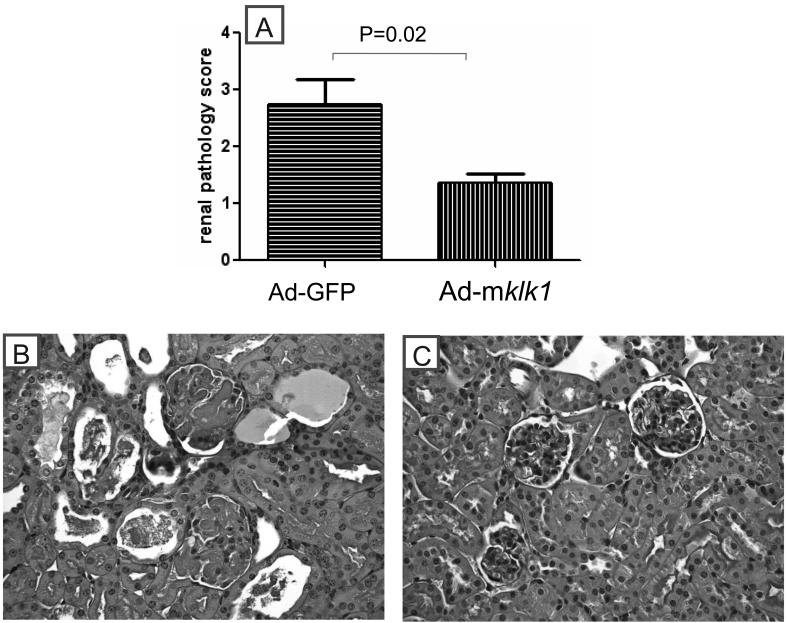
Histological analysis of kidneys confirmed that the klk gene delivery attenuated histological GN induced by anti-GBM challenge (Figure 3). B6.Sle3z mice receiving Ad-GFP vector showed moderate to severe GN with a score of 2.7±0.5, whereas the Ad-klk1 injected B6.Sle3z mice exhibited ameliorated glomerular disease with a GN score of 1.4±0.2 (P=0.02) (Figure 3A). The marked GN in the control group was also accompanied by significant glomerular proliferation and crescent formation, as exemplified by representative slides depicted in Figure 3B. Collectively, the above functional readouts and histological findings indicate that the deliberate administration of kallikreins systemically has the capacity to dampen autoantibody-initiated glomerulonephritis. In terms of the proteinuria, BUN and renal pathology, the kallikrein-treated mice did not differ significantly from unmanipulated mice (i.e., not challenged with anti-GBM Abs) (10-12).
Figure 3. Effect of klk1 gene delivery on renal pathology in anti-GBM challenged B6.Sle3z mice.
(A)Kidney specimens (collected on day21) were examined by light microscopy for evidence and grade of GN, using the WHO classification scheme. Bars represent means ± SEM; n=7 mice per group.
(B)Light-microscopic views of periodic acid-schiff-stained renal sections from Ad-vector treated B6.Sle3z mice on day 21 after anti-GBM challenge. The mice in this group exhibited typical features of anti-GBM nephritis, including glomerular proliferation, crescent formation, intracapillary hypercellularity with obliteration of capillary lumen, and tubular dilatation with casts. Shown figure is representative of sections from at least four mice in each study group. Shown are sections of the renal cortex; no differences were seen in the renal medulla (data not shown).
(C)Anti-GBM challenged B6. Sle3z mice receiving Ad-mklk1 gene transfer showed essentially normal renal histology. Shown figure is representative of sections from at least four mice in each study group.
DISCUSSION
The EAG experimental model of immune-mediated nephritis has emerged as a useful tool for dissecting out the molecular and genetic basis of lupus nephritis (9). The finding that B6.Sle3z congenic mice are more sensitive to EAG than the B6 controls has fueled studies aimed at identifying potential renal-intrinsic factors that may govern this phenotype (27). One key molecular difference that clearly distinguishes the EAG sensitive strains from the resistant strains is their renal kallikrein expression levels upon anti-GBM Ab challenge (27). Interestingly, the klk gene cluster is located within the Sle3 lupus susceptibility interval on murine chromosome 7, with interesting sequence polymorphisms, raising the possibility that these could be the genes responsible for the observed renal phenotypes in this strain. The impaired renal expression of kallikreins in B6.Sle3z and other EAG sensitive strains suggests that klk may play a protective role against nephritis (27). These earlier findings resonate well with previous genetic and pharmacological studies that also reveal a renoprotective role for kallikreins and bradykinins in nephritis following other types of insults (30-38).
In the present study, we offer the first demonstration that deliberate kallikrein gene delivery can indeed protect mice against anti-GBM induced nephritis. After systemic delivery of adenovirus containing mouse klk1, immunoreactive mouse klk1 was detected in the serum and urine of Ad-klk1 injected B6 and B6.Sle3z mice, indicating that recombinant mouse Klk1 (most likely of serum origin) was being excreted from the kidneys. Importantly, the klk1 gene transfer markedly reduced urinary protein, serum BUN levels and glomerular inflammation in the anti-GBM challenged B6.Sle3z mice, demonstrating that kallikreins can indeed be renoprotective in antibody-mediated nephritis. Though only Klk1 was used in this study, it is likely to be representative of all kallikreins given that the genes in this family are evolutionarily conserved and have 67-91% primary sequence identity (43). A second rationale for using Klk1 relates to the fact that Klk1 gene transfer has previously been demonstrated to successfully ameliorate renal disease in other animal models (35).
As reported by others, kallikreins may be exerting a protective role in nephritis by modulating several different parameters, including local blood pressure, the inflammatory milieu, redox balance and/or signaling within various cell types (39-44). Kallikreins and kinins have also been shown to improve renal function by increasing the glomerular filtration rate and renal blood flow (31, 45). Anti-GBM Abs directed to antigens on the glomerular basement membrane can trigger a rapid inflammatory reaction characterized by accumulation of polymorphonuclear leukocytes and mononuclear cells (46). It has been well documented that anti-GBM Ab induced nephritis is associated with oxidative stress in the form of ROS, which in turn can activate MAPKs, particularly p38 MAPK and JNK, and also induce apoptosis (47-52). In this context, it is worth noting that others have reported that kallikreins and kinins could also act as anti-oxidant and anti-inflammatory agents through the suppression of oxidative stress, monocyte/macrophage recruitment and renal cell apoptosis (34, 53). Others have observed that the activation of the kinin B2 receptor by kallikreins and kinins can mobilize intracellular Ca2+, enhance intracellular nitric oxide synthase (eNOS) activity and thus nitric oxide (NO) production (34, 35, 42, 53). The increased bioavailability of NO could in turn counter oxidative stress, MAPK activation and ongoing inflammation (34-37, 40-53). Hence, the renoprotective effect of kallikreins in Ab-mediated nephritis could potentially have been mediated through a wide variety of molecular mechanisms operating in parallel.
It is also worth considering the interplay of kallikreins/kinins with another multi-molecular enzyme system, the renin-angiotensin system. It is now apparent that both these systems interface at multiple levels (54-58). Both angiotensin and kinin peptides are metabolized by many different shared peptidases, including prolylcarboxypeptidases. This interaction, along with the roles of angiotensin converting enzyme, cross talk between bradykinin and angiotensin action, and the opposing effects of activation of the ANG II receptors 1 and 2 support a hypothesis that these two systems might counterbalance each other. Finally, there is evidence that ACE inhibitors, which are also therapeutically effective in lupus nephritis, could function in part by potentiating the effect of bradykinin and its analogs on B2 receptors independently of blocking peptide metabolism. Hence, some of the documented impacts of the renin-angiotensin system on nephritis need to be re-evaluated in terms of how they might impact kallikrein/kinin activity.
The observation that the NZW-derived Sle3z lupus-susceptibility locus confers susceptibility to spontaneous lupus nephritis raises the interesting possibility that polymorphisms in Klk may also confer susceptibility to spontaneous lupus nephritis. Resonating well with this observation in the mouse is the finding that SNPs in human KLK genes may also be associated with lupus nephritis in patients (27). Efforts are in progress to evaluate if long-term modulation of Klk levels in vivo can impact the severity of spontaneous lupus nephritis. The current study provides significant insights regarding the role of the kallikrein/kinin system in mediating protection against antibody-mediated nephritis. These studies also raise the interesting possibility that different end-organs may harbor an arsenal of molecules with anti-inflammatory and/or disease modulating potential as safeguards against immune-mediated disease.
REFERENCES
- 1.Hahn BH. Antibodies to DNA. N. Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 2.Pisetsky DH. Anti-DNA and autoantibodies. Curr Opin Rheumatol. 2000;12:364–368. doi: 10.1097/00002281-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kotzin JB. Systemic Lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 4.Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus: Current concepts and continuing controversies. Arthritis Rheum. 1996;39:894–903. doi: 10.1002/art.1780390605. [DOI] [PubMed] [Google Scholar]
- 5.Foster MH, Kelley VR. Lupus nephritis: update on pathogenesis and disease mechanisms. Seminars in Nephrology. 1999;19:173–81. [PubMed] [Google Scholar]
- 6.Vyse TJ, Kotzin BL. Genetic susceptibility to systemic lupus erythematosus. Annu Rev Immunol . 1998;16:261–92. doi: 10.1146/annurev.immunol.16.1.261. [DOI] [PubMed] [Google Scholar]
- 7.Kono DH, Theofilopoulos AN. Genetics of systemic autoimmunity in mouse model of lupus. Int. Rev. Immunol. 2000;19:367–87. doi: 10.3109/08830180009055504. [DOI] [PubMed] [Google Scholar]
- 8.Nose M, Nishihara M, Kamogawa J, Terada M, Nakatsuru S. Genetic basis of autoimmune disease in MRL/lpr mice: dissection of the complex pathological manifestations and their susceptibility loci. Rev in Immunogenetics. 2000;2(1):154–64. [PubMed] [Google Scholar]
- 9.Fu Y, Yong D, Mohan C. The experimental anti-GBM model as a tool for studying spontaneous lupus nephritis. Clin Immunol. 2007;124:109–118. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Xie C, Zhou JX, Mohan C. Enhanced susceptibility to nephritis in the lupus facilitating NZW strain. Arthritis Rheum. 2003;48:1080–1092. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]
- 11.Xie C, Ruchi Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172:5047–5055. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 12.Xie C, Gong Y, Qin X, Bhaskarabhatla M, Zhou Xin J, Mohan C. Strain distribution pattern of immune nephritis - a follow-up study. International Immunol. 2008;20(6):719–28. doi: 10.1093/intimm/dxn030. [DOI] [PubMed] [Google Scholar]
- 13.Mohan C. Murine Lupus Genetics: Lessons Learned. Curr Opin Rheumatol. 2001;13:352–360. doi: 10.1097/00002281-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wakeland EK. Genetic dissection of systemic lupus erythematosus. Curr Opin Immunol. 2000;11:701–707. doi: 10.1016/s0952-7915(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 15.Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of SLE pathogenesis using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- 16.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogensis: Sle2 on murine chromosome 4 leads to B-cell hyperactivity. J Immunol. 1997;159:454. [PubMed] [Google Scholar]
- 17.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan C, Morel L, Yang P, Wakeland EK. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998;41(9):1652–62. doi: 10.1002/1529-0131(199809)41:9<1652::AID-ART17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation and cell death. J Immunol. 1999;162(11):6492–502. [PubMed] [Google Scholar]
- 20.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11(2):131–9. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 22.Sobel ES, Morel L, Baert R, Mohan C, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: evidence for functional expression expression of Sle3/5 in non-T-cells. J Immunol. 2002;169:4025–4032. doi: 10.4049/jimmunol.169.7.4025. [DOI] [PubMed] [Google Scholar]
- 23.Mohan C, Morel L, Yang P, Watanabe H, Croker B, Gilkeson G, Wakeland EK. Genetic dissection of lupus pathogenesis: a recipe for nephrophilic autoantibodies. J Clin Invest. 1999;103:1685. doi: 10.1172/JCI5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying SLE-susceptibility genes derived from the SLE-prone NZM/Aeg2410 strain. Mamm. Genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Li QZ, Yu Y, Liang C, Subramanian S, Zeng Z, et al. Sle3 and Sle5 can independently couple with Sle1 to mediate severe lupus nephritis. Genes Immun. 2007;8:634–645. doi: 10.1038/sj.gene.6364426. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Li QZ, Mohan C, Wakeland EK, et al. Kallikreins as disease genes in anti-GBM disease and lupus nephritis. J Clin Invest. 2008 in press. [Google Scholar]
- 28.Moodley D, Naicker S, Naidoo S, Bhoola KD. Tissue kallikrein excretion in acute and chronic renal transplant rejection. Immunopharmacology. 1996;33:380–382. doi: 10.1016/0162-3109(96)00091-4. [DOI] [PubMed] [Google Scholar]
- 29.Zhou XJ, Laszik Z, Wang XQ, Silva FG, Vaziri ND. Association of renal injury with increased oxygen free radical activity and altered nitric oxide metabolism in chronic experimental hemosiderosis. Lab. Invest. 2000;80:1905. doi: 10.1038/labinvest.3780200. [DOI] [PubMed] [Google Scholar]
- 30.Chao J, Bledsoe G, Yin H, Chao L. The tissue kallikrein-kinin system protects against cardiovascular and renal diseases and ischemic stroke independently of blood pressure reduction. Biol Chem. 2006;387(6):665–75. doi: 10.1515/BC.2006.085. [DOI] [PubMed] [Google Scholar]
- 31.Chao J, Chao L. Kallikrein-kinin in stroke, cardiovascular and renal disease. Exp Physiol. 2004;90(3):291–298. doi: 10.1113/expphysiol.2004.028464. [DOI] [PubMed] [Google Scholar]
- 32.Chao J, Zhang J, Lin KF, Chao L. Adenovirus-mediated kallikrein gene delivery attenuates hypertension, cardiac hypertrophy and renal injury in Dahl Salt-sensitive rat. Hum Gene Ther. 1998;9:1–31. doi: 10.1089/hum.1998.9.1-21. [DOI] [PubMed] [Google Scholar]
- 33.Chao J, Zhang J, Lin KF, Chao L. Adenovirus-mediated kallikrein gene delivery reverses salt-induced renal injury in Dahl salt-sensitive rats. Kidney Int. 1998;54:1250–1260. doi: 10.1046/j.1523-1755.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 34.Bledsoe G, Crickman S, Mao J, Xia CF, Murakami H, Chao L, Chao J. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21:624–633. doi: 10.1093/ndt/gfi225. [DOI] [PubMed] [Google Scholar]
- 35.Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J. Reversal of renal fibrosis, inflammation,and glomerular hypertrophy by kallikrein gene delivery. Human Gene Therapy. 2006;17:545–555. doi: 10.1089/hum.2006.17.545. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JJ, Bledsoe G, Kato K, Chao L, Chao J. Tissue kalliakrein attenuates salt-induced renal fibrosis by inhibition of oxidative stree. Kidney Int. 2004;66:733–732. doi: 10.1111/j.1523-1755.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- 37.Schanstra JP, Neau E, Drogoz P, Gomez MAA, Novoa JML, Calise D, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakoki M, Takahashi N, Charles J, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao J, Li HJ, Yao YY, Shen B, Gao L, Bledsoe G, Chao L. Kinin Infusion Prevents Renal Inflammation, Apoptosis, and Fibrosis via Inhibition of Oxidative Stress and Mitogen-Activated Protein Kinase Activity. Hypertension. 2007;49(3):490–7. doi: 10.1161/01.HYP.0000255925.01707.eb. [DOI] [PubMed] [Google Scholar]
- 40.Bhoola KD, Figueroa CD, Worthy K. Bioregualtion of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 41.Carretero OA, Scicli AG. The renal kallikrein-kinin system. Am J Physiol. 1980;238:F247–F255. doi: 10.1152/ajprenal.1980.238.4.F247. [DOI] [PubMed] [Google Scholar]
- 42.Regoli D, Rhaleb NE, Drapeau G, Dion S. Kinin receptor subtypes. J Cardiovasc Pharmacol. 1990;15(Suppl 6):S30–S38. [PubMed] [Google Scholar]
- 43.Mason AJ, Evans BA, Cox DR, Shine J, Richards RI. Structure of mouse kallikrein gene family suggests a role in specific processing of biologically active peptides. Nature. 1983;303(5915):300–7. doi: 10.1038/303300a0. [DOI] [PubMed] [Google Scholar]
- 44.Hong Fu, Jing Li, Qi-xiong Li, Lin Xia, Lang Shao. Protective effect of ligustrazine on accelerated anti-glomerular basement membrane antibody nephritis in rats is based on its antioxidant properties. Eur J Pharmacol. 2007;563:197–202. doi: 10.1016/j.ejphar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Wolf WC, Yoshida H, Agata J, Chao L, Chao J. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney International. 2000;58:730–739. doi: 10.1046/j.1523-1755.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 46.Allison AE, Lorinda M, McCulloch AA, Jennifer Intraglomerular leukocyte recruitment during nephrotoxic serum nephritis in rats. Clin. Immunol. Immunopathol. 1990;57:441–458. doi: 10.1016/0090-1229(90)90118-a. [DOI] [PubMed] [Google Scholar]
- 47.Basnakian AG, Kaushal GP, Shah SV. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal. 2002;4:915–24. doi: 10.1089/152308602762197452. [DOI] [PubMed] [Google Scholar]
- 48.Sha SH, Schacht J. Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic Biol Med. 1999;26:341–7. doi: 10.1016/s0891-5849(98)00207-x. [DOI] [PubMed] [Google Scholar]
- 49.Stambe C, Atkins RC, Hill PA, Nikolic-Paterson DJ. Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int. 2003;64(6):2121–32. doi: 10.1046/j.1523-1755.2003.00324.x. [DOI] [PubMed] [Google Scholar]
- 50.Datta PK, Sharma M, Duann P, Lianos EA. Effect of Nitric Oxide Synthase Inhibition on Proteinuria in Glomerular Immune Injury. Exp Biol Med. 2006;231(5):576–84. doi: 10.1177/153537020623100512. [DOI] [PubMed] [Google Scholar]
- 51.Ikezumi Y, Hurst L, Atkins RC, Nikolic-Paterson DJ. Macrophage-Mediated Renal Injury Is Dependent on Signaling via the JNK Pathway. J Am Soc Nephrol. 2004;15:1775–1784. doi: 10.1097/01.asn.0000131272.06958.de. [DOI] [PubMed] [Google Scholar]
- 52.Rana A, Sathyanarayana P, Lieberthal W. Role of apoptosis of renal tubular cells in acute renal failure: therapeutic implications. Apoptosis. 2001;6:83–102. doi: 10.1023/a:1009680229931. [DOI] [PubMed] [Google Scholar]
- 53.Bledsoe G, Shen B, Yao YY, Hagiwara M, Mizell B, Teuton M, Grass D, Chao L, Chao J. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol Sci. 2008;102(2):433–43. doi: 10.1093/toxsci/kfn008. [DOI] [PubMed] [Google Scholar]
- 54.Linz W, Wiemer G, Gohlke P, Unger T, Schölkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47(1):25–49. [PubMed] [Google Scholar]
- 55.Erdös EG, Deddish PA, Marcic BM. Potentiation of Bradykinin Actions by ACE Inhibitors. Trends in Endocrinology and Metabolism. 1999;10:223–229. doi: 10.1016/s1043-2760(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 56.Tschöpe C, Seidl U, Reinecke A, Riester U, Graf K, Schultheiss HP, Hilgenfeldt U, Unger T. Kinins are involved in the antiproteinuric effect of angiotensin-converting enzyme inhibition in experimental diabetic nephropathy. Int Immunopharmacol. 2003;3(3):335–44. doi: 10.1016/S1567-5769(02)00273-4. [DOI] [PubMed] [Google Scholar]
- 57.Schmaier AH. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1–R13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 58.Buleon M, Allard J, Jaafar A, Praddaude F, Dickson Z, Ranera M-T, Pecher C, Girolami J-P, Tack I. Pharmacological blockade of B2-kinin receptor reduces renal protective effect of angiotensin-converting enzyme inhibition in db/db mice model. Am J Physiol Renal Physiol. 2008;294(5):F1249–F1256. doi: 10.1152/ajprenal.00501.2007. [DOI] [PubMed] [Google Scholar]