Abstract
Since its first identification in the late 1800s, a variety of etiologies for essential hypertension have been proposed. In this paper we review the primary proposed hypotheses in the context of both the time in which they were proposed as well as the subsequent studies performed over the years. From these various insights, we propose a current paradigm to explain the renal mechanisms underlying the hypertension epidemic today. Specifically, we propose that hypertension is initiated by agents that cause systemic and intrarenal vasoconstriction. Over time intrarenal injury develops with microvascular disease, interstitial T cell and macrophage recruitment with the induction of an autoimmune response, with local angiotensin II formation and oxidant generation. These changes maintain intrarenal vasoconstriction and hypoxia with a change in local vasoconstrictor-vasodilator balance favoring sodium retention. Both genetic and congenital (nephron number) mechanisms have profound influence on this pathway. As blood pressure rises, renal ischemia is ameliorated and sodium balance restored completely (in salt-resistant) or partially (in salt-sensitive) hypertension, but at the expense of a rightward shift in the pressure natriuresis curve and persistent hypertension.
Keywords: Fructose, hypertension, inflammation, microvascular disease, oxidative stress, salt, uric acid
Introduction
Blood pressure varies markedly, with minute to minute variations largely determined by the tone of the sympathetic nervous system (SNS) and the parasympathetic nervous system [1]. Studies using continuous blood pressure measurements have documented that the range of blood pressure readings in any given day can be marked [2,3] and presents a characteristic circadian rhythm, in which blood pressure falls during the night or while sleeping, corresponding to a decrease in SNS tone.
Although there is marked lability in normal blood pressure, most authorities have defined elevated blood pressure (hypertension) as systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or both, taken in the relaxed, sitting position. For ambulatory blood pressure monitoring, hypertension is usually defined when blood pressures are ≥ 140/90 mmHg for more than 25% of the readings for any given 24 h period [4]. The cutoff of 140/90 mmHg was selected in the early 1900s based on the fact that only 5−10% of the US population had blood pressures in that range [5]. In addition, it was recognized from the start that blood pressures in the hypertensive range were almost inevitably accompanied by small vessel disease of the arterioles (arteriolosclerosis) as well as kidneys that were grossly contracted and ‘granular’ in appearance, with glomerular, and more commonly tubular, changes on microscopic examination [6,7]. This suggests that hyper-tension should not simply be defined by an elevation in blood pressure but rather should be considered a syndrome in which microvascular disease and renal involvement are also key components.
In this review, we summarize the major hypotheses on the etiology of hypertension with special focus on renal mechanisms leading to sodium retention. We will first review how the various paradigms and hypotheses developed, and then summarize the three major currently viewed pathways.
The development of paradigms
The common observation of the triad of high blood pressure, arteriolosclerosis, and renal involvement led to controversies in the nineteenth century as to the pathogenesis of the hypertensive condition. One favored hypothesis, led by Gull and Sutton [8], was that the arteriolar injury was primary, and that this raised the vascular resistance, leading to strain on the heart (cardiac hypertrophy) and kidneys. This hypothesis was later adapted by Folkow [9], who argued that systemic vascular changes resulting in a reduction in the luminal diameter of small vessels could be a primary cause of the elevation in peripheral vascular resistance that is characteristic of most cases of essential hypertension. A second hypothesis, promoted by Sir George ‘Kidney’ Johnson [10], was that the kidney was the culprit, and that intrarenal disease slowed blood flow and raised systemic pressures that led to secondary vascular and cardiac involvement. This hypothesis was fuelled by earlier observations made by Bright [11] and others that hypertension not only accompanied chronic renal disease but was also one of its earliest manifestations. A third hypothesis was promoted by Mahomed [12] from Guy's Hospital, who had suggested that hypertension was caused by a ‘blood poison’, such as lead or uric acid, and that this led to a rise in blood pressure that then had secondary effects on the kidney, blood vessels and heart.
By the early twentieth century the introduction of the cuff sphygmomanometer by Riva Rocci, coupled with refinements and standardization of measurements by Korotkoff, Faught, and others, led not only to the acceptance of blood pressure measurement as a standard medical practice, but also to multiple studies investigating the frequency of hypertension in different populations. This led to the discovery that hypertension was primarily a condition observed in the United States and in Europe, and was extremely rare in other parts of the world, including Africa, Asia, Oceania, Australia, and South America [13]. The observation that many cases of hypertension had no clinical evidence of kidney disease and was often asymptomatic led to the term ‘benign’ hypertension to characterize those individuals [14]. In this context, some authorities suggested that the rise in blood pressure might be necessary or ‘essential’ in order to provide blood supply to its destination sites through the thickened blood vessels [15].
Additional hypotheses then developed to explain the development of hypertension. One of the major debates raged over whether hypertension might be genetically based, because an increased frequency of hypertension was noted in the families of index patients. The hypothesis of Platt [16] that there was a single gene locus was quickly dispelled by the concept that there was probably the cumulative effect of multiple genes (‘polygenic’) [17]. The concept of multiple mechanisms including genetic and environmental factors (‘mosaic hypothesis’) was also entertained [18]. This led Pickering [17] to suggest that hypertension might simply reflect the right end of a normal Gaussian curve for blood pressure in the population, and thus might not be a true ‘disease’. Nevertheless, it was clear that elevated blood pressure, although often presenting as a benign condition, was associated with a marked increased frequency of vascular, renal, and cardiac disease, as well as an increased risk of death.
In contrast to Pickering [17], others continued to focus on the potential role of the vascular and renal changes as a mechanism for the development of hypertension. Volhard [19] suggested that there were two forms of hypertension, one ‘red’ or benign form that was observed in robust, plethoric individuals who remained asymptomatic until they presented with strokes or cardiovascular disease, and a second ‘pale’ or malignant form associated with a vasoconstricted state and a more fulminating course. Volhard [19], working partly with renal pathologist Fahr [20], believed the benign form resulted from activation of the SNS that secondarily led to renal and systemic arteriolosclerotic changes that raised blood pressure, whereas the pale, malignant form was caused by vasoconstrictive substances released from the kidney. This latter concept was championed by Goldblatt [21], who thought that the cause of essential hypertension was primary disease of the preglomerular renal arterioles, leading to ischemic renal injury and the release of factors (such as renin) that would raise systemic vascular resistance and pressure. In support of his claim was the observation that the vast majority of individuals (> 90%) with essential hypertension had variable degrees of renal arteriolosclerosis and tubular changes consistent with ischemia [7]. Hypertension was strongly correlated with arteriolar changes in the kidney as opposed to other organs such as the spleen [7]. Furthermore, whereas Goldblatt [21] did not have a mechanism to induce renal microvascular disease experimentally, he was able to reduce renal blood flow by clamping the renal artery of dogs and demonstrate the rapid development of hypertension [22].
Although the hypothesis of Goldblatt [21] was attractive, several major problems led to its dismissal. First was the problem that Goldblatt [21] could provide no mechanism for why renal arteriolar disease would develop spontaneously. Second, during the 1940s there was a short period of time in which thoracolumbar sympathectomy was used as a treatment for hypertension. Although blood pressure did initially fall, in most cases this was transient, and thus the procedure was abandoned. Two of the main proponents of this procedure, Castleman and Smithwick [23], obtained a large collection of renal biopsies (> 1200) during this time of patients with essential hypertension of various duration and severity. They reported that as many as 10% of patients with essential hypertension had either no or only minimal evidence of renal arteriolosclerosis even though almost all had changes consistent with tubular ischemia [24]. Whereas Goldblatt [21] tried to rebut this observation by suggesting that it could reflect sampling error, the data nevertheless suggested that the arteriolar changes might be secondary. This latter concept was further supported by a study by Perrera [25], who showed that renal arteriolar changes became progressively more severe the longer and higher the blood pressure.
Another major area of research revolved around the potential for neural mechanisms to be responsible for hypertension. Following Volhard's suggestion that activation of the SNS might play a role in initiating essential hypertension [26], a number of studies demonstrated that many patients displayed evidence of an activated SNS (and a reduction in parasympathetic activity), including higher basal heart rate, increased blood pressure in response to stimuli (mental stress, exercise, or cold pressor test) and elevated plasma catecholamines [27-29]. The mechanisms responsible for this activation appear to be diverse, and include defective baroreceptor autoregulation [29], increased hypothalamic response to environmental stimuli [30], stimulation of renal afferent sympathetics that activate central nervous system (CNS) adrenergic pathways [31], and elevated thoracolumbar sympathetic activity [32].
Other vasoactive systems have also been proposed to play a key role in the hypertensive process. Systemic mediator systems have included evidence for inappropriate activation of the renin–angiotensin system relative to the volume (sodium) status of the patient, perhaps triggered by heterogeneous perfusion within the kidney [33]. Endothelial dysfunction associated with impaired nitric oxide release resulting in inadequate vasodilation of resistance vessels has also been shown to be present in many patients with hypertension [34]. An increase in intrarenal vasoconstrictors, such as angiotension II [35] and endothelin–endothelin A receptor [36] pathways, or a reduction in intrarenal vasodilators (kallikrein–bradykinin, endothelin–endothelin B receptor, endothelial-derived hyperpolarizing factor, epoxyeicosayrenoic acids, dopamine) have also been shown in some cases of experimental and human hypertension [37-42].
Guyton and the role of the kidney in salt-sensitive hypertension
Although the hypothesis of Goldblatt [21] was dismissed in the 1950s, the concept that the kidney might still be involved had not been abandoned. Studies by Allen [43] in the 1920s had shown that hypertension could be improved by sodium restriction, and this, coupled with the common presence of hypertension in patients with chronic renal disease, led to the theory that hypertension might relate to a defect in sodium excretion. This concept was led in particular by Dahl [44], who reported that the prevalence of hypertension in various populations often correlated with the amount of sodium ingested. While not all epidemiological studies were able to confirm a direct correlation, meta-analyses have clearly shown that sodium restriction can result in lower blood pressure, particularly in certain groups such as the African-American or aging population [45]. Furthermore, modest sodium restriction has also been shown to provide long-term cardiac benefit in the general population [46]. Some individuals with essential hypertension will have a greater fall in blood pressure with salt restriction than others, and this has led to the concept that hypertension may be further categorized as ‘salt sensitive’ or ‘salt resistant’ [47].
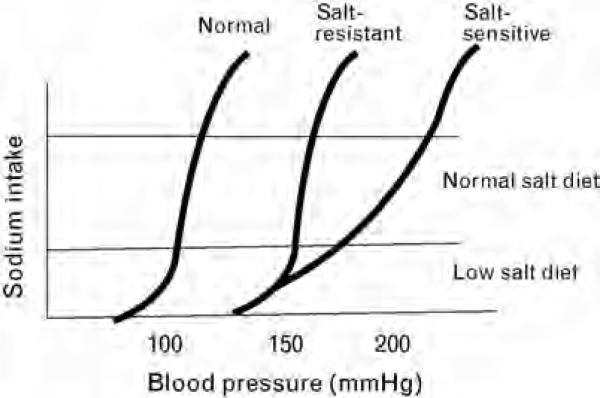
Additional insights came from studies addressing the relationship of sodium intake and excretion with blood pressure. It had been known for some time that an acute rise in blood pressure is accompanied by a reflex increase in urinary sodium excretion ‘pressure natriuresis’, and so it was proposed that hypertension might be caused by a defect in this pressure natriuresis mechanism [48,49]. Although this was first suggested by Borst and Borst-De Geus [48], it was Guyton and colleagues [49] who developed this concept into a viable hypothesis. Using mathematical and computer modeling, Guyton et al. [49] suggested that most systems that would raise blood pressure, such as activation of the SNS, would result in only transient elevations, and that permanent increases in blood pressure would require a resetting of the pressure natriuresis curve. The authors further proposed that both salt-resistant and salt-sensitive hypertension were renal dependent, but that they resulted in different types of pressure natriuresis curves (Fig. 1). Salt-resistant hyper-tension would result in a parallel shift in the pressure natriuresis curve such that sodium handling would be similar to that observed in normal individuals except that it occurs at a higher baseline blood pressure; in contrast, salt-sensitive hypertension would be associated with an exaggerated increase or decrease in blood pressure with an increased or decreased intake in sodium, respectively.
Fig. 1.
The effect of the hypertensive phenotype on the pressure natriuresis curve.
Studies by Cowley and Roman [50], Hall et al. [51] and Wang et al. [52] have since largely confirmed Guyton's paradigm. Further support for a key role for the kidney in the pathogenesis of hypertension has come from transplant studies in experimental models of hypertension [53-55] and in humans [56]. Dahl and Heine [54] demonstrated that the transplantation of a kidney from a rat with salt-sensitive hypertension to a normotensive rat will transfer the salt sensitivity. Curtis et al. [56] also showed that in humans with hypertension-induced end-stage renal disease, the transplantation of a kidney from a normotensive donor could cure the hypertension. This latter finding, as well as more recent studies [57], demonstrated that systemic vascular disease cannot be the cause of hypertension, and thus effectively rebutted the original hypotheses of Gull and Sutton [8] and Folkow [9].
The observation of the primary importance of the kidney in essential hypertension does not negate the role of non-renal mechanisms in the process. For example, the elevation in peripheral vascular resistance is mediated by vasoconstriction that is dependent on angiotensin type I receptors [58] and other mediator systems. Persistent activation of the SNS may also cause chronic hypertension. One of the best examples is caused by neurovascular compression of the brain stem, such as from an enlarged posterior inferior cerebellar artery [59]. This was probably one of the causes of hypertension in the series of case reports provided by Mosenthal [14] in a study performed in the 1920s. A decrease in vascular compliance, such as occurs with aging in larger blood vessels such as the aorta, may also contribute to the development of isolated systolic hypertension. Even in these conditions, however, it is quite possible that the kidney plays a role in maintaining the hypertensive response [60].
In recent years much attention has focused on the renal mechanisms that underlie the defect in pressure natriuresis in essential hypertension. Furthermore, it is becoming increasingly apparent that the pathogenesis of most forms of hypertension (including primary and secondary) involve an important role of the kidney. We propose that three basic renal mechanisms explain most etiologies. We will not discuss how salt retention leads to hypertension, but several excellent reviews on the sodium-dependent and volume-dependent mechanisms have been published [61-64].
The three primary renal mechanisms of hypertension
Pathway 1: glomerular filtration rate-dependent (renoprival mechanism)
After the introduction of blood pressure measurements, a strong association with acute and chronic kidney disease was observed. Hypertension was so strongly associated with kidney disease that the observation that it could occur in the absence of clinically evident renal disease was considered a major discovery [65]. Subsequent studies demonstrated that animals in which kidneys were removed (‘renoprival’) or injured rapidly developed hypertension. Similarly, an increase in the frequency of hypertension can be shown in humans as the glomerular filtration rate (GFR) falls, and this occurs even with mild dysfunction (corresponding to a GFR of 30−90 ml/min) [66].
The mechanism of hypertension in this condition is probably caused by the reduction in GFR resulting in sodium retention and volume expansion. A reduction in the filtered sodium load by itself, except with extreme reductions of GFR, should be compensated by reduced tubular sodium reabsorption in a normal functioning kidney. Therefore, one must postulate that reduced GFR must be linked with an impairment in tubular sodium handling. Potential mechanisms that could account for these effects include activation of the renal sympathetic system, the release of vasoconstrictive substances, and the loss of vasodepressor substances produced by the kidney [67,68]. Interestingly, the correction of blood volume by dialysis is often associated with a lag period before blood pressure normalizes [69]. It seems likely that this may be a result of the stimulation of vasoconstrictive substances (such as digitalis-like factors) induced by the volume expanded state [69].
It is likely that the GFR-dependent mechanisms may play an important role in the hypertension observed with acute and chronic kidney disease, with diabetes, and with aging.
Pathway 2: transport mechanisms (stimulation of sodium reabsorption in the collecting duct)
In 1955 Conn [70] reported several cases of hypertension caused by aldosterone-secreting tumors. Aldosterone's primary action is to increase sodium reabsorption via the epithelial sodium channel in the collecting duct, although it also has direct effects on vascular cells and the heart. In addition to aldosterone-secreting tumors, aldosterone may also be involved in some forms of low renin hypertension, particularly associated with obesity. For example, in addition to the regulation of aldosterone by angiotensin II and potassium, it has recently been shown that oxidized derivatives of linoleic acid can stimulate aldosterone production, and these oxidized fatty acids and aldosterone are elevated in some obese individuals with essential hypertension [71,72].
The epithelial sodium channel in the collecting duct in the pathogenesis of hypertension has also been shown to be involved in the etiology of several rare genetic forms of hypertension [73]. Hypertension was thus shown to be a consequence of a mutation resulting in elevated aldosterone levels (glucocorticoid remedial aldosteronism), of a mutation resulting in enhanced binding of the mineralocorticoid receptor on the collecting duct (syndrome of apparent mineralocorticoid excess) or the result of the increased expression of the aldosterone-regulatable epithelial sodium channel [73]. All of these conditions result in enhanced sodium reabsorption at the collecting duct.
Normally, there are extensive mechanisms for regulating sodium excretion in the kidney. The final regulation is at the level of the collecting duct. Whereas alterations in more proximal parts of the nephron may result in compensatory mechanisms to maintain sodium balance, dys-regulation of sodium transport at the collecting duct may result in the least ability to regulate sodium handling. It is perhaps not surprising that genetic mutations involving sodium reabsorption at this site are strongly associated with hypertension.
The best evidence that genetic mechanisms involving the collecting duct epithelial sodium channel are involved in essential hypertension relate to the recent discovery that certain G protein polymorphisms involved in the regulation of this channel are strongly linked with hypertension [74]. Interestingly, these polymorphisms have a higher frequency in populations living in the tropics and decrease with increasing latitude [74]; this is exactly the opposite of what has been observed for blood pressure, in that mean population blood pressures historically tended to be lower at the equator and rose with increasing latitude [75]. If one controls for latitude, however, then a strong relationship between the G protein polymorphism and blood pressure can be shown. The authors have attributed this latitude effect to negative selection because rising latitude might be associated with less sweating, more sodium retention, and higher blood pressures with greater mortality [74].
In addition to genetic alterations in epithelial sodium activity that can increase the risk of hypertension, there are also genetic alterations that could lead to reduced activity of the epithelial sodium channel that may have opposing effects [73,76]. Upregulation of the collecting duct epithelial sodium channel by intrarenal mechanisms could be another potential mechanism for hypertension, and has been shown in the spontaneously hypertensive rat [77].
Pathway 3: renal ischemia (vasoconstriction, oxidative stress and inflammation)
Although hypertension can result from a reduction in GFR, in most cases of essential hypertension the GFR is either normal or only minimally depressed. Furthermore, whereas genetic mechanisms are likely to influence the overall hypertensive phenotype [78], most studies suggest that genetics can only account for approximately 20% of the hypertension variance [79]. In addition, during the last century there has been a dramatic increase in the prevalence of hypertension, which now affects 31% of the population in the United States [80]. This is not simply because the population is living longer, as the increase in the prevalence of hypertension can be documented when controlling for age and sex [79,81]. Furthermore, the increase in hypertension has also occurred throughout the world, where it is fast approaching, and in some cases exceeding, the prevalence in the United States [82]. The increase in the frequency of hypertension from near absence in the early 1900s to rates of 30% or more after only a few generations suggests a major environmental mechanism [83].
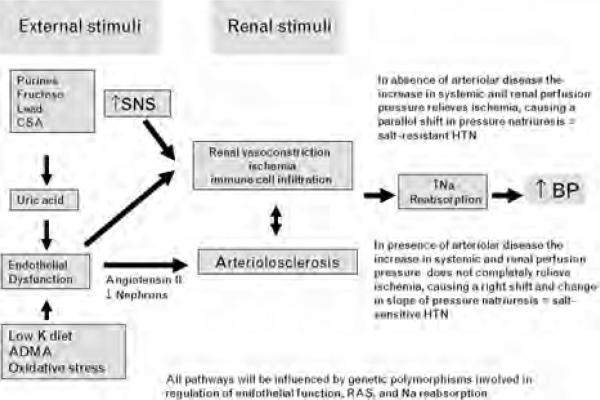
This led us to hypothesize that hypertension might represent an acquired form of renal disease that would have an effect to limit sodium excretion [59]. Following a large series of studies involving numerous animal models, strong evidence for a specific pathway was found (Fig. 2) [84-86]. In particular, the initiating mechanism in most cases appears to be renal vasoconstriction. The dominant site is the afferent arteriole. Modlinger et al. [87] and Wilcox [88] have shown that the vasoconstriction is mediated by a number of mechanisms, including oxidative stress, thromboxane, nitric oxide deficiency, and angiotensin II.
Fig. 2.
A pathway for the development of salt-resistant and salt-sensitive hypertension. ADMA, Asymmetric dimethyl arginine; BP, blood pressure; CSA, cyclosporin A, HTN, hypertension; K, potassium; Na, sodium; RAS, renin–angiotensin system; SNS, sympathetic nervous system.
We have demonstrated that the engagement of this pathway can result from a hyperactive SNS [89], activation of the renin–angiotensin system [90], endothelial dysfunction with impaired release of nitric oxide [91], hypokalemia [92], and by drugs such as cyclosporine [93]. During this early phase the kidney appears normal except for mild tubular ischemia and inflammation, and hypertension is mediated both by the effects of these vasoconstrictor systems to mediate systemic vasoconstriction as well as by mechanisms related to renal ischemia (such as renin release and enhanced sodium reabsorption). Pathologically, the kidney would appear similar to the 10% of cases noted by Castleman and Smithwick [23], in which no renal microvascular disease is present. Physiologically this is a form of salt-resistant hypertension as proposed by Guyton et al. [49], in that the rise in systemic pressure will eventually act to relieve renal ischemia and allow sodium handling to return to normal, but at the expense of an elevated blood pressure (Fig. 1).
Over time the recurrent episodes of renal vasoconstriction, particularly if coupled with local angiotensin II stimulation, can lead to the development of renal arteriolar disease. Numerous groups have noted a strong association of afferent arteriolar disease with a reduction in the lumen in individuals with hypertension [7,94]. The observation that preglomerular arteriolar disease resembling arteriolosclerosis could be rapidly induced by either angiotensin II infusion or by blocking endothelial nitric oxide synthesis [91,93] provided the long sought mechanism by Goldblatt [21] for how this vascular lesion could develop.
Intricately associated with the vascular lesion is an infiltration into the interstitium of inflammatory cells consisting of T cells and macrophages, many of which are producing oxidants and angiotensin II [89-93,95]. The important role of the immune system in mediating hypertension had previously been shown in the desoxycorticosterone acetate salt hypertensive model by Svendsen [96], who had reported that the thymus was necessary for the late salt-dependent phase. This pathway was not rediscovered until Rodriguez-Iturbe et al. [93] demonstrated that the reduction in intrarenal T cells and macrophages could also block salt-sensitive hypertension in the post angiotensin II model, and this has been subsequently shown in multiple other models by this group and others.
The mechanism by which the interstitial T cells contribute to hypertension has been shown to involve local angiotensin II generation and oxidant generation, the latter being able to reduce local nitric oxide [89-93,95,97,98]. It is likely that the intrarenal injury may also activate afferent sympathetics that activate CNS sympathetics [31]. In turn, dietary salt intake may further stimulate CNS sympathetics, activate renal efferent nerves and augment renal vasoconstriction [99,100]. Whether these intrarenal changes alter other intrarenal vasoconstrictors or vasodilators has not been well studied. Nevertheless, these changes may augment renal vasoconstriction and facilitate sodium retention (by both reducing single nephron GFR and Kf, and by stimulating sodium reabsorption) [101]. Because microvascular disease is non-uniform, the rise in systemic pressure results in some nephrons being overperfused whereas others are underperfused. Peritubular capillaries may also be damaged. The net effect is that ischemia is not completely relieved, and this leads to continued stimulation of sodium reabsorptive mechanisms by renin-dependent [33] and renin-independent mechanisms (engaging pathway 2). As a consequence, the pressure natriuresis curve is shifted to the right and flattened, and the hypertension is salt sensitive (Fig. 1). Furthermore, over time a fall in GFR may occur, thus engaging pathway 1.
As discussed, one of the key drivers of salt-sensitive hypertension appears to be the infiltrating T cells and macrophages within the renal interstitium. If the number of infiltrating cells is prevented by immunosuppressive therapy with mycophenolate mofetil (MMF) [91,93,101-103], or by blocking their activation with inhibitors of the signaling molecule nuclear factor kappa B [104], then salt-sensitive hypertension can be prevented. Whereas renal ischemia appears to be the primary mechanism leading to the accumulation of these cells, proteinuria may also play a role [105]. In the nephropathy induced by repeated injections of bovine serum albumin (‘protein overload model’), salt sensitivity develops in concert with an interstitial inflammatory infiltrate, and both the inflammation and hypertension are blocked by MMF therapy [106].
This pathway is similar to that proposed by Goldblatt in the 1930s. A key difference is that there are two phases. The first phase is driven by agents that cause systemic and intrarenal vasoconstriction such as by endothelial dysfunction, activation of the renin–angiotensin system, or a hyperactive SNS. Initially these agents only cause hypertension when they are activated (such as intermittent or continuous activation of the SNS). Over time a second phase occurs in which renal microvascular disease and inflammation perpetuates the vasoconstriction. Mechanistically this provides the explanation for why the arterioles can be normal in the early phases of hypertension. It should also be noted that not all individuals would be expected to pass from one phase to the second. We have thus found that catecholamine infusion does not induce the afferent arteriolar lesion (unpublished), whereas models driven by angiotensin II (such as those caused by angiotensin II infusion, the blockade of endothelial nitric oxide release, and cyclosporine use) are associated with the development of arteriolopathy [91,93,95,101,102]. This may be because angiotensin II but not catecholamines stimulate nicotinamide adenine dinucleotide phosphate, reduced form, oxidase and local oxidants in vascular smooth muscle cells [107], which appears to be critical in the afferent vasoconstriction and disease that occurs in models of hypertension [108]. From those studies we would suggest that individuals whose hypertension is solely driven by a hyperactive SNS may be less likely to progress to a salt-sensitive state.
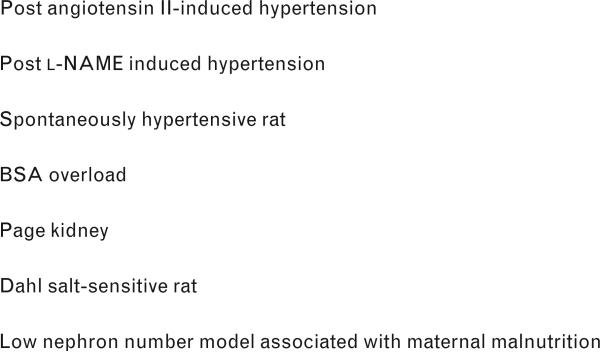
To date, this pathway of subtle renal injury as a mechanism for the development of salt-sensitive hypertension has been shown in many models of hypertension (Fig. 3). Observational studies have also suggested that MMF may be able to control hypertension in humans [109]. Future studies examining this pathway may be of extreme value in identifying how to prevent or cure this disease.
Fig. 3.
Models of salt-sensitive hypertension mediated by interstitial immune cells. BSA, bovine serum albumin; l-NAME, nitro-l-arginine methyl ester.
Low nephron numbers: a risk factor that engages renal microvascular injury and intrarenal inflammation (pathway 3)
A favored hypothesis for how hypertension may develop is that it is driven by in-utero events (fetal programming or ‘Barker–Brenner’ hypothesis) [110,111]. Epidemiological studies have found that low birth weight infants are at higher risk of developing hypertension, obesity/metabolic syndrome, and diabetes as adults [110,112]. Experimental studies in rats have confirmed that maternal malnutrition will lead to small birth weight pups that have an increased risk of developing hypertension and salt sensitivity as adults [113]. Brenner and colleagues [111] proposed this was caused by congenitally low nephron numbers, as this is commonly observed in low birth weight infants or those suffering from intrauterine growth retardation [114]. Studies in the experimental maternal malnutrition model [113] and in humans [115,116] have confirmed that hypertension is associated with lower nephron numbers.
Until recently the mechanism by which a low nephron number might cause hypertension had not been well understood. Whereas GFR could be a component, studies of individuals who have donated kidneys for transplantation have not shown any dramatic increase in the frequency of hypertension [117]. It is known that animals with reduced nephron numbers develop preglomerular arteriolar disease and tubulointerstitial inflammation [118]. This has also been shown in the maternal malnutrition model in which rat pups are born with low nephron numbers. Furthermore, in this model if the tubulointerstitial inflammation is blocked with MMF, the rats fail to develop hypertension despite the reduction in nephrons [119].
Similarly, the spontaneous hypertensive rat (SHR) has a low nephron number and this has been proposed to mediate the hypertensive response [113]. However, the SHR also has a congenital reduction in the afferent arteriolar lumen, and this is associated with renal hypoxia and tubulointerstitial inflammation [102,120]. Treatment with MMF can reduce the intrarenal inflammation and improve blood pressure [103]. Furthermore, when SHR are crossed with Wistar–Kyoto rats, the F2 hybrids can be separated with relation to either afferent arteriolar diameter or to nephron numbers. When this is done, the hypertension tracks with arteriolar narrowing, not with nephron number [121,122].
These studies suggest that low nephron numbers probably lead to hypertension because of its propensity to cause renal microvascular disease and inflammation (pathway 3). Nephron number is thus a risk factor that accelerates an underlying mechanism (renal microvascular disease) as opposed to being a cause by itself. It has recently been shown that young Caucasian individuals with essential hypertension have only half the number of nephrons compared with age-matched controls [116], whereas no relationship can be shown between glomerular number and hypertension in African Americans [115]. This may well be because in younger individuals, and particularly Caucasian individuals, the low nephron number may be a particularly strong risk factor, whereas in African American individuals other more overriding mechanisms are present.
What is causing the current epidemic of hypertension?
Currently one-third of the US population is hypertensive, representing a three to six-fold increase since the early 1900s [13]. Essential hypertension is now also common in the pediatric population, where it tracks with rising frequencies of obesity and metabolic syndrome. The marked increase in prevalence worldwide is too rapid to be accounted for solely by genetic mechanisms or by changing trends in birth weights. As such, there is probably a major environmental component driving this process.
The most likely reason for the dramatic increase in the frequency of hypertension is the marked increase in obesity. Obesity has increased from 3 to 5% of the US population in 1900 to over 30% today [123]. In turn, obesity has been shown to be associated with many prohypertensive mechanisms, including SNS activation, insulin and leptin resistance, endothelial dysfunction, elevations in plasma aldosterone, and intrarenal fat accumulation [72,124]. Many of these findings can be associated with acquired renal microvascular and inter-stitial injury, including endothelial dysfunction [91], SNS stimulation [89], and activation of the renin–angiotensin axis [93].
Our group has also suggested that specific dietary factors may also be involved in the epidemic, particularly the ingestion of fructose present in sugar and other sweeteners [125]. Fructose ingestion parallels the epidemic of obesity and hypertension [124], and fructose can cause features of the metabolic syndrome in animals [126]. Although fructose ingestion acutely causes more of a ‘white coat’ type of hypertension [127-130], it also causes microvascular disease and tubulointerstitial inflammation [131] and thus is likely to engage pathway 3. Whereas some have challenged the animal studies as they typically use high doses of fructose, this is a common experimental method to accelerate the development of a condition, and low doses of fructose also cause insulin resistance but over a greater period of time [132]. Clinical studies in humans have also shown that fructose can induce features of the metabolic syndrome rapidly if large doses are administered [133-136].
Our studies also suggest that one of the mechanisms by which fructose may cause hypertension and the metabolic syndrome is via its unique ability as a sugar to raise uric acid levels [137]. Fructose entry into cells is followed by unregulated phosphorylation by the enzyme fructokinase, resulting in rapid adenosine triphosphate depletion, cell ischemia, and uric acid generation [138]. Whereas uric acid acutely functions as an antioxidant, we have found that chronic elevations in uric acid can inhibit endothelial function, activate the renin–angiotensin system, and cause blood pressure elevation in animals [139-143]. Lowering uric acid not only prevents these hemo-dynamic and histological changes but also prevents most of the features of the metabolic syndrome, including the development of hypertension, hyperinsulinemia, hyper-triglyceridemia, and obesity [137]. Furthermore, over time elevated uric acid also causes microvascular disease and interstitial inflammation with the development of salt sensitivity (pathway 3) [143].
Studies in humans have also shown that serum uric acid levels are increasing within the population and correlate with the rise in the obesity and hypertension rates [13]. Elevated uric acid also predicts [144-146] and is commonly present in early hypertension [4], and pilot studies have suggested that lowering uric acid may lower blood pressure in new onset hypertensive subjects [147]. An ongoing clinical trial is in process to test this hypothesis (D.I. Feig, R.J. Johnson, in preparation).
The mechanism by which uric acid mediates hypertension and the metabolic syndrome is under study. Uric acid has multiple effects on endothelial cells, vascular smooth muscle cells, and adipocytes. Some of the effects include the inhibition of endothelial nitric oxide levels, the stimulation of inflammatory pathways including C-reactive protein, the stimulation of local angiotensin II production, the stimulation of nicotinamide adenine dinucleotide phosphate, oxidase, and the inhibition of adipokines [139,140,148,149]. These effects require the entry of urate into the cell. While the mechanism remains unclear, recent studies have suggested that it may involve the reaction of urate with reactive oxygen or nitrogen species with the formation of radicals. Further studies to investigate this pathway are ongoing.
Conclusion
The kidney may cause hypertension by three mechanisms that alter the physiological balance between sodium retention and sodium excretion: a reduced GFR that limits sodium excretion (first mechanism); humoral or genetic disorders resulting in the stimulation of sodium reabsorption in the distal nephron (second mechanism); or acquired mechanisms involving renal ischemia, oxidative stress and inflammation (third mechanism), or varying combinations of all three pathways. Whereas much remains to be learned, a verdict can finally be reached in relation to the original hypotheses by Gull and Sutton [8] (arteriolosclerosis hypothesis), Johnson [10] (kidney hypothesis) and Mahomed [12] (circulating factor hypothesis); all were right.
Acknowledgements
This study was supported by NIH grants DK-52121, HL-68607 and HL-079352-01.
Abbreviations
- ATP
Adenosine triphosphate
- ADMA
Asymmetric dimethylarginine
- BSA
bovine serum albumin
- CNS
central nervous system
- CSA
cyclosporine
- DOCA
deoxycorticosterone acetate
- EDHF
endothelial derived hyperpolarizing factor
- EET
epoxyeicosanoid
- Kf
glomerular ultrafiltration coefficient
- GFR
glomerular filtration rate
- MMF
mycophenolate mofetil
- NADPH
oxidase, nicotinamide adenine dinucleotide phosphate-oxidase
- L-NAME
N (G)-nitro-L- arginine methyl ester
- NO
nitric oxide
- PNS
parasympathetic nervous system
- RAS
renin angiotensin system
- SHR
spontaneous hypertensive rat
- SNS
sympathetic nervous system
- WKY
Wistar Kyoto Rat
Footnotes
Conflict of interest R.J.J. is on the Scientific Board for Nephromics Inc.
References
- 1.Conway J, Boon N, Davies C, Jones JV, Sleight P. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens. 1984;2:203–208. doi: 10.1097/00004872-198404000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bevan AT, Honour AJ, Stott FH. Direct arterial pressure recording in unrestricted man. Br Heart J. 1969;31:387–388. [PubMed] [Google Scholar]
- 3.Richardson DW, Honour AJ, Fenton GW, Stott FH, Pickering GW. Variation in arterial pressure throughout the day and night. Clin Sci. 1964;26:445–460. [PubMed] [Google Scholar]
- 4.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera G. Diagnosis and natural history of hypertensive vascular disease. Am J Med. 1948;4:416–422. doi: 10.1016/0002-9343(48)90258-7. [DOI] [PubMed] [Google Scholar]
- 6.Kimmelsteil P, Wilson C. Benign and malignant hypertension and nephrosclerosis. A clinical and pathological study. Am J Pathol. 1936;12:45–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Moritz A, Oldt M. Arteriolar sclerosis in hypertensive and nonhypertensive individuals. Am J Pathol. 1937;13:679–728. [PMC free article] [PubMed] [Google Scholar]
- 8.Gull W, Sutton H. On the pathology of the morbid state commonly called chronic Brights disease with contracted kidney ‘arterio-capillary fibrosis’. Med Chir Trans. 1872;55:325–371. doi: 10.1177/095952877205500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkow B. Structure and function of the arteries in hypertension. Am Heart J. 1987;114:938–948. doi: 10.1016/0002-8703(87)90591-6. [DOI] [PubMed] [Google Scholar]
- 10.Johnson G. The anatomy of Bright's disease: the arterio-capillary fibrosis of Sir William Gull and Dr Sutton. BMJ. 1972;I:604–605. doi: 10.1136/bmj.1.597.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bright R. Tabular view of the morbid appearances in 100 cases connected with albuminous urine. Guy's Hosp Rep. 1836;1:338–379. [Google Scholar]
- 12.Mahomed FA. The etiology of Bright's disease and the prealbuminuric state. Med Chir Trans. 1874;39:197–228. doi: 10.1177/095952877405700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Mosenthal H. Essential hypertension. Med Clin N Am. 1917;1:101–117. [Google Scholar]
- 15.Volhard F. Discussion of Bright's disease [in German]. Verh Deutsch Pathol Gesell. 1905;9:111–114. [Google Scholar]
- 16.Platt R. Heredity in hypertension. Q J Med. 1947;16:111–113. [PubMed] [Google Scholar]
- 17.Pickering G. The nature of essential hypertension. Lancet. 1959;2:1027–1028. [PubMed] [Google Scholar]
- 18.Page IH. The mosaic theory of arterial hypertension – its interpretation. Perspect Biol Med. 1967;10:325–333. doi: 10.1353/pbm.1967.0031. [DOI] [PubMed] [Google Scholar]
- 19.Volhard F, Fahr T. Bright's kidney disease: a clinical and pathology atlas [in German] Springer; Berlin: 1914. [Google Scholar]
- 20.Fahr T. Handbook of special pathological anatomy and histology [in German] F.H. Lubarsch; Berlin: 1925. Pathological anatomy of Bright's disease. [Google Scholar]
- 21.Goldblatt H. The renal origin of hypertension. Physiol Rev. 1947;27:120–165. doi: 10.1152/physrev.1947.27.1.120. [DOI] [PubMed] [Google Scholar]
- 22.Goldblatt H, Lynch J, Hanzal R, Summerville W. Studies on experimental hypertension. I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castleman B, Smithwick R. The relation of vascular disease to the hypertensive state. JAMA. 1943;12:1256–1261. doi: 10.1056/NEJM194811112392001. [DOI] [PubMed] [Google Scholar]
- 24.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 25.Perera GA. Hypertensive vascular disease; description and natural history. J Chron Dis. 1955;1:33–42. doi: 10.1016/0021-9681(55)90019-9. [DOI] [PubMed] [Google Scholar]
- 26.Lampen H. Franz Volhard and his last big subject in view of a fellow [in German]. Med Welt. 1973;24:11–17. [Google Scholar]
- 27.Julius S, Schork MA. Predictors of hypertension. Ann NY Acad Sci. 1978;304:38–58. doi: 10.1111/j.1749-6632.1978.tb25565.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G. Bjorn Folkow Award Lecture. The sympathetic nervous system in hypertension. J Hypertens. 1997;15:1553–1565. doi: 10.1097/00004872-199715120-00056. [DOI] [PubMed] [Google Scholar]
- 31.Campese VM, Park J. The kidney and hypertension: over 70 years of research. J Nephrol. 2006;19:691–698. [PubMed] [Google Scholar]
- 32.Smithwick RH. The surgical physiology of hypertension. Surg Clin North Am. 1949;29:1699–1730. doi: 10.1016/s0039-6109(16)32883-3. [DOI] [PubMed] [Google Scholar]
- 33.Sealey JE, Blumenfeld JD, Bell GM, Pecker MS, Sommers SC, Laragh JH. On the renal basis for essential hypertension: nephron heterogeneity with discordant renin secretion and sodium excretion causing a hypertensive vasoconstriction–volume relationship. J Hypertens. 1988;6:763–777. doi: 10.1097/00004872-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep. 2003;5:135–143. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noll G, Wenzel RR, Luscher TF. Endothelin and endothelin antagonists: potential role in cardiovascular and renal disease. Mol Cell Biochem. 1996;157:259–267. doi: 10.1007/BF00227908. [DOI] [PubMed] [Google Scholar]
- 37.Jose PA, Eisner GM, Felder RA. Dopamine and the kidney: a role in hypertension? Curr Opin Nephrol Hypertens. 2003;12:189–194. doi: 10.1097/00041552-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens. 2006;15:34–40. doi: 10.1097/01.mnh.0000186852.15889.1a. [DOI] [PubMed] [Google Scholar]
- 39.Ardiles LG, Figueroa CD, Mezzano SA. Renal kallikrein-kinin system damage and salt sensitivity: insights from experimental models. Kidney Int Suppl. 2003;86:S2–S8. doi: 10.1046/j.1523-1755.64.s86.2.x. [DOI] [PubMed] [Google Scholar]
- 40.Katori M, Majima M. A missing link between a high salt intake and blood pressure increase. J Pharmacol Sci. 2006;100:370–390. doi: 10.1254/jphs.crj06003x. [DOI] [PubMed] [Google Scholar]
- 41.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 42.Sarkis A, Roman RJ. Role of cytochrome P450 metabolites of arachidonic acid in hypertension. Curr Drug Metab. 2004;5:245–256. doi: 10.2174/1389200043335603. [DOI] [PubMed] [Google Scholar]
- 43.Allen F. Arterial hypertension. JAMA. 1920;74:652–655. [Google Scholar]
- 44.Dahl LK. Essential hypertension – an international symposium. Springer; Berlin: 1960. Possible role of salt intake in the development of essential hypertension. pp. 53–65. [DOI] [PubMed] [Google Scholar]
- 45.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 46.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885–894. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 48.Borst JG, Borst-De Geus A. Hypertension explained by Starling's theory of circulatory homoeostasis. Lancet. 1963;1:677–682. doi: 10.1016/s0140-6736(63)91443-0. [DOI] [PubMed] [Google Scholar]
- 49.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 50.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 51.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15:547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 52.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in Ang II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med. 1974;47:435–448. doi: 10.1042/cs0470435. [DOI] [PubMed] [Google Scholar]
- 54.Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ Res. 1975;36:692–696. doi: 10.1161/01.res.36.6.692. [DOI] [PubMed] [Google Scholar]
- 55.Rettig R, Folberth C, Kopf D, Stauss H, Unger T. Role of the kidney in the pathogenesis of primary hypertension. Clin Exp Hypertens A. 1990;12:957–1002. doi: 10.3109/10641969009073513. [DOI] [PubMed] [Google Scholar]
- 56.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N Engl J Med. 1983;309:1009–1015. doi: 10.1056/NEJM198310273091702. [DOI] [PubMed] [Google Scholar]
- 57.Kvist S, Mulvany MJ. Contrasting regression of blood pressure and cardiovascular structure in declipped renovascular hypertensive rats. Hypertension. 2003;41:540–545. doi: 10.1161/01.HYP.0000054977.07679.59. [DOI] [PubMed] [Google Scholar]
- 58.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin–angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naraghi R, Geiger H, Crnac J, Huk W, Fahlbusch R, Engels G, Luft FC. Posterior fossa neurovascular anomalies in essential hypertension. Lancet. 1994;344:1466–1470. doi: 10.1016/s0140-6736(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 60.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 61.Blaustein MP, Hamlyn JM. Endogenous ouabain: implications for cardiovascular disease diagnosis and therapy. Md Med J. 1992;41:501–504. [PubMed] [Google Scholar]
- 62.Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzani C, Ferrandi M, et al. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension. 2001;38:198–203. doi: 10.1161/01.hyp.38.2.198. [DOI] [PubMed] [Google Scholar]
- 63.Hamlyn JM, Ringel R, Schaeffer J, Levinson PD, Hamilton BP, Kowarski AA, Blaustein MP. A circulating inhibitor of (Na++ K+)ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 64.Gavras H, Gavras I. Salt-induced hypertension: the interactive role of vasopressin and of the sympathetic nervous system. J Hypertens. 1989;7:601–606. doi: 10.1097/00004872-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Mahomed FA. On chronic Bright's disease, and its essential symptoms. Lancet. 1879;I:398–404. [Google Scholar]
- 66.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 67.Campese VM, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int. 2006;69:967–973. doi: 10.1038/sj.ki.5000177. [DOI] [PubMed] [Google Scholar]
- 68.Muirhead EE. Renal vasodepressor mechanisms: the medullipin system. J Hypertens Suppl. 1993;11:S53–S58. [PubMed] [Google Scholar]
- 69.Khosla UM, Johnson RJ. Hypertension in the hemodialysis patient and the “lag phenomenon”: insights into pathophysiology and clinical management. Am J Kidney Dis. 2004;43:739–751. doi: 10.1053/j.ajkd.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 70.Conn JW. Primary aldosteronism. J Lab Clin Med. 1955;45:661–664. [PubMed] [Google Scholar]
- 71.Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 72.Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43:518–524. doi: 10.1161/01.HYP.0000116223.97436.e5. [DOI] [PubMed] [Google Scholar]
- 73.Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 74.Young JH, Chang YP, Kim JD, Chretien JP, Klag MJ, Levine MA, et al. Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet. 2005;1:e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lowenstein F. Blood-pressure in relation to age and sex in the tropics and subtropics. Lancet. 1961;1:389–392. [Google Scholar]
- 76.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 77.Kim SW, Wang W, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. Increased expression of ENaC subunits and increased apical targeting of AQP2 in the kidneys of spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F957–F968. doi: 10.1152/ajprenal.00413.2004. [DOI] [PubMed] [Google Scholar]
- 78.Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, et al. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. doi: 10.1086/510918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 80.Province MA, Kardia SL, Ranade K, Rao DC, Thiel BA, Cooper RS, et al. A meta-analysis of genome-wide linkage scans for hypertension: the National Heart, Lung and Blood Institute Family Blood Pressure Program. Am J Hypertens. 2003;16:144–147. doi: 10.1016/s0895-7061(02)03248-x. [DOI] [PubMed] [Google Scholar]
- 81.Robinson SC, Brucer M. Range of normal blood pressure. A statistical and clinical study of 11,383 persons. Arch Intern Med. 1939;64:409–444. [Google Scholar]
- 82.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases. Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 83.Johnson RJ, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Feig DI, Herrera-Acosta J. Subtle renal injury is likely a common mechanism for salt-sensitive essential hypertension. Hypertension. 2005;45:326–330. doi: 10.1161/01.HYP.0000154784.14018.5f. [DOI] [PubMed] [Google Scholar]
- 84.Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–440. doi: 10.1016/j.amjhyper.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 85.Kanellis J, Nakagawa T, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B, Johnson RJ. A single pathway for the development of essential hypertension. Cardiol Rev. 2003;11:180–196. doi: 10.1097/01.CRD.0000077361.00668.14. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 87.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, et al. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 88.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 89.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension. 1999;34:151–159. doi: 10.1161/01.hyp.34.1.151. [DOI] [PubMed] [Google Scholar]
- 90.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension. 1999;33:1013–1019. doi: 10.1161/01.hyp.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 91.Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol. 2001;281:F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 92.Suga SI, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, et al. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol. 2001;281:F620–F629. doi: 10.1152/ajprenal.2001.281.4.F620. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 94.Tracy RE, Overll EO. Arterioles of perfusion-fixed hypertensive and aged kidneys. Arch Pathol. 1966;82:526–534. [PubMed] [Google Scholar]
- 95.Andoh TF, Johnson RJ, Lam T, Bennett WM. Subclinical renal injury induced by transient cyclosporine exposure is associated with salt-sensitive hypertension. Am J Transplant. 2001;1:222–227. doi: 10.1046/j.1600-6135.ajt10305.x. [DOI] [PubMed] [Google Scholar]
- 96.Svendsen UG. The importance of thymus on the degree of increased blood pressure and vascular disease in mice with DOCA and salt hypertension. Acta Med Scand Suppl. 1976;602:19–21. doi: 10.1111/j.0954-6820.1977.tb07635.x. [DOI] [PubMed] [Google Scholar]
- 97.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 98.Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, et al. Angiotensin II, interstitial inflamation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006;291:F1281–F1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 99.DiBona GF. Renal neural mechanisms in salt-sensitive hypertension. Blood Press Suppl. 1995;2:81–87. [PubMed] [Google Scholar]
- 100.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 101.Franco M, Tapia E, Santamaria J, Zafra I, Garcia-Torres R, Gordon KL, et al. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol. 2001;12:2263–2271. doi: 10.1681/ASN.V12112263. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 103.Vanegas V, Ferrebuz A, Quiroz Y, Rodriguez-Iturbe B. Hypertension in Page (cellophane-wrapped) kidney is due to interstitial nephritis. Kidney Int. 2005;68:1161–1170. doi: 10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kappa B prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ. Interstitial inflammation, sodium retention, and the pathogenesis of nephrotic edema: a unifying hypothesis. Kidney Int. 2002;62:1379–1384. doi: 10.1111/j.1523-1755.2002.kid561.x. [DOI] [PubMed] [Google Scholar]
- 106.Alvarez V, Quiroz Y, Nava M, Pons H, Rodriguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 107.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand. 2003;179:217–223. doi: 10.1046/j.0001-6772.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 109.Herrera J, Ferrebuz A, Macgregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 110.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 112.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 113.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 114.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 115.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 116.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 117.Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, Papajcik D, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 118.Tapia E, Franco M, Sanchez-Lozada LG, Soto V, Avila-Casado C, Santamaria J, et al. Mycophenolate mofetil prevents arteriolopathy and renal injury in subtotal ablation despite persistent hypertension. Kidney Int. 2003;63:994–1002. doi: 10.1046/j.1523-1755.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 119.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 120.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron PO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59:230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 121.Black M, Briscoe T, Constantinou M, Kett M, Bertram J. Is there an association between adult blood pressure and nephron number or surface area? Kidney Int. 2004;65:582–588. doi: 10.1111/j.1523-1755.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 122.Skov K, Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand. 2004;181:397–405. doi: 10.1111/j.1365-201X.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- 123.Helmchen LA, Henderson RM. Changes in the distribution of body mass index of white US men, 1890−2000. Ann Hum Biol. 2004;31:174–181. doi: 10.1080/03014460410001663434. [DOI] [PubMed] [Google Scholar]
- 124.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Fructose-induced hyperuricemia as a casual mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2006;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 126.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 127.Brands MW, Garrity CA, Holman MG, Keen HL, Alonso-Galicia M, Hall JE. High-fructose diet does not raise 24-h mean arterial pressure in rats. Am J Hypertens. 1994;7:104–109. doi: 10.1093/ajh/7.1.104. [DOI] [PubMed] [Google Scholar]
- 128.D'Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague–Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 129.Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, Morris M. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006;130:41–50. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Verma S, Bhanot S, McNeill JH. Sympathectomy prevents fructose-induced hyperinsulinemia and hypertension. Eur J Pharmacol. 1999;373:R1–R4. doi: 10.1016/s0014-2999(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 131.Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 132.Blakely SR, Hallfrisch J, Reiser S, Prather ES. Long-term effects of moderate fructose feeding on glucose tolerance parameters in rats. J Nutr. 1981;111:307–314. doi: 10.1093/jn/111.2.307. [DOI] [PubMed] [Google Scholar]
- 133.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 134.Israel KD, Michaelis OEt, Reiser S, Keeney M. Serum uric acid, inorganic phosphorus, and glutamic-oxalacetic transaminase and blood pressure in carbohydrate-sensitive adults consuming three different levels of sucrose. Ann Nutr Metab. 1983;27:425–435. doi: 10.1159/000176714. [DOI] [PubMed] [Google Scholar]
- 135.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980;33:273–278. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- 136.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr. 1989;49:832–839. doi: 10.1093/ajcn/49.5.832. [DOI] [PubMed] [Google Scholar]
- 137.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 138.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972;21:713–721. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 139.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 140.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 141.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 142.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 143.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 144.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, et al. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 145.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 146.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 147.Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, Johnson RJ. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004;66:281–287. doi: 10.1111/j.1523-1755.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 148.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 149.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classical antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]