Abstract
We have identified a novel population of cells in the subventricular zone (SVZ) of the mammalian brain that expresses β4 tubulin (βT4) and has properties of primitive neuroectodermal cells. βT4 cells are scattered throughout the SVZ of the lateral ventricles in adult human brain and are significantly increased in the SVZs bordering demyelinated white matter in multiple sclerosis brains. In human fetal brain, βT4 cell densities peak during the latter stages of gliogenesis, which occurs in the SVZ of the lateral ventricles. βT4 cells represent <2% of the cells present in neurospheres generated from postnatal rat brain but >95% of cells in neurospheres treated with the anti-mitotic agent Ara C. βT4 cells produce oligodendrocytes, neurons, and astrocytes in vitro. We compared the myelinating potential of βT4-positive cells with A2B5-positive oligodendrocyte progenitor cells after transplantation (25,000 cells) into postnatal day 3 (P3) myelin-deficient rat brains. At P20, the progeny of βT4 cells myelinated up to 4 mm of the external capsule, which significantly exceeded that of transplanted A2B5-positive progenitor cells. Such extensive and rapid mature CNS cell generation by a relatively small number of transplanted cells provides in vivo support for the therapeutic potential of βT4 cells. We propose that βT4 cells are an endogenous cell source that can be recruited to promote neural repair in the adult telencephalon.
Introduction
Strategies for cell replacement represent a promising direction for the treatment of neurodegenerative diseases. The best example of CNS cell replacement and repair is the generation of myelinating oligodendrocytes in lesions of multiple sclerosis (MS). Unfortunately, many MS lesions do not remyelinate. Myelin repair requires the generation of new oligodendrocytes from oligodendrocyte progenitor cells (OPCs). OPCs are present in MS lesions (Wolswijk, 1998; Lucchinetti et al., 1999; Chang et al., 2000; Wilson et al., 2006), and some chronic MS lesions contain a limited number of new oligodendrocytes (Chang et al., 2002). OPC proliferation, however, is limited (Temple and Raff, 1986; Barres and Raff, 1994). Primitive cell populations located in the subventricular zone (SVZ) of the lateral ventricles are capable of producing transient amplifying cells that can generate significant numbers of new committed progenitor cells (Loeffler and Roeder, 2002; Joseph and Morrison, 2005). Therapeutic stimulation of endogenous stem cells, therefore, has great potential for promoting oligodendrocyte production and remyelination in individuals with MS.
Cells with characteristics of stem cells can be isolated from the SVZ of the mammalian brain (Doetsch et al., 1999; Kukekov et al., 1999; Roy et al., 2000; Menn et al., 2006). One population of such cells in the SVZ expresses glial fibrillary acidic protein (GFAP) and can generate multipotent neurospheres in vitro (Doetsch et al., 1997; Ganat et al., 2006). GFAP-positive cells are not the only source of neural precursors in the SVZ. The diverse spatial and temporal origin of oligodendrocyte lineage cells suggests that the mammalian CNS harbors several populations of stem cells with regionally and functionally specific potentials (Spassky et al., 1998; Kessaris et al., 2006; Menn et al., 2006; Yue et al., 2006).
A common model for studying primitive neural precursors in vitro is the neurosphere assay (Reynolds and Weiss, 1992; Reynolds and Rietze, 2005). When plated on adhesive surfaces, neurospheres generate neurons, astrocytes, and oligodendrocytes. Although neurosphere production was originally thought to be a characteristic of stem cells, transient amplifying cells and progenitor cells can also generate neurospheres (Doetsch et al., 2002; Belachew et al., 2003; Goldman and Sim, 2005). An assay system that selects primitive neural precursors from progenitor cells would represent a significant advance in stem cell research.
Here we have identified a population of cells within the SVZ of human brain that expresses β4 tubulin (βT4). The density of βT4 cells peaks during the latter stage of gliogenesis in the developing human brain and then decreases to adult densities shortly after birth. βT4 cells are increased adjacent to demyelinated lesions in MS brains. βT4 cells are also present in neurospheres derived from the perinatal rat brain and can be enriched to >95% homogeneity under growth-limiting conditions. Neurospheres derived from βT4 cell-enriched cultures produce oligodendrocytes, neurons, and astrocytes in vitro and myelinating oligodendrocytes when transplanted into the myelin-deficient (md) rat brain. Collectively, these results provide the first characterization of a primitive neural precursor cell that is capable of cell replacement in the mammalian brain.
Materials and Methods
Human tissue.
Human tissue studies were approved by the Cleveland Clinic Institutional Review Board. MS brains were obtained from prospectively consented donors and characterized as described previously (Trapp et al., 1997, 1998; Chang et al., 2002). The adult control tissues were obtained from autopsies of patients without neurological disease performed at the Cleveland Clinic. Additional clinical details are listed in Tables 1 and 2. Developmental studies were performed on autopsy tissues from the Cleveland Clinic (17 postnatal months, death caused by acute myocardial infarction secondary to congenital heart disease) and Akron Children's Hospital (19 weeks after conception, other demographics unknown).
Table 1.
Characteristics of MS tissues examined
| Pt # | MS type | Disease duration | EDSS | Sex/age | Race | PMI (h) | # Blocks a |
|---|---|---|---|---|---|---|---|
| 1 | SPMS | 15 years | 9.5 | M/53 | C | 17.0 | 2 |
| 2 | RRMS | 8 months | 6.0 | M/43 | C | 2.8 | 1 |
| 3 | SPMS | 23 years | 8.0 | M/46 | C | 3.0 | 5 |
| 4 | PPMS | 15 years | 6.5 | F/57 | C | 5.5 | 2 |
| 5 | SPMS | 33 years | 9.5 | M/56 | C | 3.0 | 5 |
| 6 | SPMS | 9 years | 8.0 | F/63 | C | 4.6 | 3 |
| 7 | SPMS | 35 years | 9.5 | F/61 | B/AA | 4.8 | 1 |
| 8 | SPMS | 25 years | 7.5 | M52 | C | 4.8 | 1 |
a Every tissue block contained a periventricular demyelinated lesion. Seven blocks from four brains (1–4) also contained adjacent nonlesion areas.
SPMS, Secondary progressive multiple sclerosis; RRMS, relapsing remitting MS; PPMS, primary progressive MS; EDSS, expanded disability status scale; M, male; F, female; C, Caucasian; B/AA, black/African American; PMI, postmortem interval.
Table 2.
Characteristics of adult control tissues examined
| Pt # | Cause of death | Sex/age | Race | PMI (h) | # Blocks |
|---|---|---|---|---|---|
| 1 | Disseminated intravascular coagulation | F/47 | B/AA | 15 | 2 |
| 2 | Acute myocardial infarction | F/65 | B/AA | 7 | 2 |
| 3 | Cardiopulmonary arrest | F/53 | B/AA | 36 | 3 |
| 4 | Acute pericardial hemorrhage | M/65 | C | 11 | 7 |
Every tissue block contained periventricular tissue. C, Caucasian; B/AA, black/African American; F, female; M, male; PMI, postmortem interval.
Cell culture.
Rodent studies were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. In primary neurosphere culture, ∼15 postnatal day 4 (P4) rat pups were used for each experiment. Each pup was anesthetized by hypothermia and decapitated. The head was sterilized with 70% isopropanol and transferred to a laminar flow hood equipped with a dissecting microscope. The skull was opened, and a 2 mm coronal slice was excised from the anterior cerebrum and discarded. The next 1–1.5 mm coronal slice was excised and transferred to a Sylgard-coated (Dow Corning) Petri dish containing minimal essential medium (MEM) (Invitrogen) on ice. Under the dissecting microscope, sections were attached to the dish with minutien pins (Fine Science Tools), and the lateral and medial SVZs were dissected using fine forceps and a dissecting knife. Dissected tissue was transferred to a Petri dish containing MEM. This process was repeated for each pup used. The combined dissected SVZs were minced with a scalpel blade, transferred to a 15 ml Falcon tube (final MEM volume of 1.1 ml), and 400 μl of trypsin (final concentration of 0.05%; Cellgro/Mediatech) and 500 μl of DNase (final concentration of 0.8 U/μl; Sigma-Aldrich) were added. The mixture was incubated for 12–15 min at 37°C in a CO2 incubator, agitating the tube every 5 min. The trypsin reaction was then quenched with 300 μl of fetal bovine serum (FBS) (Hyclone). Plugged Pasteur pipettes (fire polished to approximately one-third diameter of the original size) were pre-wet with MEM, and cells were then separated by quick and gentle trituration (7–10 times) to yield a suspension of small aggregates. Tubes were fully filled with MEM and centrifuged at 166 × g for 4 min at room temperature. The supernatant was then aspirated, and pellets were resuspended by triturating ∼10 times in ∼1000 μl of neurosphere medium (defined below) with the same polished Pasteur pipette used previously. The suspension was divided into three uncoated 75 cm2 flasks (approximately five pup brain SVZs per flask) in a final volume of 15 ml/flask and incubated for 4 d at 37°C, 5% CO2 with no medium changes. Neurosphere medium was composed of DMEM/F-12 (Cellgro/Mediatech) supplemented with the following: 2 mm l-glutamine (GlutaMAX; Invitrogen), 100 IU/ml penicillin, 100 μg/ml streptomycin (Mediatech), 1% N2 supplement (Invitrogen), 20 ng/ml epidermal growth factor (EGF) (Sigma-Aldrich), and 20 ng/ml FGF2 (R & D Systems).
Secondary neurosphere culture.
Coverslips (12 mm circular; Fisher Scientific) were coated with 0.1% poly-l-lysine (P1274; Sigma-Aldrich) for 40–60 min at room temperature, rinsed with distilled water three times, transferred to 24-well dishes, and air dried for at least 5 min. Each flask of primary neurospheres was pelleted by centrifugation in a 15 ml Falcon tube (4 min at 166 × g). Supernatant was discarded, and pellets were transferred with a plugged Pasteur pipette into a single Falcon tube for digestion (total final volume of 1.6 ml). Trypsin (400 μl, same stock as above) was added and incubated for 15 min at 37°C. Digestion was quenched by adding 300 μl of FBS. Digested primary neurospheres were then disaggregated using a fire-polished pipette with a diameter slightly larger (approximately two-thirds size of original pipette) than that used for triturating SVZ tissue. Pellets were triturated ∼15 times, resulting in small aggregates. The tube was then filled with medium and spun for 4 min at 166 × g. Supernatant was removed, and the pellet was triturated 5–10 times in ∼0.5 ml of neurosphere medium. Cells were counted, and the suspension was diluted to a concentration of ∼150,000–200,000 cells per 70–80 μl. This volume was plated into the center of the coverslips. After incubation for 20 min at room temperature, 500 μl of preheated neurosphere medium containing 10 μm cytosine β-d-arabinofuranoside hydrochloride (Ara C) (Sigma) was added and incubated at 37°C, 5% CO2 for 2 d. The medium was then replaced with fresh preheated Ara C-containing medium for an additional 2 d. After 4 total days of Ara C treatment, coverslips were gently rinsed twice with 800 μl of freshly prepared and preheated neurosphere medium. After this, 800 μl of cold neurosphere medium was added, and dishes were placed on ice for 15–20 min. Medium was then carefully jetted at the edge of the coverslip using a 1000 μl pipette, until the confluent layer of cells on the coverslip detached from the glass surface. (A population of cells remained adherent to the coverslip after this treatment. Immunocytochemistry showed that the majority were GFAP-positive astrocytes.) The suspended cells were transferred to a 15 ml Falcon tube and centrifuged for 4 min at 166 × g. The supernatant was aspirated, 1 ml of neurosphere medium was added, and the pellet was gently resuspended by trituration (three to four times). This suspension was transferred to an uncoated 75 cm2 flask in a total volume of 15 ml and incubated for 3 d. Differentiation of secondary neurospheres was achieved by plating secondary neurospheres onto poly-l-lysine-coated coverslips in neurosphere medium for 7 d.
A2B5 immunopanning.
A2B5-positive cells were isolated by immunopanning of P4 dissociated forebrain cells as described by Barres et al. (1992). Briefly, 100 mm dishes were coated with 5 μg/ml goat anti-mouse IgM antibody (Cappel) in 20 ml of 0.05 m Tris/HCl, pH 9.5, at 4°C overnight. The plates were washed three times with PBS. An A2B5 hybridoma supernatant was prepared from Clone 105 from the American Type Culture Collection by the Lerner Research Institute Hybridoma Core. Six milliliters of a 1:3 dilution of supernatant stock were added to each plate, incubated at room temperature for 2 h, and rinsed three times with PBS. Dissociated cells from two P4 rat forebrains were diluted in 24 ml of DMEM/F-12, divided into four coated plates, and incubated at 37°C, 5% CO2 for 30 min. The fluid was removed, plates were washed three times with DMEM/F-12, and adherent cells were dislodged by trituration. These cells were centrifuged, resuspended in DMEM/F-12, and quantified. Cell concentration was adjusted to ∼25,000 cells per 5 μl for transplantation. Aliquots of immunopanned cells were also immediately plated onto coverslips for double labeling with A2B5 and polysialic acid-neural cell adhesion molecule (PSA-NCAM) antibodies.
Transplantation.
md rats (gift from Dr. Wendy B. Macklin, University of Colorado Denver School of Medicine, Aurora, CO) were transplant recipients of βT4 secondary neurospheres or A2B5-positive cells. P3 rats were anesthetized by hypothermia, and ∼25,000 βT4 cells (120 secondary neurospheres) or immunopanned A2B5-positive cells in a volume of 5 μl of DMEM/F-12 were stereotactically injected into the left striatum (0.4 mm anterior to bregma, 2.3 mm lateral to midline, and 3.0 mm deep). After 17 d, rats were transcardially perfused with 4% paraformaldehyde. Serial 30 μm coronal sections of the transplanted cerebral hemispheres were cut, and every fourth section was immunofluorescently stained for proteolipid protein (PLP). Sections spaced at 240 μm intervals were imaged using confocal microscopy, and myelin areas/volumes were measured directly in NIH ImageJ software. Three-dimensional reconstructions were generated using Reconstruct software (Fiala, 2005) and assembled in Photoshop software (Adobe Systems) to provide graphical representations of myelin distributions.
Immunostaining.
Tissue immunohistochemistry was performed on paraformaldehyde-fixed free-floating sections (Trapp et al., 1997, 1998; Chang et al., 2002). Sections used for double-labeling experiments were incubated with two primary antibodies for 3–5 d, followed by fluorescently labeled and biotinylated secondary antibodies for 1–2 h. Antibodies and dilutions used on tissue sections were as follows: class IV β tubulin (1:10,000; Sigma), Tuj1 (1:1000; Covance), GFAP (1:10,000; Dako), Iba1 (1:4000; gift from Dr. Shinichi Kohsaka, National Institute of Neuroscience, Tokyo, Japan), NG2 (1:250; Millipore Bioscience Research Reagents), PLP (1:400; Agmed), PSA-NCAM (1:200; BD Pharmingen), goat anti-rat IgG biotinylated (1:1000; Vector Laboratories), goat anti-mouse IgG biotinylated (1:1000; Vector Laboratories), goat anti-mouse IgG Alexa (1:1000; Invitrogen), goat anti-rabbit FITC (1:1000; Invitrogen), goat anti-mouse IgG1 FITC (1:1000; Invitrogen), goat anti-mouse IgG2a Alexa (1:1000; Invitrogen), goat anti-rabbit IgG Alexa (1:1000; Invitrogen), goat anti-rabbit IgG biotinylated (1:1000; Vector Laboratories), and goat anti-mouse IgM biotinylated (1:1000;Vector Laboratories).
Neurospheres from suspension cultures were attached to coverslips for 15 min before fixation with 4% paraformaldehyde for 30 min, treatment with 10% goat normal serum/0.1% Triton X-100/PBS (1 h), and sequential incubation with primary and appropriate fluorescently labeled secondary antibodies. Staining of bromodeoxyuridine (BrdU)-treated cultures included pretreatment with 2N HCl (1 h) and 0.1 m sodium borate, pH 8.6 (30 min), followed by application of FITC-conjugated monoclonal anti-BrdU antibody (1:20; Abcam) overnight at 4°C. The following other primary and secondary antibodies and dilutions we used included the following: class IV β tubulin (1:15,000; Sigma), Tuj1 (1:200; Covance), GFAP (1:10,000; Dako), O4 (1:5; gift from Dr. Robert Miller, Case Western Reserve University School of Medicine, Cleveland, OH), A2B5 (1:40; gift from Dr. Robert Miller), NG2 (1:100; Millipore Bioscience Research Reagents), PLP (1:1000; Agmed), PSA-NCAM (1:100; BD Pharmingen), goat anti-rat IgG biotinylated (1:1000; Vector Laboratories), goat anti-mouse IgG Alexa (1:1000; Invitrogen), goat anti-rabbit FITC (1:1000; Invitrogen), goat anti-mouse IgM FITC (1:500; Southern Biotechnology Associates), and goat anti-rabbit IgG Alexa (1:1000; Invitrogen). 4′,6-diamidino-2-phenylindole (DAPI) was included in the mounting medium (Vectashield; Vector Laboratories) for nuclear detection.
Microscopy.
Bright-field and epifluorescence images were obtained using a Leica DMR microscope (W. Nuhsbaum Inc.) equipped with an Optronix Magnafire digital camera and processed using Photoshop software (Adobe Systems). Double immunofluorescence was evaluated by confocal microscopy. Confocal microscopy was performed on a Leica TCS-NT microscope using 63× [1.4 numerical aperture (NA)] and 40× (1.0 NA) lenses or on a Leica SP5 confocal microscope using 63× (1.4 NA), 40× (1.25 NA), and 10× (0.4 NA) lenses. Myelin volumes were measured from serial 30 μm coronal sections of rat brain, spaced as 240 μm intervals, and immunofluorescently stained for PLP. Areas with myelin were imaged as single confocal sections at 10× (pixel density, 2048 × 2048) on the SP5 confocal microscope. In NIH Image J software, regions of interest that included the myelin were created and then automatically thresholded to count only fluorescent pixels. Myelin areas per section were interpolated across serial sections to provide an estimate of myelin volume in each brain. For three-dimensional reconstructions, selected slices were imaged at 2.5× in an epifluorescence microscope, and myelin was traced and reconstructed using Reconstruct software (Fiala, 2005).
Statistical analyses.
Nonpaired Student's t tests were used for all statistical comparisons (α = 0.05). In Figure 1 E, βT4 cells in human SVZ were counted, and data were expressed with respect to length of SVZ examined (cells per millimeter). Twenty periventricular demyelinated lesions from eight MS brains and 14 periventricular areas from four control brains were evaluated. Seven lesions from four of the brains had adjacent nonlesion areas for analysis. For comparison of MS and control βT4 cells, all counts from each brain were combined (n = 8 for MS and 4 for control). The percentage of βT4-positive cells was determined in control and Ara C-treated cultures by counting βT4-positive and DAPI-positive cells in 10 randomly selected fields using standard fluorescence microscopy. Data from three independent experiments were used for statistics. In supplemental Figure S1C (available at www.jneurosci.org as supplemental material), the percentage of βT4-positive cells was determined in control and Ara C-treated cultures by counting βT4-positive and DAPI-positive cells in 10 randomly selected fields using standard fluorescence microscopy. Data from three independent experiments were used for statistics.
Figure 1.
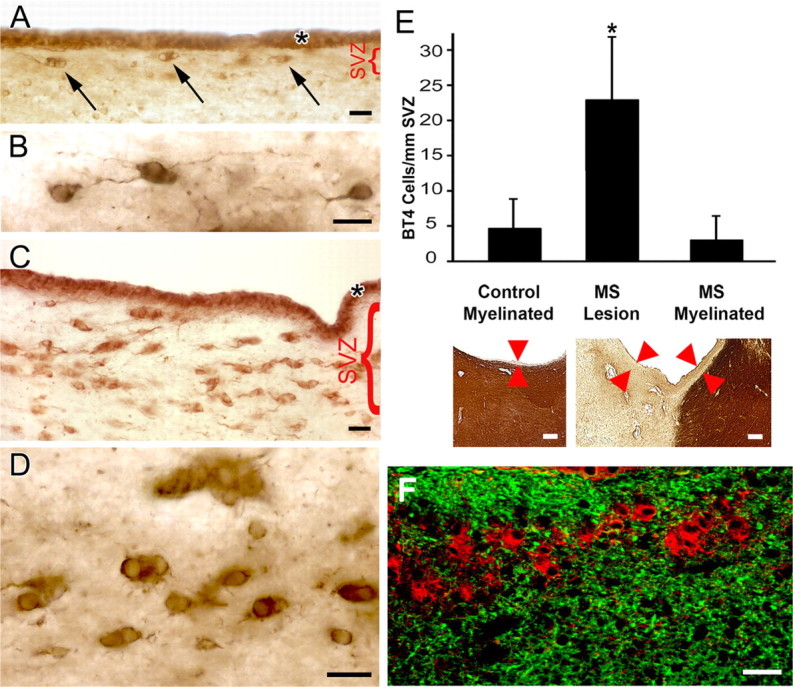
Antibodies to βT4 identify a population of undifferentiated cells in the SVZ of the adult human brain. The SVZ in control brain contains sparse cells that label intensely with antibody to βT4 (A, arrows). βT4-positive cells in the SVZ have scant cytoplasm and occasional thin processes (B). The SVZ adjacent to demyelinated lesions in MS brain contains numerous βT4 cells that are often in doublets and clusters (C, D). The asterisks in A and C show that ciliated ependymal cells are also βT4-positive. The bar graph in E demonstrates that the number (mean ± SD) of βT4 cells in SVZ adjacent to lesions of MS is significantly increased (*) compared with SVZs bordering myelinated white matter (p < 0.001) or in control (p < 0.001) brains. The images below show PLP immunostaining of a control specimen (left) and an MS specimen (right) that contains a demyelinated lesion on the left and adjacent myelinated white matter on the right. For βT4 cell quantification, the SVZ is defined as the distance between the ependyma and PLP-immunopositive myelinated white matter (arrowheads). This distance is commonly larger in MS brains compared with control brains. Data are expressed as number of cells in the SVZ divided by total length of SVZ present on the slide. F shows that βT4 cells (red) do not express the astrocytic marker GFAP (green). Scale bars: A–D, F, 20 μm; E, 200 μm.
Results
βT4-positive cells in the human SVZ
We previously detected βT4 in rat oligodendrocytes and discussed in detail the tissue distribution and difficulties in ascribing cell-specific functions of β tubulin isotypes (Terada et al., 2005). Application of βT4 antibodies to adult human brain sections demonstrated three distinct cell populations: oligodendrocytes (which stained weakly), ependymal cells, and a previously unidentified cell population in the SVZ (Fig. 1 A, arrows). We compared the distribution of βT4 in tissue sections from normal adult brain and from brains of individuals with MS. In adult control brains, βT4 antibodies intensely stained a population of cells that lined the SVZ of the lateral ventricles (Fig. 1 A, arrows). We call this population of cells “βT4 cells.” βT4 cells often appeared as doublets (Fig. 1 A), had sparse perinuclear cytoplasm, and, when viewed at higher magnification, had thin processes (Fig. 1 B). Compared with control brains, βT4 cells were more abundant in SVZs that bordered demyelinated areas in MS brains (Fig. 1 C), and doublets and clusters of cells were more frequent (Fig. 1 D). The average number of βT4 cells in the SVZ of adult control brain was 4/mm SVZ length and was not different from that in SVZs bordering nonlesion white matter in sections from MS brains (3 cells/mm SVZ length; p = 0.47) (Fig. 1 E). βT4 cell density in SVZs that bordered demyelinated regions of the MS brains was 23 cells/mm SVZ length and was significantly increased compared with both adjacent nonlesion areas (p < 0.001) and control brain SVZs (p < 0.001). These data support the concept that βT4 cells represent a dynamic cell population in the adult human SVZ that can expand locally in response to demyelination and oligodendrocyte destruction.
βT4-positive cells in the human SVZ do not express astrocytic, microglial, oligodendrocytic, or neuronal markers
To phenotype βT4-positive cells, sections of human brain SVZ were stained for βT4 and markers specific for or enriched in characterized CNS cells. βT4 cells did not express detectable levels of GFAP (Fig. 1 F), a protein expressed by a subpopulation of neuroectodermal stem cells and mature astrocytes (Doetsch et al., 1997). βT4 cells did not colabel with markers for microglia (Iba1) (Fig. 2 A) or mature oligodendrocytes (PLP) (Fig. 2 B), and the distribution and morphology of βT4-positive cells (Fig. 2 C) was different from OPCs expressing NG2 (Fig. 2 D) and neuronal progenitor cells expressing PSA-NCAM (Fig. 2 E) (Alvarez-Buylla and Garcia-Verdugo, 2002; Bonfanti, 2006). A subset of ependymal cells expressed βT4 (Fig. 1 A,C, asterisks), consistent with previous observations of this tubulin isotype in ciliated cells (Woo et al., 2002).
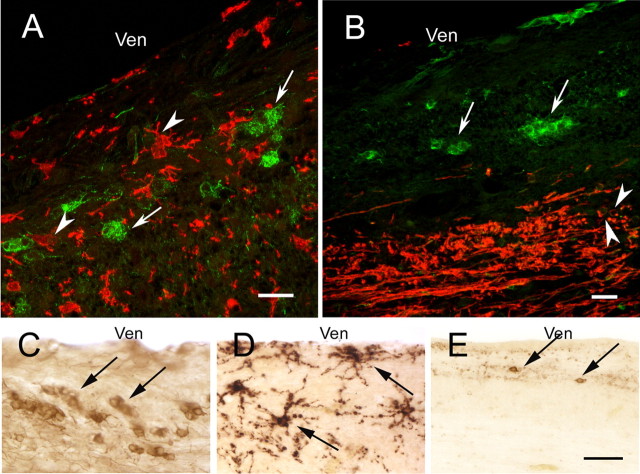
Figure 2.
βT4 cells in the subventricular zone of the adult human brain do not express markers specific for characterized CNS cells. βT4 cells (A, B, β4 tubulin, green, arrows) do not express markers specific for microglia (A, Iba1, red, arrowheads) or oligodendrocytes (B, PLP, red, arrowheads). In serially cut sections stained for individual antigens, the morphology and distribution of βT4 cells (C, arrows) are distinct from NG2-positive oligodendrocyte progenitor cells (D, arrows) or PSA-NCAM-positive committed neuronal progenitor cells (E, arrows). Ven, Lateral ventricle. Scale bars: A, B, 20 μm; C–E, 50 μm.
βT4 cells have a dynamic spatial and temporal distribution in the developing telencephalon
The combination of location and immunophenotype described above suggests that βT4 cells represent a pre-progenitor cell related to oligodendrogenesis and, as such, may have a dynamic distribution in the developing human brain. Generation of telencephalic glial progenitor cells occurs in several waves during mammalian brain development (Kessaris et al., 2006). It begins ventrally in the medial ganglionic eminence and progresses dorsally so that, in the latter stages, the SVZ of the entire lateral ventricular area contributes to gliogenesis. The density and distribution of SVZ βT4 cells in sections from fetal human brain were correlated with latter stages of gliogenesis. At 19 weeks after conception (Fig. 3 A–D), for example, the density of SVZ βT4-positive cells is high in the most ventral (inferior) regions of the lateral ventricles (Fig. 3 B), intermediate in the septal (Fig. 3 C, top) and callosal (Fig. 3 D, top) walls, and very low in the expanded SVZ (Fig. 3 A,C,D, asterisks), which overlies the caudate nucleus and is enriched in Tuj1-positive neuronal progenitors. Double labeling of this region demonstrates that βT4 cells are distinct from Tuj1-positive cells (Fig. 3 E). SVZ βT4 cells do not express detectable levels of GFAP, consistent with adult human brain (Fig. 3 F). By 17 postnatal months, SVZ βT4 cells are present in all regions of the lateral ventricles (Fig. 3 G), in which their density is slightly higher (∼9 cells/mm SVZ) than in normal adult brain. In summary, βT4 cell density and distribution peak during the latter stage of gliogenesis in the developing human brain and then decrease to adult densities shortly after birth.
Figure 3.
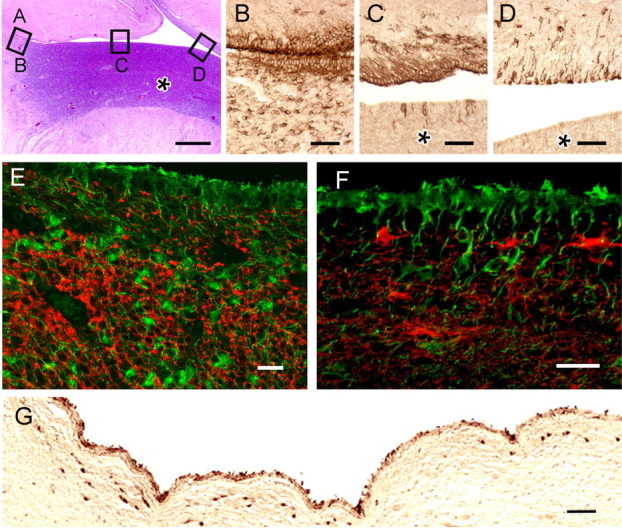
βT4 cells have a dynamic spatial and temporal distribution in the developing human telencephalon. A, Hematoxylin and eosin-stained section of cerebrum surrounding lateral ventricle at 19 weeks after conception. Left is inferior (ventral), and top is medial. Asterisk (B, C, D) indicates the expansion of the SVZ overlying the caudate nucleus that is present at this age. Boxes identify the regions shown in B–D. βT4 cells are most abundant inferiorly (B) and more frequent in the septal (C, top) and callosal (D, top) walls compared with the lateral wall (C, D, bottom). E, F, βT4 cells do not show evidence of neuronal or astroglial phenotypes (E, βT4, green, Tuj1, red; F, βT4, green, GFAP, red). G, At 17 postnatal months, SVZ βT4 cells have a morphology and distribution similar to adult brain. Scale bars: A, 1 mm; B–D, 50 μm; E, F, 20 μm; G, 100 μm.
βT4-positive cells are a slowly proliferating primitive neural precursor population in the rodent periventricular forebrain
To further elucidate the properties of βT4 cells, we investigated their morphology and activity in neurospheres generated from neonatal rat brain SVZ (Fig. 4 A). Periventricular tissue from P4 rat brain was dissociated, placed into a standard neurosphere culture medium for 4 d, plated onto coverslips, and assayed for the presence of βT4 cells. βT4-positive cells were present in a small portion of primary neurospheres; when present, they represented the vast majority of the cells in the individual neurospheres (Fig. 4 A,B). In general, βT4 cell-enriched neurospheres were smaller than non-βT4 cell neurospheres; βT4 cells represented <2% (1.33 ± 2.06%) of the total primary neurosphere cells. We next investigated whether the βT4 cells were actively dividing as they formed primary neurospheres. After a 20 h BrdU pulse, nuclei in many cells in βT4-negative neurospheres were labeled (Fig. 4 C). In contrast, ∼95% of the βT4-positive neurospheres contained no BrdU-labeled cells; the remaining 5% had one or two BrdU-positive βT4 cells (Fig. 4 C), suggesting that βT4-containing neurospheres formed by homophilic cell adhesion and not by proliferation.
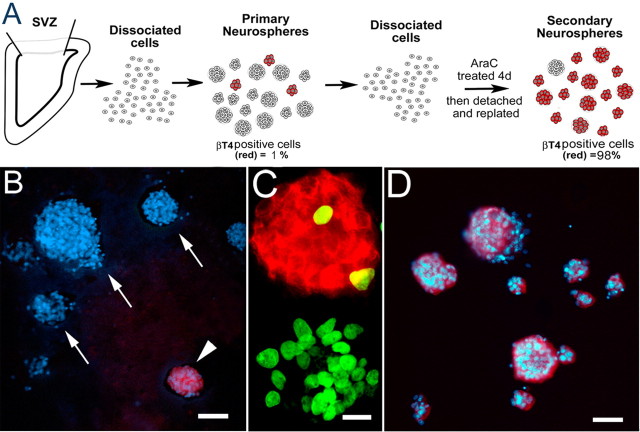
Figure 4.
Ara C treatment of neurosphere cells enriches for βT4-positive cells. A outlines the procedure for isolating neurospheres enriched in βT4-positive cells. B, Primary neurospheres stained for DAPI (blue) and βT4 (red) demonstrate that there are few βT4-positive neurospheres (arrowhead) and many βT4-negative neurospheres (arrows). C, After a 20 h BrdU pulse, many of the cells in βT4-negative neurospheres are dividing, whereas the vast majority of the βT4-positive neurospheres contained few to no BrdU-labeled cells (βT4, red; BrdU, green). D, Secondary neurospheres stained for DAPI (blue) and βT4 (red) show an abundance of βT4-positive cells after 4 d of Ara C treatment, in sharp contrast to the primary neurospheres (B). Scale bars: B, D, 50 μm; C, 20 μm.
The low level of βT4 cell proliferation and high rate of βT4-negative cell proliferation provided a mechanism for βT4 cell enrichment. Primary neurospheres were dissociated and grown in the presence or absence of the mitotic inhibitor Ara C for 4 d (Fig. 4 A). Treatment with Ara C resulted in a dramatic enrichment of βT4 cells. The marked proliferation of βT4-negative cells diluted βT4 cells to <0.03% of the total cells in the absence of Ara C (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In contrast, after 4 d of treatment with Ara C, 83.2% of the dissociated cells were βT4 positive (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). This represents a 2700-fold enrichment in plated βT4 cells (p < 0.001). This enrichment was enhanced when Ara C-treated cells were detached from the coverslips and placed into suspension culture for 4 d in the presence of EGF and FGF2, producing neurospheres. These secondary neurospheres were then attached to coverslips and stained for βT4 and nuclei. More than 95% of the secondary neurospheres were βT4 cell positive (Fig. 4 D). In these βT4 cell-positive secondary neurospheres, βT4 cells represented 98.9 ± 1.9% of the cells, a significant increase in purity compared with βT4 cells in primary neurospheres, which represented only 1.33 ± 2.06% (n = 40; p < 0.001). βT4 cell-enriched secondary neurospheres were relatively resistant to enzymatic and mechanical dissociation protocols, a property of purified cultures of embryonic stem cells (Thomson et al., 1998) and primordial germ cells (Shamblott et al., 1998). These observations support the hypothesis that the βT4 cell-enriched neurospheres were composed of slowly dividing primitive neural precursors and not transient amplifying or committed progenitor cells.
Secondary neurospheres generate neurons, astrocytes, and oligodendrocytes in vitro
To define the molecular characteristics of βT4 cells, primary and βT4 cell-enriched secondary neurospheres were adhered to coverslips, immediately fixed, and labeled with cell-type-specific antibodies. Similar to human brain tissue sections, βT4 cells did not express markers specific for astrocytes, OPCs (supplemental Fig. S2, available at www.jneurosci.org as supplemental material), oligodendrocytes, or neurons (data not shown). However, plating on coverslips for 6 h allowed migration of βT4 cells away from the neurosphere. After losing this contact inhibition, the βT4 cells incorporated BrdU and underwent cell division in 24 h (Fig. 5 A). One of the characteristics of organ-specific neural stem cells or primitive cells is the capability to produce all cell types of that organ. Therefore, we examined parallel cultures after seven days. βT4 cell-enriched secondary neurospheres generated Tuj1-positive neurons (Fig. 5 B), O4-positive oligodendrocytes (Fig. 5 C), and GFAP-positive astrocytes (Fig. 5 D). The proportion of these three cell types generated varied with media composition. Standard neurosphere media (N2, EGF/FGF) favored generation of astrocytes, whereas more oligodendrocytes and neurons were generated in the presence of sonic hedgehog (data not shown).
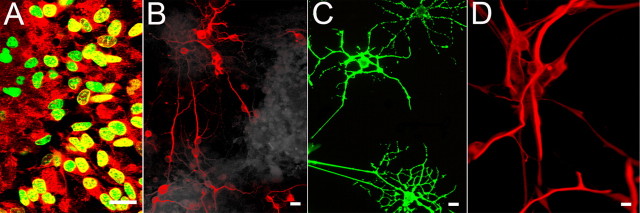
Figure 5.
βT4-enriched secondary neurospheres generate neurons, oligodendrocytes, and astrocytes in vitro. When attached to a substrate, secondary neurospheres proliferate and differentiate into mature CNS cells. A, Numerous βT4 cells (red) at the edge of plated secondary neurospheres incorporate BrdU (green). Seven days after plating, βT4-positive secondary neurospheres produce neurons (B, Tuj1), oligodendrocytes (C, O4), and astrocytes (D, GFAP). Scale bars, 20 μm.
Secondary neurospheres produce myelinating oligodendrocytes in vivo
To test the potential of βT4 cells to respond to in vivo cues and produce oligodendrocytes, 120 βT4 cell-enriched secondary neurospheres (25,000 βT4 cells) were injected into the right striatum of P3 md rat pups. The md rat has a mutation in the PLP gene (Boison and Stoffel, 1989), severe hypomyelination (Dentinger et al., 1982), and no detectable PLP-surrounding axons (Learish et al., 1999). The presence of PLP around axons after transplantation, therefore, reflects myelination by progeny of the βT4-positive secondary neurosphere cells. Because md rats die by P25, transplanted rats were killed at P20 and examined for PLP-positive myelin sheaths. During this 17 d time span, progeny of the injected βT4 cells migrated up to 4 mm and produced oligodendrocytes, which then myelinated axons throughout the external capsule on the injected side of the brain (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). To establish the extent of βT4 cell-derived myelination, seven βT4 cell-transplanted md rat brains were serially sectioned and stained for PLP. The three-dimensional distribution of PLP-positive myelin internodes was reconstructed and quantified (Fig. 6 A–D) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). PLP-positive myelin was present anterior, posterior, caudal, and rostral to the injection site at rostrocaudal distances of up to 4.0 mm. At sites closest to the injection site, thousands of axons in the external capsule were myelinated (Fig. 6 I). At sites distal to the injection site, premyelinating PLP-positive oligodendrocytes and oligodendrocytes in early stages of myelination were abundant (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), indicating that the repair potential of transplanted βT4 cell progeny had not been exhausted during the 17 d postinjection period. This extent of mature CNS cell generation and differentiation by a relatively small number of transplanted cells in a 17 d time span is unprecedented and provides in vivo support for the therapeutic potential of purified populations of βT4 cells.
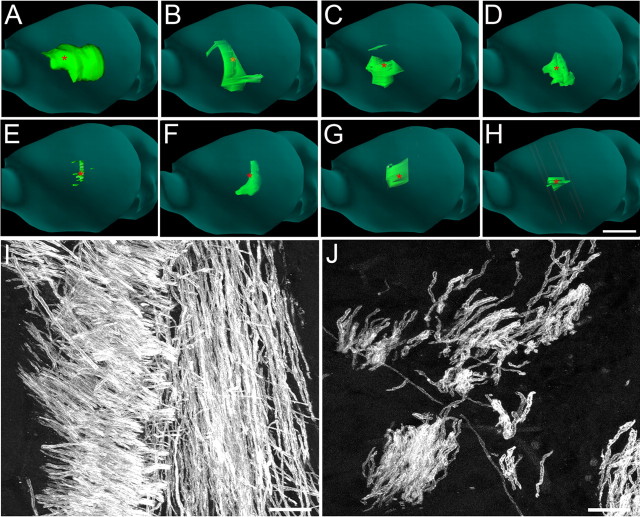
Figure 6.
βT4-enriched secondary neurospheres showed greater potential for myelination than A2B5-positive cells when transplanted into md rat brains. A–D, Representative serially sectioned and reconstructed P20 md rat brains stained for PLP (green) after injection of 120 βT4 cell-positive secondary neurospheres (∼25,000 βT4 cells) on P3. PLP-positive myelin was present anterior, posterior, caudal, and rostral to the injection site (marked by red asterisks) at rostrocaudal distances up to 4 mm (4 of 7 brains shown). E–H, Representative serially sectioned and reconstructed P20 md rat brains stained for PLP (green) after injection of 25,000 A2B5-positive cells on P3 (4 of 7 brains shown). Compared with secondary neurosphere transplantation, A2B5-positive cell transplantation produced less PLP-positive myelin, which was mostly scattered about the injection site. I, Thousands of myelinated axons were present in the external capsule close to the injection site of βT4-positive secondary neurospheres. J, Fewer myelinated axons were observed after A2B5-positive cell transplantation. Scale bars: A–H, 5 mm; I, J, 10 μm.
We next compared the myelination potential of transplanted βT4 cell-positive secondary neurospheres with transplanted A2B5-positive cells. The A2B5 monoclonal antibody recognizes a group of sialogangliosides expressed by immature neuroectodermal cells and is commonly used to isolate OPCs. A2B5-positive cells obtained from P4 wild-type rat forebrains are a mixture of PSA-NCAM-negative oligodendrocyte progenitors (77%) and PSA-NCAM-positive cells (23%) that include oligodendrocyte pre-progenitors (Grinspan and Franceschini, 1995; Ben-Hur et al., 1998) and possibly immature neurons (Windrem et al., 2004; Bonfanti, 2006). Seven md rat pups received intracerebral injections of 25,000 A2B5-positive cells. Brains were analyzed for PLP-positive myelin at P20 as described above. In the A2B5-positive cell transplanted brains, myelination was detected (Fig. 6 E–H,J) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material), but A2B5 cells produced only 37% of the myelin volume (0.06 ± 0.02 mm3) compared with md brains transplanted with βT4 cell-positive secondary neurospheres (0.18 ± 0.03 mm3; p = 0.014), and the rostrocaudal extent of myelination (1.75 ± 0.23 mm) was <60% that of βT4 cells (2.98 ± 0.24 mm; p = 0.003).
Transplantation of wild-type cells into md rat brain permits identification of myelinating oligodendrocytes originating from the transplanted cells. Neurons and astrocytes derived from the transplanted cells cannot be distinguished from endogenous md rat cells. Transplantation of βT4 neurospheres derived from enhanced green fluorescent protein-expressing rat cells (Inoue et al., 2005) resulted in astrocyte, but not neuron, production (data not shown). This is not surprising because neurogenesis is mostly complete at the time of transplantation.
Discussion
We describe a novel cell population in the human and rodent CNS SVZ. It is a newly identified source for producing mature oligodendrocytes in the CNS. These cells, identified by labeling with βT4 antibodies, express a number of characteristics commonly associated with primitive neuroepithelial cells. First, βT4 cells have a low basal rate of proliferation and can be enriched 2700-fold through antimitotic removal of transient amplifying and progenitor cells from rat brain primary neurospheres. Second, βT4 cells form neurospheres by homophilic adhesion and become contact inhibited, but they retain the capacity to produce oligodendrocytes, neurons, and astrocytes when attached to a substrate. Third, when transplanted into md rat brains, their progeny display an unprecedented potential to myelinate and to replace defective CNS myelin. Although it remains to be determined whether βT4 cells have the canonical stem cell property of continuous self-renewal, their in vitro properties, persistence in the SVZ of the adult CNS, and remarkable capacity to produce myelinating oligodendrocytes in a pathological environment identifies them as a novel and primitive neural precursor cell.
We exploited two fundamental stem cell characteristics, low basal rates of proliferation and homophilic adhesion during dissociation, to purify βT4 cells from rodent brain. By selectively killing dividing cells in primary neurospheres, we exploited the low basal rate of βT4 cell division to obtain enriched populations of βT4 cells in vitro. Our BrdU incorporation studies support that βT4 cell-enriched neurospheres are not formed by βT4 cell proliferation. After enrichment in secondary neurospheres, βT4 cells were resistant to dissociation by enzymes, Ca2+ chelators, and/or mechanical dissociation, characteristics shared with embryonic stem cells and primordial germ cells (Shamblott et al., 1998; Thomson et al., 1998), making it impossible to examine βT4 cells in single-cell colony or clonal assays. These observations support our interpretation that βT4 cell-enriched secondary neurospheres form by homophilic adhesion. Homophilic adhesion contact inhibits βT4 cell division and differentiation when secondary neurospheres are formed and maintained in suspension. However, when plated on adhesive surfaces, this contact inhibition is partially released as most BrdU-positive βT4 cells (after a 20 h pulse) were migrating away from the secondary neurospheres.
The extent of oligodendrocyte generation and myelination that we describe in the md rat brain is unprecedented, considering that only 25,000 βT4 cells were transplanted and brains were examined after only 17 d. In addition, production of myelinating oligodendrocytes was still occurring at the time of perfusion (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), indicating that the repair capabilities of cells generated from transplanted βT4 cells were still active. Compared with A2B5-positive progenitor cells, transplanted βT4-positive cells generated 270% more myelin over a significantly greater brain volume. Achieving a rapid and wide distribution of myelin is important for transplantation therapies. For example, transplantation of human fetal A2B5-positive cells into the shiverer mouse mutant results in extensive myelination (Windrem et al., 2004), but multiple transplantation sites are required to rescue the phenotype (Windrem et al., 2008). The precise mechanism by which a wider spatial distribution of myelin produced from βT4 cell transplants is unknown. Our in vitro results predict that exposure of the neurosphere to extracellular matrix provides signals that stimulate migration away from the neurosphere and proliferation via an intermediate transient amplifying cell before differentiation into OPCs. This enables a cascade because OPCs also have the capacity for migration and proliferation.
Our studies identify βT4 cells as a distinct and previously undefined population of precursor cells in the SVZ of the mammalian CNS. They do not express markers specific for committed oligodendrocyte progenitor cells (NG2 or A2B5) or mature neurons or oligodendrocytes. Previous studies have characterized a stem cell of the fetal and adult SVZ that expresses GFAP (Garcia-Verdugo et al., 1998; Merkle and Alvarez-Buylla, 2006), a protein also expressed by differentiated astrocytes. We did not observe coexpression of GFAP and βT4 in vivo or in vitro. It may be, however, that βT4 and GFAP are expressed at different stages of stem cell activation or maturation. βT4 is the third cytoskeletal protein to be enriched in a distinct immature CNS cell population. β3 tubulin is enriched in cells committed to the neuronal lineage (Lee et al., 1990; Menezes and Luskin, 1994), and GFAP-δ is an intermediate filament protein isoform detected in stem cells but not in astrocytes in which GFAP-α is expressed (Roelofs et al., 2005). However, no cell-specific function has been identified for these proteins, and their cytoplasmic distribution does not permit cell enrichment by panning or fluorescence-activated cell sorting. To date, no surface marker has been identified for the GFAP-positive stem cell. AC133 is a glycosylated epitope on human CD133/prominin-1 that has been used a marker for hematopoietic and cancer stem cells (Weigmann et al., 1997; Yin et al., 1997; Fargeas et al., 2007; Mizrak et al., 2008). In human tissue and rat neurospheres, βT4 cells were not labeled by multiple commercially available antibodies to CD133 or prominin polypeptides. In addition, prominin-1 mRNA is not enriched in secondary versus primary neurospheres (R. Dutta, C. Wu, and B. D. Trapp, unpublished data). Therefore, we have no evidence for a relationship of βT4 cells and either GFAP- or CD133-positive SVZ cells.
The developmental appearance of βT4 cells peaks during the latter stages of gliogenesis, which originate from the SVZ of the lateral ventricles. In vitro, βT4 cells are present in neurospheres generated from early postnatal SVZ but not from primary and secondary neurospheres produced from embryonic day 14 rat medial ganglionic eminence (data not shown), an early source of OPCs (Kessaris et al., 2006). In the human fetal brain, βT4 cell density also peaks during the latter stage of gliogenesis, and βT4 cells are present at adult levels by the time most cerebral myelin has formed. It is possible, therefore, that a subpopulation of βT4 cells responsible for the latter stage of gliogenesis from the lateral ventricles remains in the SVZ of the adult brain, in which they are positioned to be activated in response to insults requiring new cell production.
One consistent observation in the adult CNS is that a variety of perturbations, including focal ischemia, contusion injuries, and demyelination, result in the mobilization of precursor or stem cells from localized origins (Iwai et al., 2006; Leker, 2006; Mazurová et al., 2006; Park et al., 2006; Nait-Oumesmar et al., 2007). When βT4 cell densities are increased in SVZs bordering MS lesions, they often appear as doublets or in clusters, suggesting proliferation and symmetrical division of βT4 cells. We have not been able to detect the proliferation marker Ki-67 in βT4 cells in control or MS tissue sections (data not shown). This may reflect intrinsically low rates of proliferation (in control brains) or tightly temporally defined proliferation periods (in MS brains), depending on mitogen availability. Indeed, βT4 cells in primary and secondary neurospheres had low rates of division in basal neurosphere medium.
Regardless of whether βT4 cells are true stem cells or primitive precursors, we propose that they represent a cellular source for the latter stages of CNS myelination and for neural repair in the adult CNS. The extensive myelination after transplantation of βT4 cells into the md rat and the increased density of SVZ βT4 cells bordering the lesions of MS support βT4 cell activation, proliferation, and differentiation in the setting of oligodendrocyte deficiency and dysfunction. The potential of stem cell therapeutics to treat neurodegenerative diseases will require additional characterization of stem cells in human brain. We describe here an SVZ cell in the mammalian brain with accepted characteristics of stem cells. These include a low rate of proliferation, adhesion after enrichment, appropriate spatial, temporal, and quantitative distributions in developing and diseased human brain, and the ability to produce differentiated rat neural cells in vitro and in vivo. Additional studies, including the purification and expansion of human βT4 cells, are required before considering therapeutic applications for diseases of myelin.
Footnotes
This work was supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant R01 NS 29818 and by a postdoctoral fellowship from the National Multiple Sclerosis Society (C.W.).
References
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Stoffel W. Myelin-deficient rat: a point mutation in exon III (A–C, Thr75–Pro) of the myelin proteolipid protein causes dysmyelination and oligodendrocyte death. EMBO J. 1989;8:3295–3302. doi: 10.1002/j.1460-2075.1989.tb08490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Dentinger MP, Barron KD, Csiza CK. Ultrastructure of the central nervous system in a myelin deficient rat. J Neurocytol. 1982;11:671–691. doi: 10.1007/BF01262431. [DOI] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Huttner WB, Corbeil D. Nomenclature of prominin-1 (CD133) splice variants: an update. Tissue Antigens. 2007;69:602–606. doi: 10.1111/j.1399-0039.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo . J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Sim F. Neural progenitor cells of the adult brain. Novartis Found Symp. 2005;265:66–80. discussion 82–97. [PubMed] [Google Scholar]
- Grinspan JB, Franceschini B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor cells. J Neurosci Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- Inoue H, Ohsawa I, Murakami T, Kimura A, Hakamata Y, Sato Y, Kaneko T, Takahashi M, Okada T, Ozawa K, Francis J, Leone P, Kobayashi E. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun. 2005;329:288–295. doi: 10.1016/j.bbrc.2005.01.132. [DOI] [PubMed] [Google Scholar]
- Iwai M, Ikeda T, Hayashi T, Sato K, Nagata T, Nagano I, Shoji M, Ikenoue T, Abe K. Temporal profile of neural stem cell proliferation in the subventricular zone after ischemia/hypoxia in the neonatal rat brain. Neurol Res. 2006;28:461–468. doi: 10.1179/016164105X49283. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Morrison SJ. Toward an understanding of the physiological function of mammalian stem cells. Dev Cell. 2005;9:173–183. doi: 10.1016/j.devcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- Learish RD, Brüstle O, Zhang SC, Duncan ID. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann Neurol. 1999;46:716–722. [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Leker RR. Manipulation of endogenous neural stem cells following ischemic brain injury. Pathophysiol Haemost Thromb. 2006;35:58–62. doi: 10.1159/000093545. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Roeder I. Tissue stem cells: definition, plasticity, heterogeneity, self-organization and models—a conceptual approach. Cells Tissues Organs. 2002;171:8–26. doi: 10.1159/000057688. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- Mazurová Y, Rudolf E, Látr I, Osterreicher J. Proliferation and differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum. Neurodegener Dis. 2006;3:12–18. doi: 10.1159/000092087. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Höglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, Gullans S, Jensen FE, Sidman RL, Ourednik V, Snyder EY. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal “reporter” neural stem cells. Exp Neurol. 2006;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres: re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Roelofs RF, Fischer DF, Houtman SH, Sluijs JA, Van Haren W, Van Leeuwen FW, Hol EM. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia. 2005;52:289–300. doi: 10.1002/glia.20243. [DOI] [PubMed] [Google Scholar]
- Roy NS, Benraiss A, Wang S, Fraser RA, Goodman R, Couldwell WT, Nedergaard M, Kawaguchi A, Okano H, Goldman SA. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321–331. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, Ikenaka K, Macklin W, Cerruti I, Zalc B, Thomas JL. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Terada N, Kidd GJ, Kinter M, Bjartmar C, Moran-Jones K, Trapp BD. Beta(IV) tubulin is selectively expressed by oligodendrocytes in the central nervous system. Glia. 2005;50:212–222. doi: 10.1002/glia.20175. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, 2nd, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K, Jensen-Smith HC, Ludueña RF, Hallworth R. Differential synthesis of beta-tubulin isotypes in gerbil nasal epithelia. Cell Tissue Res. 2002;309:331–335. doi: 10.1007/s00441-002-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, Lu QR. A critical role for dorsal progenitors in cortical myelination. J Neurosci. 2006;26:1275–1280. doi: 10.1523/JNEUROSCI.4717-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]