Abstract
Purpose
This study was designed to provide preliminary data regarding the safety and efficacy of high-dose humanized anti-IL-2 receptor (daclizumab) therapy for the treatment of active intermediate, posterior or panuveitis.
Methods
Five patients were recruited into this non-randomized, prospective pilot study of high-dose intravenous induction daclizumab therapy given at doses of 8 mg/kg at day 0 and 4 mg/kg at day 14. Patients who did not meet a safety endpoint at the 3-week follow-up evaluation were given the option of continuing therapy with subcutaneous daclizumab at 2 mg/kg every 4 weeks for 52 weeks. The primary outcome assessed was a 2-step decrease in vitreous haze at day 21. Secondary outcomes evaluated included best-corrected visual acuity, retinal thickness as measured by optical coherence tomography, retinal vascular leakage assessed by fluorescein angiography, anterior chamber and vitreous cellular inflammation.
Results
4 male patients and 1 female patient were enrolled. Diagnoses included birdshot retinochoroidopathy (2 patients), Vogt-Koyanagi-Harada's disease, bilateral idiopathic panuveitis and bilateral idiopathic intermediate uveitis. By the 4th week, four of five patients demonstrated a 2-step decrease in vitreous haze. The other participant did not meet this criterion until week 20, but all 5 patients maintained stability in vitreous haze grade throughout their follow-up periods. At enrollment, mean visual acuity (10 eyes in five patients) was 69.2 ETDRS letters and following treatment was 78.2 letters (p < 0.12). Anterior chamber cell, vitreous cell, and vitreous haze also improved in the majority of eyes. Adverse events were generally mild except for one episode of left-lower lobe pneumonia requiring hospitalization and treatment.
Conclusion
This is the first demonstration that high-dose daclizumab can reduce inflammation in active uveitis. Daclizumab was well-tolerated but there may be a potential increased risk of infection associated with immunosuppression. All five patients demonstrated a decrease in vitreous haze and measures of intraocular inflammation at final follow-up. The results of this small, nonrandomized pilot study support the consideration of high-dose daclizumab therapy in cases of active posterior uveitis.
Keywords: daclizumab, posterior uveitis, panuveitis, intermediate uveitis, interleukin-2
Introduction
Daclizumab is a humanized blocking monoclonal antibody that is directed against an epitope found on the alpha subunit of the interleukin (IL)-2 receptor (CD25), localized on activated T cells and other cells of the immune system [1]. Daclizumab was first shown to be effective in the reduction of acute rejection episodes in patients undergoing renal transplantation [1-2] and has since been utilized for other solid organ transplants including heart [3], lung [4-5], pancreatic[6-7], and hepatic allografts [8-9]. Besides its use in solid organ transplantation, daclizumab has been utilized for the treatment of a subset of patients with human T-cell leukemia virus-1 (HTLV-1) associated T-cell leukemia because of the elevated levels of IL-2 receptor found on leukemic cell populations [10].
We have previously demonstrated the successful use of intravenous daclizumab as a glucocorticoid and cyclosporin A sparing agent in the treatment of patients with noninfectious intermediate and posterior uveitis. Patients who required combination systemic immunosuppressive medications to control their disease were successfully tapered off these medications and maintained on daclizumab monotherapy [11]. A subsequent study demonstrated that subcutaneous administration of daclizumab was equally efficacious [12].
While our earlier studies had demonstrated the utility of daclizumab as a corticosteroid and calcineurin inhibitor-sparing agent, we wished to address the question of whether daclizumab might be beneficial in the treatment of patients with active intermediate and posterior uveitis. The primary focus of this feasibility study is to determine whether high-dose daclizumab is effective in the acute reduction of vitreous haze in active, intermediate, posterior and panuveitis of noninfectious origin.
Materials and Methods
This study was a prospective, open-label, non-randomized, phase II pilot study of daclizumab treatments for participants with active, sight-threatening, non-infectious uveitis. The study was conducted at the Clinical Center of the National Institutes of Health under an Investigational New Drug (IND) application. The study protocol was reviewed and approved by the Institutional Review Board of the National Eye Institute and all procedures conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
Inclusion and exclusion criteria
Inclusion criteria included a diagnosis of active non-infectious intermediate, posterior, or panuveitis with ≥ Grade 1 (1+) vitreous haze in at least one eye using the SUN criteria and evidence of retinal vascular leakage or cystoid macular edema (CME) [13], best-corrected distance visual acuity (BCVA) in the poorer seeing eye of 20/400 or better (i.e. Early Treatment of Diabetic Retinopathy Study – ETDRS logMAR <1.34), and the participant did not plan to undergo elective ocular surgery (e.g., cataract extraction).
Exclusion criteria included patients who had received IL-2 or IL-2 receptor-directed therapy within 90 days, lens or media opacities that would hinder evaluation and grading of the posterior segment, pregnant or lactating patients, history of active herpes or varicella infection within 6 months or chickenpox exposure within 21 days before enrollment, known history of human immunodeficiency virus (HIV) infection, current enrollment in another trial involving the use of immunotherapy for a non-uveitic condition or any investigational therapy within 30 days, significant systemic infection requiring treatment, history of cancer within five years, or any other non-ocular comorbid conditions with significant risks to health or the patient's ability to follow-up in the study protocol.
All participants, male or female, with reproductive potential and who were sexually active agreed to use double-barrier contraception methods throughout the course of the study (minimum of 52 weeks) and for an additional 6 weeks after completion of the protocol treatment period.
Medication dosing and administration
All enrolled patients received an initial induction regimen of intravenous (IV) daclizumab, 8 mg/kg on Day 0 followed by a second IV dose of 4 mg/kg on Day 14 ± 1 day, provided a safety endpoint had not already been met. Daclizumab was provided by PDL BioPharma (Redwood City, CA). Participants who showed improvement without serious adverse events, and who did not experience a ≥ 3 line drop (15 letters) in visual acuity during the induction treatments, had the option to receive extended treatments of 2 mg/kg subcutaneous (SC) daclizumab treatments at 4-week intervals for up to one year. Meeting the safety failure criterion (i.e. ≥ 15 letter loss of visual acuity) or having a serious adverse affect directly attributed to daclizumab therapy was cause for termination from further daclizumab therapy.
Ophthalmic and medical evaluation
All patients underwent baseline medical and ophthalmologic examinations. Ophthalmic examination included best-corrected visual acuity measurement using the standardized ETDRS refraction protocol at 4 meters, intraocular pressure by applanation tonometry, slit lamp biomicroscopic examination, dilated funduscopic examination, fluorescein angiography, and stereoscopic fundus photography. Imaging with optical coherence tomography (OCT-3) was performed and mean central retinal thickness (area A1 with a 1-mm diameter) was recorded at interval visits. Medical examination included physical examination and laboratory measurements including the following values: hemoglobin, hematocrit, platelets, leukocytes, serum electrolytes (sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine), serum bilirubin, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase.
Primary and secondary outcome assessment
The primary outcome measure was the reduction of vitreous haze by at least two steps (e.g. 2+ to Trace, or 1+ to 0) from baseline at Day 21 in both eyes. Participants were considered treatment failures if at any time during the study, a ≥3 line (≥15 letters or >0.30 logMAR) decrease in best-corrected visual acuity (BCVA, ETDRS method) was observed when compared to baseline.
Secondary outcome measures included distance BCVA, ocular inflammation grades for anterior chamber (AC) cells and vitreous cells, the amount of retinal vascular leakage measured by fluorescein angiography, the presence or extent of cystoid macular edema (CME) determined by optical coherence tomography and/or visualized by fluorescein angiography, and weighted grading score of immunosuppressive medications (i.e., immunosuppression load). During the maintenance phase of the protocol (i.e. following day 28), concomitant systemic immunosuppressive medications could be tapered as clinically indicated. However, medications were not tapered during the initial 21-day induction phase.
Safety assessment and adverse event reporting
Safety outcomes were tabulated by observing the nature, severity and frequency of systemic toxicities, adverse events (AE), and infections throughout the study. Safety assessments were made routinely during the study, with a review of the previous visit interval performed at each scheduled visit. Each participant was encouraged to report any apparent adverse events between scheduled visits, and returned for additional evaluations and appropriate treatment between scheduled visits if needed. Safety failure criterion that would result in suspension and potentially permanent withdrawal of study treatments included a drop of ≥ 3 lines (≥ 15 letters) from baseline visual acuity, a serious adverse event, drug reaction or complication (whether related or unrelated to daclizumab) with an impact on visual function or any other organ system that would preclude continuation of study treatment (e.g. hypersensitivity, allergic response, or serious drug reactions), and pregnancy.
Adverse medical events were evaluated and treated by an Internal Medicine service when clinically indicated, and appropriate consultations were obtained if necessary. Following an evaluation by the Internal Medicine service, an evaluation of the possible relatedness of the AE to the administration of daclizumab was determined and recorded.
Statisical analysis
Descriptive statistics were performed using Microsoft Excel and GraphPad Prism software. The Wilcoxon matched-pairs signed-ranks test was used to determine whether a difference existed between BCVA and immunosuppressive load before and after daclizumab therapy.
Results
Five uveitis patients with poorly controlled intraocular inflammatory disease participated in this pilot study.
Demographic and enrollment characteristics
The demographic information and baseline characteristics of the patients are summarized in Table 1. Mean age ± standard deviation at enrollment was 37.6 ± 12.8 years. Diagnoses included two patients with birdshot retinochoroidopathy, one patient with Vogt-Koyanagi-Harada's disease, one patient with idiopathic bilateral, granulomatous panuveitis, and one patient with idiopathic bilateral intermediate uveitis.
Table 1. Demographic information and baseline characteristics.
| Participant | Gender | Race | Age at Enrollment | Diagnosis | Medications at Enrollment | Medications at Final Follow-up |
|---|---|---|---|---|---|---|
| 1 | M | W | 30 | Birdshot retinochoroidopathy | None | None |
| 2 | F | W | 43 | Intermediate uveitis | Pred 3 mg/day, MMF 2000 mg/day | MMF 1000 mg/day |
| 3 | M | AA | 20 | Bilateral, idiopathic panuveitis | MTX 20 mg/day, Pred 20 mg/day | None |
| 4 | M | W | 53 | Birdshot retinochoroidopathy | MMF 2000 mg/day | MMF 1000 mg/day |
| 5* | M | A | 42 | Vogt-Koyanagi-Harada disease | MMF 1000 mg/day, Pred 10 mg/day | MMF 1000 mg/day Pred 10 mg/day |
Abbreviations: M = male, F = female, W = white, AA = African-American, A = Asian, Pred = prednisone, MTX = methotrexate, MMF = mycophenolate mofetil
Patient 5 did not complete entire 52-week study follow-up. However, his disease was quiescent at the time of discontinuation from the study.
Primary and secondary outcome assessment
Using the SUN criteria for evaluation of intermediate uveitis, vitreous haze was measured using an indirect ophthalmoscope and a 20D lens [13], using a set of standardized photographs for comparison [14]. The readings were performed by two ophthalmologists who examined the patients independently of the principal investigator. Any differences in observations were adjudicated with the help of a third observer.
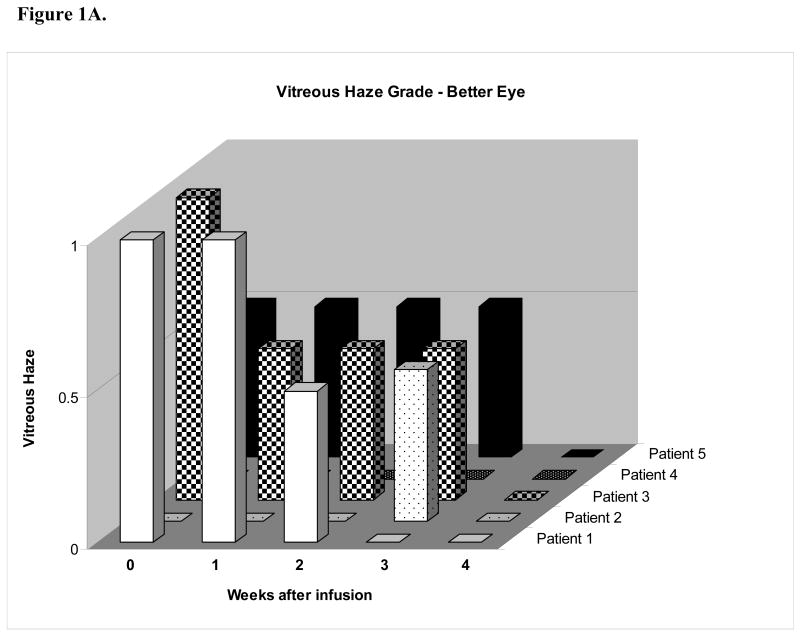
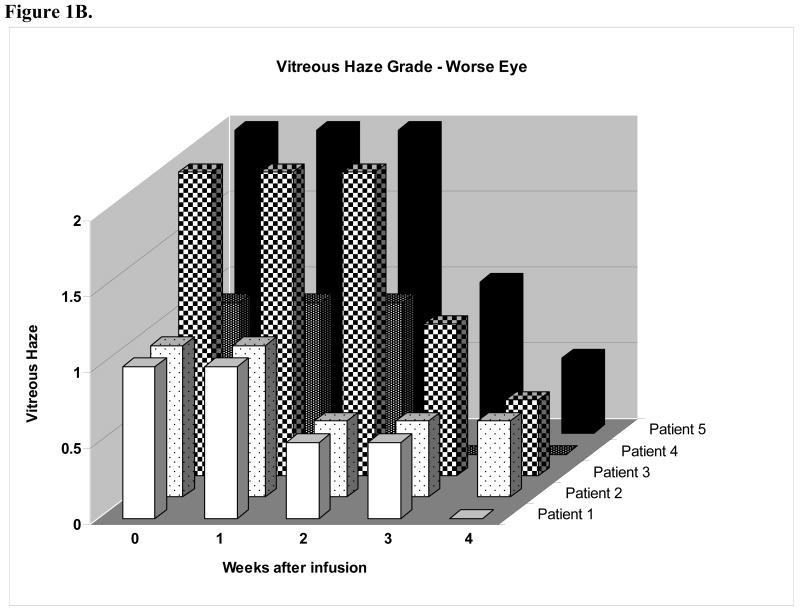
The vitreous haze results are presented in two graphs (Figures 1A-B). The score for the patients' eye with less vitreous haze appears in the “better” eye graph, while the eye with greater haze appears in the “worse” eye graph. Of the five patients who participated in the study, two patients (patients 1 and 3) demonstrated vitreous haze of grade 1 or greater severity in both eyes and were eligible for the primary outcome assessment in both eyes. Patient 1 demonstrated decreased vitreous haze in both eyes; however a two-step decrease was seen in only eye (i.e. a one-step decrease was seen in the opposite eye). Patient 3 demonstrated a one-step decrease in vitreous haze in both eyes at the primary outcome assessment and a two-step decrease in both eyes at week four. All of the “better” eyes had no evidence of vitreous haze by four weeks after the start of the daclizumab infusions, while also undergoing a taper of their immunosuppressive medication. Of “worse” eyes with greater haze at the start of the study, all had either no haze or trace haze four weeks after the first infusion.
Figure 1.
(A) Vitreous haze vs. weeks after infusion in eye with less vitreous haze (better eye) graph demonstrates a 2-step decrease in vitreous haze in two patients (patients 1 and 3). (B) Vitreous haze grade vs. weeks after infusion in eye with greater vitreous haze (worse eye) demonstrates decreased vitreous haze in 5 of 5 patients with four eyes demonstrating a 2-step decrease in vitreous haze at week 4.
Mean visual acuity was 69.2 ETDRS letters at baseline and was 78.2 letters following therapy (p < 0.12, Wilcoxon matched-pairs signed-ranks test). The left eye of patient 4, who underwent cataract extraction, was excluded from the statistical analysis.
Anterior chamber cells and vitreous cells were also evaluated at the baseline and interval follow-up visits. Five of five patients experienced a decrease in anterior chamber cell in one or both eyes at the 4-week follow-up examination. Specifically, 8 of 9 eyes with anterior chamber inflammation showed a decrease in anterior chamber cells. Of note, 7 of 8 of the eyes demonstrated a significant decrease in AC cell as defined by SUN criteria (i.e. either trace to grade 0 cell or a 2-step decrease in AC cell). At the 12-month follow-up, four of four patients who completed the follow-up period demonstrated grade 0 cell. The only exception was patient 5 who did not complete the entire 12-month course of SC daclizumab therapy (Table 2).
Table 2. Ocular inflammation grades.
| Baseline AC Grade | Final AC Grade | Baseline Vitreous Cell Grade | Final Vitreous Cell Grade | |||||
|---|---|---|---|---|---|---|---|---|
| Patient | OD | OS | OD | OS | OD | OS | OD | OS |
| 1 | Trace | 0 | 0 | 0 | 3 | Trace | 1 | 0 |
| 2 | Trace | Trace | 0 | 0 | Trace | Trace | 0 | 0 |
| 3 | 2 | Trace | 0 | 0 | 2 | 1 | 1 | 0 |
| 4 | Trace | Trace | 0 | 0 | Trace | 0 | 0 | 0 |
| 5* | 1 | 2 | 1 | 1 | 1 | 2 | Trace | 1 |
Abbreviations: AC - Anterior chamber, OD – Right eye, OS – Left eye
Patient 5 elected to withdraw from the study at the 28-week follow-up visit due to travel constraints.
Five of ten eyes with vitreous cells at baseline showed a decrease in vitreous cellularity at the 4-week follow-up examination. Vitreous cells in the other five eyes remained unchanged at this time point. In patient 1, both eyes demonstrated a 2-step or greater reduction in vitreous cells at this visit. At final follow-up, six of eight eyes in four patients who completed the follow-up period demonstrated reductions in vitreous cells with 4 eyes demonstrating a 2-step or greater reduction in vitreous cells or a reduction of vitreous cells from trace to grade 0. (Table 2).
Of the five patients, one patient (Subject 1) presented with retinal vascular leakage on his initial fluorescein angiogram. At his day 21 visit, the retinal vascular leakage was improved bilaterally with decreased perivascular staining and leakage in the late phases of fluorescein angiography. This effect was sustained at the 6-month time point with almost complete resolution of retinal vascular leakage at his 12-month follow-up evaluation. Of some concern, however, was the occurrence of a 2.5 disc diameter intra-retinal macular hemorrhage observed OD with a decrease in visual acuity to 20/40 at the 12-month evaluation. Neither increased retinal vascular leakage nor any evidence of choroidal neovascularization observed, and the retinal hemorrhage resolved without any known visual sequelae. His visual acuity remains 20/20 at the 24 month follow-up visit.
The presence or absence of CME was also evaluated in all patients (Supplemental Table 1). CME was observed in four eyes in two patients (Patients 1 and 2) at the baseline evaluation. At the final follow-up visit (52 week visit), CME was not observed in either patient.
Immunosuppressive load was also calculated at baseline, and the 6 and 12 month visits using previously accepted criteria.15 Immunosuppressive load (Mean ± SD) at baseline for all patients was 2.6 ± 1.7 (Range 0-4). At the final follow-up visit, mean immunosuppressive load was 1.4 ± 1.5 (Range 0-3). No significant difference was demonstrated between baseline and final immunosuppressive load (p < 0.25, Wilcoxon matched-pairs signed-ranks test). However, three of five patients were able to decrease their baseline immunosuppressive medications.
Safety assessment and adverse event reporting
All patients tolerated the infusions well. No patient stopped the treatment or had adverse events that could be directly associated with daclizumab. However, several adverse events were noted and are listed in Table 3. Patient 3 developed a viral syndrome with left lower lobe pneumonia and gastrointestinal complaints requiring hospitalization, intravenous fluids, and empiric levofloxacin therapy. Diagnostic workup included sputum, blood, and stool cultures, which were negative for viral and bacterial etiologies of the pneumonia. This occurred 4 months following his initial high-dose induction regimen and 4 weeks subsequent to a dose of subcutaneous maintenance daclizumab. Patient 2 developed an episode of dizziness, insomnia, and poor energy, and she was subsequently hospitalized for a medical evaluation. No evidence of acute medical illness was found during the workup and her symptoms were attributed to anxiety and not directly associated with daclizumab.
Table 3. Adverse events.
| Participant Number | Description | Infection | Related to Agent | Severity | Treatment Required | Hospitalized | Outcome |
|---|---|---|---|---|---|---|---|
| 001 | Severe abdominal cramping | No | Possibly | Moderate | No | No | No residual effects |
| 002 | Follicular conjunctivitis | No | Not Related | Moderate | Treatment | No | No residual effects |
| 002 | Shortness of breath | No | Not Related | Mild | Treatment | No | No residual effects |
| 002 | Itching lesions on chest | No | Not Related | Moderate | Treatment | No | Persistent condition |
| 002 | Upper respiratory infection | Yes | Not Related | Moderate | Treatment | No | No residual effects |
| 002 | Anxiety | No | Not Related | Mild | No | Yes | No residual effects |
| 003 | Decreased hearing left ear | No | Not Related | Mild | No | No | Persistent condition |
| 003 | Left lower lobe pneumonia | Yes | Not Related | Severe | Treatment | Yes | With sequelae |
| 003 | Stomach ache | No | Remotely | Mild | No | No | No residual effects |
| 004 | Abdominal incision – related to patient's gastric bypass surgery | No | Not Related | Moderate | Treatment | No | Persistent condition |
| 004 | Hand tremors | No | Not Related | Mild | No | No | Persistent condition |
| 004 | Headache | No | Not Related | Mild | Treatment | No | No residual effects |
| 004 | Rash on hands | No | Not Related | Mild | Treatment | No | Persistent condition |
Discussion
The development of an experimental autoimmune uveitis model has been of tremendous help in elucidating potentially important mechanisms that lead to human uveitis [16]. One major result of these studies has been the use of various biologic agents, mostly monoclonal antibodies, directed against specific points in the immune cascade. Daclizumab is one such agent that demonstrated efficacy in the treatment of uveitis in a non-human primate model prior to its use in patients with uveitis [17].
The mechanism of action of daclizumab remains under investigation. Ragheb et al suggested that the medication had a major effect on interferon gamma (IFN-γ) production by T cells. Specifically, daclizumab inhibited the production of IFN-γ by activated human peripheral blood mononuclear cells (PBMCs) via its impact on both IL-12-dependent and IL-12-independent pathways [18]. Li and colleagues have found that a CD56bright population of regulatory natural killer cells expands in the peripheral blood of patients receiving this dosing schedule and this daclizumab-induced expansion seems associated with clinical regression of the disease in treated patients [19]. Others have reported similar observations in patients with multiple sclerosis [20]. Interestingly, the CD56bright cells were found to secrete large amounts of the immunoregulatory cytokine IL-10, but this was not observed in CD56dim cells [19]. Amadi-Obi et al found that IL-2 appears to promote the development of T-helper type 17 cells (TH17), which were found in elevated levels in the PBMCs of patients with uveitis and scleritis [21]. Based on their findings, one possible mechanism of daclizumab therapy is to inhibit maturation of TH17 cells, a new subgroup of T-helper cells involved in chronic inflammatory diseases that has recently received much attention in uveitis and by the wider immunologic community as well [22].
The efficacy of daclizumab as a corticosteroid-sparing agent in patients with intermediate and posterior uveitis has been demonstrated previously. In a phase I/II clinical trial of daclizumab, eight of ten patients with noninfectious intermediate, posterior or panuveitis were completely tapered off their other immunosuppressive medications while receiving intravenous daclizumab at a dose of 1 mg/kg/infusion [11].
Subsequent studies have replicated these findings in a variety of uveitic syndromes including birdshot retinochoroidopathy [23] childhood uveitis [24], ocular cicatricial pemphigoid, scleritis, and panuveitis [25]. In a multicenter, interventional case series of patients with uveitis treated with subcutaneous daclizumab, 10 of 15 patients (67%) were able to taper their immunosuppressive medications while on daclizumab, achieving their primary endpoint of a 50% reduction in immunosuppressive medication [26].
A longer term (> 4 years), phase I/II single armed interventional study using IV daclizumab and a short-term Phase II study evaluating the use of a subcutaneous daclizumab formulation has also been conducted. In this cohort of patients, seven of ten patients were maintained on daclizumab and were able to taper their immunosuppressive medications [12]. Of the patients who were unable to be successfully transferred to daclizumab therapy, we observed one patient whose disease was not as well-controlled as the others. We theorized that saturation of the CD25 receptor, both in the peripheral blood and in more sequestered areas, such as the lymph nodes, was necessary to obtain good immunosuppression. Patients receiving the doses of daclizumab administered previously (1-2 mg/kg) theoretically may not had have their CD25 receptors in lymph nodes totally saturated although CD25 receptor saturation in peripheral blood lymphocytes was documented by immunophenotyping. Interestingly, one patient receiving daclizumab who had a lymph node biopsy for an unrelated medical workup revealed that indeed a small number of CD25+ T cells did not have all their receptors saturated [12].
Based on the experience of one of us (TW) in treating lymphomas with high numbers of CD25 receptors, we theorized that a higher initial dose of medication could saturate these cells even in more sequestered areas, followed by the standard daclizumab dose. This theory was evaluated as the hypothesis in this pilot clinical trial in which patients with active uveitis were treated initially with high dose intravenous daclizumab followed by a lower subcutaneous dose. The findings from this trial reveal that this therapeutic regimen appears to decrease active intraocular inflammatory disease of the posterior segment of the eye.
We did not observe secondary adverse events that could be directly associated with the higher dose of daclizumab, but only a small number of patients received the medication. Daclizumab was well-tolerated by the patients in the study; however, the episode of presumed viral pneumonia in one patient is an important consideration, as there may be a potential increased risk of infection associated with immunosuppression.
While the power of any small pilot study is limited, our findings suggest that high-dose daclizumab is efficacious and well-tolerated in patients with active uveitis. All five patients treated with the induction regimen of high-dose daclizumab demonstrated a decrease in vitreous haze, which has been accepted as a marker for vitreous inflammation established by the uveitis community. Secondary outcomes including visual acuity, anterior chamber and vitreous cellular inflammation, and immunosuppressive load were also improved during the observation period. If these results are confirmed by subsequent studies, including an ongoing study in children with uveitis (RBN), it would widen the clinical indications for this medication.
Supplementary Material
Figure 2.
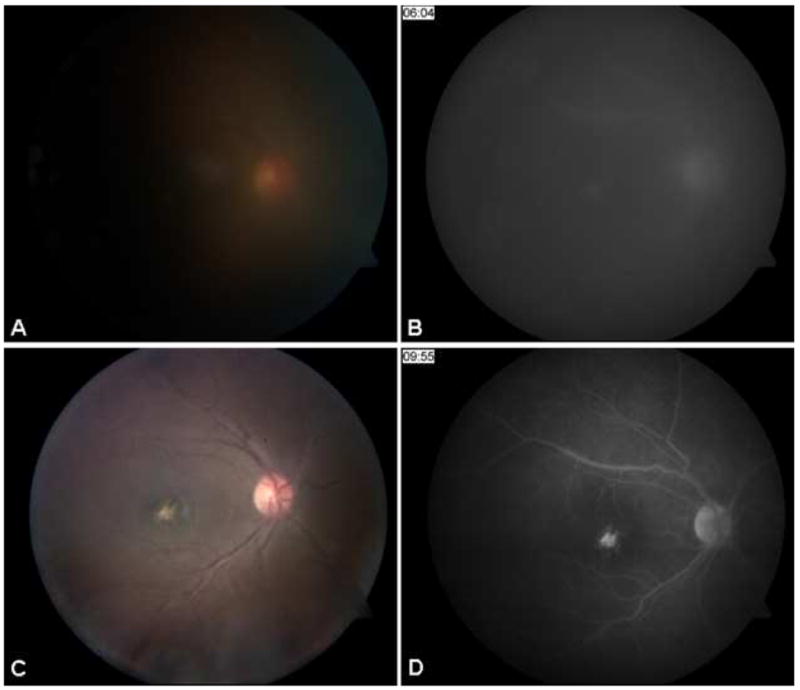
Fundus photo (A) and venous phase fluorescein angiogram (B) of patient 3 at baseline demonstrates 2+ vitreous haze and mild hyperfluorescence of optic nerve and fovea OD. Following administration of induction doses of high-dose daclizumab therapy and subcutaneous daclizumab every four weeks, fundus photograph (C) and venous phase angiogram (D) at final follow-up demonstrate resolution of vitreous haze, decreased optic nerve hyperfluorescence and staining of a macular scar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338(3):161–5. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 2.Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Phase III Daclizumab Study Group. Results of 3-year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation. 2001;72(5):839–45. doi: 10.1097/00007890-200109150-00017. [DOI] [PubMed] [Google Scholar]
- 3.Vlad G, Ho EK, Vasilescu ER, Fan J, Liu Z, Cai JW, et al. Anti-CD25 treatment and FOXP3-positive regulatory T cells in heart transplantation. Transpl Immunol. 2007;18(1):13–21. doi: 10.1016/j.trim.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Mullen JC, Oreopoulos A, Lien DC, Bentley MJ, Modry DL, Stewart K, et al. A randomized, controlled trial of daclizumab vs anti-thymocyte globulin induction for lung transplantation. J Heart Lung Transplant. 2007;26(5):504–10. doi: 10.1016/j.healun.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Lischke R, Simonek J, Davidova R, Schutzner J, Stolz AJ, Vojacek J, et al. Induction therapy in lung transplantation: initial single-center experience comparing daclizumab and antithymocyte globulin. Transplant Proc. 2007;39(1):205–12. doi: 10.1016/j.transproceed.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasaiah SB, Light JA, Sasaki TM, Currier CB. A comparison of daclizumab to ATGAM induction in simultaneous pancreas-kidney transplant recipients on triple maintenance immunosuppression. Clin Transplant. 2000;14(4 Pt 2):409–12. doi: 10.1034/j.1399-0012.2000.14040902.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transpl. 2005;11(9):1064–72. doi: 10.1002/lt.20490. [DOI] [PubMed] [Google Scholar]
- 9.Figueras J, Prieto M, Bernardos A, Rimola A, Suarez F, de Urbina JO, et al. Daclizumab induction and maintenance steroid-free immunosuppression with mycophenolate mofetil and tacrolimus to prevent acute rejection of hepatic allografts. Transpl Int. 2006;19(8):641–8. doi: 10.1111/j.1432-2277.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26(25):3699–703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- 11.Nussenblatt RB, Fortin E, Schiffman R, Rizzo L, Smith J, Van Veldhuisen P, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci U S A. 1999;96(13):7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussenblatt RB, Thompson DJ, Li Z, Chan CC, Peterson JS, Robinson RR, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21(3):283–93. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 15.Buggage RR, Levy-Clarke G, Sen HN, Ursea R, Srivastava SK, Suhler EB, et al. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behcet's disease. Ocul Immunol Inflamm. 2007;15(2):63–70. doi: 10.1080/09273940701299370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140(5):1490–5. [PubMed] [Google Scholar]
- 17.Guex-Crosier Y, Raber J, Chan CC, Kriete MS, Benichou J, Pilson RS, et al. Humanized antibodies against the alpha-chain of the IL-2 receptor and against the beta-chain shared by the IL-2 and IL-15 receptors in a monkey uveitis model of autoimmune diseases. J Immunol. 1997;158(1):452–8. [PubMed] [Google Scholar]
- 18.McDyer JF, Li Z, John S, Yu X, Wu CY, Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-gamma production. J Immunol. 2002 Sep 1;169(5):2736–46. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005 May 1;174(9):5187–91. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 20.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103(15):5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120(2):247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Kiss S, Ahmed M, Letko E, Foster CS. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005;112(6):1066–71. doi: 10.1016/j.ophtha.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher M, Quinones K, Cervantes-Castaneda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthalmol. 2007 Oct;91(10):1341–4. doi: 10.1136/bjo.2007.124081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaliodis GN, Chu D, Foster CS. Treatment of ocular inflammatory disorders with daclizumab. Ophthalmology. 2003;110(4):786–9. doi: 10.1016/S0161-6420(02)01932-2. [DOI] [PubMed] [Google Scholar]
- 26.Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology. 2005;112(5):764–70. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.