Abstract
Objective
To assess the quality of clinical care provided to patients with HIV in Felege Hiwot Referral Hospital.
Approach and design
Normative evaluation based on Donabedian's structure–process–outcome model of health care quality. Cross-sectional study design was employed to gather data in September 2007.
Setting
Felege Hiwot Referral Hospital is a government hospital in North West Ethiopia. The hospital is providing clinical care for patients infected with HIV free of patient charge since 2005.
Measures
The evaluation used 10 process and 5 outcome indicators of quality measured by reviewing 351 randomly selected patient records and interview with 368 patients. Resource inventory was conducted to assess the availability of trained staff, laboratory facilities and drugs required for provision of HIV care.
Results
All resources recommended by the national antiretroviral therapy (ART) Implementation Guideline including trained staff, laboratory facilities and drugs were continuously available, except for a shortage of cotrimoxazole. Despite this, important components of care and treatment recommended by national treatment guidelines were not delivered for significant portion of patients. The study showed that only 45.9% of patients eligible for cotrimoxazole prophylactic therapy (CPT) and 76.8% of patients eligible for ART were actually taking CPT and ART, respectively. Compliance with national guidelines to monitor patients was also found to be a major problem.
Conclusion
Availability of resources alone does not ensure the quality of HIV care and treatment. The study results indicate a need for regular monitoring and improvement of processes and outcomes of care in the Ethiopian Health System.
Keywords: quality measurement, quality improvement, quality indicators
Introduction
Like in other Sub-Saharan African Countries, HIV/AIDS is a major public health, social and economic problem in Ethiopia. As part of the multi-sectoral response to mitigate the impact of the pandemic and through the support of PEPFAR and Global Fund, the government is providing medical care services to People Living with HIV/AIDS free of charge [1–3].
Time and again, researches have revealed that medical care prolongs and improves the quality of life of patients infected with HIV/AIDS. However, the outcomes of clinical care for patients depend not only on attending a clinical care provider but also on appropriate execution of recommended components of care including prevention and treatment of opportunistic infections, use of Highly Active Antiretroviral Therapy (HAART) and laboratory and clinical follow-up of patients [4–8].
Felege Hiwot Referral Hospital is one of the three referral hospitals in Amhara National Regional State of Ethiopia. The hospital uses the national guidelines for the management of opportunistic infections and ART to manage HIV-infected patients [4, 5]. By the end of August 2007, a total of 6396 patients were registered for HIV care out of which 5031 (78.7%) started ART. Out of those patients who started ART, 3225 (64.1%) were reported as taking antiretroviral drugs actively in the hospital while the remaining 1806 (35.9%) failed to follow-up, transferred out to other health facilities or died [9].
To date, in Ethiopia, there exists neither an established system of quality management nor a comprehensive study assessing the quality of HIV clinical care. But some studies conducted outside Ethiopia showed that quality of medical care is indeed a major problem that needs to be addressed systematically [10–14]. A specific study conducted to assess adherence of patients with HAART in Addis Ababa also showed that adherence to antiretroviral drugs is a problem even though it is better when compared with that of patients in more urban settings [15].
Objective
To assess the quality of clinical care provided to patients infected with HIV in Felege Hiwot Referral Hospital.
Indicator selection
Quality of care indicators were set based on national guidelines for the treatment of patients with HIV/AIDS in Ethiopia [4, 5] after a thorough review on the quality of HIV/AIDS care literature. The selected indicators were again reviewed by physicians working on HIV/AIDS control program as well as HIV care-providing facilities for their local relevance. After all these procedures, a total of 15 indicators were selected. Ten indicators were on the process of care while the rest five indicators were on outcomes of care.
Measurement
Donabedian's structure–process–outcome model of health care quality was employed as a framework for the study [14, 16]. Data on the structure, processes and outcomes of care were collected cross-sectionally in September 2007. The measures included:
Availability of resources required to provide HIV clinical care service
Compliance of HIV clinical care practice with national guidelines
Outcomes of care including behavioral, immunologic and clinical conditions
Resource inventory was conducted to assess the availability of resources, including trained staff, laboratory facilities, antiretroviral drugs and opportunistic infection prophylactic drugs. An inventory tool prepared based on the resource requirements of the national program for health facilities providing ART was used for this purpose.
The process and outcome indicators were assessed by reviewing 351 randomly selected patient records. Of the total records reviewed, 233 (66.4%) were patients on ART and the remaining 118 (33.6%) were on chronic care before ART initiation, which was based on the actual proportion of patients in the two categories of care. In addition, an exit interview was conducted with 368 patients visiting the HIV clinic during the data collection period to assess satisfaction of patients. Interviews were conducted by trained data collectors using a satisfaction questionnaire adapted from the HIVQUAL project of New York State Department of Health AIDS Institute [17], which included 29 satisfaction measuring variables against a five-level satisfaction score. SPSS version 12.0.1 software was used to analyze the data.
Inclusion and exclusion criteria
Patients who have been in care and treatment for at least 6 months were eligible for record review. With the purpose of assessing recent practices, patients who had no clinic visit during the 1-year period prior to data collection were excluded. Patients who transferred in or out to other health facilities were also excluded from the study to keep focus on quality practice in the study hospital. For the patient interview, patients who visited the clinic for the first time were excluded as they may not have adequate prior experience with the hospital to provide meaningful answers to the questions.
Results
Resource availability
HIV clinical care in Felege Hiwot Referral Hospital is provided in a separate clinic. Daily, the ART unit is staffed with two general practitioners and seven nurses who took part in in-service trainings on HIV/AIDS clinical care provision based on the national guideline. The HIV clinic uses laboratory services from two sources: the hospital laboratory department and the Regional Health Research Laboratory, which is located in the hospital's compound. The hospital's laboratory provides routine laboratory tests, including HIV testing, microscopy for TB diagnosis and stool examination. For other HIV/AIDS-related laboratory tests including CD4 measurement, liver function test, renal function test and hematology analysis, it uses the Regional Research Laboratory. All the required laboratory tests, based on the National ART Implementation Guideline, are available in either the hospital's laboratory unit or the regional laboratory.
During the 6-month period prior to data collection, the hospital's drug store lacked stock of the first line antiretroviral drugs, Stavudine and Zidovudine. The cumulative period without stock of these drugs was for 2 and 8 days, respectively. But the drug supply was not interrupted in the dispensing unit. There was no stock of cotrimoxazole in either the store or the dispensary for 2 months prior to and during the data collection period.
Background characteristics of study subjects for chart review
Table 1 presents the background characteristics of study population. The review included 351 records of patients. The median duration in care and treatment was found to be 15.5 months (IQR: 12.13 months). One hundred twenty-one (34.5%) were enrolled within 1 year prior to the data collection period while the remaining 230 (65.5%) have been following care for more than 1 year.
Table 1.
Background characteristics of study subjects for chart review, Felege Hiwot Referral Hospital, September 2007
| Variable | Category | Number | Percent |
|---|---|---|---|
| Sex | Male | 159 | 45.3 |
| Female | 192 | 54.7 | |
| Total | 351 | 100.0 | |
| Age (completed years) | <5 | 9 | 2.6 |
| 5–14 | 14 | 4.0 | |
| ≥15 | 328 | 93.4 | |
| Total | 351 | 100.0 | |
| Marital status | Married | 97 | 27.6 |
| Never married | 34 | 9.7 | |
| Widowed | 28 | 8.0 | |
| Divorced | 50 | 14.2 | |
| Not documented | 142 | 40.5 | |
| Total | 351 | 100.0 | |
| Educational status | No education | 92 | 26.2 |
| Primary education | 29 | 8.3 | |
| Secondary education | 70 | 19.9 | |
| Tertiary education | 18 | 5.1 | |
| Not documented | 142 | 40.5 | |
| Total | 351 | 100.0 | |
| Occupation | Civil servant | 26 | 7.4 |
| Farmer | 12 | 3.4 | |
| Housewife | 19 | 5.4 | |
| Merchant | 19 | 5.4 | |
| Daily labor | 39 | 11.1 | |
| Others | 24 | 6.8 | |
| Not documented | 212 | 60.4 | |
| Total | 351 | 100.0 | |
| Address (Zone) | Bahir Dar (The city where the hospital is located) | 200 | 57.0 |
| Outside of Bahir Dar | 151 | 43.0 | |
| Total | 351 | 100.0 | |
| On HIV treatment / ART | Yes | 233 | 66.4 |
| No | 118 | 33.6 | |
| Total | 351 | 100.0 |
Compliance of HIV clinical care with national guidelines
Compliance with national guidelines [4, 5] for the provision of HIV clinical care was assessed based on 10 selected process indicators on 351 patient records, of which 233 (66.4%) were on ART and the remaining 118 (33.6%) were patients in care prior to ART initiation. The performance of the hospital in regard to the selected process indicators is presented in Table 2 and findings revealed major problems in adherence with national guidelines for HIV clinical care provision.
Table 2.
Summary of performance on process indicators of quality, Felege Hiwot Referral Hospital, September 2007
| Processes indicators | Number of eligible patients | Achievement, P (95% CI) |
|---|---|---|
| Care during enrollment to HIV clinic | ||
| Proportion of patients who received their first-time CD4 count within 2 weeks after first HIV clinic visit | 121 | 90.9% (85.8–96.0) |
| Opportunistic infection prevention | ||
| Proportion of patients whose CD4 count is <350 cells/µl who currently are on cotrimoxazole prophylactic therapy | 236 | 45.9% (39.5–52.3) |
| ARV therapy and follow-up | ||
| Proportion of patients with continued care | 351 | 70.9% (66.1–75.7) |
| Proportion of patients who had CD4 count measured at least once during the last 6 months | 351 | 20.8% (16.6–25.0) |
| Proportion of patients eligible for ARV who are currently on ARV | 267 | 76.8% (71.7–81.9) |
| Proportion of patients on ART who are started on ART within 2 weeks after clinical eligibility is confirmed | 233 | 86.3% (82.5–91.1) |
| Proportion of patients on NVP who had LFT at least once within 1 month after initiation of NVP-based ART | 179 | 0.0% |
| Proportion of patients on ART for whom adherence is measured on last three visits | 218 | 28.0% (22.1–33.9) |
| Record completeness | ||
| Proportion of patients with previous ARV regimen change for whom reason for change in regimen is documented | 26 | 53.8% (35.3–72.3) |
| Proportion of patients on ARV who had registered own and contact address | 351 | 41.9% (36.7–47.1) |
According to the national guidelines, 267 (76.1%) of the 351 sample patients were eligible for ART. Among these, only 205 (76.8%) were actually taking ART while 34 (12.7%) of them never started ART and the rest 28 (10.5%) failed to continue treatment after initiation. Of the 118 patients who were in care before ART initiation, 34 (28.8%) were actually eligible for ART but had not yet started it.
Out of the 351 patients included in this study, 236 (67.2%) had a most recent CD4 count of less than 350 cells/µl that makes them eligible for CPT according to the national guidelines. The result showed that only 179 (75.8%) of these eligible patients were prescribed CPT during their last HIV clinic visit. Furthermore, the clinic was able to maintain continuity of care only for 146 (81.6%) of those patients who were prescribed with CPT during their last visit. Exit interviews with patients attending the HIV clinic showed that only 74.3% of patients prescribed with CPT during their previous clinic visit took their prophylactic drugs. Continuity of care was assessed against this standard and was found to be maintained for only 249 (70.9%) patients. It was 88.0% for patients taking ART and 37.3% for patients in care before ART initiation.
According to the national guideline on management of patients with HIV infection [4], a patient with HIV is expected to visit HIV clinic at least once every 6 months prior to ART initiation and every 3 months after ART initiation. Continuity of care was assessed against this standard and was found to be maintained for only 249 (70.9%) patients. It was 88.0% for patients taking ART and 37.3% for patients in care before ART initiation.
Behavioral and health outcomes
Achievement of desired behavioral and health outcomes was measured by the level of patients' adherence to prescribed drugs, immunologic status of patients, clinical improvement (improvement in functional status of bedridden or ambulatory patients) and satisfaction of patients. Table 3 presents the findings on specific outcome indicators of care and treatment.
Table 3.
Summary of performance on outcome indicators of quality, Felege Hiwot Referral Hsopital, September 2007
| Outcome indicators | n | Achievement, P (95% CI) |
|---|---|---|
| Adherence to medications | ||
| Proportion of patients on ARV with at least 95% (good) reported adherence on last visit | 346 | 97.7% (96.1–99.3) |
| Proportion of patients on cotrimoxazole prophylaxis with at least 95% reported adherence on last visit | 279 | 65.2% (59.6–70.8) |
| Immunologic and clinical response to HIV care | ||
| Proportion of patients with CD4 count greater than 200 cells/µl | 331 | 60.4% (55.1–65.7) |
| Proportion of either bedridden or ambulatory patients who have improvement in functional status | 92 | 71.7% (62.5–80.9) |
| Patient satisfaction | ||
| Average of patient satisfaction score | 368 | 78.0% (73.8–82.2) |
Adherence to antiretroviral drugs and to CPT was measured by interviewing patients on the number of doses missed during 1-week period prior to the date of interview. If a patient was taking 95% or more of prescribed pills, this was defined as good adherence. The result from the interview showed that 338 (97.7%) of patients on ART and 182 (65.2%) of those on CPT had good adherence to their respective prescribed drugs. Among respondents of the interview who were prescribed with CPT, it was found that 72 (25.8%) of them were not taking the drugs at all.
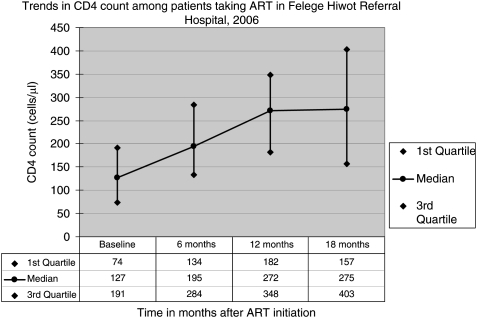
Out of the 331 patients who had their CD4 count measured at least once, first-time CD4 count was greater than 200 cells/µl for 150 (45.3%) of the patients. After varying period in care and/or treatment, most recent CD4 count was greater than 200 cells/µl for 200 (60.4%) of them. Among patients who were taking ART, CD4 measurements at baseline, 6, 12 and 18 months after ART were found documented for 199, 69, 53 and 23 patients, respectively. The result of this study showed an increase in median CD4 count with duration on ART. Fig. 1 presents the trend in median CD4 count with duration on ART. As shown in the figure, the median baseline CD4 count was found to be 275 cells/µl (IQR: 157, 403 cells/µl) at 18 months of treatment when compared with that of baseline CD4 count which was 127 cells/µl (IQR: 74, 191 cells/µl).
Figure 1.
CD4 count of patients on HAART across time after ART initiation, Flege Hiwot Referral Hospital, September 2007.
Patients’ self-reported functional status was one of the patient monitoring measures recommended by the national treatment guideline. Working, ambulatory and bed ridden were the categories used for this purpose. Among the study subjects, functional status was assessed and documented during enrollment to care for 246 (70.1%) of them. Out of these, 154 (62.6%) were working; 77 (31.3%) were ambulatory and 15 (6.1%) were bedridden. Of the total 92 patients who were either bedridden or ambulatory, 66 (71.7%) showed improvement from ambulatory to working, bedridden to ambulatory or bedridden to working.
On the basis of the patient satisfaction survey, the average level of satisfaction was 3.9 out of 5 points (78.0%). Average rate of satisfaction was relatively higher for variables related with provider–patient interaction for which the average satisfaction score was 4.4 out of 5 points. On the other hand, the satisfaction score was relatively lower for affordability of care which was mainly related with the non-medical cost of visiting the HIV clinic. The average satisfaction score for this dimension was 3.2 out of 5 points. Table 4 presents the number of satisfaction questions under each satisfaction dimension and the level of satisfaction of patients on each of satisfaction dimensions.
Table 4.
Summary of satisfaction levels by dimensions of satisfaction, Flege Hiwot Referral Hospital, September 2007
| Dimensions of patient satisfaction | Average number of respondents | Number of questions | Mean satisfaction score |
|---|---|---|---|
| Availability of resources | 345 | 7 | 3.4 |
| Perceived quality of care | 317 | 7 | 4.0 |
| Health worker–patient interaction | 320 | 8 | 4.4 |
| Accommodation and time management | 312 | 5 | 4.0 |
| Affordability of care | 365 | 2 | 3.2 |
| Average of all indices | 327 | 29 | 3.9 |
Discussion
This evaluation study used an indicator-driven approach for the measurement of HIV care quality and has addressed three components of quality—structure, process and outcome. The results show that a significant portion of patients attending HIV clinic in the study hospital were missing important components of care recommended by the national guidelines. Although there were important improvements in many patients’ immunologic and clinical conditions, achievement of these desired patient outcomes needed further improvement. The presence of all the required resources, except an observed shortage of cotrimoxazole, to provide HIV care reveals a vast opportunity for the hospital to improve process and outcome indicators of quality. A relatively better quality of care was provided to patients on enrollment in care with 90.9% of the patients received their first CD4 count within 2 weeks after first HIV clinic visit. For the remaining components of care expected to be provided as follow up care, the hospital failed to provide recommended care for significant portion of its HIV-infected patients.
A number of studies have shown that CPT prevents the occurrence of fatal opportunistic infections among patients with HIV infection [18–21]. World Health Organization and UNAIDS also recommended the use of CPT for patients with symptomatic HIV infection [22]. Considering these recommendations, the national CPT guideline has made a recommendation to prescribe CPT for HIV-infected patients with CD4 count of less than 350 cells/µl [5]. In this respect, the finding from this evaluation study showed that out of the sample patients who were eligible for CPT, only about 45.9% were actually on CPT. This considerably low achievement was because of failure to prescribe CPT for eligible patients during clinic visits, lower rate of continuity of care and poor compliance of patients with CPT.
Timely initiation of appropriate ART is one of the basic components of chronic HIV care which determines the outcome of HIV clinical care. Many studies have proved that antiretroviral therapy reduces morbidity and mortality among patients with HIV [6, 7, 23, 24]. The Ethiopian standard on the time to start ART is based on the World Health Organization's recommendation for resource-limited settings [25]. According to the national ART guideline, patients need to be started on ART if they have clinical stage IV disease, clinical stage III disease with CD4 count less than 350 cells/µl or those with CD4 count less than 200 cells/µl irrespective of their clinical stage [4].
According to this study, the study hospital was able to keep 76.8% of eligible patients on ART. Nearly 13% of the eligible patients were never started on ART and the remaining 10.5% failed to follow-up after starting ART. This showed that there are HIV patients who are eligible but have not yet been put on ART. In concrete terms, more than a quarter (28.8%) of HIV-infected patients who were in care before ART initiation did not start ART although they should have been started according to the recommendation in the national treatment guideline. This finding appears to be an issue for discussion among HIV/AIDS program managers who are determined to expand the service to health centre level, with the aim of universal access to ART, probably without due attention to the quality of care in the existing delivery sites.
Continuity of care was maintained for 88.0% for patients in care after ART initiation and 37.3% before ART initiation. Those patients who lost to follow-up require putting in place a strong system of patient-tracing mechanism. Unfortunately, documentation of patients' own and contact address was poor; only 41.9% of patients had a complete address documented in their records, making tracing of patients difficult.
Time and again, studies have shown that outcome of HAART is strongly associated with adherence to antiretroviral drugs [26–30]. This study showed that good adherence to antiretroviral drugs was achieved in 97.7% of the patients. This result was higher compared with a similar measure in a more urbanized setting of Addis Ababa where the proportion of patients with good adherence was 81.2% which was considered even better when compared with the situation in the developed world [15]. However, adherence to CPT was low; only 65.2% of patients prescribed with CPT had good adherence to CPT during the week prior to the data collection day and 25.8% were not taking the drug at all even though it was prescribed by their providers.
The overall process of care for patients with HIV is to keep patients protected from opportunistic infections, by improving their immunological status [4]. A patient's CD4 count less than 200 cells/µl is a strong marker for the possible occurrence of opportunistic infections [31]. The last CD4 count among the sample HIV-infected patients was found to be greater than 200 cells/µl in 60.4% (n = 351) of them when compared with that of first-time CD4 count which showed that only 45.3% of the patients had CD4 count of greater than 200 cells/µl. CD4 measures at baseline, 6, 12 and 18 months after ART initiation also showed improvement in immunologic status of patients with time on ART. Despite this improvement, there is a huge gap for improvement to keep all patients with CD4 count of at least 200 cells/µl. This finding should alert relevant stakeholders to give emphasis on prophylactic therapies and work towards early enrollment of patients to care and treatment.
Quality of care provided to patients was found to vary across different components of care. The best care provided was during first time enrollment of patients during which 90.9% of patients received their first-time CD4 measurement within 2 weeks of enrollment. For most of the rest care components expected to be provided during follow-up, the system was able to provide recommended care only for less than half of the patients. This finding is comparable to a study in the USA, which showed that patients are more likely to receive the recommended HIV care better during their first visit than in follow-up care [11].
The patient record was the main source of process indicators used in this evaluation study. The accuracy and completeness of the patient record may result in either underestimating or overestimating some of the indicators. To date, there are no studies done in Ethiopia to compare the reliability of methods to measure health care quality, so the direction of bias introduced by inherent limitations of chart abstraction is not well understood in our context. However, some studies outside the country have shown that using chart review may underestimate quality indicators by a margin of 5.4 and 10.6% when compared with measures through the use of clinical vignettes and standardized patients, respectively [32]. If this holds true in our setting, the use of standardized patients would have increased the values of the measured process indices by up to 10%, but most of the indicators would still fall below the expected level of performance.
Conclusions
Felege Hiwot Referral Hospital has adequate resources to provide HIV/AIDS clinical care. However, important components of care recommended by the national guidelines were found to be missed for significant proportions of patients. Although immunologic and clinical improvements were observed among patients attending the hospital, there is still a huge gap to maximize benefits to all eligible patients. The presence of these gaps despite the availability of adequate human and material resources shows that availability of resources alone will not ensure the quality of clinical services to patients with HIV infection signifying the need to continuously monitor and improve process and outcome measures of quality.
References
- FMOH/FHAPCO. AIDS in Ethiopia: sixth report. http://www.etharc.org/aidsineth/publications/AIDSinEth6th_en.pdf. (12 December 2006, date last accessed)
- World Health Organization. Progress on Global Access to HIV Antiretroviral Therapy: A Report on ‘3 by 5’ and Beyond. Geneva: World Health Organization; 2006. [Google Scholar]
- UNAIDS. 2006 Report on the global AIDS epidemic. 2006.
- Federal HIV/AIDS Prevention and Control Office/Federal Ministry of Health. Guidelines for management of opportunistic infections and antiretroviral treatment in adolescents and adults in Ethiopia. http://www.etharc.org/arvinfo/ethOIARTguideline_2007.pdf. (11 November 2007, date last accessed)
- Federal MOH/Federal HAPCO. Guideline for cotrimoxazole prophylaxis in HIV/AIDS care and treatment. http://www.etharc.org/arvinfo/cotromoxizoleguide.pdf. (12 May 2006, date last accessed)
- Palella FJ, Jr, oria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Lundgren JD. Starting highly active antiretroviral therapy: why, when and response to HAART. J Antimicrob Chemother. 2004;54:10–3. doi: 10.1093/jac/dkh290. [DOI] [PubMed] [Google Scholar]
- Liu C, Weber K, Robison E, et al. Assessing the effect of HAART on change in quality of life among HIV-infected women. AIDS Res Ther. 2006;3:6. doi: 10.1186/1742-6405-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHAPCO. Monthly ART update as of September 10, 2007. 2007.
- Wilson IB, Landon BE, Marsden PV, et al. Correlations among measures of quality in HIV care in the United States: cross sectional study. Br Med J. 2007;335:1085. doi: 10.1136/bmj.39364.520278.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to Medicare beneficiaries: a profile at state and national levels. J Am Med Assoc. 2000;284:1670–6. doi: 10.1001/jama.284.13.1670. [DOI] [PubMed] [Google Scholar]
- Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? 1998. Milbank Q. 2005;83:843–95. doi: 10.1111/j.1468-0009.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian A. An Introducation to Quality Assurance in Health Care. New York: Oxford University Press; 2003. [Google Scholar]
- Tadios Y, Davey G. Antiretroviral treatment adherence and its correlates in Addis Ababa, Ethiopia. Ethiop Med J. 2006;44:237–44. [PubMed] [Google Scholar]
- Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83:691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Health AIDS Institute, Johns Hopkins University. HIV clinical resource: adult and adolescent indicators. 2006. http://www.hivguidelines.org/Content.aspx?pageID=36. (15 July 2006, date last accessed)
- McNaghten AD, Hanson DL, Jones JL, et al. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS. 1999;13:1687–95. doi: 10.1097/00002030-199909100-00012. [DOI] [PubMed] [Google Scholar]
- Grimwade K, Sturm AW, Nunn AJ, et al. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS. 2005;19:163–8. doi: 10.1097/00002030-200501280-00008. [DOI] [PubMed] [Google Scholar]
- Walker AS, Mulenga V, Ford D, et al. The impact of daily cotrimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007;44:1361–7. doi: 10.1086/515396. [DOI] [PubMed] [Google Scholar]
- Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization, UNAIDS. Antibiotic recommendation for African HIV patients. 2000. 2007.
- Enrico Girardia. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–91. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. J Am Med Assoc. 2001;286:2560–7. doi: 10.1001/jama.286.20.2560. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Scaling up antiretroviral therapy in resource limitted settings: treatment guidelines for a public health approach. 2007. http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf .
- Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–8. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–10. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. J Am Med Assoc. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]