Abstract
The prefrontal cortex r regulates behavior, cognition, and emotion by using working memory. Prefrontal functions are impaired by stress exposure. Acute, stress-induced deficits arise from excessive protein kinase C (PKC) signaling, which diminishes prefrontal neuronal firing. Chronic stress additionally produces architectural changes, reducing dendritic complexity and spine density of cortico-cortical pyramidal neurons, thereby disrupting excitatory working memory networks. In vitro studies have found that sustained PKC activity leads to spine loss from hippocampal-cultured neurons, suggesting that PKC may contribute to spine loss during chronic stress exposure. The present study tested whether inhibition of PKC with chelerythrine before daily stress would protect prefrontal spines and working memory. We found that inhibition of PKC rescued working memory impairments and reversed distal apical dendritic spine loss in layer II/III pyramidal neurons of rat prelimbic cortex. Greater spine density predicted better cognitive performance, the first direct correlation between pyramidal cell structure and working memory abilities. These findings suggest that PKC inhibitors may be neuroprotective in disorders with dysregulated PKC signaling such as bipolar disorder, schizophrenia, post-traumatic stress disorder, and lead poisoning—conditions characterized by impoverished prefrontal structural and functional integrity.
Keywords: bipolar disorder, post-traumatic stress disorder, working memory, chelerythrine, lead poisoning
The prefrontal cortex is the most highly evolved brain region and is essential for the intelligent regulation of attention, action, and emotion by representational knowledge. The ability to represent information is often referred to as working memory or the “mental sketch-pad”. Networks of prefrontal cortical neurons interconnect on dendritic spines to maintain information in working memory (1, 2). However, the prefrontal cortex is also the brain region that is most sensitive to the detrimental effects of stress. Even mild acute, uncontrollable stress rapidly impairs prefrontal functions in animals (3–5) and humans (6), whereas regions such as the hippocampus require more severe or prolonged stress to show functional changes (7, 8). Acute stress impairs prefrontal cortical function through a cascade of events: There is a large increase in catecholamine release in the prefrontal cortex, which in turn leads to high levels of cyclic adenosine monophosphate (cAMP) and protein kinase C (PKC) intracellular signaling, which reduce prefrontal neuronal firing, and rapidly impair working memory (9, 10).
When stressors are repeated over many days and weeks, there are further architectural changes in the prefrontal cortex: a retraction of dendrites and a loss of spine density (11–15). These dendritic changes are associated with impaired prefrontal cortical attentional set-shifting in rats and, it is likely, in human subjects as well (16, 17). Dendritic changes in the prefrontal cortex can occur with repeated exposure to even mild stressors (18, 19), indicating that this region is remarkably sensitive to chronic stress as well as to acute stress. Dendritic changes are evident in layer II/III pyramidal cells, which form the networks subserving representational knowledge (1), and particularly in distal apical dendrites (11). However, the molecular changes underlying this spine loss are not yet understood.
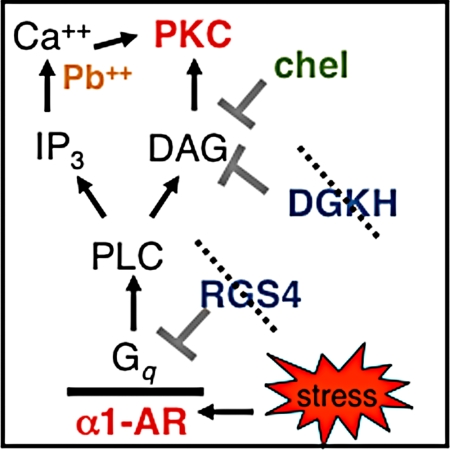
It is likely that many molecular “culprits” contribute to architectural changes in prefrontal neurons during chronic stress. One likely candidate contributing to dendritic spine loss is PKC intracellular signaling, as in vitro studies of hippocampal cell cultures have shown that elevated PKC signaling induces spine collapse through disruption of the actin cytoskeleton (20). Excessive PKC signaling also has immediate clinical relevance, as either genetic or environmental insults can overactivate this pathway. As illustrated in Fig. 1, several molecules that normally inhibit PKC signaling are genetically altered and/or dramatically reduced in patients with mental illness who have impaired prefrontal function. The bipolar disorder genome has recently identified alterations in diacylglycerol (DAG) kinase eta (DGKH) (21, 22), which encodes for the kinase that normally inhibits DAG activation of PKC signaling. Loss of function of DGKH in bipolar disorder would lead to excessive PKC signaling, as has been reported previously (23). Another key factor is regulator of G protein signaling 4 (RGS4), which normally inhibits Gq signaling (Fig. 1). RGS4 levels are greatly reduced in the prefrontal cortex of patients with schizophrenia (24). It is noteworthy that pharmacological treatments for both bipolar disorder and schizophrenia indirectly inhibit PKC signaling: Lithium indirectly reduces PKC activity by inhibiting inositol monophosphatase, whereas atypical antipsychotics block α1 adrenoceptors (α1-ARs) and 5HT2 receptors that are coupled to Gq signaling (25–29).
Fig. 1.
Stress results in elevated catecholamine release in the prefrontal cortex. Increased noradrenergic signaling activates the PI-PKC cascade via Gq-coupled α1-AR. RGS4 and diacylglycerol kinase, including DGKH, inhibit this pathway. Genetic studies indicate that RGS4 and DGKH are compromised in schizophrenia and bipolar disorder, which would lead to overactivation of PKC signaling. Lead (Pb++) mimics Ca2+, a necessary cofactor for the activation of PKC. Thus, PKC is likely dysregulated in stress-related disorders such as PTSD as well as in lead poisoning, bipolar disorder, and schizophrenia. PKC dysregulation may be related to pathology and symptomology as overactive PKC leads to prefrontal cognitive deficits in primates and rodents and spine loss in vitro. CHEL inhibits PKC isoforms activated by this cascade by blocking the phorbol ester binding site.
Environmental factors can also activate PKC signaling and result in prefrontal impairment. For example, lead can mimic calcium (Ca2+) and potently activate PKC (Fig. 1). Lead poisoning is associated with prefrontal deficits including altered attention regulation and behavioral disinhibition in animals (30, 31) and humans (32–34). Exposure to a traumatic stressor can lead to post-traumatic stress disorder (PTSD), which is associated with weakened medial prefrontal function (35). Importantly, PTSD, lead poisoning, schizophrenia, and bipolar disorder are all associated with prefrontal gray matter loss (10), and where studied, dendritic spine loss from prefrontal pyramidal neurons (36). Recent data indicate that lithium can rescue prefrontal gray matter in bipolar disorder (37–40) and restore stress-induced dendritic retraction in rat hippocampi (8), suggesting that PKC signaling may contribute to architectural changes in patients with mental illness. Thus, there is immediate clinical significance to determining whether PKC signaling contributes to prefrontal dendritic spine loss in vivo.
The current study used the chronic restraint stress paradigm previously shown to alter prefrontal dendritic morphology and function in rats (11, 13, 17), and examined whether chronic treatment with the PKC inhibitor, chelerythrine (CHEL), before daily stress would rescue prefrontal cognition and spine density. Systemic administration of CHEL has previously been shown to be effective in reversing the biochemical and cognitive effects of acute stress on PKC signaling (9). The current study reports that treatment with daily CHEL rescued both working memory and dendritic spine density from the detrimental effects of chronic stress exposure.
Results
PKC Inhibition Rescues Stress-Induced Working Memory Impairment.
Rats were exposed to a restraint stress paradigm (21 days, 6 h/day) that induces distal apical dendritic retraction and spine loss in layer II/III pyramidal neurons in the medial prefrontal cortex (11, 13, 17). Half of the stressed animals received vehicle and half received CHEL (1.0 mg/kg, s.c.), daily, before stress exposure. Nonstressed controls also received vehicle or CHEL daily. All rats had been pretrained on a spatial delayed alternation task in a T maze to an equivalent level of performance, and were then tested prior to drug/stress treatment each day. This task requires working memory as well as behavioral inhibition and the ability to overcome distraction, and necessitates an intact medial prefrontal cortical function in rats (41). Rats were assessed for cognitive ability 10 times over the 21 days and were rapidly anesthetized and killed following the last test session. The prelimbic cortex was dissected and processed by using the rapid Golgi technique for analysis of apical dendritic morphology and spine density in layer II/III pyramidal cells.
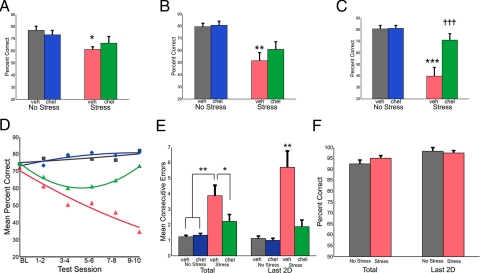
Exposure to stress induced a dramatic and progressive loss of delayed alternation performance in vehicle-treated rats, growing more pronounced over the duration of the stress (Fig. 2 A–D). As animals were tested before the daily stress session, these impairments reflect the accumulating effects of chronic stress rather than an immediate response to an acute stress. Inhibition of PKC intracellular signaling with daily CHEL treatment (administered after testing) rescued delayed alternation performance in stressed rats. Repeated-measures ANOVA indicated significant group effects for the entire 10 sessions (F (3, 27) = 15.25, P < 0.0001) as well as for the early (first two test sessions, F (3, 27) = 3.21, P = 0.039), middle (middle two test sessions, F (3, 27) = 8.13, P = 0.001) and late (last two consecutive test sessions, F (3, 27) = 22.65, P < 0.0001) phases of the stress. Tukey HSD tests indicated that, for each of these four time points, vehicle-treated stressed rats performed significantly worse than nonstressed rats, whereas CHEL-treated stressed rats were at no time significantly different from nonstressed rats. The beneficial effect of CHEL treatment was most pronounced in the late phase of the stress when dendritic changes are observed (11, 13, 17). Specifically, stressed rats receiving CHEL performed significantly better than stressed rats receiving vehicle over the last two days of the stress paradigm (stress+vehicle, 34.8 ± 6.5% correct; stress+chel, 73.0 ± 5.7% correct; P < 0.0001; Fig. 2C).
Fig. 2.
PKC inhibition rescues stress-induced working memory impairment. (A–C) Mean spatial delayed alternation (working memory) performance over the first (A), middle (B), and last (C) two test sessions. Daily CHEL treatment rescued working memory impairment in the late phase of chronic stress (C). (D) Mean working memory performance is shown for test sessions as indicated (quadratic fit). BL, baseline; nonstress vehicle (gray squares); nonstress CHEL (blue diamonds), stress vehicle (red triangles); stress CHEL (green triangles). (E) Perseveration was measured by mean number of consecutive entries into the same choice arm per test session. Stressed, vehicle-treated rats (red) made significantly more perseverative errors than stressed rats treated with CHEL (green) and nonstressed vehicle (gray) or CHEL-treated (blue) rats. Similar to working memory performance (C), perseveration, measured as mean consecutive errors within a test session, increased over the duration of the stressor, with the greatest number of errors occurring in the last two testing days, at the end of the 21-day stress period. Spatial discrimination was evaluated in a separate group of rats exposed to the chronic stress paradigm (red) or handled daily (gray). (F) Spatial discrimination was assessed as a control task. Performance was unaffected by stress, and performance did not change or deteriorate over the course of the 21 days of stress, suggesting that the deficits observed in spatial delayed alternation performance are specific to the operations of the prefrontal cortex. Different from nonstress+vehicle: *, P < 0.05; **, P < 0.01; ***, P < 0.0001; different from stress+vehicle: = = = P < 0.0001; error bars denote SEM.
Impaired working memory performance was accompanied by an increase in perseveration in stressed, vehicle-treated rats. Perseveration was defined as the mean number of consecutive errors (CE) per test session. ANOVA indicated significant group effects for the entire 10 sessions (F (3, 27) = 9.46, P < 0.0001) as well as for the last two days of testing (F (3, 27) = 16.02, P < 0.0001; Fig. 2E). Perseveration is consistent with PFC dysfunction (42, 43) and was most pronounced when the most significant stress-induced working memory deficits were observed (Fig. 2C). Similar to overall working memory performance, perseveration was rescued in stressed animals treated with CHEL (10 sessions: stress+vehicle, 3.88 ± 0.67 CE; stress+chel, 2.21 ± 0.45 CE; Tukey HSD, P < 0.0001; last 2 sessions: stress+vehicle, 5.71 ± 1.05 CE; stress+chel, 1.87 ± 0.43 CE; Tukey HSD, P < 0.0001; Fig. 2E).
To address the specificity of the stress response to prefrontal cognitive abilities, a separate group of rats was exposed to the same stress paradigm but tested on a spatial discrimination task in the same test apparatus. The spatial discrimination task requires similar motivational, locomotor, and spatial abilities as delayed alternation but does not require an intact prefrontal cortex (44). Chronic stress exposure had no effect on performance of this control task, indicating that deficits observed on the delayed alternation task represent prefrontal-specific cognitive deficits (Fig. 2F).
Stress-Induced Atrophy of the Apical Dendrite.
Dendritic morphology and spine density were evaluated in five-layer II/III pyramidal neurons from the prelimbic cortex of each cognitively characterized animal. The apical dendrite was completely reconstructed to allow for measurements of dendritic length and branching. Soma size and distance from pial surface was not significantly different between groups.
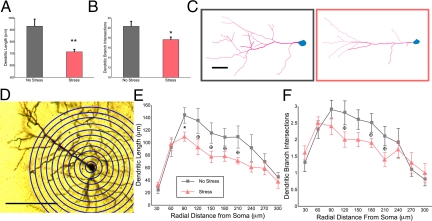
Comparisons of vehicle-treated groups replicated earlier findings (11, 13, 14). Stressed, vehicle-treated animals had reduced apical dendritic length (no-stress, 931.0 ± 59.6 μm; stress, 715.4 ± 20.8 μm; F (1, 12) = 11.27; P = 0.006; Fig. 3A), and reduced apical dendritic branch intersections (no-stress, 20.33 ± 1.02 intersections; stress, 17.64 ± 0.53 intersections; F (1, 12) = 6.22; P = 0.028; Fig. 3B). Also similar to previous reports (13, 14), sholl analysis indicated atrophy at 90 μm from the soma and a trend of reductions in more distal portions of the apical dendrite (Fig. 3 D–F). This replication indicates that training, testing, and husbandry variations do not mitigate the stress response. Further, these results indicate that Golgi impregnation and intracellular cell loading (13, 17) yield comparable results.
Fig. 3.
Layer II/III pyramidal cells from the prelimbic cortex of cognitively trained rats show expected trend of chronic, stress-induced apical dendritic atrophy. (A and B) Chronic stress reduced apical dendritic length (A) and branch intersections (B). (C) Representative reconstructions of apical dendrites from nonstressed (gray) and stressed (red) neurons are shown (scale bar = 50 μm). (D) Apical dendritic length and branch intersections were quantified in consecutive 30-μm bins radiating from the center of the soma by using sholl analysis. (E) Similar to previous reports, stressed rats (red triangles) showed reduced apical branch length at 90 μm from the center of the soma, and a trend of reduced length in more distal regions relative to nonstressed rats (gray squares). (F) Although branch intersections were not significantly reduced, there was a trend of stress-reducing branch intersections in distal regions as well. *, P < 0.05; B trend, P < 0.10; error bars denote SEM.
Although group effects for dendritic length and branch intersections did not reach significance, total apical dendritic length tended to be increased in the stressed rats receiving CHEL (Fig. S1). Given that PKC modulates actin (20, 45), we hypothesized that it would specifically rescue spines but not dendrites. It is possible that when large numbers of spines are affected (see Fig. 4 A and C), changes in the associated dendrites are triggered as well.
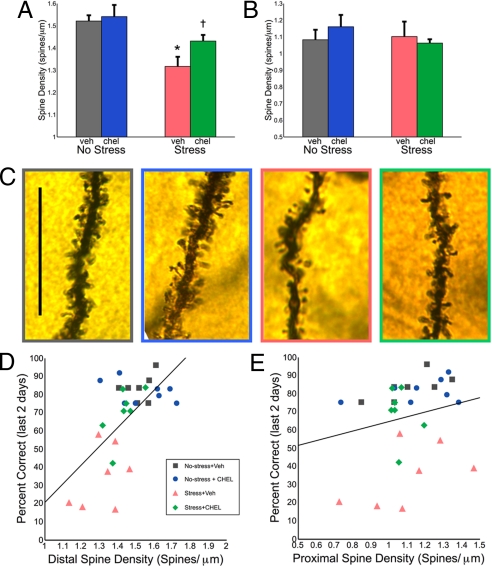
Fig. 4.
Distal dendritic spines are critical sites for stress and working memory performance and are protected by PKC inhibition. (A) Apical spine density was reduced in stressed, vehicle-treated rats 200 μm from the soma. This spine loss was rescued by daily CHEL treatment. (B) Stress and CHEL treatment did not affect proximal apical spine density on more proximal (<70 μm) apical branches. (C) Representative images of distal dendritic segments are shown (nonstress vehicle, gray; nonstress CHEL, blue; stress vehicle, red; stress CHEL, green; scale bar = 25 μm). Distal apical spine density predicts working memory performance. (D and E) Pearson's test indicated that working memory performance on the last two days of stress significantly correlated with distal apical spine density (r = 0.636, P < 0.0001) (D) but not with proximal spine density (r = 0.222, P = 0.248) (E). Different from nonstress+vehicle: *, P < 0.05; different from stress+vehicle: = P < 0.05; error bars denote SEM.
PKC Inhibition Rescues Chronic Stress-Induced Distal Dendritic Spine Loss.
Dendritic spines were quantified in five regions of the dendrite (see Methods). Similar to previous reports (11, 19), stress significantly reduced apical spine density 200 μm from the center of the soma (no-stress, 1.52 ± 0.03 spine/μm; stress, 1.32 ± 0.04 spines/μm; Fig. 4A) but did not have significant effects on more proximal portions of the apical dendrite (e.g., <70 μm from the center of the soma; Fig. 4B) nor on the basal dendrite. Comparisons across all four groups by using repeated-measures ANOVA indicated group differences for distal apical spines only (F (3, 25) = 6.407; P = 0.002). As illustrated in Fig. 4 A and C, CHEL-treated, stressed rats had a significantly higher density of apical dendritic spines 200 μm from the center of the soma than did vehicle-treated, stressed rats (stress+vehicle, 1.32 ± 0.04 spines/μm; stress+CHEL, 1.43 ± 0.03 spines/μm; P = 0.043).
CHEL was most effective in reversing stress-related cognitive deficits in the late phase of the stress paradigm (Fig. 2 C and D) when these morphological measures were obtained. To determine whether there was a correlation between spine density and cognitive performance, Pearson's tests were used. We observed a highly significant correlation between mean apical dendritic spine density (averaged across all five neurons within one animal) and mean delayed alternation performance over last two days of the study (r = 0.636, P < 0.0001, Fig. 4D). Stressed rats with the lowest spine density had the worst cognitive performance, whereas those receiving CHEL—and the nonstressed rats—had higher spine density and better cognitive performance. Spine density in more proximal regions of the apical dendrite did not correlate with behavior (r = 0.222, P = 0.248, Fig. 4E). Interestingly, proximal basal spine density, which was unaffected by stress or CHEL treatment, showed a significant, albeit weaker, relationship with spatial delayed alternation performance (r = 0.431, P = 0.02). The magnitude of the correlation between cognitive performance and proximal basal spine density was less than one-half the magnitude of the correlation between cognitive performance and distal apical spine density (Fig. 4D).
Discussion
The current study used a chronic stress paradigm to begin to examine the molecular mechanisms underlying dendritic spine loss in the prefrontal cortex. Loss of prefrontal gray matter and dendritic spines in patients with mental illness likely contributes to profound prefrontal deficits, and the stress model provides an excellent opportunity to study the signaling events that contribute to these devastating changes. The present study focused on the role of PKC intracellular signaling, as experiments have shown that sustained increases in PKC activity lead to spine loss in hippocampal cultures (20). We found that daily, systemic treatment with the PKC inhibitor CHEL rescued prefrontal spine density and working memory from the detrimental effects of chronic stress exposure.
Chronic Stress Impairs Prefrontal Cortex Structure and Function.
This study used a chronic restraint stress paradigm that has been demonstrated to induce dendritic atrophy and decrease dendritic spine density in rat prefrontal cortical neurons (11, 13, 17). Although the animals in this study were exposed to numerous factors that could potentially mitigate the effects of stress—daily handling, injections, food restriction, cognitive training, and testing—we observed patterns of dendritic and spine changes that precisely replicated earlier studies. This indicates that effects of chronic stress on prefrontal morphology are robust, and morphological changes can be captured by using either Golgi impregnation, which is optimal for samples requiring extensive analysis and light exposure, or intracellular cell loading methods, which have the advantage of providing three-dimensional information.
Layer II/III pyramidal neurons form cortical networks that allow the representation of rules and goals and the intelligent regulation of behavior (1). Thus, the loss of spines and dendritic material from these neurons is expected to have great consequences for cognitive performance. Indeed, Liston et al. (16, 17) observed a correlation of attentional, perceptual set-shifting and dendritic morphology in rat dorsal medial prefrontal cortices, and has recently reported similar reductions in set-shifting ability and fMRI measures of prefrontal connectivity in humans subjected to chronic mild stress. Given that spines are likely a critical anatomical substrate for the reverberating excitatory networks underlying working memory, we examined the relationship between spine density and spatial working memory performance. We observed a highly significant correlation between apical dendritic spine density 200 μm from the soma—the most stress sensitive region of the dendrite—and cognitive performance over the last two days of the study. Conclusions are limited because of the correlative nature of this study. Although we cannot conclude that the loss of spines causes cognitive changes, these findings suggest that protecting prefrontal regions vulnerable to stress may be key to rescuing prefrontal cortical regulation of behavior and cognition. These findings may encourage postmortem spine-counting studies, similar to the rigorous anatomical analyses performed by Glantz and colleagues (36, 46), examining spine pathology in the prefrontal cortex of patients with bipolar disorder, PTSD, and lead poisoning.
Role of PKC Signaling.
PKC activity is increased in the prefrontal cortex during stress exposure (9). The working memory deficits induced by acute stress exposure have been blocked by six different PKC inhibitors in both appetitive (9) and aversive (47, 48) working memory paradigms. Studies of hippocampal cultures have shown that PKC phosphorylation of a myristoylated, alanine-rich C kinase substrate (MARCKS) results in the disruption of filamentous actin cross-linking, spine instability, and spine collapse (20). These in vitro findings suggested that PKC activity may similarly contribute to spine loss in vivo during chronic stress. CHEL inhibits stress-induced PKC activity in the prefrontal cortex (9, 49) and has the unique advantage of being the only PKC inhibitor that crosses the blood–brain barrier following systemic administration. This was key in manipulating processes induced by chronic stress, as the requirement for daily infusions into prefrontal cortex would have induced extensive gliosis and scarring, and thus interfered with investigation of the morphology of the region under analysis. Because CHEL was administered systemically, it is likely that it altered stress effects in other brain regions as well. For example, stress-sensitive regions such as the hippocampus and amygdala are also regulated by PKC (10) and interact with the prefrontal cortex. Thus, future studies of CHEL effects on these and other brain regions would be of interest.
The finding that CHEL protected prefrontal spine density and working memory suggests that PKC plays a prominent role in dendritic changes during chronic stress. High levels of PKC activity during chronic stress may arise from increased α1-AR stimulation (Fig. 1), as chronic stress sensitizes noradrenergic axons in prefrontal cortex (50–52). PKC activity could also be increased by stress-induced release of serotonin acting via 5HT2 receptors, and/or glucocorticoids acting via nongenomic mechanisms (53), although these mechanisms have not been demonstrated in prefrontal cortex.
It is unlikely that PKC dysregulation alone accounts for the stress-related deficits that we observed. Stress affects the nervous system in myriad ways, altering hormones, neurotransmitter functions, cytoskeletal architecture, and cell signaling, including growth factors (54). For example, previous work from our lab has shown that cAMP hyperpolarization-activated cyclic nucleotide-gated (HCN) channel signaling is also critical to the cellular and cognitive operations of the prefrontal cortex (55, 56). There is mounting evidence that cAMP and PI-PKC pathways potentiate each other. Ca2+ activates adenylyl cyclase, increasing cAMP (57), and cAMP facilitates IP3 receptor binding (58). Such feed-forward interactions have the potential to amplify the stress response.
Clinical Relevance.
Loss of prefrontal gray matter in patients with mental illness is related to severe prefrontal dysfunction, which is in turn related to impaired social, emotional, and cognitive functioning (59, 60). Similarly, lead poisoning is associated with impaired regulation of attention and social behavior (e.g., criminality, out-of-wedlock pregnancy), and with loss of gray matter in the prefrontal cortex (31–33, 61). The current results suggest that loss of prefrontal gray matter in schizophrenia, bipolar disorder, PTSD, or lead poisoning likely involves excessive PKC signaling and that treatments directly inhibiting PKC may help rescue prefrontal gray matter and cognitive function. Genetic vulnerabilities such as loss of function mutations in DGK or RGS4 may predispose patients with bipolar disorder or schizophrenia to increased PKC signaling, thus lowering the threshold for dendritic atrophy. This idea is supported by the recent findings that lithium, which indirectly inhibits PKC activity, restores prefrontal gray matter in patients with bipolar disorder (37–40). In PTSD, the experience of a traumatic stress and subsequent repeated, conditioned stressors would produce chronic elevation of stress-signaling pathways. Indeed, PTSD patients, like rats exposed to chronic stress, have hypernoradrenergic signaling (62). In this regard, it is of interest that a blockade of α1-ARs can help to reduce symptoms of PTSD (63), although it is not known if this helps rescue prefrontal cortical gray matter. Finally, an environmental toxin—lead—can mimic Ca2+, resulting in elevated PKC activity (64–66). The current results suggest that an increase in PKC signaling may contribute to a loss of prefrontal cortical gray matter in subjects with lead poisoning. As lead remains in the body for a lifetime, PKC inhibitors may be helpful in restoring gray matter in children exposed to lead poisoning.
Methods
Cognitive Assessment.
Animal care and use were approved by the Yale Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines. Male Sprague–Dawley rats (250–350 g) were housed, maintained on a restricted diet, and trained on the spatial delayed alternation task in a T maze as described previously (9). Further details can be found in the SI Text. Testing occurred before daily stress to dissociate sustained from acute effects of the stress. Rats were tested 10 times (approximately every two to three days) throughout the 21-day stress period. The last two days of testing followed the 20th and 21st day of stress. Performance was evaluated as the number of trials correct out of 12. Group differences were compared by using one-way, repeated-measures ANOVAs, with test session as the repeated measure. For spatial delayed alternation, significant effects were evaluated with Tukey HSD posthoc tests and performance over two consecutive test sessions was averaged and fit to a quadratic curve to illustrate performance over time. Correlations were analyzed with Pearson's correlation tests. P < 0.05 was considered statistically significant.
Chronic Stress.
Rats in the stress group were restrained in Plexiglas tubes daily, following testing and drug treatment, for 6 h (10:00 a.m.–4:00 p.m.) for 21 consecutive days, a paradigm known to reliably produce dendritic atrophy and spine loss in the prelimbic cortex (11, 13). Animals were monitored to prevent undue distress. Animals in the no-stress group were handled daily. All animals were maintained on a restricted diet where food was offered after the cessation of stress to control for motivational effects.
PKC Inhibition.
CHEL (LC Laboratories) mixed in bacteriostatic (1.0 mg/kg, s.c.) was used to inhibit PKC. Either intraprefrontal cortex infusions or systemic administration of CHEL reverses stress- or age-related working memory impairments in rats (9, 49). CHEL was the favored treatment because it is the only PKC inhibitor available that penetrates the brain following systemic administration. All animals were injected after behavioral testing and 20 min before stress onset, eliminating the confound of acute treatment drug or stress treatments influencing task performance.
Morphological Analyses.
Immediately following the last spatial delayed alternation test session, rats were decapitated under isoflurane anesthesia. Tissue was prepared by using the rapid Golgi kit (FD Neurotechnologies) according to manufacturer's instructions. Following a 14 day incubation period, tissue was sliced coronally (200 μm) and mounted on gelatin-coated slides. Tissue from one animal in the no-stress+vehicle group and one animal in the stress+chel group was damaged and therefore not suitable for morphological analyses.
Pyramidal cell bodies lying in layer II/III of prelimbic cortex (were reconstructed in three dimensions at 80–100× magnification (40–60× objective lens, with 1.0–2.0 turret magnification) by using a microscope equipped with a motorized stage, video camera system, and Neurolucida morphometry software (MicroBrightField). Neurons were reconstructed and spines were quantified by one of two blinded experimenters. The two experimenters achieved >90% reliability on spine number and dendrite reconstruction on reliability tests administered three times throughout the 18 months of reconstruction. Inclusion criteria for neurons are listed in the SI Text. Five neurons for each rat included in the cognitive characterization study were assessed—with the exception of two animals—in which only four neurons within the prelimbic cortex met inclusion criteria.
Spines were quantified in five locations on each reconstructed neuron: (i) apical branch(es) lying 200 μm from the soma; (ii) the first apical branch 20–70 μm from the soma; (iii) the apical trunk); (iv) proximal basal branch (0–30 μm from soma); and (v) distal basal branch (30–60 μm from soma). Additional details can be found in the SI Text.
Dendritic length and branch intersections were evaluated by performing sholl analyses in 30-μm bins, as described in earlier studies (13, 14). Total dendritic length and branch intersections represent the sum of the sholl output up to 300-μm radial distance from the soma, which was the greatest extent of the apical dendrite achieved by all neurons included in the analyses.
Statistical Analyses.
Group differences in spine density, dendritic length and dendritic branch intersections were compared by using a mixed ANOVA design, with neurons as the repeated, within-subject measure, and group as the between-subjects measure. Significant effects were evaluated with Tukey HSD posthoc tests and a targeted model to test for differences between stressed animals with and without drug treatment.
Correlations were analyzed with Pearson's correlation tests comparing mean spine density (the average of the five neurons within each animal), and mean performance over the last two days of testing. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank M.F. Yeckel and V. Pieribone for comments; A. Begovic and T. Sadlon for technical assistance; and L. Selemon, P. Hof, and A. Duque for discussion and technical advice. This study was supported by U.S. Public Health Service Grants P50MH068789 and RL1AA017536 within consortium U54RR024350 (to A.F.T.A.) and K25NS044316 (to P.K.M.), and by a National Science Foundation Graduate Research Fellowship (to A.B.H.).
Footnotes
Conflict of interest statement: Drs. Arnsten and Hains, together with Yale University, have a patent and patent applications, and are pursuing a license agreement to develop CHEL for the treatment of PTSD, bipolar disorder, and related illnesses.
See Commentary on page 17613.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908563106/DCSupplemental.
References
- 1.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatr. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 5.Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qui S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 7.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 8.Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum SG, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 10.Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 11.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 12.Radley JJ, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 15.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 16.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 19.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Baum AE, Akula N, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnsten AFT, Manji HK. Mania: A rational neurobiology. Future Neurol. 2008;3:125–131. [Google Scholar]
- 23.Manji HK, Lenox RH. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 24.Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi Y, Pandey GN. Effects of treatment with haloperidol, chlorpromazine, and clozapine on protein kinase C (PKC) and phosphoinositide-specific phospholipase C (PI-PLC) activity and on mRNA and protein expression of PKC and PLC isozymes in rat brain. J Pharmacol Exp Ther. 1999;291:688–704. [PubMed] [Google Scholar]
- 26.Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1145–1158. doi: 10.1016/j.pnpbp.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980;137:1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- 28.Bone R, Springer JP, Atack JR. Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci USA. 1992;89:10031–10035. doi: 10.1073/pnas.89.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen BM, Lipinski JF. In vivo potencies of antipsychotic drugs in blocking alpha 1 noradrenergic and dopamine D2 receptors: Implications for drug mechanisms of action. Life Sci. 1986;39:2571–2580. doi: 10.1016/0024-3205(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 30.Alber SA, Strupp BJ. An in-depth analysis of lead effects in a delayed spatial alternation task: Assessment of mnemonic effects, side bias, and proactive interference. Neurotoxicol Teratol. 1996;18:3–15. doi: 10.1016/0892-0362(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 31.Morgan RE, et al. Early lead exposure produces lasting changes in sustained attention, response initiation, and reactivity to errors. Neurotoxicol Teratol. 2001;23:519–531. doi: 10.1016/s0892-0362(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 32.Wright JP, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nevin R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ Res. 2000;83:1–22. doi: 10.1006/enrs.1999.4045. [DOI] [PubMed] [Google Scholar]
- 34.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bremner JD, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 37.Blumberg HP, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: A cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bearden CE, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 41.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- 43.Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- 44.Divac I. Frontal lobe system and spatial reversal in the rat. Neuropsychologia. 1971;9:175–183. doi: 10.1016/0028-3932(71)90041-8. [DOI] [PubMed] [Google Scholar]
- 45.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001;58:203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- 47.Dash PK, Moore AN, Kobori N, Runyan JD. Molecular activity underlying working memory. Learn Mem. 2007;14:554–563. doi: 10.1101/lm.558707. [DOI] [PubMed] [Google Scholar]
- 48.Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennan AR, et al. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol Aging. 2007;30:782–792. doi: 10.1016/j.neurobiolaging.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miner LH, et al. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finlay JM, et al. Impact of corticotropin-releasing hormone on extracellular norepinephrine in prefrontal cortex after chronic cold stress. J Neurochem. 1997;69:144–150. doi: 10.1046/j.1471-4159.1997.69010144.x. [DOI] [PubMed] [Google Scholar]
- 52.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 53.Han JZ, Lin W, Lou SJ, Qiu J, Chen YZ. A rapid, nongenomic action of glucocorticoids in rat B103 neuroblastoma cells. Biochim Biophys Acta. 2002;1591:21–27. doi: 10.1016/s0167-4889(02)00242-2. [DOI] [PubMed] [Google Scholar]
- 54.McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 55.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 58.Soulsby MD, Wojcikiewicz RJ. Biochem J. 2005;392:493–497. doi: 10.1042/BJ20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prasad KM, Sahni SD, Rohm BR, Keshavan MS. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Psychiatry Res. 2005;140:147–155. [Google Scholar]
- 60.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Cecil KM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Southwick SM, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 63.Taylor FB, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein GW. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology. 1993;14:97–101. [PubMed] [Google Scholar]
- 65.Simons TJ. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology. 1993;14:77–85. [PubMed] [Google Scholar]
- 66.Sun X, Tian X, Tomsig JL, Suszkiw JB. Analysis of differential effects of Pb2+ on protein kinase C isozymes. Toxicol Appl Pharmacol. 1999;156:40–45. doi: 10.1006/taap.1999.8622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.