Abstract
Orexin/hypocretin neurons of the lateral hypothalamus/perifornical area project to a diverse array of brain regions and are responsive to a variety of psychostimulant drugs. It has been shown that orexin neurons are activated by systemic nicotine adminstration suggesting a possible orexinergic contribution to the effects of this drug on arousal and cognitive function. The basal forebrain and paraventricular nucleus of the dorsal thalamus (PVT) both receive orexin inputs and have been implicated in arousal, attention and psychostimulant drug responses. However, it is unknown whether orexin inputs to these areas are activated by psychostimulant drugs such as nicotine. Here, we infused the retrograde tract tracer cholera toxin B subunit (CTb) into either the basal forebrain or PVT of adult male rats. Seven to 10 days later, animals received an acute systemic administration of (−) nicotine hydrogen tartrate or vehicle and were euthanized two hours later. Triple-label immunohistochemistry/immunofluorescence was used to detect Fos expression in retrogradely-labeled orexin neurons. Nicotine increased Fos expression in orexin neurons projecting to both basal forebrain and PVT. The relative activation in lateral and medial banks of retrogradely-labeled orexin neurons was similar following basal forebrain CTb deposits, but was more pronounced in the medial bank following PVT deposits of CTb. Our findings suggest that orexin inputs to the basal forebrain and PVT may contribute to nicotine effects on arousal and cognition and provide further support for the existence of functional heterogeneity across the medial-lateral distribution of orexin neurons.
Keywords: arousal, attention, basal forebrain cholinergic system, hypothalamus
1. Introduction
The orexins (orexin A and B; also known as hypocretin 1 and 2) are neuropeptides expressed in neurons of the lateral hypothalamus and contiguous perifornical area (LH/PFA) of the mammalian brain [7,34]. Consistent with the role of these peptides in arousal and modulation of state-dependent behavior [2,38], orexin neurons are activated by a variety of different psychostimulant drugs, including d-amphetamine [12], methamphetamine [11], modafinil [38] and caffeine [27]. We have recently shown that acute nicotine administration also activates orexin neurons as measured by expression of the immediate-early gene product, Fos [30]. Nicotine also upregulates expression of orexins and orexin receptors in the rat brain [22]. These studies strongly suggest that the orexin system is a common target of acute psychostimulant treatment and may, in turn, mediate some of the behavioral, reinforcing, rewarding and addictive effects of many drugs of abuse. Indeed, orexin peptides have been strongly implicated in responses to cocaine and morphine [3,18].
While the rodent brain contains only a few thousand orexin neurons, the target projections of this relatively small group of cells are impressively diverse [32]. One way that the orexin system might accomplish its wide array of divergent functions is through stimulus-dependent activation of different target projections. Some functional and anatomical heterogeneity has already been suggested for this system, with orexin neurons of the lateral hypothalamus implicated in the rewarding properties of natural and pharmacological reinforcers while more medially-located orexin neurons may regulate arousal and stress responses [17]. Functional and connectional differences across the medial-lateral gradient of orexin neurons have also been suggested based on studies investigating Fos expression in orexin neurons as a function of behavioral state [11] and antipsychotic drug responses [12].
Rostral targets that could contribute to the effect of orexins on arousal and attention include the basal forebrain and the paraventricular nucleus of the thalamus (PVT). These areas receive moderate to dense orexin innervation [10,14,23,32] and play important roles in cortical activation in support of arousal and attention. Functionally, intrabasalis administration of OxA promotes wakefulness [9,40], increases prefrontal cortical ACh release [14] and excites cholinergic neurons [8]. Wakefulness-related release of OxA within the basal forebrain has also been reported [24].
Similarly, recent studies indicate that orexins increase firing in PVT neurons, implicating a role in wakefulness [19,20]. Collectively, these background data suggest that orexin projections to both the basal forebrain and PVT may be part of the neural substrates underlying nicotine effects on arousal, wakefulness and attention. Here, we combined neuronal tract-tracing using the retrograde tracer cholera toxin b (CTb) with Fos expression to examine the effect of acute nicotine administration on orexin neurons projecting to the basal forebrain or PVT.
2. Materials and methods
2.1 Animals and surgery
Male Sprague-Dawley rats (Harlan Laboratories, Birmingham, AL), weighing approximately 250–300g were pair-housed in an environmentally controlled animal facility on a 12:12 h light: dark cycle with lights on at 07:00. Purina rat chow and water were available ad libitum. Animals were handled daily for at least one week prior to experimentation. All experiments were conducted during the light phase, beginning at least 2 hours after light phase onset and concluding at least 2 hours prior to the beginning of the dark phase. Animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
Rats were anesthetized using sodium pentobarbital, prepared for surgery and placed in a stereotaxic apparatus. The scalp was incised to expose the skull, and a small burr hole was drilled above the target brain structure. The needle of a 1.0 μl Hamilton syringe containing the retrograde neuronal tracer cholera toxin B subunit (CTb; 1% solution in artificial cerebrospinal fluid; List Biological Laboratories, Campbell, CA) was lowered through the burr hole to the target structure. Stereotaxic coordinates, relative to Bregma [31], used for the basal forebrain were AP −0.8 mm, DV −8.0 mm and L +2.5 mm. This part of the basal forebrain lies at the intersection of the substantia innominata, ventral pallidum and rostral nucleus basalis and was chosen because it contains the cell bodies of basal forebrain cholinergic neurons that innervate rostral cortical regions such as the prefrontal cortex. Cholinergic neurons in this region receive a dense innervation by orexin fibers and respond to local orexin administration by increasing cortical acetylcholine release [14]. Stereotaxic coordinates used for the PVT were AP −2.5 mm, DV −4.9 mm and L +0.1 mm. This mid-level portion of the PVT were chosen because different rostro-caudal levels of the PVT do not appear to be differentially innervated by orexins [23] or differentially responsive to arousal-related stimuli such as footshock [6].
After positioning the needle tip 100 nl of the CTb solution was manually infused at a rate of 20 nl/min. Ten minutes after infusion was complete (in order to allow for diffusion of CTb away from the needle tract), the needle was withdrawn, the incision was sutured and the animal was returned to its home cage upon emergence from anesthesia. Each animal received a single, unilateral CTb infusions either into the basal forebrain or PVT. Daily monitoring and handling of the animals resumed the day following surgery.
2.2 Drugs and treatment
Following ten days of recovery, each rat (final usable N = 4 per drug condition for each brain region) received a single systemic injection of either (−) nicotine hydrogen tartrate (2.0 mg/kg, i.p.; equates to a free base nicotine dose of 0.7 mg/kg) or its vehicle solution (saline). This dose of acute systemic nicotine elicits a robust Fos response in orexin neurons [30]. Two hours after the injection, animals were deeply anesthetized with isoflurane and transcardially perfused with 0.1 M phosphate buffer followed by 4% paraformaldehyde/0.1 M phosphate buffer (pH 7.4). The brains were removed, postfixed overnight and transferred to 30% sucrose solution for cryoprotection. Serial coronal sections (45 μm) were cut on a cryostat and processed for immunohistochemistry.
2.3 Immunohistochemistry
A combination of single-label immunoperoxidase (Fos) and double-label immunofluorescence (orexin plus CTb) was performed on the serial coronal sections with the rostro-caudal extent of the hypothalamus. Briefly, all sections were initially incubated with a goat anti-Fos antibody (1:3000; 48 h at 4°C; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a biotinylated donkey anti-goat secondary (1:1000; 1.5 h at room temperature; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and horseradish peroxidase-conjugated streptavidin (1:1600; 1 h at room temperature; Jackson). Fos immunoreactivity was visualized by developing the sections in a nickel-cobalt intensified diaminobenzidine solution with 0.3 % hydrogen peroxide, yielding a blue-black reaction product confined to the nucleus of Fos-positive cells. After Fos staining, sections were incubated in a combined goat primary antisera directed against CTb (1:7500; List Biological Laboratories, Campbell, CA) and rabbit primary antisera directed against orexin A (1:3000; Oncogene Research Products, Cambridge, MA) at 4°C for 48 h, followed by incubation in CY2-labeled donkey anti-goat and CY3- labeled donkey anti-rabbit secondary antibodies (1:1000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 3 h at room temperature. After mounting and coverslipping, epifluorescence was visualized using a Nikon E600 microscope equipped with a mercury lamp and filters selective for the Cy2 or Cy3 wavelengths. Fos-immunoreactive nuclei (characterized by a black “spot” of appropriate shape and size either alone or surrounded by cytoplasmic CTb and/or orexin immunofluorescent signal) were clearly visible under these conditions (Figure 3) but were also verified by light microscopy with the slide in the same position. Photomicrographic images were captured with a Photometrics CoolSnap monochrome digital camera (Roper Scientific, Trenton, NJ, USA) linked to a computer equipped with IPLab software. Monochrome images were collected under each filter setting, digitally assigned a pseudocolor and imported into Adobe Photoshop where minor adjustments for contrast and brightness were made.
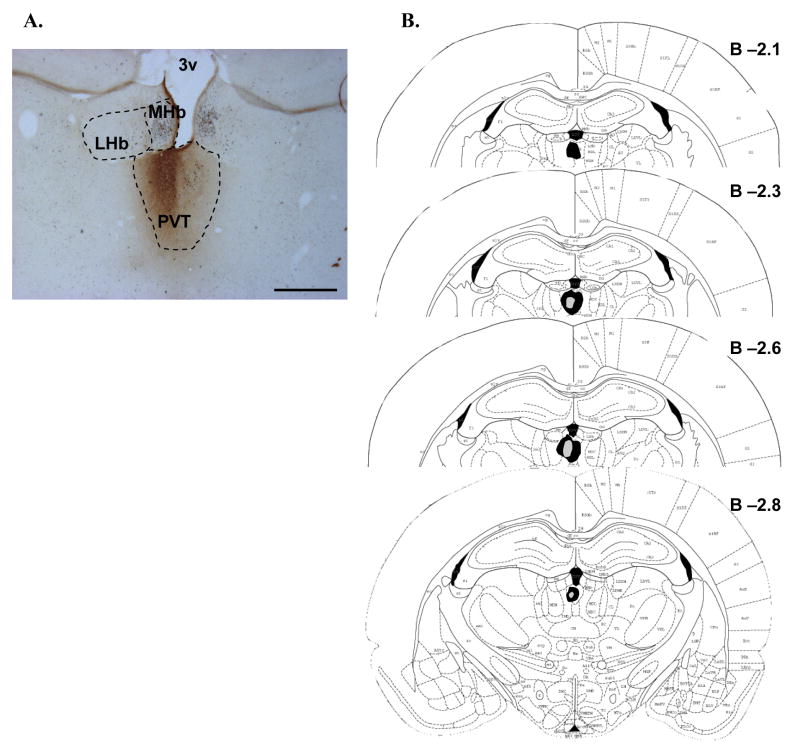
Figure 3.
CTb deposits in the PVT. A. A representative photomicrograph of the core deposit region in the PVT of one animal. CTb (brown immunoreactive product) was centered in the PVT, with minimal overlap into adjacent intralaminar thalamic nuclei. No labeling in the habenula was observed. Black immunoreactive product indicates Fos labeling on this double-immunolabeled section from a nicotine-treated animal. Scale bar equals approximately 500 μm. B. The approximate extent of the largest (black fill) and smallest (gray fill) deposits for all animals is plotted schematically at several rostro-caudal levels of the PVT. Numbers represent approximate distance (in mm) from Bregma. Although deposits generally showed a hemispheric preference, there was generally some degree of overlap across the midline. Although this preference was equally distributed between right and left hemispheres all deposits are represented in the same hemisphere for clarity. See text for further description of PVT deposit distributions. PVT, paraventricular thalamic nucleus; LHb, lateral habenula; MHb, medial habenula; 3v, third ventricle.
2.4 Cell counts and data analysis
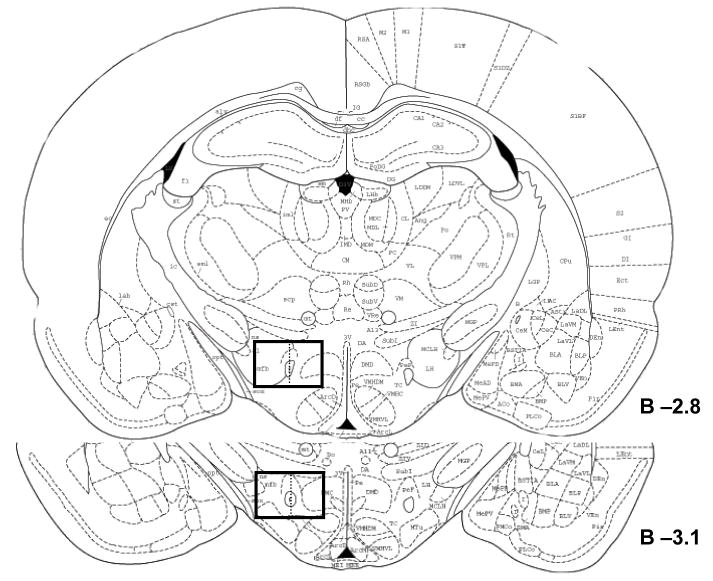
Because previous reports have suggested the response of orexin neurons to psychostimulants may differ along the medial-lateral gradient of these cells [12,17,27], the number of single (CTb)-, double (CTb plus OxA)-, or triple (CTb plus orexin plus Fos)- labeled cells were counted in both medial and lateral sectors (defined as medial or lateral to a line bisecting the fornix) of the LH/PFA using fluorescent microscopy under 20X magnification. Cell counts were conducted on the hemisphere ipsilateral to the CTb deposit at two different rostrocaudal levels of the LH/PFA corresponding approximately to 2.8 mm and 3.1 mm caudal to Bregma (Figure 1). This part of the LH/PFA contains the highest density of orexin neurons [4]. Double-label data were expressed as a percentage of CTb-labeled cells positive for orexin. Triple-labeled data were expressed as a percentage of double-labeled neurons (CTb plus OxA) positive for Fos. Data were analyzed by multivariate analysis of variance using SPSS for PC software (v. 14.0; Chicago, IL). The source of significant (P < 0.05) main effects was determined by post-hoc Bonferroni comparisons.
Figure 1.
Schematic of the area used for cell counts within the LH/PFA. Cell counts were conducted at two representative rostro-caudal levels of the LH/PFA, corresponding approximately to 2.8 and 3.1 mm caudal to Bregma. These levels contain dense clusters of orexin neurons. These counts were conducted unilaterally on the same side as the CTb deposits. Counts were restricted to the part of the LH/PFA containing the greatest numbers of orexin neurons as indicated by the boxed outlines. Data were separated into the medial LH/PFA (medial to the fornix as indicated by the dashed vertical line bisecting the box) and lateral LH/PFA (lateral to the fornix).
3. Results
3.1 CTb deposits and general characteristics of labeling
Basal forebrain CTb deposits were centered in the ventral pallidum and contiguous substantia innominata (Figure 2). Some degree of overflow was observed in surrounding basal forebrain regions such as the rostral portion of the nucleus basalis magnocellularis, ventral portions of the globus pallidus, the ventrolateral bed nucleus of the stria terminalis and the interstitial nucleus of the posterior limb of the anterior commissure.
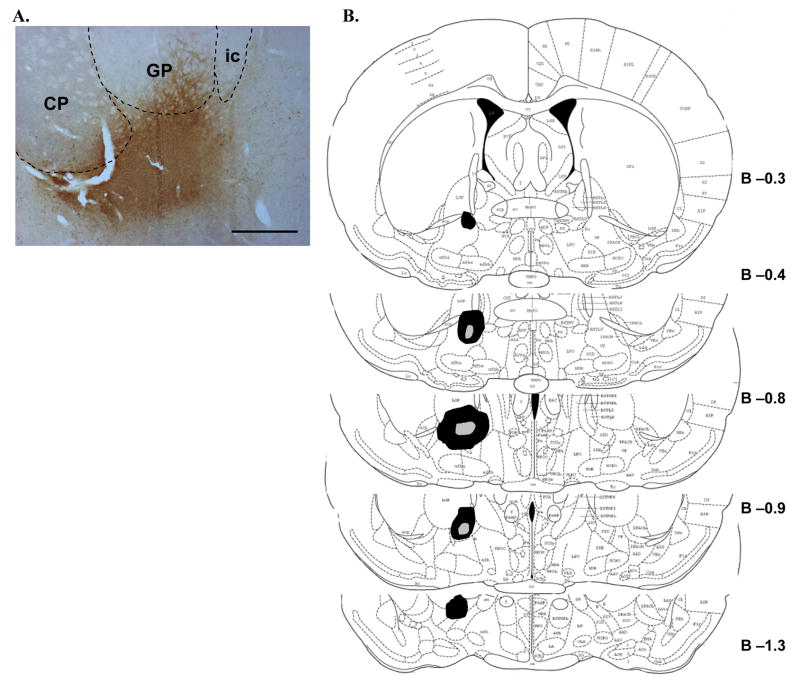
Figure 2.
CTb deposits in the basal forebrain. A. A representative photomicrograph of the core deposit region in the basal forebrain of one animal. CTb (brown immunoreactive product) was centered in the ventral pallidum/substantia innominata portion of the basal forebrain, with some overlap into adjacent ventral parts of certain basal ganglia structures. Scale bar equals approximately 500 μm. B. The approximate extent of the largest (black fill) and smallest (gray fill) basal forebrain deposits from all animals is plotted schematically at several rostro-caudal levels of the basal forebrain. Numbers represent approximate distance (in mm) from Bregma. Although half of the animals received deposits in the left hemisphere and half in the right, all deposits are represented in the same hemisphere for clarity. See text for further description of basal forebrain deposit distributions. CP, caudate-putamen; GP, globus pallidus; ic, internal capsule.
Paraventricular thalamic nucleus CTb deposits were centered in the middle and anterior portions of the PVT (Figure 3). In some cases there was minimal overlap into adjacent intralaminar nuclei, including the mediodorsal and dorsal centromedian nuclei. In all cases the habenula was largely devoid of CTb labeling. While PVT deposit sites always showed some degree of hemispheric predominance, there was frequently at least some overlap across the midline.
Peroxidase immunohistochemistry for Fos followed by immunofluorescent labeling for CTb and orexin revealed numerous single-, double- and triple-labeled neurons in the LH/PFA. These three labels were readily distinguishable from each other in triple-labeled preparations (Figure 4). Putative Fos labeling identified under epifluorescent conditions was verified at the light microscopic level by turning on the light source with the slide in the same position.
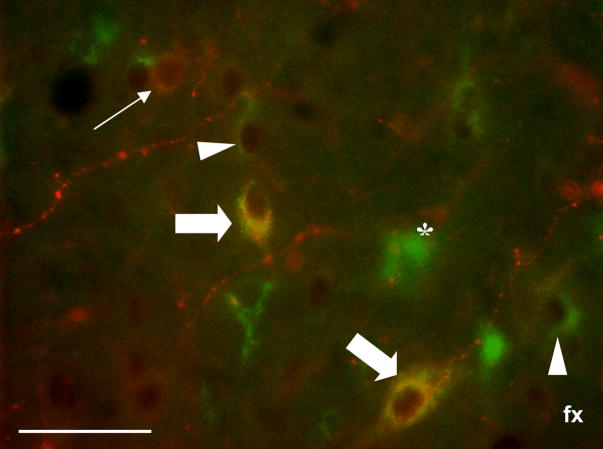
Figure 4.
Representative photomicrograph demonstrating combined immunofluorescence and immunoperoxidase triple-labeling for CTb, orexin and Fos in the LH/PFA. Green (Cy2) immunofluorescence indicates retrograde labeling (i.e., CTb), red (Cy3) labeling indicates orexin immunofluorescence and black nuclear labeling indicates Fos immunoreactive peroxidase product. Examples of single-labeled CTb-positive neurons (asterisk), retrogradely-labeled cells positive for Fos (white arrowheads), single-labeled orexin neurons (thin white arrow) and triple-labeled neurons (thick white arrows) can be clearly seen. In all cases, Fos immunoreactivity was verified with light microscopy. Scale bar equals approximately 50 μm.
3.2 Activation of basal forebrain-projecting orexin neurons by nicotine
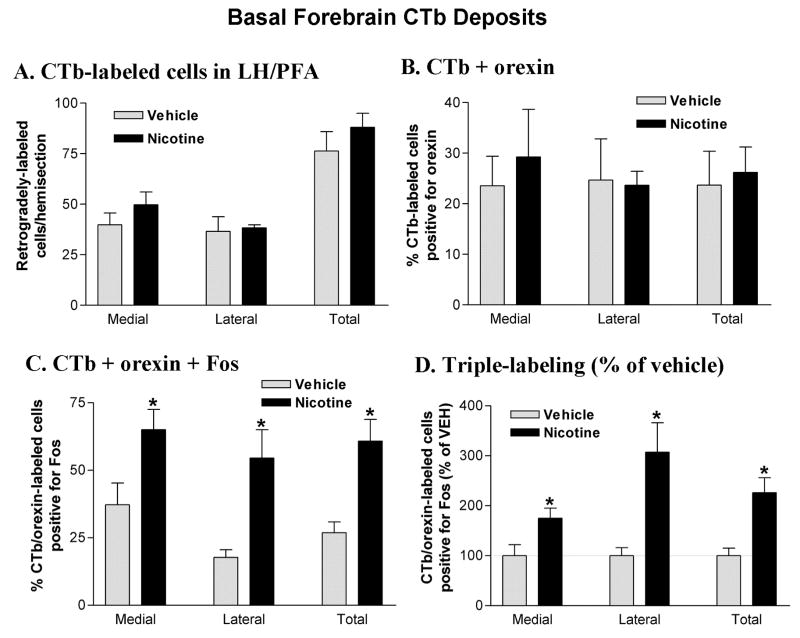
The number and distribution (medial vs. lateral) of retrogradely-labeled neurons observed in the LH/PFA did not differ as a function of treatment group (Figure 5A). The percentage of retrogradely-labeled cells positive for orexin was also similar in the two treatment groups and double-labeled cells were approximately equally distributed in medial and lateral portions of the LH/PFA (Figure 5B). For the triple-labeling studies, ANOVA revealed a significant effect of treatment on Fos expression in CTb/orexin double-labeled cells (F1,6 = 6.46; p < 0.05). Post-hoc analysis indicated that the percentage of triple-labeled cells was significantly greater in the nicotine-treated animals compared with vehicle in both sectors of the LH/PFA as well as for this region as a whole (Figure 5C). While the overall numbers of basal forebrain-projecting orexin cells activated by nicotine was similar (55–65%) in both sectors of the LH/PFA, the effect in the lateral bank was particularly robust relative to vehicle treatment (roughly a 3-fold difference; Figure 5D).
Figure 5.
Cell counts in the LH/PFA following basal forebrain CTb deposits. A. CTb-labeled neurons in the LH/PFA. Retrogradely-labeled neurons were equally distributed across medial and lateral banks of the LH/PFA and did not differ as a function of drug treatment. B. Double-labeling for CTb and orexin in the LH/PFA. A substantial portion (20–30%) of the retrogradely-labeled neurons in the LH/PFA were also immunoreactive for orexin A. Double-labeled cells were equally distributed across medial and lateral banks of the LH/PFA and did not differ as a function of drug treatment. C. Triple-labeling for CTb, orexin and Fos in the LH/PFA. Acute nicotine (2.0 mg/kg) treatment increased the percentage of CTb/orexin double-labeled neurons that were positive for Fos. This effect was significant in both medial and lateral banks of the LH/PFA. D. Triple-labeled cell counts expressed as a percentage of vehicle control values. *P < 0.05 vs. vehicle.
3.3 Activation of PVT-projecting orexin neurons by nicotine
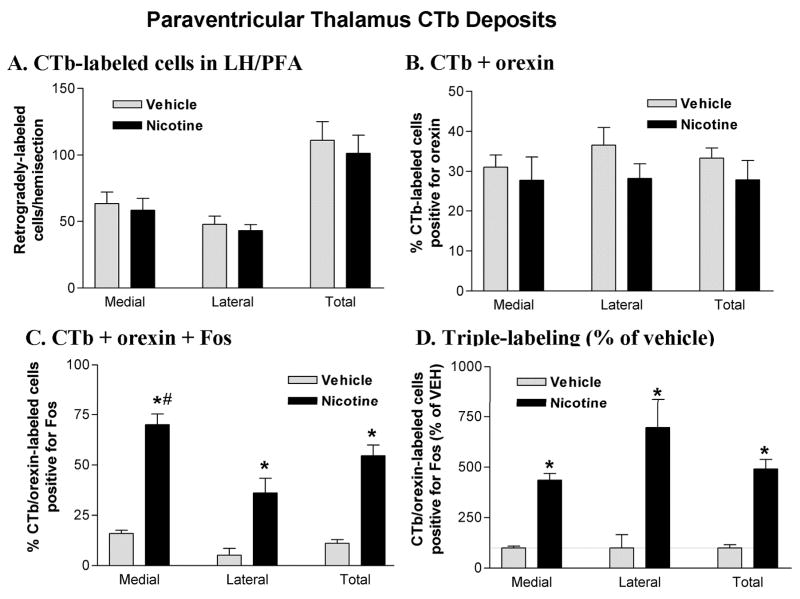
As seen in the basal forebrain CTb groups, the number and distribution of single (CTb alone) and double-labeled (CTb + orexin) neurons observed in the LH/PFA did not differ as a function of treatment group (Figure 6A&B). The percentage of retrogradely-labeled cells positive for orexin was also similar in the two treatment groups and double-labeled cells were approximately equally distributed in medial and lateral portions of the LH/PFA (Figure 6B). For the triple-labeling studies, ANOVA revealed a significant effect of treatment on Fos expression in CTb/orexin double-labeled cells (F1,6 = 6.46; p < 0.05). Post-hoc analysis indicated that the percentage of triple-labeled cells was significantly greater in the nicotine-treated animals compared with vehicle in both sectors of the LH/PFA as well as for this region as a whole (Figure 6C). Nicotine treatment also resulted in a greater percentage of triple-labeling in the medial sector of orexin neurons compared to the lateral sector (70% vs. 36%; t3 = 5.644; p = 0.11). When expressed as a percentage of vehicle control, nicotine-elicited Fos expression in PVT-projecting orexin neurons did not differ as a function of medial vs. lateral location (p > 0.05; Figure 6D).
Figure 6.
Cell counts in the LH/PFA following PVT CTb deposits. A. CTb-labeled neurons in the LH/PFA. Retrogradely-labeled neurons were equally distributed across medial and lateral banks of the LH/PFA and did not differ as a function of drug treatment. B. Double-labeling for CTb and orexin in the LH/PFA. A substantial portion (25–35%) of the retrogradely-labeled neurons in the LH/PFA were also immunoreactive for orexin A. Double-labeled cells were equally distributed across medial and lateral banks of the LH/PFA and did not differ as a function of drug treatment. C. Triple-labeling for CTb, orexin and Fos in the LH/PFA. Acute nicotine (2.0 mg/kg) treatment increased the percentage of CTb/orexin double-labeled neurons that were positive for Fos. This effect was significant in both sectors of the LH/PFA but was especially prominent in the medial bank, where approximately 70% of PVT-projecting orexin neurons were activated by nicotine. D. Triple-labeled cell counts expressed as a percentage of vehicle control values. *P < 0.05 vs. vehicle. #P < 0.05 vs. lateral.
4. Discussion
This study demonstrates that orexin efferents to both basal forebrain and PVT are robustly activated by acute nicotine treatment and suggests that orexin neurons projecting to these telencephalic regions are components of the neural substrates underlying nicotine effects on arousal and attention.
Activation of orexin neurons by acute nicotine is consistent with our previous findings [30]. However, the target projections of orexin neurons expressing Fos following nicotine treatment has not been demonstrated. Here, triple-labeled neurons (CTb + orexin + Fos) were found in both lateral and medial sectors of the LH/PFA following vehicle or nicotine treatment in animals receiving either basal forebrain or PVT retrograde tracer deposits. Consistent with a variety of previous reports [11,12,27], we found that the medial bank of orexin neurons show higher basal (or, perhaps more accurately, vehicle injection-elicited) levels of Fos expression. Lateral bank orexin neurons, however, tend to show greater relative increases in Fos expression following drug treatment. This has been interpreted in terms of a dichotomy between arousal and reward, as many of the manipulations that preferentially activate lateral orexin neurons include cues previously paired with drug or food reward whereas stressful stimuli such as footshock exert a greater effect on medial orexin neurons [18]. However, another—not incompatible—possibility is that medial bank orexin neurons may set the “background” level of orexin activity that fluctuates more broadly across the sleep wake cycle whereas the lateral bank may be more responsive to acute manipulations that link orexin activity with phenomena such as drug responses, novelty and reward. In this regard, it is interesting that acute nicotine treatment had a greater relative effect (vs. vehicle) on lateral orexin neurons that project to the basal forebrain whereas its relative effect on PVT-projecting orexin neurons was approximately the same in medial and lateral banks. Most PVT neurons appear to be responsive to orexin A and/or orexin B and the prefrontal cortex is a prominent site of projection from orexin-activated PVT neurons [19,20]. This raises the possibility that orexin inputs to the PVT may help to facilitate global cortical activation related to general arousal [37] whereas orexin projections to the basal forebrain may activate components of this region (such as the basal forebrain cholinergic system) that mediate more stimulus-specific attentional processes[15,36]. These speculations await rigorous empirical testing.
The rodent hypothalamus contains only a few thousand orexin neurons, with estimates ranging from /hemisphere. Given the widespread target projections of this system, there is almost certain to be some degree of collateralization of individual orexin neurons to innervate multiple forebrain targets. Indeed, such a phenomenon has been demonstrated for orexin projections to the basal forebrain and locus coeruleus[10]. Thus, it is possible that some orexin neurons may innervated both the basal forebrain and PVT. Several questions regarding such potential collateralization—including the degree, whether or not orexin neurons collateralizing to innervate both targets are more likely to originate from the medial or lateral banks, and whether they are differentially sensitive to nicotine treatment—await additional elegant anatomical studies combining multiple tract-tracers with pharmacological manipulations.
Nicotine may exert different effects on lateral vs. medial banks of orexin neurons innervating basal forebrain or PVT via differential effects on afferents that selectively target these parts of the orexin system. Elegant transgenic approaches have revealed several potential candidates for mediating this phenomenon, including basal forebrain cholinergic neurons and several brainstem regions[35]. Furthermore, it has been shown that other hypothalamic inputs preferentially target medial orexin neurons, whereas most brainstem inputs target the lateral bank[43]. This suggests that nicotine may exert a greater effect on lateral hypothalamic orexin neurons by preferential activation of brainstem inputs to this region.
Nicotine appears to activate orexin neurons via an α4β2-dependent mechanism [30]. Regional distribution studies of nicotinic receptor subunits indicate the presence of α4 mRNA and protein in several hypothalamic areas [39,42]. Additional studies, including demonstrations of nicotinic receptor subunit colocalization with phenotypically characterized cell body and axon terminal populations within the lateral hypothalamus/perifornical area, will be required to definitively determine the receptor mechanisms underlying nicotine-elicited activation of orexin neurons. Furthermore, the effects of chronic nicotine treatment on orexin neurons generally and orexin projections to target regions such as the basal forebrain and PVT will likely shed additional light on contribution of orexin transmission to the cognitive and reinforcing correlates of nicotine addiction.
In addition to the basal forebrain and PVT, orexin neurons provide substantial inputs to other brain regions and neurotransmitter systems that may play a role in nicotine effects on arousal and attention. There is a rich pattern of anatomical substrates for orexin interactions with brainstem dopamine and norepinephrine systems at both the cell body and terminal level [1,13,16]. Evidence also suggests that individual orexin neurons may collateralize to innervate multiple brain regions [10], further supporting a role for this system in coordinating the response of diverse neural systems to nicotine. In addition to activating both basal forebrain and PVT neurons that may project to the PFC, orexin and nicotine have been shown to directly excite the same thalamocortical synapses in this target region in a manner that correlates with attention [26]. Thus, orexinergic and nicotinic transmission may act in concert to synergistically drive basalo-cortical and thalamo-cortical transmission in a manner that facilitates cortex-dependent attention and arousal processes.
Given the widespread distribution of nicotinic acetylcholine receptors in the brain, the neurotransmitter systems and brain regions underlying nicotine effects on arousal and attention are likely diverse. Orexin neurons accounted for 25–40% (depending on sector and site of CTb deposit) of retrogradely-labeled LH/PFA neurons from the basal forebrain and PVT; thus, a majority of retrograde labeling was observed in non-orexin neurons of the LH/PFA. While this part of the hypothalamus is phenotypically heterogeneous, candidate cell populations that could contribute to these ascending projections include melanin-concentrating hormone and cocaine- and amphetamine-regulated transcript given the anatomical distribution of these neurons and their responsiveness to nicotine [4,21,25,29]. However, nicotine-elicited Fos expression appears to be targeted preferentially to orexin neurons of this area [30] suggesting that non-orexin LH/PFA neurons may not contribute substantially to nicotine effects on basal forebrain and PVT.
Patients with narcolepsy, a sleep disorder associated with loss of orexin neurons [28,41], show attentional deficits even during periods of normal wakefulness [33]. Furthermore, clinical studies suggest that tobacco use fails to enhance daytime arousal in narcoleptics [5]. Collectively, these data are consistent with a role for the orexin system in mediating certain aspects of nicotine-elicited wakefulness.
In conclusion, we have shown here that acute nicotine treatment activates orexin neurons that project to the basal forebrain and the PVT, two brain regions that are important for arousal and attention. Our findings suggest that orexin inputs to the basal forebrain and PVT may contribute to nicotine effects on arousal and cognition and provide further support for the existence of functional heterogeneity across the medial-lateral distribution of orexin neurons.
Acknowledgments
This work was supported in part by a grant from the American Federation for Aging Research to JF.
Footnotes
Conflict of interest
None.
References
- 1.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 2.Berridge CW, Espana RA. Hypocretins: waking, arousal, or action? Neuron. 2005;46:696–698. doi: 10.1016/j.neuron.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 5.Browman CP, Gujavarty KS, Mitler MM. Tobacco use by narcoleptics and daytime sleep tendency. Drug Alcohol Depend. 1984;14:23–26. doi: 10.1016/0376-8716(84)90015-2. [DOI] [PubMed] [Google Scholar]
- 6.Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 9.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 10.Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 11.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 14.Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 15.Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Harris GC, Wimmer M, Jones GA. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005 doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Jo YH, Wiedl D, Role LW. Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice. J Neurosci. 2005;25:11133–11144. doi: 10.1523/JNEUROSCI.3638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- 23.Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer PR, Kramer SF, Marr K, Guan G, Wellman PJ, Bellinger LL. Nicotine administration effects on feeding and cocaine-amphetamine-regulated transcript (CART) expression in the hypothalamus. Regul Pept. 2007;138:66–73. doi: 10.1016/j.regpep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JA, Deurveilher S, Semba K. Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience. 2003;121:269–275. doi: 10.1016/s0306-4522(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 28.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 29.Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- 30.Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 32.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieger M, Mayer G, Gauggel S. Attention deficits in patients with narcolepsy. Sleep. 2003;26:36–43. [PubMed] [Google Scholar]
- 34.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Sato-Suzuki I, Kita I, Seki Y, Oguri M, Arita H. Cortical arousal induced by microinjection of orexins into the paraventricular nucleus of the rat. Behav Brain Res. 2002;128:169–177. doi: 10.1016/s0166-4328(01)00307-2. [DOI] [PubMed] [Google Scholar]
- 38.Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shioda S, Nakajo S, Hirabayashi T, Nakayama H, Nakaya K, Matsuda K, Nakai Y. Neuronal nicotinic acetylcholine receptor in the hypothalamus: morphological diversity and neuroendocrine regulations. Brain Res Mol Brain Res. 1997;49:45–54. doi: 10.1016/s0169-328x(97)00122-8. [DOI] [PubMed] [Google Scholar]
- 40.Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- 41.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]