Abstract
Background
Hepatitis C virus (HCV) polymerase is an essential enzyme for HCV replication and has multiple inhibitor binding sites making it a major target for antiviral intervention. It is apparent that no single drug can inhibit HCV replication in humans. Hence, combinations of nucleoside analogues β-D-2′-C-methylcytidine (2′-C-MeC; NM-107) or β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine (2′-F-C-MeC; PSI-6130) with interferon-α2b (IFN-α2b) or triple combination with ribavirin (RBV) were evaluated.
Methods
Huh-7 cells containing the self-replicating subgenomic HCV replicon (Clone B) were used for drug combination studies. After drug treatment for 5 days, total cellular RNA was then extracted and both ribosomal RNA and HCV replicon RNA were amplified in a single-step multiplex real-time PCR assay. Drug interaction analyses were performed using the CalcuSyn program.
Results
Double combinations of 2′-C-MeC or 2′-F-C-MeC with IFN-α2b at all ratios tested had weighted average combination index (CIwt) values <1 indicating synergistic inhibition of HCV replication in the replicon system. For the triple combinations of IFN-α2b plus RBV with either 2′-C-MeC or 2′-F-C-MeC, the CIwt values at 1:1:1 ratio tested were 0.5 and 0.8, respectively, indicating synergistic antiviral effects. No apparent cytotoxicity effects were observed with any of the combinations tested.
Conclusion
These promising in vitro data warrant clinical investigation of the nucleosides analogues such as 2′-C-MeC or 2′-F-C-MeC in their prodrug forms, together with IFN-α2b and RBV, for successful treatment of HCV infections.
Introduction
The current standard of care for chronic hepatitis C virus (HCV) infection is a combination of pegylated interferon (IFN)-α and ribavirin (RBV), which results in a sustained virological response in about a half of infected individuals; however, this combined modality is associated with considerable side effects [1,2]. Several new HCV inhibitors have been identified and some are being evaluated in controlled clinical trials [3]. Recently, promising 2′-modified nucleoside analogues with activity against HCV have been reported; for instance, valopicitabine (NM-283), the 3′-valine ester of β-D-2′-C-methylcytidine (2′-C-MeC; NM-107), is a prodrug nucleoside that after intracellular phosphorylation to its 5′-triphosphate metabolite, selectively inhibits HCV RNA-dependent RNA polymerase [4,5]. Although valopicitabine was recently evaluated in clinical trials and the combination of valopicitabine with IFN-α (plus RBV) has led to successful viral RNA suppression in a number of HCV-infected individuals, this drug was found to have gastrointestinal toxicity leading to its discontinuation in its present form [6]. Another novel cytidine analogue, β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130, 2′-F-C-MeC has demonstrated potent and specific in vitro anti-HCV activity with no apparent cytotoxicity [7,8]. Recently, in a 14 day Phase I monotherapy study, a new prodrug of 2′-F-C-MeC named R7128 was shown to be highly potent at lowering levels of HCV RNA, with a 2.7-log reduction in the viral load of individuals infected with HCV genotype 1 who had failed prior IFN therapy [9,10]. These nucleoside analogues have demonstrated excellent anti-HCV activity in vitro in both antireplicon and infectious HCV systems [11]. Furthermore, nucleoside analogue prodrugs can be administered in combination with IFN, with or without RBV, or with other antiviral drugs for the treatment of HCV infections. Previous studies using the HCV replicon have reported that RBV may interfere with pyrimidine nucleoside metabolism [12] and that IFN has shown both cytopathic and non-cytopathic activities [13–14]. Therefore, we determined the in vitro anti-HCV activity of these 2′-Me nucleosides with IFN-α2b to assess the potential drug-drug interactions. Furthermore, the triple combination of 2′-C-MeC or 2′-F-C-MeC with IFN-α2b and RBV was also evaluated using the replicon system.
Methods
Compounds
Recombinant IFN-α2b (Intron A) was obtained from Schering Corporation, (Kenilworth, NJ, USA). The powder was diluted in sterile water and aliquots were stored at −20°C. Compounds 2′-C-MeC and RBV were synthesized in our laboratories following published procedures [15,16], whereas 2′-F-C-MeC was synthesized according to the methods of Clark et al. [16]. Stocks of 40 mM were prepared in 100% DMSO and stored at −20°C.
Cells
Subgenomic HCV replicon RNA-containing Huh7 cell line (Clone B cells; Apath LLC, St Louis, MO, USA) was kindly provided by Dr Charles M Rice [17] through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (Bethesda, MD, USA), and was maintained in exponential growth in Dulbecco’s modified Eagle’s medium supplemented with L-glutamine and 4.5 g/l D-glucose (GIBCO/Invitrogen, Technologies, Carlsbad, CA, USA). The medium was further supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1× non-essential amino acids, 100 U/ml of penicillin, 100 (μg/ml of streptomycin and 500 μg/ml of G418 (Geneticin, GIBCO/Invitrogen). Cells were maintained at 37°C in a humidified 5% CO2 and 95% air atmosphere.
Cell-based antiviral assays
The HCV replicon cells were seeded onto 96-well tissue culture plates (5,000 cells/well) in 50 μl of medium without G418. Immediately after seeding, 50 μl of test compounds, alone and in combination, were added at several concentrations in threefold dilutions. The concentrations of IFN-α2b, 2′-C-MeC, 2′-F-C-MeC and RBV used ranged from 0.02 to 99 IU/ml, 0.1 to 33 μM, 0.01 to 33 μM and 1.2 to 33 μM, respectively. After 5 days in culture, total cellular RNA was isolated using RNeasy 96 kit (Qiagen, Valencia, CA, USA), and both HCV replicon and endogenous control (TaqMan Ribosomal RNA, Applied Biosystems, Foster City, CA, USA) were amplified in a single-step multiplex real-time PCR assay (7900 HT Sequence Detection System, Applied Biosystems). To express the antiviral effectiveness of the drugs, the average threshold cycle for HCV amplification of the compounds, alone or in combination, was subtracted from the average cycle of the untreated control (ΔCt). Determination of the 50% effective concentration (EC50) and the 90% effective concentration (EQ90) in HCV replicon RNA levels of the compounds were performed as previously described by Stuyver et al. [18]. In addition, the 50% cytotoxicity concentration (CC50) of the compounds was also determined by calculating the ΔCt for rRNA [18]. Cell proliferation was also determined by 5-(3-carboxymethoxyphenyl-2-(4,5-dimethylthiazoly)-3-(4-sulfophenyl) tetrazolium salt (MTT) reduction test using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), according to the manufacturer’s instructions. Cytotoxicity was considered when the concentration of the test compounds alone or in combination inhibited cell growth by ≥50%.
Drug combination analyses
To evaluate whether the antiviral effects of two-three-drug combinations of 2′-C-MeC and IFN-α2b (with or without RBV) or 2′-F-C-MeC and IFN-α2b (with or without RBV) were synergistic, additive or antagonistic, the drug interactions were analysed using CalcuSyn (Biosoft, Ferguson, MO, USA) computer software. The program allows automated simulation of synergism and antagonism at all dose and effect levels and displays the methods of Chou and Talalay [19], including dose-effect curve, median effect plot, combination index (CI), isobologram, dose-reduction index (DRI) and the Monte Carlo algorithm plot of Belen’kii and Schinazi [20]. We chose the constant ratio drug combination design proposed by Chou and Talalay [19] because it offers several advantages, such as reduction of required data points and generation of maximal useful information on both dual and triple combinations. In addition, it allows the construction of fraction affected (Fa)-CI and Fa-DRI plots with the actual combination data points. Further more, it allows computer simulation of CI and DRI values at all effect levels. As high degree effects are more therapeutically relevant than the low degree of effects, the additional weighted average CI (CIwt) of Chou and Talalay [19] was calculated (see review [21]), which uses the formula: CIwt = [CI50 + 2CI75 + 3CI90 + 4CI95]/10, where CI50, CI75, CI90, and CI95 are the CI values at 50%, 75%, 90% and 95% inhibition, respectively.
Results
Combinations of either 2′-C-MeC or 2′-F-C-MeC with IFN-α2b (and RBV) are synergistic in replicon cells The in vitro effect of the combination of 2′-C-MeC or 2′-F-C-MeC with IFN-α2b and the triple combination of either 2′-C-MeC or 2′-F-C-MeC with IFN-α2b and RBV using the replicon system was determined. For each drug pair (or triple combination), three to four independent experiments were performed and all samples were processed in duplicate. HCV replicon (Clone B) cells were incubated with various concentrations of these compounds for 5 days and the anti-HCV activity and cytotoxicity were simultaneously measured. The activity and cytotoxicity effects of 2′-C-MeC, 2′-F-C-MeC, IFN-α2b, and RBV, alone or in combination, are shown in Tables 1 and 2.
Table 1.
Anti-HCV activity and cytotoxicity of the four compounds used singly in HCV-replicon-containing Huh-7 cells (Clone B)
| Compound | EC50* | EC90* | CC50† |
|---|---|---|---|
| 2′-C-MeC, μM | 2.4±0.8 | 8.8±1.9 | >33 |
| 2′-F-C-MeC, μM | 1.2±0.8 | 3.8±1.7 | >33 |
| IFN-α2b, IU/ml | 0.5±0.6 | 17±12 | >99 |
| RBV, μM | 8.0±3.8 | NM‡ | 19±6.1 |
All values represent the average of four to six experiments and samples were performed in duplicate ± SD.
EC50 and EC90 effective concentrations required for reducing hepatitis C virus (HCV) levels by 50% and 90%, respectively, on day 5;
CC50, cytotoxicity concentration required for reducing the rRNA levels by 50% on day 5.
Not measurable (NM) because activity at this level was similar to its toxicity. IFN, interferon; RBV, ribavirin.
Table 2.
Antiviral and cytotoxicity effects of two- to three-drug combination therapy in HCV-replicon-containing Huh-7 cells (Clone B)
| Treatment | Ratio | EC50 μM | EC90 μM | CC50, μM* |
|---|---|---|---|---|
| 2′-C-MeC + IFN-α2b | 1:3 | 0.1 | 1.3 | >33 |
| 2′-C-MeC + IFN-α2b | 1:2 | 0.4 | 1.8 | >33 |
| 2′-C-MeC + IFN-α2b | 1:1 | 0.3 | 1.6 | >33 |
| 2′-C-MeC + IFN-α2b | 2:1 | 0.02 | 0.8 | >33 |
| 2′-C-MeC + IFN-α2b + RBV | 2:1:1 | 0.1 | 4.9 | >11 |
| 2′-C-MeC + IFN-α2b + RBV | 1:1:1 | 0.2 | 2.2 | >3.6 |
| 2′-F-C-MeC + IFN-α2b | 1:10 | 0.09 | 0.6 | >33 |
| 2′-F-C-MeC + IFN-α2b | 1:5 | 0.1 | 0.8 | >33 |
| 2′-F-C-MeC + IFN-α2b | 1:1.5 | 0.2 | 1.2 | >33 |
| 2′-F-C-MeC + IFN-α2b | 1:1 | 0.02 | 0.4 | >33 |
| 2′-F-C-MeC + IFN-α2b + RBV | 2:1:1 | 0.2 | 2.2 | >11 |
| 2′-F-C-MeC + IFN-α2b + RBV | 1:1:1 | 0.09 | 1.2 | >11 |
| 2′-F-C-MeC + IFN-α2b + RBV | 1:1.5:1.5 | 0.2 | 2.6 | >3.6 |
Values represent the apparent potency of the first compound (2′-C-MeC or 2′-F-C-MeC) listed in the combination.
Cytotoxicity concentration (CC50) represents only the first compound listed in the combination, and it was tested by calculating the Δ threshold cycle of rRNA values. EC50, 50% effective concentration; EC90 90% effective concentration; IFN, interferon; RBV, ribavirin.
In the present computerized analysis for either one of the four compounds (2′-C-MeC, 2′-F-C-MeC, IFN-α2b or RBV), the linear correlation coefficient of the median-effect plot (r values) ranged from 0.85 to 0.99 and the r values for their constant ratio combinations ranged from 0.91 to 0.99 (data not shown). Therefore, the experimental results matched the mass-action law. No cytotoxicity effects of rRNA levels in the replicon cells were observed with 2′-C-MeC, 2′-F-C-MeC and IFN-α2b (or with the combinations) when tested at the highest antiviral concentrations (Tables 1 and 2). In contrast, RBV alone showed toxicity effects at concentration of 19 ±6.1 μM. Furthermore, after 5 days of incubation in an MTT assay, there was no apparent cytotoxicity for any of the combinations containing either 2′-C-MeC or 2′-F-C-MeC (up to 33 μM), IFN-μ2b (up to 33 IU/ml) and RBV (up to 11 μM; data not shown). A dose-dependent inhibition of HCV replicon replication was observed in cultures treated with each compound alone and their combinations. A 90% reduction of HCV replication was observed when tested alone with 8.8 μM, 3.8 μM and 17 IU/ml of 2′-C-MeC, 2′-F-C-MeC and IFN-α2b, respectively (Table 1). In this system, 2′-F-C-MeC was found to be greater than twofold more potent than 2′-C-MeC.
The EC90 values of the combined activity of 2′-C-MeC and IFN-α2b (with or without RBV) at all ratios ranged from 0.8 to 4.9 μM and the combined activity of 2′-F-C-MeC and IFN-α2b (with or without RBV) ranged from 0.4 to 2.6 μM (Table 2). These results indicate that both double and triple combinations of either 2′-C-MeC or 2′-F-C-MeC and IFN-α (with or without RBV) were effective against HCV replication in Clone B cells and enhanced potency over the corresponding monotherapies.
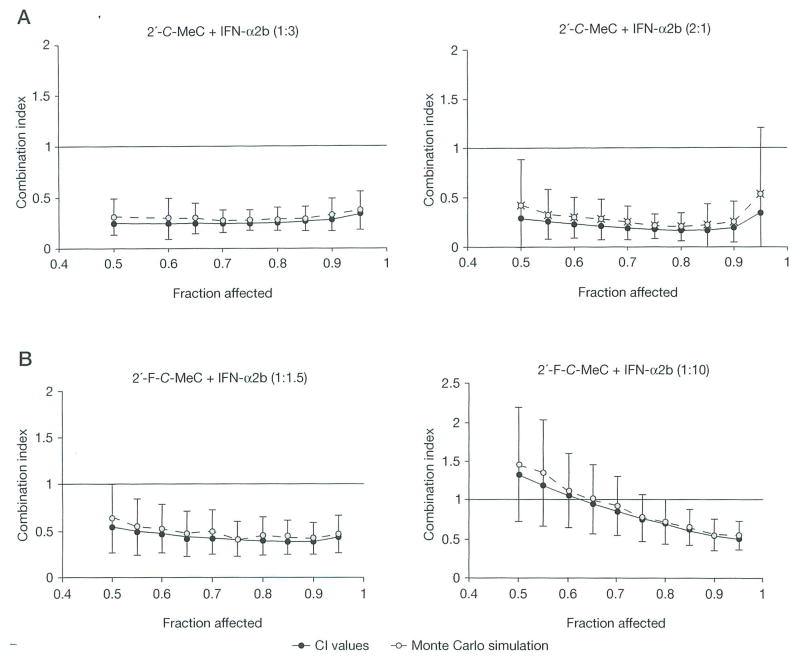
In order to determine if the interactions were additive, synergistic or antagonistic, we applied the CalcuSyn approach to the data generated from dual combinations (Table 3 and Figures 1 and 2). Double combination of 2′-C-MeC with IFN-α2b except for the 1:2 ratio showed favourable dose reduction from EC50 to EC95 (Table 3). The combination of 2′-F-C-MeC with IFN-α at ratios of 1:1.5 and 1:1 showed more favourable dose reduction (from EC50 to EC95) than at ratios of 1:10 and 1:5. In addition, all average CI values (see Methods) were significantly <1, suggesting synergy when the combination ratios of either 2′-C-MeC or 2′-F-C-MeC and IFN-α2b were analysed. These results indicate that both combinations have synergistic effects on inhibition of HCV RNA replication in the replicon system. The experimental data were also analysed using Monte Carlo simulation for the estimation of errors in CI and the results are presented in Figures 1A and 1B.
Table 3.
Computer-simulated combination index of two- to three-drug combinations at 50%, 75%, 90% and 95% inhibition of HCV replicon replication
| CI values at inhibition of |
||||||
|---|---|---|---|---|---|---|
| Treatment | Ratio | 50% | 75% | 90% | 95% | CIwt |
| 2′-C-MeC + IFN-α2b | 1:3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 |
| 2′-C-MeC + IFN-α2b | 1:2 | 1.7 | 0.6 | 0.3 | 0.3 | 0.5 |
| 2′-C-MeC +IFN-α2b | 1:1 | 0.8 | 0.5 | 0.3 | 0.3 | 0.4 |
| 2′-C-MeC + IFN-α2b | 2:1 | 0.3 | 0.2 | 0.2 | 0.4 | 0.3 |
| 2′-C-MeC + IFN-α2b + RBV | 2:1:1 | 1.4 | 0.9 | 0.9 | 1.4 | 1.1 |
| 2′-C-MeC + IFN-α2b + RBV | 1:1:1 | 0.6 | 0.5 | 0.5 | 0.6 | 0.5 |
| 2′-F-C-MeC + IFN-α2b | 1:10 | 1.7 | 0.9 | 0.6 | 0.5 | 0.7 |
| 2′-F-C-MeC + IFN-α2b | 1:5 | 1.3 | 0.8 | 0.5 | 0.5 | 0.7 |
| 2′-F-C-MeC + IFN-α2b | 1:1.5 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 |
| 2′-F-C-MeC + IFN-α2b | 1:1 | 0.7 | 0.3 | 0.3 | 0.3 | 0.3 |
| 2′-F-C-MeC + IFN-α2b + RBV | 2:1:1 | 2.3 | 1.0 | 0.8 | 1.0 | 1.1 |
| 2′-F-C-MeC + IFN-α2b + RBV | 1:1:1 | 2.2 | 0.8 | 0.5 | 0.6 | 0.8 |
| 2′-F-C-MeC + IFN-α2b + RBV | 1:1.5:1.5 | 1.1 | 0.9 | 0.9 | 1.1 | 1.0 |
Combination index (CI) values were determined for a mutually exclusive interaction using CalcuSyn program (Biosoft, Ferguson, MO, USA). CI <1, =1 or >1 indicates synergism, additive effect and antagonism, respectively. CI wt weighted average CI value was assigned as = [CI50 + 2CI75 + 3CI90, + 4CI95]/10 (see review [21]). IFN, interferon; RBV, ribavirin.
Figure 1.
Examples of fraction affected–combination index plots and Monte Carlo simulations
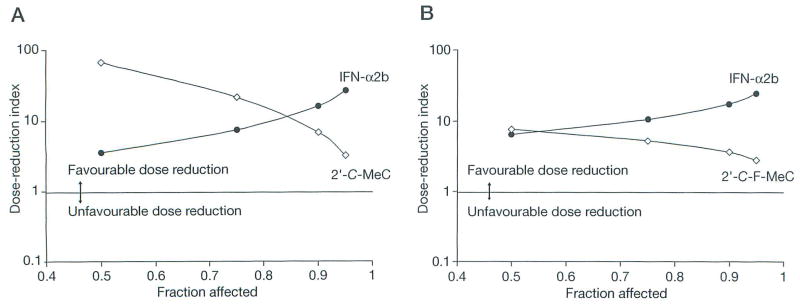
Figure 2.
Simulation of the fraction affected-dose-reduction index plot obtained using CalcuSyn computer program
Since the drug combination ratios indicated synergism and there was no overlapping toxicity in the replicon system, the DRI was determined. As shown in Figures 2A and 2B, the three drugs in the two-drug combinations allowed significant dose reductions (DRI >1) for a given effect level. Remarkably, greater DRI values of IFN-α2b (DRI >10 at EC90 and EC95) were observed, suggesting a greater dose reduction of IFN-α2b with a potential benefit when administered in combination with either 2′-C-MeC or 2′-F-C-MeC.
Next, the type of interaction obtained with triple combination of either 2′-C-MeC or 2′-F-C-MeC with IFN-α2b and RBV was determined. The CI values for 2′-C-MeC + IFN-α2b + RBV at ratio of 2:1:1 at 50% and 95% inhibition levels were 1.4, indicating moderate antagonism; however, at 75% and 90% inhibition levels, CI values were 0.9, indicating slight synergism. Overall, the CIwt of 1.1 indicated a nearly additive effect. Similarly, combination of 2′-F-C-MeC + IFN + RBV at the same ratio of 2:1:1 produced CIwt value of 1.1, which is considered to be nearly additive (CalcuSyn: Windows software for dose effect analysis program manual). In addition, the combination of 2′-F-C-MeC + IFN + RBV at ratios of 2:1:1 and 1:1:1 were antagonistic at low effect level of 50% inhibition; however, additive to synergistic effects were noted at high effect levels (≥75%) at all ratios tested. Moreover, the triple combination of IFN-α2b and RBV with either 2′-C-MeC or 2′-F-C-MeC reduced the HCV replicon replication at ratios of 1:1:1. The comparison of the weighted CI values between the two sets of triple combinations indicated that the combination of 2′-C-MeC with IFN-α2b and RBV at ratio of 1:1:1 yielded greater synergism than 2′-F-C-MeC, IFN-α2b and RBV at the same ratio, but the difference was not significant (Table 3).
Discussion
Using a robust drug interactions methodology, we determined that 2′-C-MeC or 2′-F-C-MeC demonstrated synergistic anti-HCV activities when combined with IFN-α2b alone or combined with IFN-α2b and RBV in the replicon system without any apparent cytotoxicity. The combination of RBV to the IFN treatment of chronic hepatitis C infection greatly improved responses [22], but unfortunately half of the infected individuals still do not achieve sustained clearance of HCV 12]. For this reason, newer potent antiviral agents are likely to be used in combination with these agents.
RBV is a guanosine analogue that has broad antiviral activity against several viruses, but its direct antiviral mechanisms on HCV are not completely elucidated. In vitro anti-HCV studies have demonstrated that it could have several modes of action: modulating the immune system when favouring the T helper 1 response; increasing the antiviral activity of endogenous IFN, as demonstrated in the respiratory syncytial virus system [23]; increasing the mutation frequency of HCV and error catastrophe or lethal mutagenesis, thus reducing HCV infectivity; inhibiting inosine monophosphate dehydrogenase leading to guanosine 5′-triphosphate depletion; or having a small and unlikely direct effect on HCV RNA replication through polymerase inhibition (see review [24]). We demonstrated that RBV alone at a non-toxic concentration has a very weak inhibitory effect (EC90>19±6.1μM) on intracellular HCV replicon replication, which is consistent with results of other studies [25,26]. However, the combinations of IFN-α2b and either 2′-C-MeC or 2′-F-C-MeC, at several concentration ratios, were synergistic and the addition of RBV also resulted in additive/synergistic activity. None of the combinations presented cytotoxicity (see Table 2, where CC50 of combinations containing RBV were either >3.6 or >11 μM). Our findings are in agreement with earlier in vivo studies when triple combination of NM283 plus pegylated IFN-α plus RBV were used in individuals with chronic HCV infection and resulted in a significant decline (>70%) of viral load levels [27]. Unfortunately, clinical trials of NM283 were put on hold by the Food and Drug Administration as gastrointestinal side effects were demonstrated [28]. Although the weighted average values in our study increased when comparing double with triple therapies, mainly at the ratio of 2:1:1 for 2′-C-MeC combination, which might be indicative of a negative effect of RBV on the previously observed synergism, it did show synergistic effects at ratio of 1:1:1 (see Table 3). Based on CIwt values, no significant antagonism was noted for all the combinations. In contrast to the results of the present study, a previous study that used human hepatoblastoma Huh-6 cells containing the HCV replicon showed an antagonistic effect of RBV on the anti-HCV activity of the NM-107 [12]. As the HCV replicon in the Huh-6 cell line replicates independently from ongoing cell proliferation, the toxic effects of RBV may have been masqueraded. Cell toxicity of certain compounds like RBV might be obscured at high concentrations in the HuH6 replicon cells. In fact, we tested the cytotoxicity activity of RBV using HuH6-naive cells by means of real-time PCR (effect on ribosomal RNA levels) and observed a CC50 value of 29 μM (data not shown), whereas Coelmont et al. [12] did not show toxicity of RBV at the highest concentration reported (135 μM). Therefore, the system may not solely reflect HCV replication, but the secondary effects of the drug. Moreover, in this study the addition of purine nucleoside analogues to the RBV treatment did not have any major effect on antiviral activity. However, the triple combination presented in our study with pyrimidine nucleoside analogues did have additive/synergistic effects when using equal concentrations of triple (1:1:1) combinations: CIwt values of 0.5 for 2′-C-MeC and 0.8 for 2′-F-C-MeC (Table 3). Our group [29] reported preliminary data that RBV at a higher ratio (1:5) had a CIwt value of 1.5, indicating antagonistic effects in the in vitro anti-HCV activity of 2′-C-MeC; however, the CI value at 90% inhibition ranged from 0.9 to 2.1, suggesting that the double combination could be either slight synergistic or antagonistic. Nevertheless, and most importantly, we demonstrated that RBV did not interfere with the in vitro anti-HCV activity of 2′-C-MeC metabolism (and vice versa) in Huh-7 cells and primary hepatocytes, supporting the results found in the current antiviral studies.
The combination of small-molecule inhibitors of the HCV protease or polymerase with IFN-α and RBV might act synergistically by both decreasing HCV replication and interfering with the ability of HCV to evade the mediators of IFN action. These combinations hold the promise of greatly improved rates of response to therapy of hepatitis C, and perhaps will help to decrease the doses of IFN-α and/or RBV, which are associated with frequent side effects and contraindicated in many chronically infected individuals. Furthermore, in the present study, the inhibitory effect of HCV RNA replication was increased when 2′-C-MeC was used in combination with IFN-α2b, which is in agreement with a recent Phase IIb human trial of the anti-HCV activity of a prodrug of 2′-C-MeC (NM-283) shown to produce profound synergistic effects when combined with pegylated IFN [6,30,31]. As mentioned previously, combinations of pegylated IFN-α and RBV have a relatively low sustained viral response especially in individuals infected with HCV genotype 1 [2]. Because of the high genetic heterogeneity, increased efficiency of replication of HCV and expected emergence of resistance mutations, it is likely that the future therapy for HCV will include orally bioavailable drug combinations in addition to RBV and IFN. Furthermore, it is essential to evaluate in vitro combinations beforehand to determine their potential effects on antiviral activity as well as cytotoxicity.
In summary, we demonstrate that double or triple combinations of either 2′-C-MeC or 2′-F-C-MeC with IFN-α2b alone or combined with IFN-α2b and RBV interacted synergistically in inhibiting HCV in a replicon system. Such studies provide a strong virological rationale for using 2′-C-MeC nucleoside analogues together with the current standard of care treatment for HCV infections.
Acknowledgments
This work was supported in part by NIH Grant 5P30-AI-50409 (CFAR), 5R37-AI-041980 and by the Department of Veterans Affairs. Part of this work was presented at the 42nd Annual Meeting of the European Association for the Study of the Liver 11–15 April 2007 Barcelona, Spain, and was rated in the top 10 percentile.
Footnotes
Disclosure statement
RFS is a founder and major shareholder of Pharmasset Inc., the developer of PSI-6130. All other authors have no competing interests.
References
- 1.Abergel A, Hezode C, Leroy V, et al. Peginterferon alpha-2b plus ribavirin for treatment of chronic hepatitis C with severe fibrosis: a multicentreb randomized controlled trial comparing two doses of peginterferon alpha-2b. J Viral Hepat. 2006;13:811–820. doi: 10.1111/j.1365-2893.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 2.Bronowicki JP, Ouzan D, Asselah T, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alpha-2a plus ribavirin. Gastroenterology. 2006;131:1040–1048. doi: 10.1053/j.gastro.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Carroll SS, Olsen DB. Nucleoside analogue inhibitors of hepatitis C virus replication. Infect Disord Drug Targets. 2006;6:17–29. doi: 10.2174/187152606776056698. [DOI] [PubMed] [Google Scholar]
- 4.Pierra C, Benzaria S, Amador A, et al. NM 283, an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. Nucleosides Nucleotides Nucleic Acids. 2005;24:767–770. doi: 10.1081/ncn-200060112. [DOI] [PubMed] [Google Scholar]
- 5.Pierra C, Amador A, Benzaria S, et al. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J Med Chem. 2006;49:6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- 6.Toniutto P, Fabris C, Bitetto D, Fornasiere E, Rapetti R, Pirisi M. Valopicitabine dihydrochloride:a specific polymerase inhibitor of hepatitis C virus. Curr Opin Investig Drugs. 2007;8:150–158. [PubMed] [Google Scholar]
- 7.Stuyver LJ, McBrayer TR, Tharnish PM, et al. Inhibition of hepatitis C replicon RNA synthesis by beta-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antivir Chem Chemother. 2006;17:79–87. doi: 10.1177/095632020601700203. [DOI] [PubMed] [Google Scholar]
- 8.Murakami E, Bao H, Ramesh M, et al. Mechanism of activation of beta-D-2′-deoxy-2′-fluoro-2′-c-methylcytidine and inhibition of hepatitis C virus NS5B RNA polymerase. Antimicrob Agents Chemother. 2007;51:503–509. doi: 10.1128/AAC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy R, Rodriguez-Torres M, Gane E, et al. Antiviral activity, pharmacokinetics, safety, and tolerability of R7128, a novel nucleoside HCV RNA polymerase inhibitor, following multiple, ascending, oral closes in patients with HCV genotype 1 infection who have failed prior interferon therapy. Hepatology. 2007;46 (Suppl 1):862A. [Google Scholar]
- 10.McHutchison JG, Reddy R, Rodriguez-Torres M, et al. Potent antiviral activity of the nucleoside HCV inhibitor, R7128, in prior IFN non-responders. Hep DART Frontiers in Drug Development for Viral Hepatitis; 9–13 December 2007; Lahaina, Hawaii, USA. Abstract 61. [Google Scholar]
- 11.Schinazi RF, Bassit L, Mateu G, McBrayer T, Grakoui A. Development and validation of a robust acute hepatitis C virus infectious system for novel drug evaluation in vitro. 14th International Symposium on Hepatitis C Virus & Related Viruses; 9–13 September 2007; Glasgow, Scotland, UK. Abstract P-275. [Google Scholar]
- 12.Coelmont L, Paeshuyse J, Windisch MP, De Clercq E, Bartenschlager R, Neyts J. Ribavirin antagonizes the in vitro anti-hepatitis C virus activity of 2′-C-methylcytidine, the active component of valopicitabine. Antimicrob Agents Chemother. 2006;50:3444–3446. doi: 10.1128/AAC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 14.Guo JT, Zhu Q, Seeger C. Cytopathic and noncytopathic interferon responses in cells expressing hepatitis C virus subgenomic replicons. J Virol. 2003;77:10769–10779. doi: 10.1128/JVI.77.20.10769-10779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldrup AB, Allerson CR, Bennett CF, et al. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J Med Chem. 2004;47:2283–2295. doi: 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- 16.Clark JL, Hollecker L, Mason JC, et al. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-merhylcytidine, a potent inhibitor of hepatitis C virus replication . J Med Chem. 2005;48:5504–5508. doi: 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- 17.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 18.Stuyver LJ, Whitaker T, McBrayer TR, et al. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob Agents Chemother. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Belen’kii MS, Schinazi RF. Multiple drug effect analysis with confidence interval. Antiviral Res. 1994;25:1–11. doi: 10.1016/0166-3542(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 21.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 22.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alpha-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Jamaluddin M, Wang S, et al. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol. 2003;77:5933–5947. doi: 10.1128/JVI.77.10.5933-5947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 25.Lanford RE, Guerra B, Lee H, et al. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(i)-poly(c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J Virol. 2003;77:1092–1104. doi: 10.1128/JVI.77.2.1092-1104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanabe Y, Sakamoto N, Enomoto N, et al. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon-alpha. J Infect Dis. 2004;189:1129–1139. doi: 10.1086/382595. [DOI] [PubMed] [Google Scholar]
- 27.Idenix Pharmaceuticals. [Updated 12 June 2007];Valopicitabine (NM283) produces greater suppression of HCV when added to pegylated interferon plus ribavirin. Available from: http://www.hivandhepatitis.com/hep_c/news/2007/062207_b.html.
- 28.Franciscus A. FDA: NM-283 on hold. [August 2007];HCV Advocate. 10(8) Available from: http://www.hcvadvocate.org/news/newsLetter/2007/advocate0807.html.
- 29.Hernandez-Santiago BI, Bassit L, Grier J, Schinazi RF. Anti-HCV activity and cellular pharmacology of 2′-methylcytidine alone and in combination with non-toxic concentrations of ribavirin in the HCV replicon system. Antivir Ther. 2007;12:528. [Google Scholar]
- 30.Dieterish D, Lawitz E, Nguyen T, et al. Early clearance of HCV RNA with valopicitabine (NM283) plus PEG-Interferon in treatment-naïve patients with HCV-1 infection: First results from a phase IIb trial. J Hepatol. 2006;44 (Suppl 2):S271–S272. [Google Scholar]
- 31.Stauber RE, Stadlbauer V. Novel approaches for therapy of chronic hepatitis C. J Clin Virol. 2006;36:87–94. doi: 10.1016/j.jcv.2006.02.003. [DOI] [PubMed] [Google Scholar]