Abstract
Retinoic acid (RA) induces cell cycle arrest of hormone-dependent human breast cancer (HBC) cells. Previously, we demonstrated that RA-induced growth arrest of T-47D HBC cells required the activity of the RA-induced protein kinase, protein kinase Cα (PKCα) [J. Cell Physiol. 172 (1997) 306]. Here, we demonstrate that RA treatment of T-47D cells interfered with growth factor signaling to downstream, cytoplasmic and nuclear targets. RA treatment did not inhibit epidermal growth factor (EGF) receptor activation but resulted in rapid inactivation. The lack of sustained EGFR activation was associated with transient rather than sustained association of the EGFR with the Shc adaptor proteins and activation of Erk 1/2 and with compromised induction of expression of immediate early response genes. Inhibiting the activity of PKCα, a retinoic acid-induced target gene, prevented the effects of RA on cell proliferation and EGF signaling. Constitutive expression of PKCα, in the absence of RA, decreased cell proliferation and decreased EGF signaling. RA treatment increased steady-state levels of the protein tyrosine phosphatase PTP-1C and all measured effects of RA on EGF receptor function were reversed by the tyrosine phosphate inhibitor orthovanadate. These results indicate that RA-induced target genes, particularly PKCα, prevent sustained growth factor signaling, uncoupling activated receptor tyrosine kinases and nuclear targets that are required for cell cycle progression.
Keywords: Retinoic acid, HBC cells, PKCα
Introduction
Retinoids, the natural and synthetic derivatives of vitamin A (retinol), exert antitumorigenic effects in many types of cells and inhibit proliferation of human breast cancer (HBC) cells [2]. In general, the effect of retinoic acid (RA) on HBC cell proliferation is limited to estrogen receptor positive (ER+) HBC cells and to HBC cells that express the alpha isoform of the retinoic acid receptor [1,3-11]. In contrast, most ER(−), hormone-independent cells fail to express RARα and are resistant to the antiproliferative effect of natural retinoids.
Retinoic acid receptors (RARs) and the retinoid X receptors (RXRs) are members of the steroid hormone receptor superfamily of ligand-activated transcription factors [12]. The ability of RA to regulate cell proliferation is associated with the ability of ligand-bound RARs to interfere with the function of the mitogenic transcription factor, AP1 [9,13-19]. Although in some circumstances retinoid inhibition of AP-1 function is independent of the transcriptional activity of RARs, in many other situations it is associated with retinoid-induced changes in expression of components of cellular signaling pathways [20-23]. In T-47D cells, RA-induced growth arrest is mediated by RARα [11] and requires protein kinase Cα (PKCα) activity [1].
Epidermal growth factor (EGF)-related peptides are ligands for the ErbB family of receptor tyrosine kinases [24]. There are four known ErbB family receptors: EGFR (ErbB1), ErbB2 (also called Neu, HER-2), ErbB3 and ErbB4 [24]. Mitogenic effects of EGF are mediated through ligand-induced activation of EGFR. Signal transduction from the EGFR is initiated when ligand binding stimulates receptor dimerization and tyrosine autophosphorylation on multiple carboxy-terminal tyrosines, which then act as high-affinity binding sites for effector proteins [25]. EGFR signaling pathways regulate proliferation of normal mammary epithelial cells [26-28] and of ER(+) hormone-dependent HBC cells [29].
To elucidate the mechanisms by which RA and PKCα regulate HBC cell cycle progression, we have characterized the nature of RA-induced growth arrest of T-47D HBC cells. We demonstrate that RA treatment of ER(+) T-47D HBC cells led to a state of functional mitogen deprivation and withdrawal from the cell cycle. In the presence of RA, multiple growth factors including EGF failed to induce mitogenic signaling pathways. The results indicate that RA induces PKCα expression and PKCα then prevents sustained growth factor-induced intracellular signaling. As a result, cell cycle progression in HBC cells is blocked and cells enter a G0-like state.
Material and methods
Cell culture
The T-47D human breast cancer cell line was obtained from the American Type Culture Collection (ATCC) and cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The establishment of T-47D cells constitutively expressing PKCα has been described previously [1]. Retinoids (from Biomol), Gö6976, PD98059 (all from Calbiochem) and phorbol 12-myristate 13-acetate (TPA, from Sigma) were stored as concentrated stocks in either absolute ethanol (retinoids) or DMSO, stocks of EGF (Upstate Biotech, Inc.) and Nrg-1 (Oncogene Sciences, Inc) were prepared in RPMI 1640.
Proliferation assays
Cells were plated at 1.5 × 105 cells per 60-mm tissue culture dish or 1000 cells/well in 96-well dishes. After 18–24 h (T = 0), reagents were added in fresh media. Media and reagents were replenished every 48–72 h. Cell numbers were quantified following trypsinization and counting using a standard hemocytometer or by measuring reduction of MTS (CellTiter Aqueous assay, Promega, Inc.). The number of viable cells was determined by exclusion of trypan blue dye. Each sample was counted in duplicate, and each condition was done in duplicate or triplicate.
FACS analysis
Cells were cultured in the presence and absence of reagents, solvent (ethanol or DMSO) or in 0.5% FBS RPMI. Upon completion of treatments, cells were trypsinized, counted and then centrifuged; 1 × 106 cells were resuspended in 50 μl PBS containing 2% calf serum. Cells were fixed with 1 ml cold 80% ethanol, centrifuged and resuspended in 500 μl 0.1 mg/ml propidium iodide (Sigma) with 0.6% NP-40, followed by incubation with 500 Al 2 mg/ml RNase in the dark at room temperature. Cells were filtered through Nitex mesh and analyzed at the Herbert Irving Comprehensive Cancer Center Flow Cytometry Laboratory. The cell cycle phase distribution of each sample was estimated using the ModFit software program.
Transient transfection and CAT assay
For transient assays cells were transfected with 4 μg of an AP1 reporter plasmid (AP15-tk-CAT) as a 0.5-ml calcium phosphate precipitate. After transfection, the media were replaced with fresh medium with or without 10−6 RA and incubated for 48 h. Total CAT activity was then measured in extracts prepared 48 h post-transfection. Assays were performed using an amount of extract that maintained activity in a linear range (determined to be between 1% and 50% substrate conversion). All conditions were performed in triplicate. The data are representative of two independent experiments. For AP15-tk-CAT activity in the presence of TPA, transfected cells were incubated with or without 10−6 M RA for 24 h followed by the addition of 100 ng/ml TPA. The transfected cells were then incubated in the presence or absence of both RA and TPA for an additional 24 h.
Northern (RNA) blot
Cell monolayers were washed with cold PBS, scraped off the tissue culture dish in 3 ml of PBS and centrifuged. Cell pellets were resuspended in PBS and total RNA isolated using TRI Reagent LS (Molecular Research Center, Inc.) according to the manufacturer's protocol. The final RNA was dissolved in 10 mM Tris–HCl (pH 8.0), 1 mM EDTA, 100 mM NaCl and 1% SDS. RNA was denatured by heating to 65°C for 5 min in 50% formamide, 1.1 M formaldehyde and resolved electrophoretically in 1.2% agarose gels containing 1.1 M formaldehyde and 40 mM MOPS. After electrophoresis, the gels were washed (1 × 20 min in 50 mM NaOH, 1 × 30 min in 10 × SSC and 0.1 M Tris–HCl, pH 7.5, and 1 × 60 min in 10× SSC) and transferred to nylon membranes by diffusion in 10× SSC. After transfer, RNA was covalently cross-linked to the membranes with a Stratagene UV cross-linker. Membranes were hybridized overnight at 65°C with 32P-cRNA probes. Membranes were then washed twice at 65°C in 2× SSC/1% SDS and twice in 0.2× SSC/0.1% SDS. The blots were exposed to X-ray film at −80°C with intensifying screens. Duplicate filters were probed for the level of the ribosomal protein, L30 mRNA [30] as an indicator of total amount of RNA per sample.
Immunoprecipitations and immunoblot analyses
Cells were washed in cold PBS and lysed in 20 mM Tris–HCl, pH 8, 150 mM NaCl, 10 mM sodium phosphate, 100 μM sodium orthovanadate, 100 μM ammonium molybdate, 10 mM DTT, 10% glycerol, 1% NP-40, 1 mM PMSF and 1% protease inhibitor cocktail (Sigma) at 4°C for 15 min. Lysates were cleared by centrifugation and protein concentrations were measured using Bradford's reagent. For immunoprecipitations, equal amounts of protein (usually 0.8–1 mg) were incubated with specific antibodies for 12–16 h at 4°C. Immune complexes were collected with protein A-agarose (Upstate Biotech, Inc., or Sigma) and washed several times with lysis buffer. Bound proteins were released by heating for 5 min at 95°C in sample buffer. Immunoprecipitates and cell lysates were resolved by electrophoresis on 6% or 10% sodium dodecyl sulfate-polyacrylamide gels and transferred electrophoretically to nitrocellulose membranes. Membranes were blocked in 5% BSA/PBS/0.05% Tween-20 (PBS-T) or 5% nonfat dry milk in PBS-T for 3 h at room temperature or overnight at 4°C. Membranes were washed in PBS-T and then incubated overnight at 4°C with the indicated antibody. Anti-EGFR antibodies were from Santa Cruz or from Transduction Lab; anti-PTP1C, anti-phosphotyrosine and anti-Shc antibodies were obtained from UBI; and anti-phospho- and anti-total p44/42 MAP kinase or Akt/PKB antibodies were from New England BioLabs, Inc. After incubation, membranes were washed in H2O, incubated with horseradish peroxidase-coupled anti-IgG for 1 h at room temperature and then washed in H2O. Immunoreactive proteins were visualized by enhanced chemiluminescence (ECL, Amersham).
Results
Retinoids reversibly arrest T-47D cell proliferation
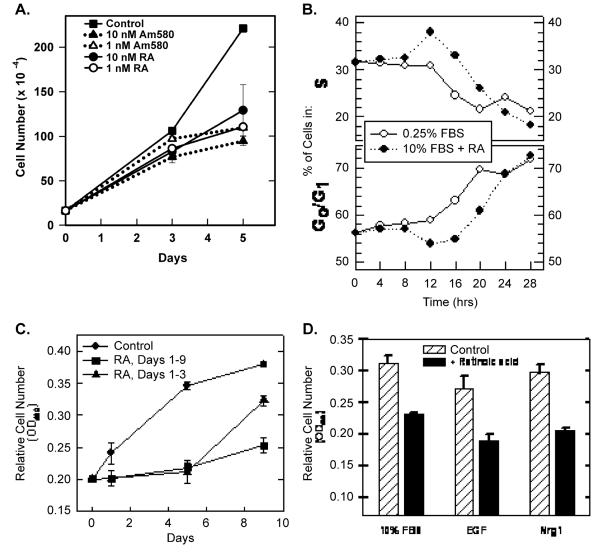
Both all trans-retinoic acid (RA) and a RARα-selective retinoid (Am580) arrested T-47D cell proliferation in a time-and concentration-dependent manner (Fig. 1A). At low concentrations (≤10 nM, where the selectivity of Am580 for RARα vs. RARβ or RARγ is <100-fold [31]), Am580 was as effective as equal concentrations of RA, implicating RARα as the receptor responsible for arresting proliferation. Retinoic acid also inhibited EGF-dependent and Nrg-1-dependent proliferation (Fig. 1B). Therefore, it is likely that retinoids disrupt a signaling pathway commonly used by multiple mitogens (see also Fig. 2, below).
Fig. 1.
Retinoids reversibly inhibit T-47D cell proliferation. (A) T-47D cells (initial plating density = 1.5 × 105 cells per 60-mm dish) were cultured in the presence of the indicated concentration of RA or the RARα-selective retinoid, Am580. Day 0 represents the time of retinoid addition. Cells were trypsinized and cell numbers determined 3 and 5 days later (data plotted ± SEM). Viability, as determined by trypan blue exclusion, was >90% at all times. (B) The effect of RA and serum deprivation on T-47D cell cycle distribution was determined at 4-h intervals by FACS analysis of propidium iodide-stained cells. The data show the change in the percentage of cells in either S (top) or G0/G1 (bottom). Serum deprivation (after 12 h) and RA treatment (after 16 h, 10−7 M) prevented entry into S. (C) T-47D cells were initially plated at 5 × 103 cells/well in 96-well dishes (six wells per condition). Relative cell numbers were determined using MTS reduction as measured by absorbance at 490 nm (data plotted ± SEM). RA (10−7 M) was added at day 0 and again at day 3 (squares and triangles). On day 5, RA was removed from half of the treated wells (triangles). (D) T-47D cells were plated at 5 × 103 cells/well in 96-well dishes in RPMI 1640 + 10% FBS. After 24 h, media were replaced with RPMI 1640 supplemented with 10% FBS, 20 ng/ml EGF or 10 ng/ml Nrg 1 in the absence or presence of 10−7 M RA. After 48 h, relative cell numbers were determined using MTS reduction (±SEM).
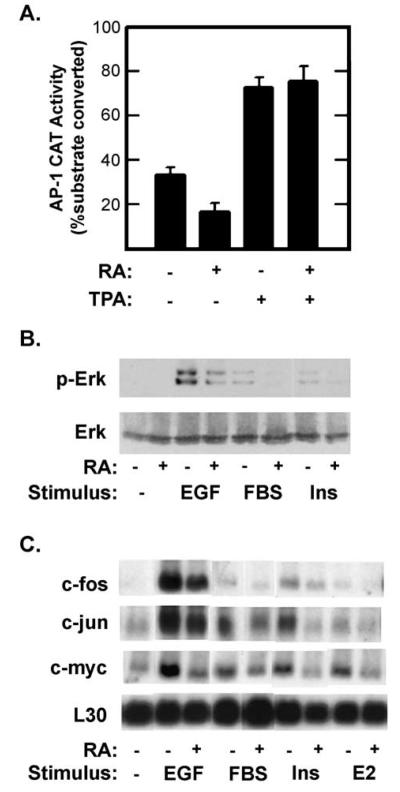
Fig. 2.
Retinoic acid inhibits growth factor signaling. (A) T-47D cells were transfected with a reporter plasmid (AP15-tk-CAT) in which the bacterial chloramphenicol acetyl transferase (CAT) gene was under the transcriptional regulation of five copies of a synthetic AP-1 element and the minimal thymidine kinase promoter. Transfected cells were cultured in the presence or absence of 1 μM RA for 48 h after which total CAT activity was measured in whole cell extracts. TPA (100 ng/ml) was added for the last 24 h before measuring CAT levels. Data are shown as the percentage of the substrate, chloramphenicol, converted to acetylated forms (±SE). Similar results were obtained using a reporter plasmid in which CAT expression was regulated by the endogenous c-jun AP1 elements (pJTx-CAT). (B) Serum-starved (0.25% FBS) T-47D cells were treated with 10−7 M RA for 36 h and then stimulated with EGF (40 ng/ml), fetal bovine serum (FBS, 10%) or insulin (Ins, 10 μg/ml) for 30 min. Activation of the Erk 1 and 2 MAPKs was measured by probing immunoblots with antibodies specific for the doubly phosphorylated, active forms of Erk 1 and 2 (top, p-Erk) or for total Erk 2 (bottom). (C) Total RNA was isolated from serum-starved T-47D cells 30 min after stimulation with insulin (10 μg/ml), 17-β estradiol (10−6 M E2), EGF (20 ng/ml) or 10% FBS or with no stimulation (−). One half of the cultures were pretreated with 10−6 M RA for 15 h. Filters were probed with the indicated 32P-labeled cRNAs. Filters were exposed overnight at −80°C with an intensifying screen (c-fos in EGF sample was exposed for 4 h). Based on scanning densitometry of films from multiple experiments, RA inhibited c-fos expression and Erk activation in response to: EGF by 73% and 68%; to FBS by 84% and 78%; and to insulin by 63% and 35%, respectively.
Retinoid-induced growth arrest was reversible. First, at no time point (up to 11 days of retinoid treatment) or retinoid concentration (up to 10−6 M) did the viability of the treated cells drop below 90% (not shown). Second, when RA was removed from the culture media, the treated cells resumed proliferation (Fig. 1C). Taken together, these data demonstrate that activating RARα reversibly arrests T-47D cell cycle progression without inducing cell death. In support of this conclusion, we demonstrated that RA-treated T-47D cells were arrested in G0 or early G1 by measuring DNA content at various times after addition of RA to the culture media or after reducing the serum content of the culture media (Fig. 1D). Between 12 and 16 h after RA (1 μM) addition or following serum reduction (from 10% to 0.25% fetal bovine serum), the percentage of the population in S phase (i.e. with a DNA content >2N but <4N) decreased from approximately 30% to less than 20% (Fig. 1D). There was a concomitant increase in the percentage of cells with a 2N DNA content (from 55% to >70%). The accumulation of cells with a 2N DNA content in a growth-arrested population is typically the result of either a block to transit through the restriction point (R) late in G1, or withdrawal of cells from the cell cycle into the quiescent state (G0) [32]. A late G1 block can be distinguished from a G0 arrest at the level of total cellular RNA (which has nearly doubled by the time cells reach R) [33,34]. FACS analysis following staining of cells with acridine orange showed a population of cells in both serum-starved and RA-treated cultures with reduced total RNA consistent with an early G1 or G0 population (data not shown).
Retinoid inhibition of AP1 transcriptional transactivation
The ability of retinoids to inhibit the proliferation of tumor cells, or to prevent de novo transformation of normal cells, correlates with their ability to inhibit the transcription factor AP1 [9,13-21,35,36]. Several mechanisms have been proposed to account for the anti-AP1 activity of retinoids. Some investigators have demonstrated that retinoids inhibit AP1 activity either by inhibiting expression of the proteins that constitute the AP1 factors or by inhibiting signaling pathways that result in posttranslational activation of preexisting AP1 [3,15,20-23,37,38]. Alternatively, some studies point to competition between activated RARs and AP1 for limiting co-activators or interference with AP1 resulting from physical interactions between ligand-bound RARs and AP1 [13,17]. The reduction in T-47D proliferation is associated with decreased steady-state levels of AP1 activity (Fig. 2A). When T-47D cells were transiently transfected with an AP1-CAT reporter plasmid, RA treatment resulted in a 50% reduction in total AP1-dependent CAT activity. Treatment of transfected cells with TPA increased AP1 activity by approximately 100% compared to untreated cells. This increase was also seen in RA-treated cells. The ability of TPA treatment to stimulate AP1 activity in the presence of RA was not consistent with either competition for limiting co-activators or physical interference of AP1 activity by ligand-bound RARs.
Retinoids inhibit mitogen signaling in T-47D cells
The above results demonstrate that retinoid-treated cells arrest in a G0/early-G1 state that closely parallels the effects of removing serum. Thus, it is possible that retinoid-treated cells are unable to respond to mitogenic compounds normally present in fetal bovine serum. To test this prediction, we assayed induction of immediate early gene expression (specifically c-fos, c-jun and c-myc) by serum, insulin, epidermal growth factor and 17β-estradiol in control cells and in cells pretreated with retinoids (Fig. 2B). T-47D cells were serum starved (0.25% FBS) overnight in the presence or absence of 10−6 M RA and then stimulated with insulin (10 μg/ml), 17β-estradiol (E2, 10−6 M), EGF (20 ng/ml) or FBS (10%) for 30 min. All four treatments resulted in an increase in mRNA levels for c-fos, c-jun and c-myc. Although the magnitude of the response varied between stimuli, EGF was the most potent stimulant and E2 was the weakest. RA pretreatment reduced or eliminated the response to each mitogen.
Mitogenic induction of these genes involves signal transduction via several protein kinase pathways that activate distinct preexisting transcription factors [39-44]. Therefore, we felt that it was most likely that retinoids were preventing signals from reaching these transcription factors. To test this prediction, we assayed the effects of retinoids on mitogen activation of several cytoplasmic protein kinases. Acute stimulation of serum-starved T-47D cells with EGF, FBS or insulin resulted in rapid activation of Erk 1/2 (Fig. 2C), and in the case of EGF, Akt/PKB as well. Pretreatment of cells with RA (1 μM, 16 h) reduced Erk activation by all stimuli tested, but did not affect EGF activation of Akt/PKB. These results clearly demonstrate that RA selectively interferes with pathways utilized by multiple growth factor receptors.
Retinoids limit the duration of mitogen signaling
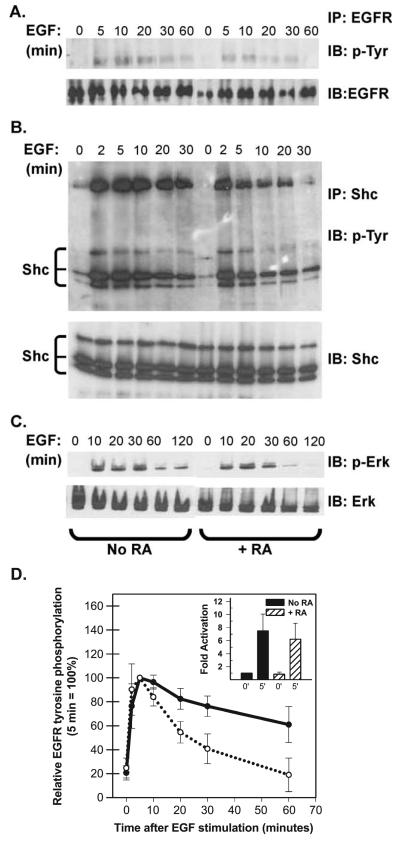
In an effort to better understand how RA treatment attenuated growth factor activation of Erk 1/2 we focused on early responses of T-47D cells to EGF stimulation. Serum-starved T-47D cells were pretreated with retinoic acid (RA, 10−7 M for 36 h) and then stimulated with EGF (40 ng/ml). At various times after EGF stimulation whole cell lysates were analyzed for the extent of EGFR tyrosine phosphor-ylation (Fig. 3A), tyrosine phosphorylation of the adaptor protein Shc (Fig. 3B) and activation of Erk 1/2 (Fig. 3C). EGF rapidly induced phosphorylation of the EGFR and all three isoforms of Shc. In the latter case, EGF stimulated a physical association between the EGFR and Shc resulting in both tyrosine phosphorylation of Shc and co-immunoprecipitation of Shc and EGFR (Fig. 3B). RA treatment did not alter the level of EGFR, Shc or Erk 1/2 expression, nor did it affect the initial (0–5 min) response to EGF. The most pronounced effect of pretreating cells with RA on EGF signaling was a shortening of the duration of the EGF signal. This was evident when looking at EGFR activation, Shc phosphorylation and Erk 1/2 activation. The data from five independent experiments measuring EGFR tyrosine phosphorylation were averaged and plotted in Fig. 3D. The average activation of the EGFR after 2–5 min was comparable in the presence and absence of RA (Fig. 3D, inset). EGFR phosphorylation returned to resting levels within 30 min in RA-treated cells, whereas in the absence of RA, EGFR phosphorylation was still elevated after 60 min. Similar results were seen when the kinetics of Erk 1/2 activation by EGF was quantified (not shown).
Fig. 3.
RA reduces the duration of EGF signaling. (A) T-47D cells were serum starved in the absence or presence of 10−7 M RA for 36 h and then stimulated with 40 ng/ml EGF. At the times indicated: (A) Cells were harvested and EGF receptor (EGFR) was recovered by immunoprecipitation (IP) and resolved by SDS-PAGE. After transfer to nitrocellulose, blots were sequentially probed with an anti-phosphotyrosine antibody (4G10, IB: pTyr) and then, after stripping, with an anti-EGFR antibody (IB: EGFR). Only the region from the gel corresponding to the approximately 175-kDa standard is shown. (B) Cells were immunoprecipitated with a polyclonal antibody directed against the adapter protein Shc. Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to nitrocellulose and the filters were probed sequentially with the monoclonal 4G10 antibody (IP: Shc, IB: p-Try) and a monoclonal anti-Shc antibody (IB: Shc). The positions of Shc isoforms and the co-immunoprecipitated EGFR (confirmed by additional immunoblotting, not shown) are indicated. (C) Or, Erk 1/2 activation was assessed by sequential immunoblotting of whole cell extracts (30 Ag/lane) with anti-phospho-Erk antibodies (IB: p-Erk) and anti-Erk antibodies (IB: Erk). (D) EGFR tyrosine phosphorylation was assessed as described above and signal strength was quantified by densitometry. The data plotted in panel D show the degree of EGFR staining with anti-phosphotyrosine antibodies after various times of EGF stimulation (averaged from five independent experiments ± SEM). The inset shows that the average fold activation at 0 and 5 min did not differ between control (=1) and RA-pretreated cells.
Retinoid effects require PKCα activity
Previously we showed that activation of RARα induces PKCα mRNA and protein expression [1,38,45], and that the increase in population doubling times of RA- or Am580-treated T-47D cells required PKCα activity [1]. The latter conclusion was reached based on experiments using an inhibitor of conventional PKC (PKCα is the only conventional PKC expressed in T-47D cells before or after retinoid treatment), and by constitutively expressing PKCα in T-47D cells. To determine if the effects of retinoids on mitogen signaling also required the activity of RA-induced PKCα we examined the effect of the inhibitor Gö6976 [46] on EGF signaling in RA-treated T-47D cells. We then compared EGF signaling between parental T-47D cells, RA-treated T-47D cells and T-47D cells stably expressing PKCα (in the absence of RA).
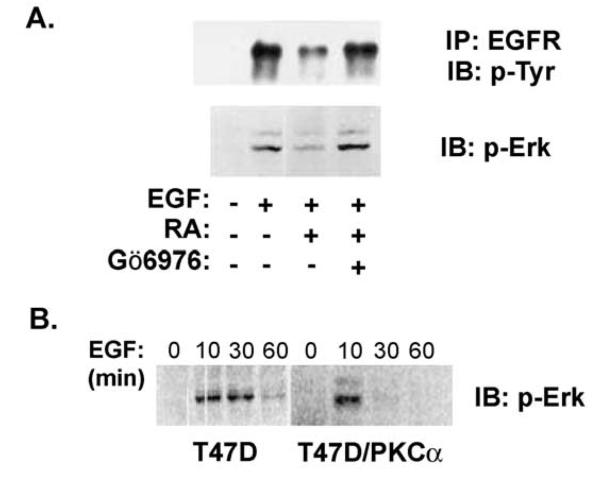
Retinoid treatment attenuated sustained EGF signaling in T-47D cells. Inhibiting PKCα activity reversed these effects of RA on EGF signaling (Fig. 4). Serum-starved T-47D cells were pretreated with RA for 36 h before stimulation with EGF. A subset of cultures was treated with the conventional PKC inhibitor Gö6976 for 30 min before EGF stimulation. EGF signaling was measured by determining the extent of EGFR tyrosine phosphorylation by immunoblotting and by measuring activated Erk 1/2 levels (Fig. 4A). As seen in Figs. 2 and 3, both tyrosine phosphorylated EGFR and phosphorylated Erk 1/2 levels were reduced in RA-treated cells (Fig. 4A). When RA-treated cells were treated with the conventional PKC inhibitor, Gö6976, before EGF stimulation, EGFR tyrosine phosphorylation and phospho-Erk 1/2 were elevated to control levels (Fig. 4A). Thus, pretreating cells with Gö6976 reversed the inhibitory effects of RA on EGF signaling, indicating that RA effects on EGF signaling require the activity of PKCα. This conclusion was supported by expressing PKCα in T-47D cells from a constitutive promoter [1]. Compared to parental T-47D cells, T-47D/PKCα cells grow at a reduced rate that approximates that seen following treatment with 10−9 M RA [1]. When assayed at the level of Erk 1/2 activation, constitutive expression of PKCα also reduced the duration but not the initial magnitude of EGF signaling (Fig. 4B).
Fig. 4.
Conventional PKC isoforms meditate retinoid effects on EGF signaling. (A) EGF activation of EGFR autophosphorylation and of Erk 1/2 was analyzed as described above. Serum-deprived T-47D cells were pretreated with 10−7 M RA for 36 h and then stimulated with EGF for 10 min. Where indicated the inhibitor of the conventional PKCs, Gö6976 (500 nM), was added 30 min before stimulation with EGF for 10 min. In each assay, Gö6976 restored EGF signaling in RA-treated cells, implicating cPKC isoforms as mediators of the RA effect. (B) EGF activation of Erk 1/2 was compared in parental T-47D cells and in T-47D cells constitutively expressing PKCα (T47D-PKCα). EGF treatment stimulated Erk phosphor-ylation in both cell lines, but in the PKCα-expressing cells p-Erk levels returned to baseline by 30 min, whereas activation in the parental cells was sustained for at least 30 min.
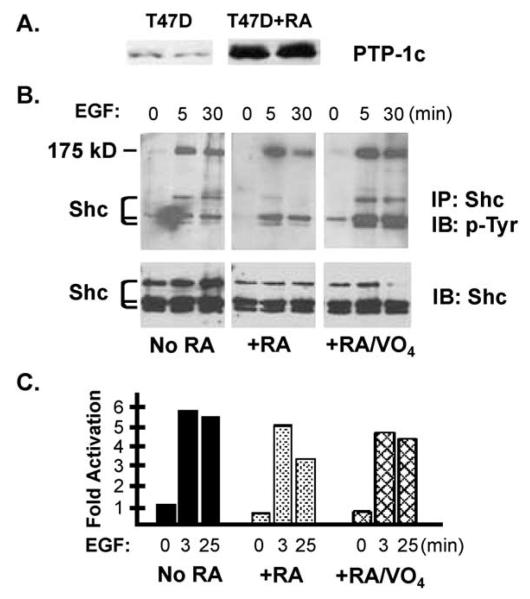
The above results demonstrate that RA, acting via PKCα, affects mitogen signaling by selectively targeting cytoplasmic signaling pathways, and at least in the case of EGF signaling, sharply limits the duration of the signal. One way to reduce the magnitude and duration of kinase signaling is to induce or activate protein phosphatases. To assess the involvement of PTPs in mediating the effects of retinoids, we determined whether orthovanadate, a nonselective inhibitor of protein tyrosine phosphatases, could reverse the RA effects on EGF signaling (Fig. 5A and B). Serum-starved T-47D cells were pretreated with RA (10−7 M for 36 h) before stimulation with (40 ng/ml) EGF. A subset of cultures was pretreated with sodium orthovanadate (100 μM) for 30 min before EGF stimulation. Treated cells were lysed after 0, 3 or 25 min, and we assayed tyrosine phosphorylation of Shc or activation of Erk 1/2. As shown above (Fig. 3), the most dramatic effect of RA was to reduce the duration of the EGF signal. After a 3-min EGF stimulation, Shc and Erk 1/2 phosphorylation were equivalent in control and RA-treated cells, but by 25 min after EGF stimulation phosphorylation in RA-treated cells had returned to resting levels. Treating cells with NaVO4 did not increase Shc phosphorylation or Erk 1/2 activity (0-min time points). Orthovanadate treatment did reverse the effect of RA pretreatment leading to sustained EGF signaling at late time points.
Fig. 5.
Phosphotyrosine phosphatase 1C is implicated in mediating retinoid effects on EGF signaling. (A) RA treatment elevates PTP-1C levels in T-47D cells. T-47D cells were cultured in the presence of 10−7 M RA for 48 and PTP-1C levels were measured by immunoblotting. (B) Serum-deprived T-47D cells (±36 h in the presence of 10−7 M RA) were stimulated for 0–25 min with 40 ng/ml EGF. Where indicated, 100 μM sodium orthovanadate (VO4) was added to the culture medium 15 min before EGF. Shc immunoprecipitates were blotted sequentially with an antiphosphotyrosine antibody and a Shc antibody. (C) Serum-deprived T-47D cells were treated as described in B. Erk activation was measured by immunoblot, and the results were quantified by scanning appropriately exposed films [expressed as the ratio of the p-Erk to total Erk signals normalized to the 0-min time points (=1)]. The means of two experiments are shown.
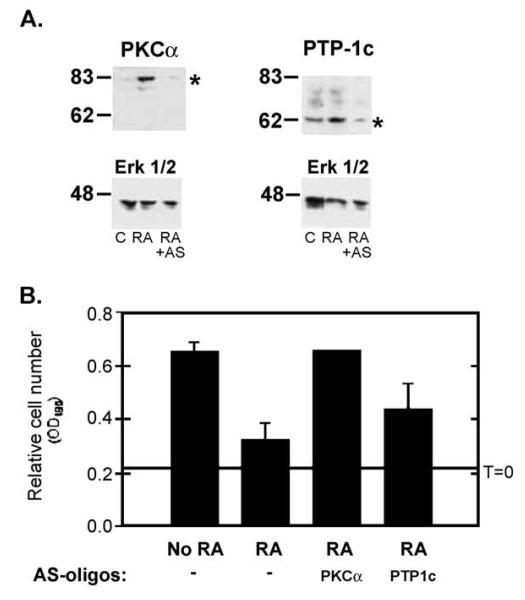
While this experiment supported the prediction that elevated tyrosine phosphatase activity could account for the RA/PKCα effects on EGF signaling, the broad action of orthovanadate prevents us from drawing conclusions about the role of specific PTPs in mediating the RA response, and the toxic nature of prolonged NaVO4 treatment prevented an assessment of phosphatase activity in the antiproliferative response to RA. To more specifically address these questions, we performed a preliminary screen looking at the effect of RA treatment on the expression of four phosphatases, the tyrosine phosphatases PTP-1C and PTP-1D, and the MAPK phosphatases, MKP1 and MKP2. Of these we observed that steady-state levels of the protein tyrosine phosphatase PTP-1C were elevated in RA-treated T-47D cells compared to untreated T-47D cells (Figs. 5A and 6A). The levels of the other three phosphatases examined were unchanged in RA-treated cells T-47D cells (data not shown). To determine if PTP-1C was responsible for the accelerated termination of EGFR signaling in RA-treated cells, we performed proliferation assays of T-47D cells treated with RA and with anti-sense oligonucleotides specific for PTP-1C. In addition, we used anti-sense oligonucleotides targeting PKCα expression. Treatment with anti-sense oligonucleotides blocked the RA-induced increase of PKCα or PTP-1C expression (Fig. 6A). Anti-sense oligonucleotides targeting PKCα completely reversed the antiproliferative effect of RA (Fig. 6B). Anti-sense oligonucleotides targeting PTP-1C partially reversed the RA effect, although this reversal was not statistically significant. These results confirm the critical role of PKCα as a mediator of the RA response. They also support a contributory, but not essential role for PTP-1C in mediating the antiproliferative effects of RA in these cells.
Fig. 6.
Phosphotyrosine phosphatase 1C is implicated in mediating retinoid effects on EGF signaling. (A) T-47D cells were grown in the presence of 10−7 M RA, or 10−7 M RA and 10 μM anti-sense oligonucleotides directed at PKCα or PTP-1C. After 3 days, whole cell lysates were resolved by SDS-PAGE and PKCα (top left), PTP-1c (top right) and Erk 1/2 levels were measured by immunoblotting (following stripping and reprobing of the same filters shown in the top panels). Exposure to anti-sense oligonucleotides prevented the RA-induced increase in PKCα (left) and PTP-1c expression without affecting Erk 1/2 levels. (B) T-47D cells were grown in the presence of 10−6 M RA, or 10−6 M RA and 10 μM anti-sense oligonucleotides directed at PKCα or PTP-1C. After 3 and 5 days (day 5 data shown) relative cell numbers were determined using MTS (OD490 ± SEM). Anti-sense oligonucleotides targeting PKCα prevented retinoid-induced growth arrest. Anti-sense oligonucleotides targeting PTP-1C resulted in a partial block to retinoid-induced growth arrest. Sequence mismatch oligonucleotides did not affect retinoid action and neither mismatch nor anti-sense oligonucleotides affected T-47D proliferation in the absence of retinoids (not shown).
Discussion
Treatment of the hormone-dependent, human breast cancer cell line, T-47D, with retinoids resulted in reversible growth arrest. The arrested cells showed reduced responses to several known mammary epithelial mitogens, notably estrogen, EGF and Nrg-1. In addition, retinoid-arrested cells expressed PKCα and PKCα activity mediated the anti-proliferative and anti-EGF signaling actions of retinoids in T-47D cells. Based on these data, we propose the following scheme. Retinoid induction of PKCα expression leads to accelerated inactivation of EGFR following ligand binding, preventing the sustained activation of downstream kinases that is necessary for expression of nuclear transcription factors that regulate progression through G1. The net result is that retinoid-treated cells do not progress through G1 and into S, even in the presence of serum growth factors. We also show that retinoid action is broadly inhibitory on one level, yet selective on another level. Thus, RA prevents sustained Erk 1/2 activation and limits c-fos expression in response to multiple stimuli, but does not prevent sustained activation of other signaling targets, notably Akt/PKB. This selectivity might allow retinoids to regulate proliferation without inducing apoptosis.
Other investigators have argued that retinoic acid blocks cell cycle progression of human breast cancer cells (both MCF-7 and T-47D cell lines) in mid-G1 [4,47-51]. Wilcken et al. [49] showed that RA treatment of T-47D cells decreased Rb phosphorylation within 16 h and that treated cells arrested in G0–G1 between 16 and 24 h. In MCF-7 cells, retinoic acid-induced cell cycle arrest was attributed to decreased Cdk2 activity [48] and decreased cyclin D3/Cdk4 [51]. Although our data do not rule out G1 targets for retinoids in T-47D cells, we believe the critical block occurs in early G1. Cyclin D1, AP1 and MEK1 are all involved in regulating the G0/G1 transition and G1 progression [52-54]. Retinoid attenuation of MAP kinase activation, immediate early gene expression, AP1 transcriptional activity and cyclin D1 expression [55] supports our hypothesis that retinoids block cell cycle progression by disrupting the mitogenic signaling that is required for early G1 progression (of cycling cells) or the transition from G0 into G1 (of quiescent cells). The retinoid block to T-47D cell cycle progression in a G0-like state is reminiscent of cell cycle changes induced in normal mammary epithelial cells by blocking EGF receptor function [28]. The extent to which the quiescent state induced by retinoids is biologically similar to quiescent, nontransformed cells is not clear. Also consistent with a G0-like arrest of RA-treated cells are recent reports that the cyclin-dependent kinase inhibitor, p27, is elevated in RA-treated cells [4,56-59]. Levels of p27 are typically high in quiescent cells and decrease following mitogen-stimulated reentry into G1 [53,60].
Retinoid treatment of T-47D cells disrupted signaling via the EGF receptor. This was not the result of decreased receptor expression, but rather a decrease in the duration of activation of the EGFR tyrosine kinase activity, and of downstream targets of EGF signaling. Similar effects were seen in cells that constitutively expressed PKCα (Fig. 4 and Ref. [1]). Support for the critical role of PKCα in mediating the negative effects on EGFR signaling came from the demonstration that the PKC inhibitor, Gö6976, largely reversed retinoid inhibition of EGF signaling (Fig. 4) and proliferation [1]. Indeed, constitutive expression of PKCα in the normally RA-resistant MDA-MB-231 cell line results in increased RA sensitivity and decreased growth factor signaling and proliferation [3]. Based on these data, we conclude that a key primary target for RA is the PKCα promoter (in which a functional retinoid response element has been identified [61]), and that the molecular basis of growth arrest is the failure of mitogens such as EGF to sustain signaling by their receptors.
Signaling from the EGFR, related family members, erbB2, erbB3 and erbB4, and other receptor tyrosine kinases is inhibited by phorbol esters [62-71]. Several effects on EGFR/erbB signaling have been associated with phorbol ester treatment of cells including PKC-dependent phosphorylation of EGFR, erbB2, and selective proteolytic cleavage of erbB4. In vitro and in vivo PKC phosphorylates serine and threonine residues in the juxtamembrane region of the EGFR, a region involved in ligand-dependent internalization and receptor down-regulation. One clear PKC site is threonine 654 [72], although the role of PKC phosphorylation of threonine 654 per se in affecting the numbers of high-affinity surface receptors and EGF signaling is unclear [69,73-77]. One possible explanation for retinoid inhibition of EGFR signaling is a PKCα-dependent phosphorylation of the EGFR in the juxtamembrane region. We have expressed both wild type and T654A mutant forms of the EGFR in T-47D cells. Although overexpression of either receptor increased EGF signaling, in neither case, was overexpression sufficient to overcome the antiproliferative effects of retinoids (Tighe and Talmage, unpublished observations).
An alternative target of PKCα is a phosphotyrosine protein phosphatase (PTP). PTPs mediate signaling by different PKC isoforms in various cell types [65,71,78-86]. A role for PTPs as mediators of PKCα effects on EGFR function is supported by several lines of evidence. First, several non-receptor tyrosine phosphatases that contain SH2 domains bind to activated EGFRs [80-83]. Binding of PTPs to the activated EGFR attenuates the EGFR signal, presumably following dephosphorylation of key tyrosines on the activated receptor [80-83]. Second, PTP-1C levels are elevated in RA-treated cells (and in PKCα expressing cells). Third, the RA effects on EGF signaling are reversed by the PTP inhibitor orthovanadate. Finally, we found that PTP-1C anti-sense oligonucleotides partially reversed the antiproliferative effect of RA on T-47D cells. However, the relatively weak effect of anti-sense PTP-1C oligonucleotides compared to orthovanadate treatment, Gö6976 treatment or anti-sense PKCα oligonucleotide treatment leaves open the question of what role this particular PTP plays in mediating the RA or PKCα effects. Therefore, additional experimental evidence is required before we can conclude that retinoid increased PTP-1C, or other PTP, activity is involved in the decreased EGF signaling.
It is now an accepted principle that retinoids inhibit cell proliferation, particularly of tumor cells, by interfering with the action of the AP1 transcription factor. Synthetic retinoids do this in the absence of measurable changes in RAR-dependent transcriptional transactivation [16,36,87,88]. It is less clear whether naturally occurring retinoids also inhibit AP1 activity by similar mechanisms. The results of this study, and of other studies point to a different mechanism that involves more traditional forms of retinoid signaling. In these examples, the effects of retinoids on AP1 are secondary to RAR activation of target genes. The products of these RAR target genes functionally interfere with AP1, both by preventing the expression of AP1's constituent subunits such as c-fos and c-jun [15,37,38] and by preventing posttranslational modification of preformed AP1 [20,21,23]. The retinoid effects on T-47D cells that are documented in this study included a decrease in AP1 activity in some, but not all circumstances. Specifically, retinoids failed to block phorbol ester activation of AP1. At the same time, retinoids were effective at inhibiting EGF-, serum-, insulin- and estrogen-induced expression of c-fos and c-jun, and activation of signaling kinases that are known to activate factors that regulate c-fos and c-jun expression, as well as activate preformed AP1. Taken together, these results indicate that retinoids disrupt a subset of the signals that utilize AP1.
In toto, these data support the conclusion that PKCα expression is a primary response to RAR activation. Equally critical to our conclusions are the data demonstrating that PKCα activity mediates both the antiproliferative effects of retinoids and the anti-EGF signaling effects of retinoids. Whether the critical substrate for PKCα is the EGFR, one of the other erbB receptors, PTP-1C or a transcription factor that subsequently up-regulates expression of PTP-1C and other targets, remains to be determined.
Acknowledgments
Grants from the US Department of Army Breast Cancer Research Program (DAMD-17-94-J-4100 and DAMD-17-97-1-7289), the American Institute for Cancer Research and the NIH (CA79737) supported this work. The authors would like to thank Mark Listerud for technical assistance.
References
- 1.Cho Y, Tighe AP, Talmage DA. Retinoic acid induced growth arrest of human breast carcinoma cells requires protein kinase C alpha expression and activity. J. Cell. Physiol. 1997;172:306–313. doi: 10.1002/(SICI)1097-4652(199709)172:3<306::AID-JCP4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- 3.Cho Y, Talmage DA. Protein kinase C-alpha expression confers retinoic acid sensitivity on MDA-MB-231 human breast cancer cells. Exp. Cell Res. 2001;269:97–108. doi: 10.1006/excr.2001.5298. [DOI] [PubMed] [Google Scholar]
- 4.Dow R, Hendley J, Pirkmaier A, Musgrove EA, Germain D. Retinoic acid-mediated growth arrest requires ubiquitylation and degradation of the F-box protein Skp2. J. Biol. Chem. 2001;276:45945–45951. doi: 10.1074/jbc.M103593200. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald P, Teng M, Chandraratna RA, Heyman RA, Allegretto EA. Retinoic acid receptor alpha expression correlates with retinoid-induced growth inhibition of human breast cancer cells regardless of estrogen receptor status. Cancer Res. 1997;57:2642–2650. [PubMed] [Google Scholar]
- 6.Okamoto K, Andreola F, Chiantore MV, Dedrick RL, De Luca LM. Differences in uptake and metabolism of retinoic acid between estrogen receptor-positive and -negative human breast cancer cells. Cancer Chemother. Pharmacol. 2000;46:128–134. doi: 10.1007/s002800000125. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh MS, Shao ZM, Chen JC, Hussain A, Jetten AM, Fontana JA. Estrogen receptor-negative breast cancer cells transfected with the estrogen receptor exhibit increased RAR alpha gene expression and sensitivity to growth inhibition by retinoic acid. J. Cell. Biochem. 1993;53:394–404. doi: 10.1002/jcb.240530417. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh MS, Shao ZM, Li XS, Dawson M, Jetten AM, Wu S, Conley BA, Garcia M, Rochefort H, Fontana JA. Retinoid-resistant estrogen receptor-negative human breast carcinoma cells transfected with retinoic acid receptor-alpha acquire sensitivity to growth inhibition by retinoids. J. Biol. Chem. 1994;269:21440–21447. [PubMed] [Google Scholar]
- 9.van der Burg B, Slager-Davidov R, van der Leede BM, de Laat SW, van der Saag PT. Differential regulation of AP1 activity by retinoic acid in hormone-dependent and -independent breast cancer cells. Mol. Cell. Endocrinol. 1995;112:143–152. doi: 10.1016/0303-7207(95)03600-c. [DOI] [PubMed] [Google Scholar]
- 10.van der Burg B, van der Leede BM, Kwakkenbos-Isbrucker L, Salverda S, de Laat SW, van der Saag PT. Retinoic acid resistance of estradiol-independent breast cancer cells coincides with diminished retinoic acid receptor function. Mol. Cell. Endocrinol. 1993;91:149–157. doi: 10.1016/0303-7207(93)90267-n. [DOI] [PubMed] [Google Scholar]
- 11.Schneider SM, Offterdinger M, Huber H, Grunt TW. Activation of retinoic acid receptor alpha is sufficient for full induction of retinoid responses in SK-BR-3 and T47D human breast cancer cells [In Process Citation] Cancer Res. 2000;60:5479–5487. [PubMed] [Google Scholar]
- 12.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 13.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 14.Pfahl M. Nuclear receptor/AP-1 interaction. Endocr. Rev. 1993;14:651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- 15.Talmage DA, Listerud M. Retinoic acid suppresses polyoma virus transformation by inhibiting transcription of the c-fos proto-oncogene. Oncogene. 1994;9:3557–3563. [PubMed] [Google Scholar]
- 16.Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994;372:107–111. doi: 10.1038/372107a0. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang-Yen HF, Zhang XK, Graupner G, Tzukerman M, Sakamoto B, Karin M, Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991;3:1206–1219. [PubMed] [Google Scholar]
- 19.Zhou XF, Shen XQ, Shemshedini L. Ligand-activated retinoic acid receptor inhibits AP-1 transactivation by disrupting c-Jun/c-Fos dimerization. Mol. Endocrinol. 1999;13:276–285. doi: 10.1210/mend.13.2.0237. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Freund R, Listerud M, Wang Z, Talmage DA. Retinoic acid inhibits transformation by preventing phosphatidylinositol 3-kinase dependent activation of the c-fos promoter. Oncogene. 1999;18:139–148. doi: 10.1038/sj.onc.1202272. [DOI] [PubMed] [Google Scholar]
- 21.Caelles C, Gonzalez-Sancho JM, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. Retinoic acid inhibits induction of cJun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Sueoka N, Hong WK, Mangelsdorf DJ, Claret FX, Kurie JM. All-trans-retinoic acid inhibits Jun N-terminal kinase by increasing dual-specificity phosphatase activity. Mol. Cell. Biol. 1999;19:1973–1980. doi: 10.1128/mcb.19.3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riese DJ, II, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 26.Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- 27.Snedeker SM, Brown CF, DiAugustine RP. Expression and functional properties of transforming growth factor alpha and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous reentry into the cell cycle. Exp. Cell Res. 1993;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- 29.Dickson RB, Lippman ME. Growth factors in breast cancer. Endocr. Rev. 1995;16:559–589. doi: 10.1210/edrv-16-5-559. [DOI] [PubMed] [Google Scholar]
- 30.Wiedemann LM, Perry RP. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol. Cell. Biol. 1984;4:2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delescluse C, Cavey MT, Martin B, Bernard BA, Reichert U, Maignan J, Darmon M, Shroot B. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol. Pharmacol. 1991;40:556–562. [PubMed] [Google Scholar]
- 32.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 33.Wallen CA, Higashikubo R, Dethlefsen LA. Murine mammary tumour cells in vitro. I. The development of a quiescent state. Cell Tissue Kinet. 1984;17:65–77. doi: 10.1111/j.1365-2184.1984.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallen CA, Higashikubo R, Dethlefsen LA. Murine mammary tumour cells in vitro. II. Recruitment of quiescent cells. Cell Tissue Kinet. 1984;17:79–89. doi: 10.1111/j.1365-2184.1984.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 35.Agadir A, Shealy YF, Hill DL, Zhang X. Retinyl methyl ether down-regulates activator protein 1 transcriptional activation in breast cancer cells. Cancer Res. 1997;57:3444–3450. [PubMed] [Google Scholar]
- 36.Lu XP, Fanjul A, Picard N, Pfahl M, Rungta D, Nared-Hood K, Carter B, Piedrafita J, Tang S, Fabbrizio E. Novel retinoid-related molecules as apoptosis inducers and effective inhibitors of human lung cancer cells in vivo. Nat. Med. 1997;3:686–690. doi: 10.1038/nm0697-686. [DOI] [PubMed] [Google Scholar]
- 37.Talmage DA, Lackey RS. Retinoic acid receptor alpha suppresses polyomavirus transformation and c-fos expression in rat fibroblasts. Oncogene. 1992;7:1837–1845. [PubMed] [Google Scholar]
- 38.Khuri FR, Cho Y, Talmage DA. Retinoic acid-induced transition from protein kinase C beta to protein kinase C alpha in differentiated F9 cells: correlation with altered regulation of proto-oncogene expression by phorbol esters. Cell Growth Differ. 1996;7:595–602. [PubMed] [Google Scholar]
- 39.Clarke N, Arenzana N, Hai T, Minden A, Prywes R. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol. Cell. Biol. 1998;18:1065–1073. doi: 10.1128/mcb.18.2.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 41.Price MA, Hill C, Treisman R. Integration of growth factor signals at the c-fos serum response element. Philos. Trans. R. Soc. London, Ser. B Biol. Sci. 1996;351:551–559. doi: 10.1098/rstb.1996.0054. [DOI] [PubMed] [Google Scholar]
- 42.Treisman R. Journey to the surface of the cell: fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Yates PR, Whitmarsh AJ, Davis RJ, Sharrocks AD. The regulation of TCF transcription factors by MAP kinase cascades. Biochem. Soc. Trans. 1997;25:153S. doi: 10.1042/bst025153s. [DOI] [PubMed] [Google Scholar]
- 45.Cho Y, Klein MG, Talmage DA. Distinct functions of protein kinase Calpha and protein kinase Cbeta during retinoic acid-induced differentiation of F9 cells. Cell Growth Differ. 1998;9:147–154. [PubMed] [Google Scholar]
- 46.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 47.Bardon S, Razanamahefa L. Retinoic acid suppresses insulin-induced cell growth and cyclin D1 gene expression in human breast cancer cells. Int. J. Oncol. 1998;12:355–359. doi: 10.3892/ijo.12.2.355. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira C, Pratt MA. CDK2 is a target for retinoic acid-mediated growth inhibition in MCF-7 human breast cancer cells. Mol. Endocrinol. 1997;11:1191–1202. doi: 10.1210/mend.11.9.9977. [DOI] [PubMed] [Google Scholar]
- 49.Wilcken NR, Sarcevic B, Musgrove EA, Sutherland RL. Differential effects of retinoids and antiestrogens on cell cycle progression and cell cycle regulatory genes in human breast cancer cells. Cell Growth Differ. 1996;7:65–74. [PubMed] [Google Scholar]
- 50.Wilcken NR, Musgrove EA, Sutherland RL. Different points of action of retinoids and anti-estrogens in G1 phase identified in synchronized T-47D breast cancer cells. Int. J. Oncol. 1997;70:291–296. doi: 10.1002/(sici)1097-0215(19970127)70:3<291::aid-ijc8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 51.Zhu WY, Jones CS, Kiss A, Matsukuma K, Amin S, De Luca LM. Retinoic acid inhibition of cell cycle progression in MCF-7 human breast cancer cells. Exp. Cell Res. 1997;234:293–299. doi: 10.1006/excr.1997.3589. [DOI] [PubMed] [Google Scholar]
- 52.Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ladha MH, Lee KY, Upton TM, Reed MF, Ewen ME. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol. Cell. Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musgrove EA, Lee CS, Buckley MF, Sutherland RL. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tighe AP. Institute of Human Nutrition, Columbia University; New York: 1999. [Google Scholar]
- 56.Wakino S, Kintscher U, Kim S, Jackson S, Yin F, Nagpal S, Chandraratna RA, Hsueh WA, Law RE. Retinoids inhibit proliferation of human coronary smooth muscle cells by modulating cell cycle regulators. Arterioscler., Thromb., Vasc. Biol. 2001;21:746–751. doi: 10.1161/01.atv.21.5.746. [DOI] [PubMed] [Google Scholar]
- 57.Hsu SL, Hsu JW, Liu MC, Chen LY, Chang CD. Retinoic acid-mediated G1 arrest is associated with induction of p27(Kip1) and inhibition of cyclin-dependent kinase 3 in human lung squamous carcinoma CH27 cells. Exp. Cell Res. 2000;258:322–331. doi: 10.1006/excr.2000.4933. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki K, Tamura S, Tachibana H, Sugita M, Gao Y, Furuyama J, Kakishita E, Sakai T, Tamaoki T, Hashimoto-Tamaoki T. Expression and role of p27(kip1) in neuronal differentiation of embryonal carcinoma cells. Brain Res. Mol. Brain Res. 2000;77:209–221. doi: 10.1016/s0169-328x(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 59.Sueoka N, Lee HY, Walsh GL, Hong WK, Kurie JM. Posttranslational mechanisms contribute to the suppression of specific cyclin:CDK complexes by all-trans retinoic acid in human bronchial epithelial cells. Cancer Res. 1999;59:3838–3844. [PubMed] [Google Scholar]
- 60.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 61.Desai DS, Hirai S, Karnes WE, Jr., Niles RM, Ohno S. Cloning and characterization of the murine PKC alpha promoter: identification of a retinoic acid response element. Biochem. Biophys. Res. Commun. 1999;263:28–34. doi: 10.1006/bbrc.1999.1307. [DOI] [PubMed] [Google Scholar]
- 62.Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J. Biol. Chem. 1996;271:18989–18995. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- 63.Ouyang X, Gulliford T, Epstein RJ. The duration of phorbolinducible ErbB2 tyrosine dephosphorylation parallels that of receptor endocytosis rather than threonine-686 phosphorylation: implications for the physiological role of protein kinase C in growth factor receptor signalling. Carcinogenesis. 1998;19:2013–2019. doi: 10.1093/carcin/19.11.2013. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang X, Gulliford T, Zhang H, Huang GC, Epstein R. Human cancer cells exhibit protein kinase C-dependent c-erbB-2 trans-modulation that correlates with phosphatase sensitivity and kinase activity. J. Biol. Chem. 1996;271:21786–21792. doi: 10.1074/jbc.271.36.21786. [DOI] [PubMed] [Google Scholar]
- 65.Morrison P, Saltiel AR, Rosner MR. Role of mitogen-activated protein kinase kinase in regulation of the epidermal growth factor receptor by protein kinase C. J. Biol. Chem. 1996;271:12891–12896. doi: 10.1074/jbc.271.22.12891. [DOI] [PubMed] [Google Scholar]
- 66.Fitzer-Attas C, Eldar H, Eisenbach L, Livneh E. The expression of PDGF-alpha but not PDGF-beta receptors is suppressed in Swiss/3T3 fibroblasts over-expressing protein kinase C-alpha. FEBS Lett. 1994;342:165–170. doi: 10.1016/0014-5793(94)80493-1. [DOI] [PubMed] [Google Scholar]
- 67.Eldar H, Zisman Y, Ullrich A, Livneh E. Overexpression of protein kinase C alpha-subtype in Swiss/3T3 fibroblasts causes loss of both high and low affinity receptor numbers for epidermal growth factor. J. Biol. Chem. 1990;265:13290–13296. [PubMed] [Google Scholar]
- 68.Lee LS, Weinstein IB. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978;202:313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- 69.Davis RJ. Independent mechanisms account for the regulation by protein kinase C of the epidermal growth factor receptor affinity and tyrosine-protein kinase activity. J. Biol. Chem. 1988;263:9462–9469. [PubMed] [Google Scholar]
- 70.Chen P, Xie H, Wells A. Mitogenic signaling from the egf receptor is attenuated by a phospholipase C-gamma/protein kinase C feedback mechanism. Mol. Biol Cell. 1996;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ho JJ, Farrelly ER, Kim YS. Phorbol ester reduces phosphor-ylation of epidermal growth factor receptor in pancreatic cancer cells by activation of a tyrosine phosphatase. Biochem. Biophys. Res. Commun. 1999;265:728–733. doi: 10.1006/bbrc.1999.1656. [DOI] [PubMed] [Google Scholar]
- 72.Hunter T, Ling N, Cooper JA. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984;311:480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- 73.Davis RJ, Czech MP. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc. Natl. Acad. Sci. U. S. A. 1985;82:1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Downward J, Waterfield MD, Parker PJ. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J. Biol. Chem. 1985;260:14538–14546. [PubMed] [Google Scholar]
- 75.Livneh E, Dull TJ, Berent E, Prywes R, Ullrich A, Schlessinger J. Release of a phorbol ester-induced mitogenic block by mutation at Thr-654 of the epidermal growth factor receptor. Mol. Cell. Biol. 1988;8:2302–2308. doi: 10.1128/mcb.8.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lund KA, Lazar CS, Chen WS, Walsh BJ, Welsh JB, Herbst JJ, Walton GM, Rosenfeld MG, Gill GN, Wiley HS. Phosphorylation of the epidermal growth factor receptor at threonine 654 inhibits ligand-induced internalization and down-regulation. J. Biol. Chem. 1990;265:20517–20523. [PubMed] [Google Scholar]
- 77.Magun BE, Matrisian LM, Bowden GT. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J. Biol. Chem. 1980;255:6373–6381. [PubMed] [Google Scholar]
- 78.Errasfa M, Stern A. Inhibition of epidermal growth factor-dependent protein tyrosine phosphorylation by phorbol myristate acetate is mediated by protein tyrosine phosphatase activity. FEBS Lett. 1994;339:7–10. doi: 10.1016/0014-5793(94)80374-9. [DOI] [PubMed] [Google Scholar]
- 79.Langgut W, Ogilvie A. Silencing of the epidermal growth factor receptor in the absence of the ligand requires phospholipase C activity. FEBS Lett. 1995;372:173–176. doi: 10.1016/0014-5793(95)00971-b. [DOI] [PubMed] [Google Scholar]
- 80.Ostman A, Bohmer FD. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–266. doi: 10.1016/s0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- 81.Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, Bohmer FD. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J. Biol. Chem. 1998;273:24839–24846. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- 82.Tenev T, Keilhack H, Tomic S, Stoyanov B, Stein-Gerlach M, Lammers R, Krivtsov AV, Ullrich A, Bohmer FD. Both SH2 domains are involved in interaction of SHP-1 with the epidermal growth factor receptor but cannot confer receptor-directed activity to SHP-1/SHP-2 chimera. J. Biol. Chem. 1997;272:5966–5973. doi: 10.1074/jbc.272.9.5966. [DOI] [PubMed] [Google Scholar]
- 83.Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Bohmer FD. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C. J. Biol. Chem. 1995;270:21277–21284. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- 84.Kuo ML, Huang TS, Lin JK. Preferential requirement for protein tyrosine phosphatase activity in the 12-O-tetradecanoylphorbol-13-acetate-induced differentiation of human colon cancer cells. Biochem. Pharmacol. 1995;50:1217–1222. doi: 10.1016/0006-2952(95)00261-w. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida K, Kufe D. Negative regulation of the SHPTP1 protein tyrosine phosphatase by protein kinase C delta in response to DNA damage. Mol. Pharmacol. 2001;60:1431–1438. doi: 10.1124/mol.60.6.1431. [DOI] [PubMed] [Google Scholar]
- 86.Zhao Z, Shen SH, Fischer EH. Phorbol ester-induced expression, phosphorylation, and translocation of protein-tyrosine-phosphatase 1C in HL-60 cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5007–5011. doi: 10.1073/pnas.91.11.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kizaki M, Dawson MI, Heyman R, Elster E, Morosetti R, Pakkala S, Chen DL, Ueno H, Chao W, Morikawa M, Ikeda Y, Heber D, Pfahl M, Koeffler HP. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood. 1996;87:1977–1984. [PubMed] [Google Scholar]
- 88.Zhang Y, Huang Y, Rishi AK, Sheikh MS, Shroot B, Reichert U, Dawson M, Poirer G, Fontana JA. Activation of the p38 and JNK/ SAPK mitogen-activated protein kinase pathways during apoptosis is mediated by a novel retinoid. Exp. Cell Res. 1999;247:233–240. doi: 10.1006/excr.1998.4350. [DOI] [PubMed] [Google Scholar]