Abstract
Global descriptors of the cognitive phenotype of Turner syndrome are well established and are thus commonly referred to. For example, Turner syndrome is a proposed etiology of the nonverbal learning disability – because of reported relative strengths in verbal skills, and relatively weaker nonverbal skills – particularly in arithmetic, select visuospatial skills, and processing speed. This profile is observed throughout and beyond the school age years. Reliance on this gross level description of the cognitive profile (e.g., nonverbal learning disability) may be helpful as a starting point when determining whether an individual with Turner syndrome has educational needs, but it carries limited practical significance when determining the specific nature of these needs. The limitations stem from the fact that the severity of the cognitive profile is highly variable among individuals with Turner syndrome; that the “nonverbal” difficulties are specific rather than widespread; and that any individual with Turner syndrome may also manifest cognitive characteristics independent of Turner syndrome. In view of the increased risk for specific cognitive difficulties, a detailed assessment prior to the onset of formal schooling (or at the time of diagnosis, when diagnosis occurs after 5 years of age) can play an important role in determining school readiness and potential need for educational support among individual girls with Turner syndrome.
Keywords: Turner syndrome, Dyscalculia, Mathematics learning disability, Nonverbal learning disability
1. Introduction
That Turner syndrome is associated with a cognitive phenotype is not news. Shaffer's [1] initial report of the cognitive phenotype was followed by subsequent studies replicating and expanding upon his, and later Waber's [2], findings that Verbal IQ scores are significantly higher than Performance IQ scores among girls with Turner syndrome. This “V–P split” is now considered a hallmark of the Turner syndrome phenotype. Indeed, results from studies conducted over the last four decades have demonstrated remarkable consensus in this finding. Yet this consensus does not mean, as it may be misinterpreted to indicate, that all girls with Turner syndrome will show the phenotype, or that all girls with Turner syndrome who have this profile will have comparable degrees of relative verbal strengths and nonverbal weaknesses. Moreover, the V–P split does not provide sufficient specific information about the cognitive strengths and weaknesses, many of which researchers still do not fully understand.
Research goals over recent years have included efforts to fine-tune the description of the cognitive phenotype for Turner syndrome. Group data support the notion of relatively strong language skills, although this language strength is not global; there is evidence that oral fluency skills are impaired [2,3] despite average to above average performance on most other verbal tasks. Similarly, relatively weaker skills in mathematics, visuospatial skills, and executive functions are not global within each of those domains. As such, reliance on a broad general descriptor of this profile (such as nonverbal learning disability) diminishes the emphasis on more specific characteristics. As such, the use of an umbrella term can be of limited practical significance when dealing with an individual who has Turner syndrome. In fact, the severity of the cognitive profile can be highly variable among individuals with Turner syndrome; also, reference to “nonverbal” difficulties is global whereas, among girls with Turner syndrome, some nonverbal difficulties are specific rather than widespread.
The full story of the phenotype deserves a more detailed summary than that of the V–P split and nonverbal learning disability. In the review that follows, the story begins with an account of the phenotypic variability seen across individuals with Turner syndrome, even in the IQ score distribution and discrepancy. Following a review of IQ scores, I review results of current efforts to further specify the cognitive deficits in mathematics, visuospatial, and executive function skills, and then briefly discuss the implications of findings to date for individuals with Turner syndrome.
2. IQ scores: just the beginning of the story
Turner syndrome typically leads to a slight decrease in scores of overall intelligence, except in cases of mental retardation associated with a ring chromosome karyotype [14]. The ring karyotype is infrequent, so most girls with Turner syndrome have full scale IQ scores that are well within the average range, normally distributed, and differ only slightly from scores observed in the general population. The data summarized in Table 1 reflects the minimal degree to which full scale IQ scores are affected by Turner syndrome, as reported in a sample of studies that have been carried out within the last 40 years.
Table 1.
IQ scores from a sample of studies of Turner syndrome from 1962 to present
| Study | N | Age (years) | IQ range | IQ group [mean (S.D.)] | |
|---|---|---|---|---|---|
| Alexander et al. [4] | 18 | 10–24 | 74–130 | 101.0 | Not reported |
| Elliott et al. [5] | 6 | 11–15 | 70–126 | 99.0 | (14.7) |
| Inozemtseva et al. [6] | 15 | 8–19 | 71–120 | 98.3 | (15.2) |
| Keysor et al. [7] | 11 | 12–20 | 65–126 | 96.5 | (18.0) |
| Mazzocco [8] | 29 | 5–16 | 72–135 | 95.0 | (13.5) |
| Mazzocco et al. [9] | 25 | 7–11 | 64–136 | 102.6 | (14.5) |
| Pennington et al. [10] | 10 | Mean=23 | 81–106 | 96.7 | (7.4) |
| Rovet et al. [11] | 45 | 7–16 | Not reported | 94.5 | (16.8) |
| Russell et al. [12] | 50 | 7–16 | Not reported | 92.7 | (13.5) |
| Shaffer [1] | 20 | 5–30 | Not reported | 97.7 | (10.5) |
| Temple and Carney [13] | 15 | 8–12 | 74–130 | 95.0 | Not reported |
Additional data that demonstrate the effects of Turner syndrome on intelligence are drawn from our comparison of IQ scores of girls with Turner syndrome with the IQ scores obtained from the mother of each girl. The scores that appear in Fig. 1 are from girls who have participated in my research program, and who (as a group) are described in greater detail elsewhere [15,16]. Scores in Fig. 1 are limited to those of girls for whom Wechsler scores were obtained for both her and her biological mother. [Although paternal IQ was of equal interest to maternal IQ, IQ data were not available from a sufficient number of fathers to include in this comparison.] In view of the well established heritability of intelligence [17], we would expect these pairs of mother–daughter scores to be quite similar to one another. This is precisely the pattern observed in Fig. 1: the variations in full scale IQ scores among girls with Turner syndrome parallels the variation observed in their own mothers (who, of course, do not have Turner syndrome). Note that all of the IQ scores included in Fig. 1 fall within the average range of 85 to 115, that many are above average, and that the girls' scores – while lower than the mothers' scores on average – vary with regard to both the magnitude and direction of the mother–child discrepancy. It is clear from Fig. 1 that mother's scores partially predict daughter scores.
Fig. 1.
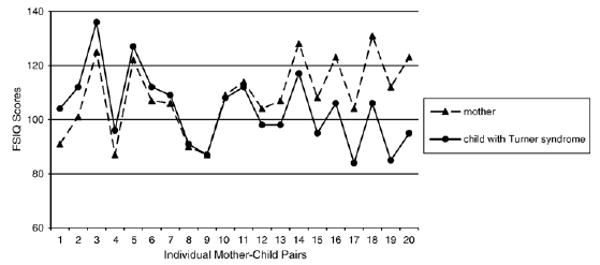
Full scale IQ scores of 20 individual girls with Turner syndrome and their own mothers. The paired set of data points appears in order of smallest to largest discrepancy between mothers (▲) and their own daughters (●). The girls ranged in age from 7 to 10 years at the time of their IQ assessment.
Full scale IQ scores reveal little about the Turner syndrome phenotype. The specificity reported over decades of research studies is in Verbal IQ (VIQ) vs. Performance IQ (PIQ) differences. When comparing VIQ and PIQ scores of girls with Turner syndrome to their own mothers, it is apparent where the specific cognitive phenotypic features emerge. As seen in Fig. 2, there is no consistent difference between mother and daughter VIQ scores, for either the direction or magnitude of the mother–daughter discrepancy. For PIQ scores, the direction is consistent: girls with Turner syndrome score at the same level or below that of their mothers, never higher than their mothers as does sometimes occurs on the VIQ scale (although not at a level that is statistically significant). The difference in mother–child discrepancies cannot be attributed to age differences, because both sets of scores are age-referenced against published normative data [18].
Fig. 2.
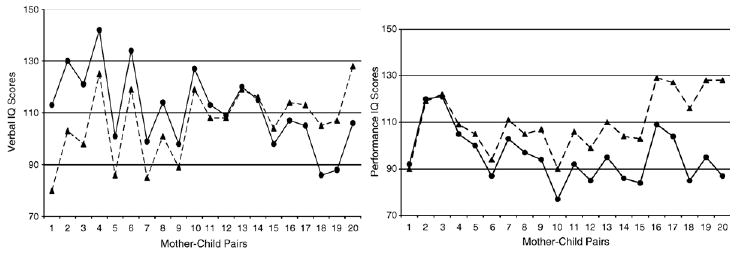
Verbal (VIQ) and Performance IQ (PIQ) scores of 20 individual girls with Turner syndrome and their own mothers. The paired set of data points appears in order of smallest to largest discrepancy between mothers (▲) and their own daughters (●).
Despite the consistency in direction of the discrepancy for mother vs. child PIQ score, there certainly is no consistency in the magnitude of the discrepancy. Although scientists and clinicians well understand the variability indicated by standard deviation values that typically (but do not always) accompany reported means, how often is this information conveyed when describing the cognitive phenotype associated with Turner (or the anticipated cognitive phenotype) to the parent of an individual with a diagnosis of Turner syndrome? Fig. 3 depicts the discrepancy between VIQ and PIQ scores among the girls whose data also appear in Figs. 1 and 2. It is apparent, from Fig. 3, that the extent to which girls with Turner syndrome have weaker nonverbal vs. verbal skills varies from basically no difference to a remarkable and substantial difference of 40 points. This is part of the missing piece of the story of the Turner syndrome phenotype. Thus, when conveying information about the Turner syndrome cognitive phenotype to individuals, their families, and their teachers, an accurate discussion of the “V–P split” would include reference to the consistency in direction of the VIQ–PIQ discrepancy, the inconsistency in magnitude of this discrepancy, and the full range of manifestations of the discrepancy across individuals. It is as important to highlight the possibility of a remarkable discrepancy (more than two standard deviations, or 40 points) as it is to indicate the possibility of no discrepancy. The anticipated discrepancy for an individual should be discussed only when it has been assessed using standardized testing.
Fig. 3.
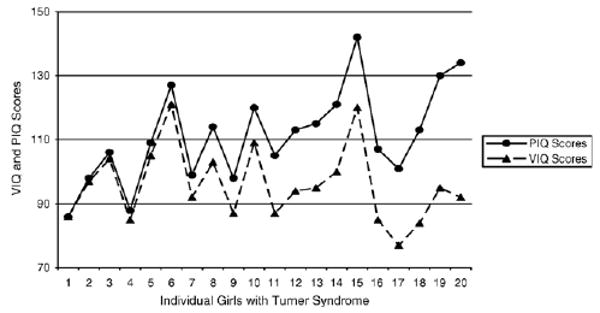
Verbal and Performance IQ score discrepancies vary across individual girls with Turner syndrome.
The V–P split is the finding that has led to the frequent reference to nonverbal learning disability in girls with Turner syndrome. Do all girls with Turner syndrome have a learning disability, and if so, in what areas?
3. Mathematics disabilities and difficulties
3.1. Mathematics achievement
A primary component of a nonverbal learning disability is difficulty with mathematics [19]. Difficulty with mathematics is also reported for school age girls with Turner syndrome [8,11,15,16,20]. In view of the lack of consensus regarding what cognitive features underlie mathematics difficulties or disabilities [21], it is not surprising that the global description of poor mathematics performance is an insufficient explanation for why girls with Turner syndrome have difficulty with mathematics, or what aspects of mathematics are most challenging for girls with Turner syndrome. That the difficulties are present is undeniable, based on group data. For example, in an ongoing longitudinal study of the Turner syndrome phenotype, we found that scores on measures of Verbal reasoning (analogous to a Verbal IQ score), obtained from the Stanford Binet Fourth Edition (SB-IV) [22] are significantly higher than scores reported for the Test of Early Mathematics Ability Second Edition (TEMA-2; [23]), even at kindergarten or first grade [15]. Among these young primary school age students, the discrepancy between verbal reasoning and TEMA-2 scores was significant for the group of girls with Turner syndrome (n = 20), Wilcoxon tied Z value = −3.24, p=.001, but not for girls in an age- and grade-matched comparison group, p =.73. Elsewhere we have noted the consistency with which the direction of this difference is observed, and the relative consistency in the overall magnitude of the discrepancy between these two scores [8,16].
3.2. Mathematics disability
The verbal vs. mathematics performance difference implicates lower math vs. verbal skills, but it does not necessarily implicate poor math performance because perhaps girls with Turner syndrome – as a group – have above average verbal reasoning skills relative to age appropriate mathematics ability. This explanation fails to surface from published studies. Instead, the prevalence of mathematics learning disability (MLD) among girls with Turner syndrome exceeds the estimated prevalence in the general population (∼6% to 10%). Rovet [20] found that 55% of the 7- to 16-year-old girls with Turner syndrome who participated in her study met criteria for MLD, vs. 7% of her comparison group. Similar rates have been reported for girls in primary school, for which 43% of girls with Turner syndrome and 10% of girls without Turner syndrome meet criteria for MLD [15].
Meeting criteria for MLD at one point in time does not definitively mean that the child will continue to meet these criteria. Persistence over time is an important criterion for establishing MLD. Based on recent findings, when girls with Turner syndrome meet criteria for MLD in their early school years, evidence of MLD is more likely to persist than to dissipate by the end of third grade [16]. The recurrence rate between kindergarten and grade 3 is as high for girls with Turner syndrome as for children from the general population [16]. Although the persistent rates do not differ significantly between these two participant groups, it is worth noting that the reported persistence rate for Turner syndrome (84%) emerges over two visits during 4 years of primary school (grades kindergarten to 3), whereas the rate for the general population (70%) is based on four visits over 4 years. That is, children with Turner syndrome had fewer opportunities to demonstrate persistently meeting criteria for MLD than did children from the comparison group, and yet the frequency of persistence was as high (if not higher) than that for children without Turner syndrome [16].
It is thus well established that individuals with Turner syndrome are at higher risk for MLD, and that, as a group, these individuals have difficulty with mathematics. What is the source of these difficulties, and what are the sources that have been ruled out?
First, the observed math deficits reported for girls with Turner syndrome appear to be unrelated to difficulty in number sense or overall calculation accuracy in females with Turner syndrome [16,24–26]. Indeed, poor math achievement occurs despite relative strengths in simple arithmetic, number comprehension and production, number comparison and estimation accuracy [24,26].
Second, visuospatial skills also appear to be unrelated to mathematics difficulties, despite reported deficits in visuospatial skills in girls with Turner syndrome (described below). Item analyses and correlational studies have been carried out to evaluate the potential link between math and spatial skills in school age girls with Turner syndrome. The item analyses have failed to reveal difficulty on specific, spatially oriented problems (e.g., geometry, shape matching) among girls with Turner syndrome [11,15,16], although item analyses have differentiated other populations from their peers (such as girls with fragile X syndrome [15,16]. An error analysis study also failed to identify more spatially relevant math calculation errors in girls with Turner syndrome (such as more alignment errors), relative to their peers, except when the peer group was girls with fragile X [8]. Correlational studies, which have demonstrated a positive association between math performance and several visuospatial skills among girls with fragile X syndrome have not revealed consistent support for such associations among girls with Turner syndrome [8,9]. Together, these findings support Rovet's [11] conclusion that arithmetic deficits in girls with Turner syndrome are independent of spatial skills performance levels.
In the absence of poor number sense or underlying spatial deficits, what does characterize math performance in girls with Turner syndrome? Processing speed appears to play a significant role, as girls with Turner syndrome are slower on arithmetic fact retrieval, and on response times during calculations [24,25,27] especially for larger addends [26,27]. Response times are slower on estimation problems [24,28], even for very small quantities that typically developing children tend to process instantly, or subitize, rather than count [24]. More research is needed to determine the nature of these processing deficits, and whether or how specific aspects of mathematics are differentially challenging for girls with Turner syndrome as mathematics curriculum demands increase during the school age years.
4. Spatial skills, executive functions, and attention
4.1. Visuospatial deficits
Despite reports of visuospatial deficits in Turner syndrome, several attempts to specify deficits in only a subset of visuospatial skills have failed [2,29]. Relative to their peers, girls with Turner syndrome have difficulty with both visuoperception and visuo-constructional tasks [13]. Visuoperception deficits are apparent on object identification and location identification tasks, and yet appear associated with poor visual working memory [29]. The global deficits should not be taken to mean that all visuospatial skills are impaired; in fact, in our work we have not found differences in accuracy on measures of shape matching, or in capturing the gestalt of visual arrays on tests of visual short term memory [9]. However, like others, we have found less accurate performance on tests of object identification, object location, and visual memory for objects; and slower response times on select visuospatial tasks [9]. Others have also reported slower response times on visual spatial tasks by girls with Turner syndrome [29].
4.2. Working memory and executive function
It is unclear whether visual working memory difficulties and slowed response times are further reflection of the processing speed deficits reported in both early and recent studies [2,3]. In one recent study of working memory and executive function, we administered a timed task with varying degrees of working memory loads. We found that although third graders with Turner syndrome took significantly longer than their peers to complete a basic naming task, response times did not differ significantly when working memory demands were increased: response times increased with working memory demands, but for both participant groups. However, the girls with Turner syndrome made more than twice as many errors as their peers on the naming tasks, when working memory demands increased [30]; this means that for them, the increase in response time across tasks did not lead to maintaining a relatively high degree of accuracy, as it did for the peer comparison group. In other words, girls with Turner syndrome were less accurate despite taking as much time to complete the timed task as did their peers.
Temple and colleagues [31] also reported working memory deficits in 8 to 12 year olds with Turner syndrome. They found that girls with Turner syndrome were impaired on executive function tasks that, like the task reported on above, involved speeded responses. However, they found no deficits on planning or set maintenance tasks.
4.3. Attention deficits in Turner syndrome
Early studies of attention skills in girls with Turner syndrome failed to reveal either widespread attention difficulties or specific deficits in sustained attention or impulsivity [32]. Yet the variability in attention and impulsivity observed in girls with Turner syndrome was interpreted as an indicator of atypical attention deficit disorder [33]. This notion has received additional support from a recent report that showed both an increased incidence of attention-deficit hyperactivity disorder (ADHD; 24%), and a higher frequency of the hyperactive/impulsive subtype of ADHD, in girls with Turner syndrome vs. children in the general population [12]. It remains to be seen whether ADHD in girls with Turner syndrome has similar neuropsychological profiles and treatment response patterns to those reported for children with typical ADHD. Treatment implications are complicated by the growth retardation that occurs with Turner syndrome.
5. Conclusions: the story in its proper context
The Turner syndrome cognitive phenotype is well described at a global level. The cognitive phenotype is characterized by specific deficits in visuospatial and executive skills, visual working memory, and mathematics. Response fluency (long response times) is a remarkably persistent finding across tasks. We can improve upon this phenotype description by specifying the evidence to date: Evidence for highly variable levels of phenotype severity, and for specific difficulties within each of several cognitive domains; findings that phenotypic features emerge by kindergarten if not sooner, and that the deficits that do emerge persist over time [16].
Several relevant bodies of research were not included in this brief review. For example, the cognitive features reviewed herein are aligned with results of neuroimaging studies, such as findings of parieto-occipital and basal ganglia abnormalities [34–36] including parietal and occipital–parietal hypometabolism that is observed among girls with Turner syndrome who also have learning difficulties [5]. Moreover, there is evidence that heterogeneity in the cognitive deficits observed in Turner syndrome are related to gene mapping [37] or specific karyotypes [31], and that select deficits show improvement with estrogen therapy [38]. There is an ongoing controversy regarding potential imprinting (parent of origin effects) on the Turner syndrome cognitive phenotype. For instance, whereas Skuse reports imprinting effects on social cognition [39], Russell and colleagues found no effects of imprinting on ADHD behaviours [12]. Future studies will serve to further address each of these factors and to contribute to efforts to delineate the processes that underlie the cognitive phenotype. Also, as most studies of the cognitive phenotype are based on cross sectional studies, questions remain concerning the developmental trajectory of the cognitive phenotype associated with Turner syndrome. We have begun to report on longitudinal studies [16] and will continue this line of research.
There are important implications to be drawn from the existing data. There is an increased risk for specific cognitive difficulties, including mathematics learning disability and ADHD, but not in all individuals with Turner syndrome. Math difficulties do appear among most girls with Turner syndrome, and manifestation of these and other cognitive difficulties occur as early as kindergarten (if not earlier). In order to promote appropriate early intervention, screening is advisable for all girls with Turner syndrome, particularly prior to formal schooling as a means to determine school readiness and potential needs for educational support. Both longitudinal and cross sectional studies suggest that the cognitive difficulties associated with Turner syndrome persist throughout development, so educational support needs may continue well beyond the elementary school years. Among the most frequent findings across studies is slowed response times, as seen on tests of oral fluency, arithmetic, visuospatial processing, and working memory. Thus, for many girls with Turner syndrome, adjusting time demands on school tasks may be a necessary means by which to assess level of conceptual mastery in a given subject area.
In view of the variability observed in the Turner syndrome cognitive phenotype, care should be taken to individualize profile descriptions and recommendations for intervention whenever applying research findings to an individual. Such efforts will also aid in the identification of learning difficulties not associated with Turner syndrome, such as dyslexia, that may be overlooked simply because they fail to be included in the global descriptions that are subject to misinterpretation when applied to individuals.
Acknowledgments
I would like to acknowledge the support of the participants and their families, research coordinators Gwen F. Myers and Kathleen Devlin, and research assistant Elizabeth Romanow. This work was supported by NIH grant HDR0103461-01-09 awarded to Dr. Mazzocco.
References
- 1.Shaffer JW. A specific cognitive deficit observed in gonadal aplasia (Turner's syndrome) J Clin Psychol. 1962;18:403–406. doi: 10.1002/1097-4679(196210)18:4<403::aid-jclp2270180404>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Waber DP. Neuropsychological aspects of Turner's syndrome. Dev Med Child Neurol. 1979;21(1):58–70. doi: 10.1111/j.1469-8749.1979.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 3.Temple CM. Oral fluency and narrative production in children with Turner's syndrome. Neuropsychologia. 2002;40(8):1419–1427. doi: 10.1016/s0028-3932(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 4.Alexander D, Ehrhardt AA, Money J. Defective figure drawing, geometric and human, in Turner's syndrome. J Nerv Ment Dis. 1966;142(2):161–167. doi: 10.1097/00005053-196602000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Elliott TK, et al. Positron emission tomography and neuropsychological correlations in children with Turner's syndrome. Dev Neuropsychol. 1996;12(3):365–386. [Google Scholar]
- 6.Inozemtseva O, et al. Syntactic processing in Turner's syndrome. J Child Neurol. 2002;17:668–672. doi: 10.1177/088307380201700903. [DOI] [PubMed] [Google Scholar]
- 7.Keysor CS, et al. Physiological arousal in females with fragile X or Turner syndrome. Dev Psychobiol. 2002;41(2):133–146. doi: 10.1002/dev.10060. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocco MMM. A process approach to describing mathematics difficulties in girls with Turner syndrome. Pediatrics. 1998;102(2 Pt 3):492–496. [PubMed] [Google Scholar]
- 9.Mazzocco MMM, Bhatia NS, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychol. 2006;12(2):87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- 10.Pennington BF, et al. The neuropsychological phenotype in Turner syndrome. Cortex. 1985;21(3):391–404. doi: 10.1016/s0010-9452(85)80004-6. [DOI] [PubMed] [Google Scholar]
- 11.Rovet J, Szekely C, Hockenberry MN. Specific arithmetic calculation deficits in children with Turner syndrome. J Clin Exp Neuropsychol. 1994;16(6):820–839. doi: 10.1080/01688639408402696. [DOI] [PubMed] [Google Scholar]
- 12.Russell HF, et al. Increased Prevalence of ADHD in Turner Syndrome with no evidence of imprinting effects. J Pediatr Psychol. doi: 10.1093/jpepsy/jsj106. in press. [DOI] [PubMed] [Google Scholar]
- 13.Temple CM, Carney RA. Patterns of spatial functioning in Turner's syndrome. Cortex. 1995;31(1):109–118. doi: 10.1016/s0010-9452(13)80109-8. [DOI] [PubMed] [Google Scholar]
- 14.Van Dyke DL, et al. Ullrich–Turner syndrome with a small ring X chromosome and presence of mental retardation. Am J Med Genet. 1992;43:996–1005. doi: 10.1002/ajmg.1320430617. [DOI] [PubMed] [Google Scholar]
- 15.Mazzocco MMM. Math learning disability and math LD subtypes: evidence from studies of Turner syndrome, fragile X syndrome, and neurofibromatosis type 1. J Learn Disabil. 2001;34(6):520–533. doi: 10.1177/002221940103400605. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MM, et al. Mathematics learning disability in girls with Turner syndrome or fragile X syndrome. Brain Cogn. 2006;61(2):195–210. doi: 10.1016/j.bandc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Plomin R, et al. DNA markers associated with high versus low IQ: the IQ Quantitative Trait Loci (QTL) Project. Behav Genet. 1994;24(2):107–118. doi: 10.1007/BF01067815. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence: WASI. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 19.Rourke BP. Introduction: the NLD syndrome and the white matter model. In: Rourke BP, editor. Syndrome of Nonverbal Learning Disabilities. The Guilford Press; New York: 1995. pp. 1–25. [Google Scholar]
- 20.Rovet JF. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil. 1993;26(5):333–341. doi: 10.1177/002221949302600506. [DOI] [PubMed] [Google Scholar]
- 21.Mazzocco MMM. Challenges in identifying target skills for math disability screening and intervention. J Learn Disabil. 2005;38(4):318–323. doi: 10.1177/00222194050380040701. [DOI] [PubMed] [Google Scholar]
- 22.Thorndike RL, Hagen EP, Sattler JM. Stanford–Binet Intelligence Scale: Guide for Administering and Scoring. Fourth. The Riverside Publishing Company; Chicago, IL: 1986. [Google Scholar]
- 23.Ginsburg HP, Baroody AJ. Test of Early Mathematics Ability. Second. PRO-ED; Austin, TX: 1990. [Google Scholar]
- 24.Bruandet M, Molko N, Cohen L, Dehaene S. A cognitive characterization of dyscalculia in Turner syndrome. Neuropsychologia. 2004;42:288–298. doi: 10.1016/j.neuropsychologia.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Rovet J, Ireland L. Behavioral phenotype in children with Turner syndrome. J Pediatr Psychol. 1994;19(6):779–790. doi: 10.1093/jpepsy/19.6.779. [DOI] [PubMed] [Google Scholar]
- 26.Temple CM, Marriott AJ. Arithmetical ability and disability in Turner's syndrome: a cognitive neuropsychological analysis. Dev Neuropsychol. 1998;14(1):47–67. [Google Scholar]
- 27.Molko N, et al. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40(4):847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- 28.Butterworth B, et al. Language and the origins of number skills: karyotypic differences in Turner's syndrome. Brain Lang. 1999;69:486–488. [Google Scholar]
- 29.Buchanan L, Pavlovic J, Rovet J. A reexamination of the visuospatial deficit in Turner syndrome: contributions of working memory. Dev Neuropsychol. 1998;14(23):341–367. [Google Scholar]
- 30.Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT) Dev Neuropsychol. 2005;28(3):755–777. doi: 10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- 31.Temple CM, Carney RA, Mullarkey S. Frontal lobe function and executive skills in children with Turner's syndrome. Dev Neuropsychol. 1996;12(3):343–363. [Google Scholar]
- 32.Williams J, Richman L, Yarbrough D. A comparison of memory and attention in Turner syndrome and learning disability. J Pediatr Psychol. 1991;16(5):585–593. doi: 10.1093/jpepsy/16.5.585. [DOI] [PubMed] [Google Scholar]
- 33.Williams JK, Richman LC, Yarbrough DB. Comparison of visual–spatial performance strategy training in children with Turner syndrome and learning disabilities. J Learn Disabil. 1992;25(10):658–664. doi: 10.1177/002221949202501005. [DOI] [PubMed] [Google Scholar]
- 34.Cutter WJ, et al. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol Psychiatry. 2006;59(3):273–283. doi: 10.1016/j.biopsych.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Reiss AL, et al. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol. 1995;38(5):731–738. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DG, et al. X-chromosome effects on female brain: a magnetic resonance imaging study of Turner's syndrome. Lancet. 1993;342(8881):1197–1200. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- 37.Ross JL, et al. The Turner syndrome-associated neurocognitive phenotype maps to distal Xp. Am J Hum Genet. 2000;67(3):672–681. doi: 10.1086/303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross JL, et al. Use of estrogen in young girls with Turner syndrome: effects on memory. Neurology. 2000;54(1):164–170. doi: 10.1212/wnl.54.1.164. [DOI] [PubMed] [Google Scholar]
- 39.Skuse DH, et al. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387(6634):705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]


