Abstract
Transcription factor activating enhancer-binding protein 4 (AP-4) is a basic helix-loop-helix protein that binds to E-box elements. AP-4 has received increasing attention for its regulatory role in cell growth and development, including transcriptional repression of the human homolog of murine double minute 2 (HDM2), an important oncoprotein controlling cell growth and survival, by an unknown mechanism. Here we demonstrate that AP-4 binds to an E-box located in the HDM2-P2 promoter and represses HDM2 transcription in a p53-independent manner. Incremental truncations of AP-4 revealed that the C-terminal Gln/Pro-rich domain was essential for transcriptional repression of HDM2. To further delineate the molecular mechanism(s) of AP-4 transcriptional control and its potential implications, we used DNA-affinity purification followed by complementary quantitative proteomics, cICAT and iTRAQ labeling methods, to identify a previously unknown E-box-bound AP-4 protein complex containing 75 putative components. The two labeling methods complementarily quantified differentially AP-4-enriched proteins, including the most significant recruitment of DNA damage response proteins, followed by transcription factors, transcriptional repressors/corepressors, and histone-modifying proteins. Specific interaction of AP-4 with CCCTC binding factor, stimulatory protein 1, and histone deacetylase 1 (an AP-4 corepressor) was validated using AP-4 truncation mutants. Importantly, inclusion of trichostatin A did not alleviate AP-4-mediated repression of HDM2 transcription, suggesting a previously unidentified histone deacetylase-independent repression mechanism. In contrast, the complementary quantitative proteomics study suggested that transcription repression occurs via coordination of AP-4 with other transcription factors, histone methyltransferases, and/or a nucleosome remodeling SWI·SNF complex. In addition to previously known functions of AP-4, our data suggest that AP-4 participates in a transcriptional-regulating complex at the HDM2-P2 promoter in response to DNA damage.
Transcription factor activating enhancer binding-protein 4 (AP-4)1 is a ubiquitously expressed transcription factor belonging to the basic helix-loop-helix (bHLH) superfamily and binds to the consensus E-box sequence 5′-CAGCTG-3′ (1, 2). It was first identified as a cellular factor that binds to the simian virus 40 enhancer sequence and interacts synergistically with AP-1 to increase viral late gene transcription in vitro (2). Since its discovery, AP-4 has become recognized for its important role in modulation of cellular functions via regulation of genes involved in viral production (2–6), cell growth and survival (7–12), immune response (13–15), and angiogenesis (16). Sequence analysis has revealed that, unlike other bHLH proteins, AP-4 contains several protein-protein-interacting domains, including a bHLH domain and two distinct leucine repeat (LR) domains (1). The activity of many proteins is controlled by their cooperation and interplay within protein complexes. Likewise, the multiple protein-protein interaction domains within AP-4 suggest that it may achieve gene-specific transcriptional regulation by dynamic interaction with a wide variety of transcription factors or cofactors (1, 17). For example, AP-4 represses neuron-specific PAHX-AP1 transcription by forming a protein complex with the transcription corepressor geminin (18), and it activates the transcriptional activity of dopamine β-hydroxylase by interacting with GATA-3 and SP1 (19).
The oncoprotein, human homolog of murine double minute 2 (HDM2), has been recognized as an important molecule in regulating cell proliferation and DNA damage response (20). It is well established that HDM2 and p53 form a negative autoregulatory feedback loop, in which p53 activates HDM2 transcription, and HDM2 acts as a negative regulator of p53 (21–23). However, there is considerable evidence suggesting that HDM2 has tumorigenic properties independent of p53, implicating HDM2 as a potential target for cancer therapy (20). Inhibition of HDM2 has been reported to result in tumorigenic inhibition and chemotherapeutic sensitization in various human cancers (24). A recent study has shown that HDM2 activity was down-regulated upon AP-4 overexpression, possibly via transcriptional repression (25). However, how AP-4 regulates HDM2 transcription remains elusive because AP-4 response element has not been identified in the HDM2 promoter (25). On the other hand, AP-4 has been shown to repress gene transcription by forming protein complexes with transcription repressors (18); therefore implying the possibility that it may rely on a similar mechanism to repress HDM2 transcription. To uncover the repressive mechanism at the molecular level, identification of protein complexes associated with AP-4 at the HDM2-P2 promoter is necessary. Elucidation of the mechanism of HDM2 transcriptional repression by AP-4 may also lead to a better understanding of the regulatory network between AP-4 and HDM2 as well as the potential role of AP-4 as a target for cancer therapy.
Despite the technological advances of mass spectrometry for protein characterization, identification of specific DNA-bound protein complexes has proven to be a challenge using the classical single-step DNA-affinity isolation (26). Transcription factors that bind to specific promoters only account for <0.01% of the total cellular protein (27). Thus, the low abundance of transcription factors necessitates purification from nuclear extracts prepared from a large number of cultured cells to achieve the 10,000- to 100,000-fold enrichment, which is required to obtain sufficient amounts of protein for further chemical and functional analyses (28). However, nonspecific protein-DNA interactions inevitably arise from the binding of positively charged proteins to the DNA sequence of interest and mask identification of the sequence-specific components of DNA-binding protein complexes. Quantitative proteomics using stable isotope labeling methods, such as ICAT (29) or iTRAQ (30), in combination with single-step DNA-affinity purification can circumvent these problems via quantitative comparison of the extent of enrichment between wild-type (WT) and mutant (MU) DNA sequence-bound proteins. The comparison of isotopically labeled protein abundance enriched during DNA pull-down experiments allows discrimination of specific DNA-protein complex components from contaminating proteins originating from the purification background (26). These isotopic labeling strategies have been successfully used to study the dynamics of transcriptional complex formation during erythroid differentiation (31), to identify the dynamic components of the large RNA polymerase II pre-initiation complex (32, 33) as well as the TATA-binding protein (TBP) transcription complex (34), to identify the transcription factor Six4 bound to the muscle creatine kinase enhancer (35), and to identify transcription factor MAZ as a regulator of muscle-specific genes (36). Therefore, we utilized quantitative proteomics to facilitate identification of potential AP-4 complexes at the HDM2 promoter.
To explore the potential role of AP-4 in regulating HDM2 transcription, we investigated AP-4-mediated repression of HDM2 transcription by examining binding of AP-4 and associated proteins to the HDM2 promoter. First, we showed that AP-4 bound to a previously uncharacterized E-box in HDM2-P2 promoter and demonstrated that AP-4 repressed HDM2 transcription in a dose-dependent and p53-independent manner. Next, we characterized AP-4 complex bound to the HDM2-P2 promoter using single-step DNA-affinity purification in combination with complementary quantitative proteomics using cICAT and iTRAQ labeling. The two complementary methods allowed quantitative identification of many transcriptional repressors/corepressors, histone-modifying enzymes, and, in particular, several DNA damage-response proteins in the AP-4 complex; proteins in the latter category suggest a potential function of AP-4 in the DNA damage response. Furthermore, Western blotting confirmed that cICAT- and iTRAQ-labeling methods identified distinct DNA-enriched AP-4-associated proteins, highlighting the importance of complementary quantitative proteomics for identifying protein complex components. Together with DNA pull-downs and luciferase assays using incremental truncations of AP-4, the quantitative proteomics approach allowed us to conclude that AP-4 represses HDM2 transcription via an HDAC- and p53-independent mechanism and to identify many of the accessory proteins involved in AP-4-mediated repression.
EXPERIMENTAL PROCEDURES
Chemicals
Monomeric acrylamide/bisacrylamide solution (40%, 29:1) was purchased from Bio-Rad. Trypsin (modified, sequencing grade) was obtained from Promega (Madison, WI). The BCA and Bradford protein assay reagent kits were obtained from Pierce. SDS, Tris, urea, ammonium persulfate, iodoacetamide, and N,N,N′,N′-tetramethylenediamine (TEMED) were purchased from Amersham Biosciences. EDTA and methanol were purchased from Merck (Darmstadt, Germany). Bromphenol blue, β-mercaptoethanol, Tris (2-carboxyethyl)-phosphine hydrochloride (TCEP-HCl), triethylammonium bicarbonate (TEABC), ammonium bicarbonate (ABC), phosphate-buffered saline, potassium hydroxide (KOH), potassium chloride (KCl), poly(deoxyinosinic-deoxycytidylic) acid, glycerol, magnesium chloride hexahydrate, dithiothreitol, HEPES, imidazole, methyl methanethiosulfonate, Triton X-100, trifluoroacetic acid, trichostatin A (TSA), and high pressure liquid chromatography (HPLC)-grade ACN were purchased from Sigma-Aldrich. Formic acid (FA) and potassium dihydrogen phosphate (KH2PO4) were purchased from Riedel de Haen (Seelze, Germany). Water was purified using a Milli-Q® Ultrapure Water Purification System from Millipore (Billerica, MA). Nonidet P-40 was obtained from Calbiochem. Tris borate-EDTA (TBE) buffer was obtained from Amresco (Solon, OH).
Cell Lines
HCT116 p53+/+ and HCT116 p53−/− cells lines, which were generous gifts from Professor Bert Vogelstein (Johns Hopkins University), were maintained at 37 °C in Dulbecco's modified Eagle's medium (HyClone, Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and antibiotic-antimycotic (Invitrogen), which contained a final concentration of 100 units/ml of penicillin (base), 100 μg/ml of streptomycin (base), and 0.25 μg/ml of amphotericin B.
Plasmids
To construct the AP-4 bacterial expression plasmid pTriEx-4-AP-4 encoding a His6 tag at the N terminus, full-length AP-4 cDNA was amplified by reverse transcription (RT)-PCR from HeLa mRNA and cloned into the pTriExTM-4-neo plasmid (Novagen, Madison, WI).
To generate various plasmids expressing incremental truncations of AP-4 without any tag, including pcDNA-AP-4 (full-length), pcDNA-ΔN99 (residues 100–339), pcDNA-ΔN142 (residues 143–339), pcDNA-ΔN179 (residues 180–339), pcDNA-ΔC51 (residues 1–289), pcDNA-ΔC89 (residues 1–244), pcDNA-ΔC159 (residues 1–179), pcDNA-ΔC197 (residues 1–142), and pcDNA-ΔC239 (residues 1–99), various AP-4 cDNA fragments were amplified by PCR with appropriate oligonucleotide primers using pTriEx-4-AP-4 as the template. PCR products were subcloned into the pcDNATM 3.1/myc-His (−) A vector (Invitrogen). A stop codon (TGA) was included in all reverse PCR primers to exclude the vector-born C-terminal myc and His tags.
For the construction of various plasmids expressing incremental truncations of AP-4 encoding an N-terminal FLAG tag, pcDNA-AP-4 (full-length) and the truncation mutants described above were individually digested with EcoRI and HindIII restriction endonucleases (New England Biolabs, Beverly, MA). The restriction fragments were subcloned into the pCMV-Tag 2B expression plasmid (Stratagene, La Jolla, CA).
The luciferase reporter plasmid containing the HDM2-P2 promoter in pGL3-Basic (Promega), designated as hdm2luc01-WT, was constructed as described (37). To generate the reporter plasmid containing the mutant E-box located at −29 to −34 bp from the transcription start site (hdm2luc01-MU), hdm2luc01-WT plasmid was subjected to overlap extension PCR (38) using the mutagenic primers 5′-TCTCGAATTCGGGCTATTTAAACCATGC-3′ (forward) and 5′-GCCCGAATTCGAGACAAGTCAGGACTTA-3′ (reverse) (sequence mutations are underlined). All plasmid constructs described above were verified by DNA sequencing.
Antibodies
To generate in-house rabbit anti-AP-4, His-tagged AP-4 (His-AP-4) was first purified from inclusion bodies treated with 6 m urea following isopropyl 1-thio-β-d-galactopyranoside (IPTG) induction of Escherichia coli Tuner(DE3)pLacI (Novagen) cells transformed with pTriEx-4-AP-4 or by His-affinity chromatography using TalonTM superflow resin (Clontech, Palo Alto, CA). His-AP-4 was refolded on-column, eluted with 100 mm imidazole, and further purified using a HiTrapTM Heparin HP column (Amersham Biosciences). Rabbit polyclonal anti-AP-4 antibody was generated by IgMedica Biotech (Taipei, Taiwan) using 1 mg of purified His-AP-4 as antigen. The antibody was further purified using Protein G chromatography and dialyzed against phosphate-buffered saline containing 0.1% (v/v) Triton X-100.
Other antibodies including goat anti-AP-4 (SC-18593X), goat anti-CTBP1 (SC-5961), goat anti-actin (SC-1615), mouse anti-NFATc2 (SC-7296), mouse anti-APEX1 (SC-17774), mouse anti-USF2 (SC-81421), rabbit anti-PCNA (SC-7907), and rabbit anti-HMGB1 (SC-33199) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-CTCF (07–729), rabbit anti-SP1 (17–601), rabbit anti-histone H3 (06–755), rabbit anti-acetyl-histone H3 (09–599), mouse anti-HDAC1 (05–614), and mouse anti-HDAC2 (05–814) antibodies were purchased from Millipore. Mouse anti-p53 (OP43T) antibody was purchased from Calbiochem. Mouse anti-FLAG M2 antibody was purchased from Stratagene.
Electrophoretic Mobility Shift Assays (EMSA)
EMSA were performed using the LightShift Chemiluminescent EMSA kit (Pierce) following the manufacturer's procedure. Complementary double-stranded oligonucleotides corresponding to the −43 to −20 region of the HDM2-P2 promoter containing WT (sense strand: 5′-biotin-ACTTGTCTCCAGCTGGGGCTATTT-3′) or MU (sense strand: 5′-biotin-ACTTGTCTCGAATTCGGGCTATTT-3′) E-box sequences were prepared by Bio Basic Inc. (Ontario, Canada). For EMSA reactions, 10 μg of nuclear extract was incubated with 20 fmol of double-stranded biotin-labeled WT oligonucleotide in a 20-μl reaction containing 10 mM Tris (pH 7.5), 50 mM KCl, 1 mM dithiothreitol, 50 ng/μl poly(deoxyinosinic-deoxycytidylic) acid, 2.5% (v/v) glycerol, 0.05% (v/v) Nonidet P-40, and 1 mM MgCl2 at room temperature for 20 min. For antibody super-shift experiments, 1 μg of normal goat serum or goat anti-AP-4 was added to the reactions and further incubated on ice for 30 min. Reactions were subjected to 4% non-denaturing PAGE analysis in TBE (45 mM Tris base, 45 mM boric acid, and 1 mM EDTA, pH 8.0), transferred to nylon membranes (Pierce), and DNA-bound proteins were visualized using the enhanced chemiluminescence (ECL) detection system (Millipore).
Chromatin Immunoprecipitation (ChIP)
ChIP analysis was performed using the EZ-ChIPTM kit (Millipore). Briefly, HCT116 cells were fixed in 1% (v/v) formaldehyde for 10 min at room temperature. Cross-linked DNA was extracted and sheared by sonication using a Bioruptor (Cosmo Bio, Tokyo, Japan). Sheared DNA (amounts equivalent to that from 2 × 106 cells) was subjected to immunoprecipitation using 5 μg of indicated antibodies. After reversal from cross-linking and purification, fragmented DNA (2-μl aliquots) was subjected to 32 cycles of PCR amplification using the following primer pairs designed to amplify a region corresponding to −116 to +10 bp of the HDM2-P2 promoter: 5′-GACTCAGCTTTTCCTCTTGAGC-3′ (forward) and 5′-CTGAACACAGCTGGGAAAATG-3′ (reverse). The PCR reaction products were resolved via 2% agarose gel electrophoresis.
Luciferase Assays
HCT116 p53+/+ and HCT116 p53−/− cells were cultured in 12-well culture plates and transfected using GeneJuice Transfection Reagent (Novagen) following the manufacturer's instruction. In brief, 8 × 104 cells/well were cotransfected with 1 μg of hdm2p2luc01-WT or hdm2p2luc01-MU and indicated amounts of pcDNA-AP-4 plasmid. Empty vector pcDNATM 3.1/myc-His (−) A was added to each reaction such that a constant amount of DNA was transfected. As an internal control, 50 ng of pRL-TK vector (Promega) expressing Renilla Luciferase under control of thymidine kinase promoter was included in each reaction. After 48 h of transfection, the cells were harvested, and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to that from Renilla luciferase, and the data represent mean-fold activity (±S. D.) relative to control transfections. All experiments were performed in triplicate.
DNA Pull-down Assay
Complementary double-stranded oligonucleotides corresponding to the −43 to −20 region of the HDM2-P2 promoter containing a WT (sense strand: 5′-biotin-GATCACTTGTCTCCAGCTGGGGCTATTT-3′) or MU (sense strand: 5′-biotin-GATCACTTGTCTCGAATTCGGGCTATTT-3′) E-box sequence was prepared by Bio Basic Inc. MatInspector (39) was used to verify that disruption of the E-box in the MU DNA did not introduce new transcription factor binding sites. Double-stranded oligonucleotides were conjugated with MagnaBindTM streptavidin beads (Pierce). The concentration of conjugated DNA was 6 fmol/μg of MagnaBind streptavidin beads. Nuclear extracts from 1 × 107 HCT116 p53+/+ cells (∼200 μg) were incubated at a 250:1 (w/w) ratio with poly(deoxyinosinic-deoxycytidylic) acid in binding buffer containing 20 mM HEPES-KOH (pH 7.9), 1.5 mM MgCl2, 100 mM NaCl, 0.1% (v/v) Nonidet P-40, and protease inhibitor mixture (Calbiochem) at room temperature for 10 min, followed by addition of 10-μg MagnaBind streptavidin beads conjugated with either WT or MU oligonucleotides (∼60 fmol DNA) at room temperature for 30 min. Bead-bound complexes were washed three times with the binding buffer and eluted using SDS sample buffer (65 mM Tris, pH 6.8, 2% SDS, 10% (v/v) glycerol, 0.05% bromphenol blue, and 0.3 m β-mercaptoethanol) at 95 °C for 5 min.
For cICAT and iTRAQ analyses, large-scale DNA pull-downs were performed as above except nuclear extracts from ∼4 × 109 HCT116 p53+/+ cells (∼140 mg of total protein) were harvested, and equal amounts (∼70 mg each) were incubated with 25 nmol of bead-bound WT or MU oligonucleotide. Bound proteins were eluted using 100 mM Tris-HCl (pH 7.3), 5 mM EDTA, 0.05% SDS, and 8 M urea at room temperature for 30 min. Eluted protein concentrations were determined using the BCA assay reagent (Pierce).
cICAT Labeling and Fractionation by SCX Chromatography
For cICAT labeling, 200 μg of the eluted protein from large-scale DNA pull-downs prepared using WT or mutant DNA was labeled with light or heavy cICAT reagents, respectively (Applied Biosystems, Foster City, CA). The cICAT labeling reactions were performed as described (40). In brief, each protein sample was reduced with 1.25 mM TCEP-HCl and subsequently labeled with the cICAT reagents at 37 °C for 2 h. Labeled samples were combined and subjected to trypsin digestion (20:1 (w/w) protein:trypsin) at 37 °C for 16 h. The digested cICAT-labeled peptide mixtures were resuspended in buffer A (5 mM K2HPO4, pH 3.0, and 25% ACN) and fractionated by SCX chromatography using a 2.1 × 200 mm polysulfoethyl A SCX column (Poly LC, Columbia, MD). Gradient elution was performed at a flow rate of 1 ml/min using 0–25% buffer B (5 mM K2HPO4, pH 3.0, 25% ACN, and 350 mM KCl) over 30 min followed by 25–100% buffer B over 20 min. The cICAT-labeled peptides in the SCX fractions (retention time: 20–60 min) were further purified by avidin-affinity chromatography (Applied Biosystems). The purified cICAT-labeled peptides were dried by vacuum centrifugation, dissolved in cleaving reagents, and incubated in the dark at 37 °C for 2 h. After desalting using a ZipTipC18 (Millipore), peptides were dried and subjected to duplicate LC-MS/MS analyses.
iTRAQ Labeling and Fractionation by SCX Chromatography
For iTRAQ labeling, 200 μg of eluted protein from large-scale DNA pull-downs prepared using WT or MU DNA beads were first subjected to gel-assisted digestion (41) to remove any buffer components that would interfere in the downstream iTRAQ labeling procedure. In brief, each sample was chemically reduced using 5 mM TCEP-HCl and alkylated using 10 mM methyl methanethiosulfonate at room temperature for 30 min. The alkylated protein sample was mixed with 17.5 μl of acrylamide/bisacrylamide solution (40%, v/v, 29:1), 2.5 μl of 10% (w/v) ammonium persulfate, and 1.07 μl of TEMED to form a gel matrix. The resulting gel was cut into small pieces and washed several times using 25 mM TEABC containing 50% (v/v) ACN. The gel samples were further dehydrated using 100% ACN and dried under vacuum. Trypsin digestion (20:1 (w/w) protein:trypsin) of gel samples was performed in 25 mM TEABC at 37 °C for 16 h. Peptides were extracted from the gel using 50% ACN containing 5% (v/v) FA, vacuum dried, and reconstituted in 30 μl of 25 mM TEABC.
To label peptides with the iTRAQ reagents (Applied Biosystems), one unit of labeling reagent (defined as the amount of reagent required to label 100 μg of protein) was reconstituted in 70 μl of ethanol. In this study, products isolated from duplicate WT DNA-bead reactions were labeled with iTRAQ114 and iTRAQ115, whereas products isolated from duplicate MU DNA-bead reactions were labeled with iTRAQ116 and iTRAQ117, respectively. After incubation at room temperature for 1 h, labeled peptides were pooled, resuspended in buffer A (1 ml final volume), and fractionated by SCX chromatography as described above.
LC-ESI-MS/MS Analysis
Vacuum-dried cICAT- and iTRAQ-labeled samples were reconstituted in 10 μl of buffer A (0.1% (v/v) FA in H2O) and analyzed by nanoLC-MS/MS. Samples were injected into a 20 mm × 100 μm capillary trap column (Magic C18; Michrom BioResources, Auburn, CA), separated using a 100 mm × 75 μm capillary column (Magic C18; Michrom BioResources), and eluted using a linear gradient of 12–32% buffer B (0.1% (v/v) FA in ACN) over 50 min at 200 nl/min. An HP 1100 solvent delivery system (Hewlett-Packard, Palo Alto, CA) was used with post-column flow splitting interfaced to a QSTAR Pulsar i mass spectrometer (Applied Biosystems). Peptide fragmentation by collision-induced dissociation was performed automatically using information-dependent acquisition (Analyst QS v1.1, Applied Biosystems). The method applied one 1-s TOF MS scan and automatically switched to three 1.5-s product ion scans (MS/MS) when a target ion reached an intensity threshold of 20 counts. TOF MS scanning was performed over the range 400–1600 m/z using a Q1 transmission window of 380 amu (100%). Product ion scans were performed over the range 110–1600 m/z at low resolution utilizing Q2 transmission windows of 90 amu (25%), 165 amu (25%), 330 amu (25%), and 660 amu (25%).
Data Processing and Quantitative Analysis
For protein identification, data files from LC-ESI-MS/MS were batch searched against the Swiss-Prot sequence database v54.2 using the MASCOT v2.2.1 program (Matrix Science, London, UK). The peak list from MS/MS spectra generated by QSTAR Pulsar i was extracted using Analyst QS v1.1 with the charge state set to 2+, 3+, and 4+. MS and MS/MS centroid parameters were set to 50% height percentage and a merge distance of 0.1 amu. For MS/MS grouping, the averaging parameters were used by rejecting spectra with less than five peaks or precursor ions with less than 30 counts per second. The precursor mass tolerance for grouping was set to 0.01 Da, and both the maximum and minimum number of cycles per group was set to 1. Search parameters for peptide and MS/MS mass tolerance were ± 0.5 Da and ± 0.3 Da, respectively, with allowance for two missed cleavages from the trypsin digestion. Variable modifications for cICAT analysis were ICAT-C (Cys), ICAT-C:13C (9) (Cys), and oxidation (Met), whereas the variable modifications for iTRAQ analysis were deamidation (Asn, Gln), oxidation (Met), iTRAQ (N-terminal), iTRAQ (Lys), and methyl methanethiosulfonate (Cys). Peptides were considered identified if the MASCOT individual ion score was higher than the MASCOT identity score (p < 0.05). The false discovery rate for peptide matches above the cut-off threshold in this study was 2.18% for cICAT and 2.04% for iTRAQ. Under the indicated false discovery rate, proteins with scores ≥25 (for cICAT) and ≥40 (for iTRAQ) were confidently assigned.
For protein quantification, the ASAPRatio program (42) and the Multi-Q program (43) were used for cICAT and iTRAQ, respectively. First, the raw data files from QSTAR Pulsar i were converted to .mzXML format by the mzwiff v4.0.2 program (Seattle Proteome Center, Seattle, WA), and the MASCOT search results were exported in .xml data format. After the data conversions, ASAPRatio and Multi-Q were used to select unique cICAT- and iTRAQ-labeled peptides with confident MS/MS identification to detect signature ions (m/z = light and heavy for cICAT; 114, 115, 116, and 117 for iTRAQ), to apply a statistical method for the identification of outliers to be excluded from protein quantification, and to perform automated quantitation of peptide abundance (42, 43). All the differentially enriched proteins and corresponding peptides were manually verified. To calculate average protein ratios, the ratios of quantified unique cICAT and iTRAQ peptides were weighted according to their peak areas (for cICAT) or peak intensities (for iTRAQ).
Western Blotting
To measure histone H3 hyper-acetylation, HCT116 p53+/+ cells were transfected as described in luciferase assays. At 24-h post-transfection, the cells were treated with the indicated amounts of TSA and further cultured for 8 h, 24 h, and 48 h, then harvested and washed three times with ice-cold phosphate-buffered saline. Total cell lysates were prepared by resuspending the cells in the SDS sample buffer, sonicating using a Bioruptor, and heating at 95 °C for 5 min. For verification of proteins associated with HDM2-P2 promoter sequences, DNA pull-down samples were prepared as described above. Protein samples were subjected to 10% SDS-PAGE analysis and transferred to polyvinylidene fluoride membranes (Millipore). The membranes were incubated with blocking buffer containing 5% (w/v) non-fat milk in TBS with 0.05% (v/v) Tween 20 (TTBS) at room temperature for 1 h, washed with TTBS three times at room temperature for 5 min, and then incubated with the antibodies in blocking buffer at 4 °C for 16 h with gentle agitation. Membranes were washed four times in TTBS at room temperature for 5 min and incubated with a 1:5000 dilution of secondary antibody (goat anti-mouse, donkey anti-goat, or goat anti-rabbit) conjugated with horseradish peroxidase (Santa Cruz Biotechnology) in blocking buffer. The resulting membranes were washed four times in TTBS at room temperature for 5 min, and immunopositive bands were visualized using the ECL detection system.
RESULTS
Identification of an HDM2-P2 Promoter E-box Responsible for p53-independent Transcriptional Repression by AP-4
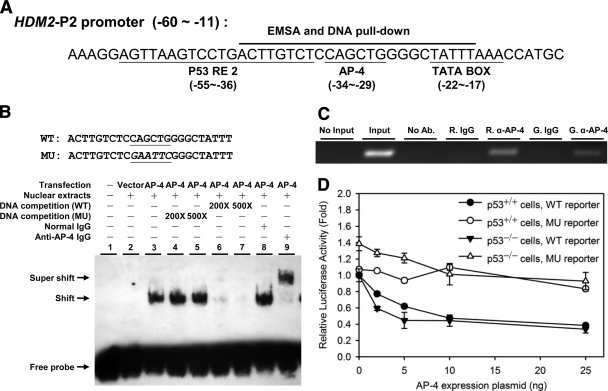
It is well established that transcription of HDM2 is mediated by a constitutive P1 promoter and an inducible P2 promoter (44). In response to DNA damage, p53 induces HDM2 transcription by binding to the p53 responsive element (RE) located in the P2 promoter (44). In contrast, a UV-responsive cis-element immediately downstream of the p53 RE was reported to mediate repression of HDM2 transcription in response to UV damage prior to p53-mediated activation (45, 46). Examination of the HDM2-P2 promoter revealed a putative E-box sequence (−29 to −34 bp relative to the transcription start site, CAGCTG) located within this UV-responsive cis-element (Fig. 1A) (21). In previous studies, AP-4 is highly expressed in colorectal carcinomas (12). In addition, transfection of HCT116 p53−/− cells, a p53-null derivative from the colorectal cancer cell line HCT116 (47), with an AP-4 expression plasmid resulted in a decrease of HDM2 protein levels (25). Therefore, we chose the HCT116 cell line as our model system.
Fig. 1.
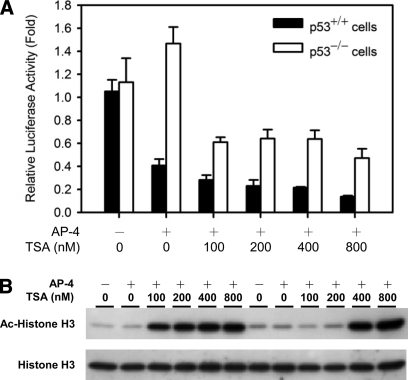
Identification of a functional AP-4 binding site in the HDM2-P2 promoter. A, DNA sequence within the HDM2-P2 promoter containing a putative E-box (37). The oligonucleotide sequence used for EMSAs and DNA pull-downs is indicated. B, EMSA analysis of HDM2-P2 E-box oligonucleotide binding by AP-4; biotin-labeled HDM2-P2 E-box oligonucleotides were incubated with HCT116 p53−/− nuclear extracts transiently transfected with pcDNATM 3.1/myc-His (−) A empty vector (lane 2) or pcDNA-AP-4 plasmid (lanes 3–9). Excess unlabeled competitor DNA containing the WT (lanes 6 and 7) or MU (lanes 4 and 5) E-box was added to reactions to determine binding specificity. Identification of AP-4 in the complex was determined by addition of normal goat IgG (control, lane 8) or goat anti-AP-4 antibody (super shift, lane 9) to binding reactions. WT and MU sense strand oligonucleotide sequences are shown at the top. C, ChIP analysis demonstrating AP-4 binding at the HDM2-P2 promoter in vivo. Rabbit anti-AP-4 (R. α-AP-4) or goat anti-AP-4 (G. α-AP-4) antibody was used to precipitate DNA-cross-linked AP-4 from AP-4-overexpressing HCT116 cells prior to PCR amplification; normal rabbit (R. IgG) or goat (G. IgG). IgG was used as a negative control. D, luciferase assay demonstrating E-box-mediated repression of the HDM2-P2 promoter. Increasing amounts of pcDNA-AP-4 plasmid were transiently cotransfected with hdm2luc01-WT reporter (wild-type AP-4 E-box; filled symbols) or hdm2luc01-MU reporter (mutant E-box; open symbols) in HCT116 p53+/+ cells (circles) or HCT116 p53−/− cells (triangles). After 48 h, total cell lysates were prepared and assayed for luciferase activity. Values represent mean-fold change ± S. D. (n = 3).
First, EMSA were performed to examine AP-4 binding to the predicted E-box sequence. Fig. 1B indicates that nuclear extracts from HCT116 p53−/− cells transiently over-expressing AP-4 shifted the WT E-box derived from the HDM2-P2 promoter (lane 3). Addition of unlabeled oligonucleotide containing WT (5′-CAGCTG-3′) E-box sequence into the reaction competed the observed EMSA shift (lanes 4–7) whereas no change was observed for oligonucleotide containing the MU (5′-GAATTC-3′) E-box, demonstrating that the AP-4-containing nuclear extract specifically associated with the identified E-box. To investigate whether the EMSA shift was AP-4-dependent, anti-AP-4 antibody was added to the EMSA reaction. As shown in lane 9 of Fig. 1B, a supershifted band was observed, indicating that AP-4 was present in the complex bound to the E-box of the HDM2-P2 promoter in vitro.
To assess whether AP-4 bound to the identified E-box in vivo, ChIP assays were performed using rabbit or goat anti-AP-4. As shown in Fig. 1C, ectopically expressed AP-4 bound to the E-box region of endogenous HDM2-P2 promoter. In addition, co-transfection of AP-4 dose-dependently repressed the luciferase activity of the WT HDM2-P2 promoter reporter, whereas the mutated E-box sequence did not support repression (Fig. 1D). Importantly, no difference between HCT116 p53+/+ and p53−/− cells was observed in the inhibition of HDM2 transcription, indicating that the observed repression by AP-4 was p53-independent (Fig. 1D). Together these data indicated that AP-4 represses HDM2 transcription by binding to the newly identified E-box of the HDM2-P2 promoter in a p53-independent manner.
Identification of HDM2-P2 Promoter-bound AP-4 Complex Components Using Complementary Quantitative Proteomics
Biochemical analyses have shown that AP-4 contains multiple protein-protein interaction domains that potentially promote either homo- or hetero-dimerization (1, 18). To identify proteins specifically associated with AP-4 and that participate in AP-4-mediated repression of HDM2, we adapted a single-step DNA-affinity purification strategy using nuclear extracts and HDM2-P2 promoter sequences containing the identified WT (5′-CAGCTG-3′) or MU (5′-GAATTC-3′) E-box sequences followed by quantitative comparison. It has been well documented that a combination of different isotope labeling methods targeting different amino acid residues, such as cICAT tagging on cysteine residues and iTRAQ tagging on N-terminal and lysine residues, provides a complementary and sequence-dependent analysis of interacting partners within a protein complex (48, 49). To identify as many AP-4 partners as possible, complementary cICAT and iTRAQ labeling strategies followed by two-dimensional LC-MS/MS analysis were performed to identify E-box-bound AP-4-interacting proteins.
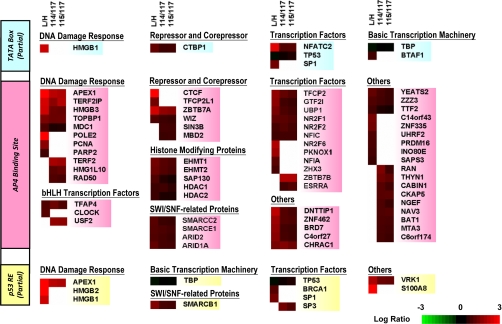
After the single-step DNA affinity purification, proteins were eluted from magnetic beads, conjugated with WT or MU DNA, and subjected to cICAT and iTRAQ labeling. For cICAT labeling, proteins eluted from WT or MU DNA beads were labeled with light (L) or heavy (H) cICAT reagents, respectively. Because iTRAQ allows parallel four-plexed analysis, two replicate batches of proteins eluted from WT DNA beads were labeled with iTRAQ114 and iTRAQ115 reagents, and two replicate batches of proteins eluted from MU DNA beads were labeled with iTRAQ116 and iTRAQ117 reagents. The ratio of iTRAQ116/iTRAQ117 can provide additional information of experimental errors during DNA pull-down, trypsin digestion, and iTRAQ labeling. The iTRAQ116/iTRAQ117 ratio determined in this study ranges from 0.95 to 1.32 (supplemental Table 2). After duplicate LC-MS/MS analysis, 542 and 879 proteins were quantified using cICAT and iTRAQ labeling methods, respectively. The false discovery rate (p < 0.05) for peptide matches above the cut-off threshold was 2.18% for cICAT and 2.04% for iTRAQ. As expected, the majority of the quantified proteins interacted with both DNA sequences in a nonspecific manner (enrichment ratio ≈1), highlighting the challenge of inherent background complexity in the characterization of DNA-protein complexes (26). Notably, the identified E-box lies adjacent to the TATA box and the p53 RE in the HDM2-P2 promoter (Fig. 1A). Therefore, among the nonspecific-binding proteins, those associated preferentially with the TATA box and p53 RE, such as TBP and p53, were identified. Because TATA box- and p53 RE-associated proteins were recruited independently of the E-box mutation, these proteins had enrichment ratios close to 1.0. Based on the three standard deviations model for the determination of fold-change thresholds (p = 0.01) (40, 41, 43), a protein enrichment ratio of ≥1.6-fold using WT DNA beads was considered statistically significant. Under this cut-off threshold, 75 proteins were considered putative AP-4-interacting proteins among the 1085 quantified proteins. Surprisingly, only 35 proteins were commonly identified by both labeling methods; 22 distinct proteins were identified using cICAT whereas 18 distinct proteins were identified using iTRAQ (Table I). The data presented herein strongly support the complementary nature of cICAT and iTRAQ labeling methods (48, 49).
Table I. List of the potential AP-4-interacting proteins differentially bound to the HDM2-P2 promoter E-box sequence.
Proteins from HCT116 p53+/+ cell nuclear extracts enriched on the HDM2-P2 promoter E-box sequence (enrichment ratio ≥ 1.6) were identified by quantitative proteomics using cICAT and iTRAQ labeling methods and were categorized according to their known molecular function(s). Complete information of enriched proteins labeled by cICAT and iTRAQ was summarized in supplemental Tables 1 and 2 respectively.
| Protein name | Accession number | Gene name | Mass | cICAT |
iTRAQ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scorea | Peptideb | WT/MUc | Scorea | Peptideb | WT1/MU2d | WT2/MU2d | ||||
| bHLH transcription factor | ||||||||||
| Activating enhancer-binding protein 4 | Q01664 | TFAP4 | 38702 | 59 | 2 | 1.96 ± 0.07 | 199 | 3 | 2.46 ± 0.60 | 1.96 ± 0.39 |
| Circadian locomoter output cycles protein kaput | O15516 | CLOCK | 95244 | 64 | 1 | 2.04 ± N/A | N/A | N/A | N/A | N/A |
| Upstream stimulatory factor 2 | Q15853 | USF2 | 36932 | N/Ae | N/A | N/A | 108 | 4 | 3.36 ± 0.90 | 3.22 ± 0.71 |
| Transcription factor | ||||||||||
| Nuclear factor of activated T-cells, cytoplasmic 2 | Q13469 | NFATC2 | 100083 | 65 | 2 | 6.46 ± 2.10 | 88 | 3 | 2.00 ± 0.58 | 2.05 ± 0.49 |
| Alpha-globin transcription factor CP2 | Q12800 | TFCP2 | 57220 | 1019 | 32 | 3.32 ± 1.58 | 522 | 9 | 2.26 ± 0.34 | 2.00 ± 0.33 |
| General transcription factor II-I | P78347 | GTF2I | 112346 | 141 | 3 | 3.15 ± 0.82 | 757 | 28 | 1.74 ± 0.33 | 1.63 ± 0.36 |
| Upstream-binding protein 1 | Q9NZI7 | UBP1 | 60453 | 903 | 40 | 2.70 ± 0.45 | 582 | 12 | 1.88 ± 0.19 | 1.73 ± 0.19 |
| COUP transcription factor 1 | P10589 | NR2F1 | 46126 | 316 | 5 | 2.22 ± 2.13 | 143 | 3 | 2.13 ± 0.56 | 1.94 ± 0.49 |
| COUP transcription factor 2 | P24468 | NR2F2 | 45542 | 228 | 2 | 1.78 ± 0.05 | 136 | 1 | 1.99 ± N/A | 1.78 ± N/A |
| Nuclear factor 1 C-type | P08651 | NFIC | 55640 | 85 | 3 | 1.69 ± 0.18 | 175 | 2 | 1.73 ± 0.06 | 1.75 ± 0.05 |
| Nuclear receptor subfamily 2 group F member 6 | P10588 | NR2F6 | 42952 | 78 | 3 | 4.28 ± 0.27 | N/A | N/A | N/A | N/A |
| Homeobox protein PKNOX1 | P55347 | PKNOX1 | 47577 | 37 | 1 | 3.69 ± N/A | N/A | N/A | N/A | N/A |
| Stimulatory protein 1 | P08047 | SP1 | 80644 | 92 | 1 | 2.59 ± N/A | N/A | N/A | N/A | N/A |
| Nuclear factor 1 A-type | Q12857 | NFIA | 55909 | 75 | 2 | 2.10 ± 0.21 | N/A | N/A | N/A | N/A |
| Breast cancer type 1 susceptibility protein | P38398 | BRCA1 | 207592 | 38 | 1 | 2.03 ± N/A | N/A | N/A | N/A | N/A |
| Zinc fingers and homeoboxes protein 3 | Q9H4I2 | ZHX3 | 104592 | 60 | 2 | 1.82 ± 1.12 | N/A | N/A | N/A | N/A |
| Zinc finger and BTB domain-containing protein 7B | O15156 | ZBTB7B | 57990 | N/A | N/A | N/A | 67 | 2 | 4.25 ± 0.74 | 4.19 ± 0.95 |
| Transcription factor Sp3 | Q02447 | SP3 | 81876 | N/A | N/A | N/A | 110 | 2 | 2.60 ± 0.07 | 1.98 ± 0.03 |
| Steroid hormone receptor ERR1 | P11474 | ESRRA | 55404 | N/A | N/A | N/A | 50 | 1 | 1.76 ± N/A | 1.99 ± N/A |
| DNA damage response protein | ||||||||||
| DNA-(apurinic or apyrimidinic site) lyase | P27695 | APEX1 | 35532 | 766 | 25 | 8.10 ± 3.21 | 782 | 26 | 4.58 ± 1.43 | 4.18 ± 1.62 |
| Telomeric repeat-binding factor 2-interacting protein 1 | Q9NYB0 | TERF2IP | 44233 | 66 | 3 | 5.73 ± 2.57 | 93 | 2 | 3.18 ± 0.76 | 4.39 ± 2.67 |
| High mobility group protein B3 | O15347 | HMGB3 | 22965 | 85 | 2 | 5.51 ± 0.32 | 132 | 4 | 2.45 ± 0.30 | 2.33 ± 0.24 |
| DNA topoisomerase 2-binding protein 1 | Q92547 | TOPBP1 | 170571 | 40 | 1 | 2.77 ± N/A | 41 | 1 | 2.43 ± N/A | 2.45 ± N/A |
| Mediator of DNA damage checkpoint protein 1 | Q14676 | MDC1 | 226529 | 278 | 16 | 1.92 ± 1.35 | 427 | 10 | 1.28 ± 0.21 | 1.34 ± 0.44 |
| High mobility group protein B2 | P26583 | HMGB2 | 24019 | 497 | 1 | 10.01 ± N/A | N/A | N/A | N/A | N/A |
| DNA polymerase epsilon subunit 2 | P56282 | POLE2 | 59499 | 34 | 1 | 8.66 ± N/A | N/A | N/A | N/A | N/A |
| High mobility group protein B1 | P09429 | HMGB1 | 24878 | 603 | 9 | 5.37 ± 0.48 | N/A | N/A | N/A | N/A |
| Proliferating cell nuclear antigen | P12004 | PCNA | 28750 | 202 | 8 | 3.12 ± 1.43 | N/A | N/A | N/A | N/A |
| Poly(ADP-ribose) polymerase 2 | Q9UGN5 | PARP2 | 66164 | 45 | 2 | 1.73 ± 0.11 | N/A | N/A | N/A | N/A |
| Telomeric repeat-binding factor 2 | Q15554 | TERF2 | 55517 | N/A | N/A | N/A | 188 | 7 | 3.96 ± 0.63 | 3.64 ± 0.40 |
| High mobility group protein 1-like 10 | Q9UGV6 | HMG1L10 | 24203 | N/A | N/A | N/A | 48 | 1 | 2.08 ± N/A | 2.31 ± N/A |
| DNA repair protein RAD50 | Q92878 | RAD50 | 153797 | N/A | N/A | N/A | 83 | 4 | 1.96 ± 0.27 | 1.81 ± 0.25 |
| Repressor and corepressor | ||||||||||
| Zinc finger and BTB domain-containing protein 7A | O95365 | ZBTB7A | 61401 | 886 | 15 | 5.66 ± 1.90 | 56 | 2 | 3.32 ± 0.26 | 3.09 ± 0.17 |
| C-terminal-binding protein 1 | Q13363 | CTBP1 | 47505 | 40 | 1 | 2.27 ± N/A | 177 | 5 | 1.68 ± 0.38 | 1.71 ± 0.12 |
| Protein wiz | O95785 | WIZ | 178563 | 117 | 10 | 1.96 ± 0.40 | 801 | 26 | 1.68 ± 0.30 | 1.61 ± 0.25 |
| CCCTC binding factor | P49711 | CTCF | 82732 | 34 | 1 | 9.68 ± N/A | N/A | N/A | N/A | N/A |
| Transcription factor CP2-like protein 1 | Q9NZI6 | TFCP2L1 | 54593 | 331 | 2 | 2.61 ± 0.38 | N/A | N/A | N/A | N/A |
| Paired amphipathic helix protein Sin3b | O75182 | SIN3B | 132983 | N/A | N/A | N/A | 87 | 3 | 1.77 ± 0.49 | 1.74 ± 0.71 |
| Methyl-CpG-binding domain protein 2 | Q9UBB5 | MBD2 | 43228 | N/A | N/A | N/A | 147 | 3 | 1.64 ± 0.19 | 1.68 ± 0.25 |
| SWI·SNF-related protein | ||||||||||
| SWI·SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 | Q12824 | SMARCB1 | 44113 | 69 | 4 | 2.97 ± 0.73 | 317 | 13 | 1.84 ± 0.29 | 1.77 ± 0.26 |
| AT-rich interactive domain-containing protein 1A | O14497 | ARID1A | 241892 | 34 | 3 | 2.25 ± 0.25 | 367 | 10 | 1.79 ± 0.28 | 1.76 ± 0.24 |
| SWI·SNF-related matrix-associated actin-dependent regulator of chromatin subfamily C member 2 | Q8TAQ2 | SMARCC2 | 132797 | 1529 | 51 | 2.07 ± 0.39 | 1430 | 30 | 1.64 ± 0.40 | 1.61 ± 0.36 |
| SWI·SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 | Q969G3 | SMARCE1 | 46621 | 71 | 4 | 2.03 ± 0.12 | 1142 | 28 | 1.88 ± 0.30 | 1.76 ± 0.28 |
| AT-rich interactive domain-containing protein 2 | Q68CP9 | ARID2 | 197268 | 1226 | 42 | 1.95 ± 0.51 | 1357 | 50 | 1.80 ± 0.34 | 1.67 ± 0.30 |
| Histone-modifiying protein | ||||||||||
| Euchromatic histone-lysine N-methyltransferase 1 | Q9H9B1 | EHMT1 | 138166 | 1231 | 58 | 2.60 ± 1.02 | 226 | 7 | 2.05 ± 0.43 | 1.91 ± 0.42 |
| Euchromatic histone-lysine N-methyltransferase 2 | Q96KQ7 | EHMT2 | 132287 | 870 | 37 | 2.45 ± 0.56 | 922 | 23 | 2.02 ± 0.32 | 1.83 ± 0.31 |
| Histone deacetylase complex subunit SAP130 | Q9H0E3 | SAP130 | 110255 | 52 | 3 | 1.93 ± 0.25 | 79 | 2 | 1.37 ± 0.05 | 1.22 ± 0.12 |
| Histone deacetylase 1 | Q13547 | HDAC1 | 55068 | 175 | 5 | 1.66 ± 0.22 | 808 | 15 | 1.71 ± 0.55 | 1.47 ± 0.28 |
| Histone deacetylase 2 | Q92769 | HDAC2 | 55329 | 164 | 8 | 1.66 ± 0.19 | 631 | 8 | 1.29 ± 0.21 | 1.28 ± 0.20 |
| Basic transcription machinery | ||||||||||
| Transcription termination factor 2 | Q9UNY4 | TTF2 | 129508 | 43 | 2 | 1.73 ± 0.02 | 261 | 14 | 1.30 ± 0.22 | 1.21 ± 0.18 |
| TATA-binding protein-associated factor 172 | O14981 | BTAF1 | 206756 | 97 | 4 | 1.70 ± 0.10 | N/A | N/A | N/A | N/A |
| Others | ||||||||||
| Deoxynucleotidyltransferase terminal-interacting protein 1 | Q9H147 | DNTTIP1 | 36990 | 481 | 12 | 4.80 ± 1.30 | 132 | 5 | 2.01 ± 0.63 | 1.83 ± 0.99 |
| Serine/threonine-protein kinase VRK1 | Q99986 | VRK1 | 45447 | 124 | 3 | 4.14 ± 0.61 | 278 | 11 | 2.42 ± 0.42 | 2.28 ± 0.40 |
| Zinc finger protein 462 | Q96JM2 | ZNF462 | 161426 | 95 | 3 | 3.62 ± 0.61 | 76 | 2 | 2.02 ± 0.20 | 1.90 ± 0.17 |
| Bromodomain-containing protein 7 | Q9NPI1 | BRD7 | 74092 | 223 | 8 | 3.33 ± 0.95 | 207 | 8 | 1.77 ± 0.38 | 1.69 ± 0.24 |
| UPF0609 protein C4orf27 | Q9NWY4 | C4orf27 | 39383 | 111 | 5 | 3.10 ± 1.37 | 240 | 8 | 1.80 ± 0.45 | 1.72 ± 0.46 |
| Chromatin accessibility complex protein 1 | Q9NRG0 | CHRAC1 | 14701 | 330 | 9 | 2.67 ± 1.22 | 47 | 1 | 2.92 ± N/A | 2.50 ± N/A |
| YEATS domain-containing protein 2 | Q9ULM3 | YEATS2 | 150688 | 97 | 4 | 2.26 ± 0.43 | 198 | 7 | 1.82 ± 0.38 | 1.62 ± 0.31 |
| ZZ-type zinc finger-containing protein 3 | Q8IYH5 | ZZZ3 | 101960 | 94 | 3 | 1.96 ± 0.20 | 82 | 3 | 1.77 ± 0.10 | 1.60 ± 0.18 |
| Protein S100-A8 | P05109 | S100A8 | 10828 | 56 | 1 | 8.91 ± N/A | N/A | N/A | N/A | N/A |
| Uncharacterized protein C14orf43 | Q6PJG2 | C14orf43 | 114918 | 158 | 6 | 2.67 ± 0.14 | N/A | N/A | N/A | N/A |
| Zinc finger protein 335 | Q9H4Z2 | ZNF335 | 144802 | 57 | 2 | 2.55 ± 0.92 | N/A | N/A | N/A | N/A |
| E3 ubiquitin-protein ligase UHRF2 | Q96PU4 | UHRF2 | 89928 | 182 | 5 | 1.92 ± 0.22 | N/A | N/A | N/A | N/A |
| PR domain zinc finger protein 16 | Q9HAZ2 | PRDM16 | 140172 | 44 | 2 | 1.85 ± 0.02 | N/A | N/A | N/A | N/A |
| Coiled-coil domain-containing protein 95 | Q8NBZ0 | INO80E | 26462 | 295 | 14 | 1.76 ± 0.52 | N/A | N/A | N/A | N/A |
| SAPS domain family member 3 | Q5H9R7 | SAPS3 | 97608 | 40 | 1 | 1.63 ± N/A | N/A | N/A | N/A | N/A |
| GTP-binding nuclear protein Ran | P62826 | RAN | 24408 | N/A | N/A | N/A | 45 | 1 | 2.86 ± N/A | 1.68 ± N/A |
| Thymocyte nuclear protein 1 | Q9P016 | THYN1 | 25681 | N/A | N/A | N/A | 138 | 1 | 2.52 ± N/A | 2.19 ± N/A |
| Calcineurin-binding protein cabin-1 | Q9Y6J0 | CABIN1 | 246197 | N/A | N/A | N/A | 51 | 1 | 2.07 ± N/A | 1.75 ± N/A |
| Cytoskeleton-associated protein 5 | Q14008 | CKAP5 | 225366 | 46 | 0 | N/A | 173 | 3 | 2.07 ± 0.14 | 2.12 ± 0.38 |
| Neuron navigator 3 | Q8IVL0 | NAV3 | 255461 | N/A | N/A | N/A | 40 | 1 | 1.93 ± N/A | 2.01 ± N/A |
| Ephexin-1 | Q8N5V2 | NGEF | 82445 | N/A | N/A | N/A | 40 | 1 | 1.93 ± N/A | 1.63 ± N/A |
| Spliceosome RNA helicase BAT1 | Q13838 | BAT1 | 48960 | 157 | 0 | N/A | 214 | 1 | 1.88 ± N/A | 1.83 ± N/A |
| Metastasis-associated protein MTA3 | Q9BTC8 | MTA3 | 67461 | N/A | N/A | N/A | 95 | 2 | 1.82 ± 0.26 | 1.74 ± 0.07 |
| Uncharacterized protein C6orf174 precursor | Q5TF21 | C6orf174 | 103136 | N/A | N/A | N/A | 152 | 7 | 1.78 ± 0.17 | 1.67 ± 0.17 |
a Mascot protein score.
b The number of nondegenerate (unique) peptide used for quantification.
c cICAT ratios, WT and MU indicate proteins enriched on WT and MU DNA beads, respectively.
d iTRAQ ratios, WT1 and WT2, indicate proteins enriched on WT DNA beads, whereas MU1 and MU2 indicate proteins enriched on MU DNA-beads, respectively.
e Not available.
Next, molecular functions and protein-protein interactions of the 75 differentially enriched proteins were further explored using Ingenuity Pathways Analysis (Ingenuity® Systems). To differentiate proteins preferentially bound to the E-box from those bound to the adjacent TATA box and p53 RE (Fig. 1A), TBP and p53 were included in the analysis. As shown in functional categorization summarized in Fig. 2, only a relatively small number of proteins were categorized as p53 and/or TBP direct-interacting proteins (8 proteins for TBP alone, 5 proteins for p53 alone, and 2 proteins for both TBP and p53). For example, the known TBP-interacting protein NFATC2 was significantly enriched in both cICAT and iTRAQ experiments (50). After filtering the previously documented p53- and TBP-interacting proteins, the majority of the 75 enriched proteins were categorized as putative AP-4-interacting proteins. Interestingly, 13 known DNA damage-responsive proteins, including APEX1, BRCA1, HMGB1/2/3, TERF2, TERF2IP, PCNA, POLE2, and RAD50, were the most significantly enriched proteins, implying a role for the AP-4 complex in regulating HDM2-P2 transcription during the DNA damage response (Fig. 2 and Table I). In addition to AP-4, 19 transcription factors, including the known AP-4-interacting protein SP1 (19), and 7 transcriptional repressors or corepressors, including CTCF and ZBTB7A, were also enriched; most of them have not previously been reported to interact, directly or indirectly, with AP-4. Furthermore, five histone-modifying proteins that function as transcriptional repressors were enriched. Among them, the histone methyltransferases EHMT1 and EHMT2 showed substantial enrichment, and histone deacetylases HDAC1 and HDAC2, which have been reported to associate with AP-4 in human immunodeficiency virus type 1 (HIV-1) (6), were moderately enriched in this study (Fig. 2 and Table I). Finally, two E-box binding bHLH transcription factors, USF2 and CLOCK, were also enriched (Fig. 2 and Table I).
Fig. 2.
Heatmap representation of nuclear proteins differentially enriched on the HDM2-P2 promoter E-box. Positively enriched proteins (enrichment ratio ≥ 1.6) identified from cICAT and iTRAQ labeling methods are classified in columns according to their known molecular functions. Proteins known to interact directly with p53 (yellow, bottom) and TBP (blue, top) were assigned to their corresponding partial binding site sequences; others were categorized as potential AP-4-interacting proteins (red, middle). For the cICAT experiment, proteins enriched on WT or MU DNA beads were labeled with light (L) or heavy (H) cICAT reagents, respectively. For iTRAQ experiments, proteins enriched on WT DNA beads were labeled with iTRAQ114 and iTRAQ115 reagents, whereas proteins enriched on MU DNA beads were labeled with iTRAQ116 and iTRAQ117 reagents, respectively.
To verify the advantage of complementary quantitative proteomics in the identification of AP-4 complex components, selected proteins exclusively enriched by the cICAT-labeling method, including CTCF, HMGB1, PCNA, and SP1, or those exclusively enriched by the iTRAQ-labeling method, including USF2, were further validated by Western blotting (Fig. 3). Several substantially enriched proteins common to both labeling methods were also examined, including AP-4, APEX1, and NFATC2. As expected, these proteins exhibited the highest WT DNA-bead enrichment ratios, confirming their specific recruitment by AP-4 to the HDM2-P2 promoter E-box (Fig. 3). Moderate enrichment of HADC1 and HDAC2 by AP-4 was also confirmed by Western blotting, consistent with the proteomic data. Finally, the binding of p53 to WT and MU DNA beads was the same (enrichment ratio ≈ 1.0) (Fig. 3), further supporting p53-indepdendent transcriptional repression of HDM2 by AP-4.
Fig. 3.
Validation of AP-4 binding partners from cICAT and iTRAQ analyses by Western blotting. Proteins enriched from HCT116 p53+/+ nuclear extracts by DNA pull-down (compare WT and MU) were analyzed by Western blotting using antibodies against selected proteins indicated at the left. The relative ratios of protein binding to WT and MU DNA-bead quantified by cICAT and iTRAQ analyses are shown at right. N/A, not available.
Functional Domains of AP-4 Contribute to the Repression of HDM2 Transcription and AP-4 Complex Formation
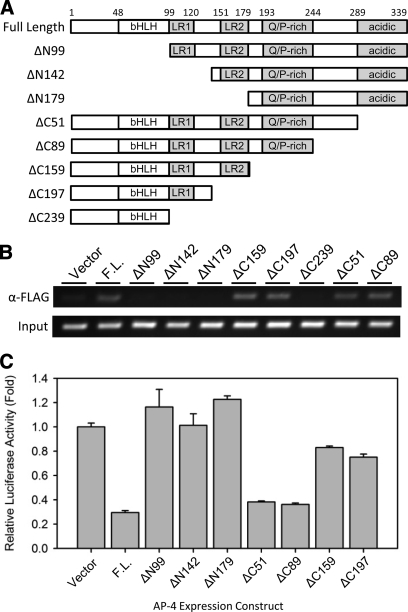
In the above proteomic study, the enriched proteins may have been recruited either by virtue of direct DNA interactions or through protein-protein interactions with AP-4. Thus, we attempted to identify the functional domain(s) of AP-4 responsible for association with selected putative AP-4-interacting proteins and for repression of HDM2 transcription. As shown in Fig. 4A, AP-4 contains several known domains, including a bHLH domain (residues 48–99), two distinct LR domains, LR1 (residues 99–120) and LR2 (residues 151–179), a Q/P-rich domain (residues 193–244), and an acidic domain (residues 289–339) (1). The bHLH domain is necessary for direct sequence-specific DNA binding (1), and both LR domains are required for the formation of the stable AP-4 homodimer (1) as well as for heterocomplex formation with GATA3 (19). The functions of the C-terminal Q/P-rich and acidic domains remain unknown; although they have been hypothesized to function as transcriptional activation domains (1).
Fig. 4.
Analysis of HDM2-P2 promoter binding and transcriptional repression using AP-4 truncation mutants. A, schematic representation of full-length AP-4 and AP-4 truncation mutant expression constructs. The AP-4 residue positions are indicated at the top. bHLH, basic helix-loop-helix domain; LR1, leucine repeat domain 1; LR2, leucine repeat domain 2; Q/P-rich, glutamine- and proline-rich domain. B, ChIP analysis demonstrating binding of FLAG-tagged full-length AP-4 and AP-4 truncation mutants at the HDM2-P2 promoter in vivo. Anti-FLAG antibody was used to precipitate DNA-cross-linked FLAG-AP-4 from HCT116 p53+/+ cells transfected with the indicated FLAG-AP-4 expression construct prior to PCR amplification. C, luciferase assay examining E-box-mediated repression of the HDM2-P2 promoter by AP-4 or AP-4 truncation mutants. HCT116 p53+/+ cells were cotransfected with hdm2luc01-WT reporter plasmid and the full-length (F.L.) pcDNA-AP-4 expression construct or the indicated AP-4-truncation mutant expression construct. After 48 h, the cells were harvested and subjected to luciferase assays. Values represent mean-fold activity relative to control (Vector)-transfected cells ± S. D. (n = 3).
We first identified domain(s) responsible for HDM2 transcription repression using incremental AP-4 truncation mutants. As shown in Fig. 4, AP-4 truncation mutants conferred varying degrees of transcriptional suppression. The ChIP analysis shown in Fig. 4B revealed, as expected, that truncation of the N-terminal region containing the DNA binding bHLH domain (ΔN99, ΔN142, ΔN179) of AP-4 resulted in failure of HDM2-P2 promoter binding, whereas all AP-4 C-terminal truncation mutants (ΔC51, ΔC89, ΔC159, ΔC197, ΔC239) except ΔC239 were able to bind to the HDM2-P2 promoter. The failure of ΔC239 to bind to the HDM2-P2 promoter was because of the instability of the truncated protein (data not shown) and (Ref. 6). Luciferase reporter assays revealed that among the mutants capable of promoter binding, C-terminal truncation including deletion of the Q/P-rich domain resulted in the most substantial relief of gene repression (Fig. 4C).
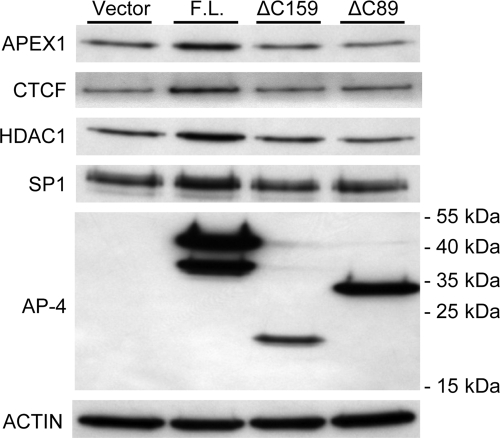
We next investigated whether the Q/P-rich domain of AP-4 is responsible for association with the AP-4-interacting proteins enriched in the quantitative proteomic study, including APEX1, CTCF, HDAC1, and SP1. Plasmids encoding various AP-4 truncation mutants were transfected into HCT116 p53+/+ cells, and DNA pull-down assays were performed using transfected cell nuclear extracts. As shown in Fig. 5, overexpression of full-length AP-4 led to increased recruitment of APEX1 to WT DNA beads compared with the AP-4 C-terminal truncation mutants ΔC89 or ΔC159. Notably, the same level of recruitment of APEX1 by the two AP-4 mutants and the endogenous AP-4 in the vector-only control was achieved, suggesting the considerable recruitment of APEX1 by endogenous AP-4 in the DNA pull-down assays. We also observed moderately increased recruitment of CTCF, HDAC1, and SP1 by the full-length AP-4 onto the WT DNA beads. These data confirmed the specific interaction between AP-4 and the selected proteins identified in the quantitative proteomics study. Taken together with the observation that deletion of the AP-4 acidic region (mutants ΔC89 or ΔC159) resulted in loss of recruitment of the selected proteins of interest, these data demonstrate that the acidic region of AP-4 is critical for interaction with APEX1, CTCF, HDAC1, and SP1.
Fig. 5.
Comparison of AP-4 and AP-4 truncation mutant binding partners by Western blotting. HCT116 p53+/+ cells were transfected with empty vector, full-length (F.L.) AP-4 expression vector, or the indicated AP-4 truncation mutant expression constructs (top). After 48 h, nuclear extracts were prepared and subjected to DNA pull-down assays using WT DNA beads followed by Western blotting analysis using antibodies against selected proteins indicated at left. As a control, nuclear extracts were subjected to Western blotting using anti-actin antibody.
HDAC-independent Repression of HDM2 Transcription by AP-4
Among the previously reported mechanisms of mammalian transcriptional regulation, histone acetylation by HAT and deacetylation by HDAC are of critical importance because they modulate the accessibility of transcription factors to their DNA binding sites (51); a common feature of mammalian transcriptional repression is promoter-specific recruitment of HDAC. In our study, HDAC1 and HDAC2 were moderately enriched on WT DNA beads (Table I and Fig. 3), and HDAC1 was confirmed to interact with the C-terminal acidic region of AP-4 by Western blot analysis (Fig. 5). To examine whether HDAC is involved in AP-4-mediated repression of HDM2 transcription, we cotransfected HCT116 p53+/+ cells with the HDM2-P2 luciferase reporter plasmid and the AP-4 expression plasmid in the presence of TSA, a specific inhibitor of HDAC. As shown in Fig. 6, incubation with TSA for 8 or 24 h resulted in dose-dependent hyper-acetylation of histone H3. To our surprise, however, TSA did not relieve AP-4-dependent repression of HDM2 transcription (Fig. 6A). Because TSA is cytotoxic after prolonged treatment (52), measurement of luciferase activity from cells treated with TSA for more than 24 h was not possible. Although DNA pull-downs revealed specific recruitment of HDAC1 by AP-4 (Figs. 3 and 5), transient transfection assays suggested that AP-4 modulates HDM2 expression through an HDAC-independent mechanism.
Fig. 6.
Effect of TSA on AP-4-mediated repression of HDM2-P2 transcription. HCT116 p53+/+ and p53−/− cells were cotransfected with the wild-type HDM2-P2 reporter (hdm2luc01-WT) and the pcDNA-AP-4 expression construct. After 24 h, the indicated amounts of TSA were added to transfection reactions and incubated for additional 8 h or 24 h. Cells were harvested and subjected to (A) luciferase assays or (B) Western blotting using antibodies against acetyl-histone H3 or histone H3.
DISCUSSION
MS-based Comprehensive Identification of DNA-bound Protein Complex Using Complementary Isotopic Labeling Methods
In this study, we reported the first identification of AP-4 protein complex containing 75 putative components recruited to the HDM2-P2 promoter E-box sequence. Our data also demonstrated the first example to apply single-step DNA affinity purification from crude nuclear extracts followed by complementary quantitative proteomics, including cICAT and iTRAQ labeling methods, to achieve comprehensive identification of protein complex components. Quantitative proteomics using stable isotope labeling methods in combination with affinity purification has been widely used to characterize DNA-bound protein complexes (26). For example, ICAT labeling in combination with single-step immunoprecipitation (31) or DNA affinity purification (32–36) has been used to identify DNA-bound protein complexes and to study the dynamics of the transcriptional machinery.
In principle, ICAT method detects only cysteine-labeled peptides enriched via biotin-avidin affinity purification, allowing significant reduction of sample complexity to enhance the probability of identifying low-abundance proteins such as transcription factor complexes (29, 33). However, cysteine abundance is relatively low in proteins, yielding low recovery of labeled proteins during biotin-avidin affinity purification (53, 54). The introduction of the iTRAQ method (30), which labels N-terminal and lysine residues, allows more sensitive and global analysis of protein complex components. These complementary labeling methods have been successfully applied to identify cancer markers in endometrial tissues, in which cICAT labeling enhances identification of signaling factors and low-abundance proteins, whereas iTRAQ labeling enhances transcription factor identification (49). In a recent proteomic study of butyrate-treated colorectal cancer cells, Tan et al. (55) also reported that cICAT and iTRAQ complementarily identified different proteins. In the present study, about half (35 out of 75) of the enriched proteins were detected and quantified by both labeling methods, whereas 22 and 18 of the 75 proteins were quantified only by cICAT and iTRAQ labeling, respectively. Because the complexity of the samples enriched via protein-DNA complex formation from nuclear extracts was greatly reduced compared with whole-cell proteomic analysis, our proteomics results did not reveal any distinct functional category for these uniquely identified proteins by each labeling method. Nevertheless, our study demonstrates the efficacy of complementary isotope labeling for identification of DNA-bound protein complex components.
Sequence-specific DNA-protein Complex Isolation Revealed by Biochemical versus MS-based Approaches
Purification of sequence-specific DNA binding transcription factors and their stably or dynamically associating protein partners faces the challenge of obtaining specific protein complex with minimal nonspecific interference. In classical biochemical approach, unbiased identification of sequence-specific DNA binding proteins requires several chromatographic separation steps followed by a final DNA affinity chromatography using cognate recognition sequence as a ligand. In addition to cell organelle differential centrifugation, several chromatographic steps, such as gel-filtration, heparin-affinity, or phosphocellulose P11, have been widely used as a prefractionation step prior to DNA affinity purification, allowing isolation of highly purified transcription factors (56, 57). In a recent study of Yaneva and Tempst (58), a prefractionation of crude nuclear extracts using phosphocellulose P11 chromatography followed by DNA affinity purification has been successfully applied for MALDI-TOF and TOF/TOF MS-based identification of transcription factors. However, the multi-step biochemical purification procedure is typically laborious and suffers from low purification yield as well as potential disruption of protein-protein interaction by stringent washing/elution conditions, e.g. high salt washes, hampering the dynamics studies of transcription factor complex in response to environmental stimuli.
In contrast, quantitative proteomics using stable isotope labeling in combination with single-step DNA affinity purification from crude nuclear extracts has been developed to characterize novel transcription factor binding to a DNA sequence of interests (35, 36) and to study the dynamics of transcription factor complex (32–34, 59, 60). By taking the advantages of quantitative comparison on the recruited proteins from wild-type versus mutant DNA sequences, specific DNA-binding proteins can be effectively discriminated from nonspecific co-purifications. Compared with commonly used biochemical multi-step purification methods, in general, the “brute-force” single-step DNA affinity purification followed by quantitative proteomics offers advantages of relatively simple purification process and less amount of starting materials. By applying appropriate washing conditions, such as stringent negative/positive selection using WT and MU DNA sequences (35, 36) or mild washes using binding buffer (32–34, 59, 60), candidate transcription factor or its associated protein complex binding to functional DNA elements can be identified. Despite these successes, there are several drawbacks regarding the MS-based approach. Biochemical validations are still required to exclude false-positive and false-negative identifications (60), In addition, these approaches are usually applicable in few expert laboratories with sophisticated instrumentation and bioinformatic support.
Taken together, therefore, optimal purity for characterization of transcription factor and its association complex may be achieved by combined use of classical biochemical purifications, DNA affinity purification, and mass spectrometric identification. When the protein abundance is a critical issue, quantitative proteomics approaches utilizing isotopic labeling methods offer an alternative with better sensitivity to identify unknown binding partners or to study the dynamic changes of proteins complex from a known transcription factor.
Functional Implications of AP-4 Protein Complex on HDM2 Repression
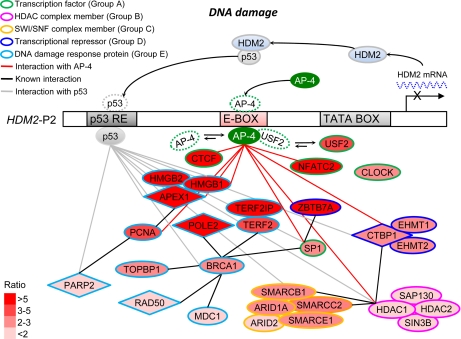
Based on our data and previous studies, we propose potential mechanisms of HDM2 transcription repression by AP-4 are presented in Fig. 7. The differentially recruited proteins in the AP-4 complex were categorized into five groups according to known molecular functions and protein-protein interactions: transcription factors (group A), HDAC complex members (group B), SWI·SNF complex members (group C), transcriptional repressors (group D), and DNA damage response proteins (group E). The transcriptional repressors can be further categorized as active repressors, which bind specific DNA sequences to recruit corepressors for inhibition of target gene expression, or as passive repressors, which likely sequester bHLH activator proteins through nonfunctional heterodimer formation. The potential mechanisms of AP-4-mediated HDM2 repression are elaborated as follows:
Fig. 7.
Schematic diagram depicting possible mechanisms of AP-4-mediated HDM2 transcription repression revealed by complementary quantitative proteomics. Proteins from HCT116 p53+/+ cell nuclear extracts with enriched binding to the WT HDM2-P2 promoter sequence were evaluated via complementary quantitative proteomics. Resulting proteins of interest are depicted in the diagram at different distances from AP-4 according to their enrichment ratio (varying shades of red). Molecular functions are indicated by the outline colors.
I. Passive Repression by Competition or Coordination with Other Transcription Factors
It is well recognized that mammalian transcription repression can be mediated by passive repressor proteins (51). Passive repressor proteins inhibit transcription by binding to and forming inactive heterodimers with transcriptional activators or by sequestering co-activators required for transcription activation (51). For example, transcription factor MafG forms homodimer and competes with MafG/p45 heterodimer for binding to Maf recognition element, resulting in the repression of Maf recognition element-dependent gene activation, a feature characteristic of passive repression (61, 62). AP-4 has also been shown to function as a passive repressor by competing with TBP for TATA box binding (4), or by competing with other bHLH transcription factor complexes for E-box binding (63). In the present quantitative proteomic study, TBP remained unchanged. However, two bHLH transcription factors, USF2 and CLOCK, were enriched (Fig. 7, group A), suggesting that they may participate in the regulation of HDM2 transcription by competing with AP-4 for E-box binding (Fig. 7). It will be of interest to determine whether AP-4 and USF2 or other bHLH transcription factors regulate HDM2 transcription in a competitive manner.
Other transcription factors, such as CTCF, NFATC2, and SP1, were also significantly enriched in our quantitative proteomic study (Fig. 7, group A). Although the oligonucleotides used in the DNA affinity purification did not contain any binding sequence for these transcription factors, a distal NFAT binding site responsible for HDM2 transcription repression has been identified (64). In addition, sequence analysis by MatInspector program (39) revealed several putative SP1 and CTCF binding sites distal to the HDM2-P2 promoter E-box (Chr. 12, contig NC_000012, 67488540–67489062, 523 bp) (37) (Table II). These observations raise the possibility that regulation of HDM2 transcription may be coordinated by AP-4 and other transcription factors. For example, distant interaction of AP-4 and SP1 via mutual interaction with GATA-3 was demonstrated to regulate the transcription of the dopamine β-hydroxylase gene (19). It is also noted that a transcriptional repressor ZBTB7A, which is an SP1-interacting protein (65), is also significantly enriched. Our data suggest that AP-4 may coordinate association of these transcription factors with a repressor molecule(s) to regulate HDM2 transcription.
Table II. Putative transcription factor binding sites in HDM2-P2 promoter analyzed by the MatInspector program.
The DNA sequence of the HDM2-P2 promoter (Chr. 12, contig NC_000012, 67488540–67489062, 523 bp) was analyzed by MatInspector using MatInspector library version 7.1 with an optimized matrix threshold of 0.80.
| Family/Matrixa | Optimized matrix similarity thresholdb | Positionc | Strandd | Matrix similaritye | Sequence |
|---|---|---|---|---|---|
| AP-4 | |||||
| V$AP4R/V$AP4.01 | 0.85 | −40 ∼ −24 | (−) | 0.939 | agcccCAGCTGgagaca |
| CTCF | |||||
| V$CTCF/V$CTCF.01 | 0.80 | −305 ∼ −281 | (+) | 0.820 | ctctcgcggcggtgGGGGtgggggt |
| V$CTCF/V$CTCF.01 | 0.80 | −247 ∼ −223 | (+) | 0.815 | ggtcacgggggccgGGGGctgcggg |
| NFATC2 | |||||
| V$NFAT/V$NFAT.01 | 0.95 | −118 ∼ −100 | (−) | 0.965 | agaGGAAaagctgagtcaa |
| SP1 | |||||
| V$SP1F/V$SP1.01 | 0.88 | −327 ∼ −313 | (+) | 0.911 | aggaGGGCgggattt |
| V$SP1F/V$SP1.01 | 0.88 | −243 ∼ −229 | (+) | 0.896 | acggGGGCcgggggc |
| V$SP1F/V$SP1.01 | 0.88 | −152 ∼ −138 | (+) | 0.911 | gtctGGGCgggattg |
| V$SP1F/V$SP1.02 | 0.85 | −407 ∼ −393 | (+) | 0.851 | cgggGCGCggggcgc |
a Matrix name of transcription factor in MatInspector library version 7.1.
b Similarity threshold between identified DNA sequence and the matrix of transcription factor binding sequence.
c Position of identified DNA sequence from transcriptional start site.
d Orientation of identified DNA sequence.
e Similarity between the identified DNA sequence and the matrix of transcription factor binding sequence.
II. Active Repression by Direct Recruitment of Repressor Molecules
Transcriptional repression can also be mediated by active repressors, which target chromatin organization via histone deacetylation or methylation (51). For example, MafG, in addition to passive repression, can actively repress target gene expression by recruiting HDAC (66). Similarly, AP-4 has also been reported to repress transcription by recruiting active repressor proteins such as the HDAC complex (Fig. 7, group B), as exemplified by repression of PAHX-AP1 expression in nonneuronal brain cells (18) and repression of HIV-1 gene expression in T lymphocytes (6). However, our findings suggest that HDM2 repression by AP-4 occurs via an HDAC-independent mechanism in the colorectal cancer cell line HCT116, indicating that HDAC-independent repression by AP-4 may be tissue-specific. Bimodal repression has been reported for other transcription factors such as SHARP-1 (67) and NRSF (68, 69). Transcriptional repression by NRSF is mediated by an N-terminal domain that recruits HDAC1 and Sin3B (68) or by a C-terminal domain that recruits a corepressor complex consisting of Co-REST and SMARCC2 (69), which belong to the SWI·SNF nucleosome remodeling complex. Interestingly, SMARCC2, as well as four other components of the SWI·SNF complex, were also enriched on the WT HDM2-P2 promoter sequence by AP-4 (Fig. 7, group C). Notably, the enrichment ratio of SWI·SNF complex components was higher than that of HDAC complex components (HDAC1, HDAC2, Sin3B, and SAP130). Furthermore, it has been shown that histone H3-Lys-9 methyltransferases, EHMT1 and EHMT2, participate in transcription repression by formation of facultative heterochromatin (51). As shown in Fig. 7 (group D), EHMT1 and EHMT2, members of the CTBP1 complex, were also enriched on the WT HDM2-P2 promoter. Thus, our data suggest that HDAC-independent HDM2 repression by AP-4 occurs via recruitment of active repressors such as histone methylases or the SWI·SNF complex (Fig. 7).
III. Functional Involvement of AP-4 in DNA Damage Response
Our proteomic study revealed that DNA damage response proteins were the most substantially enriched group of proteins (Fig. 7, group E). Interestingly, a putative UV-responsive region encompassing the AP-4 binding site identified herein has been reported to repress HDM2 transcription in response to high doses of UV radiation (45, 46). Among the enriched DNA damage-responsive proteins, RAD50 has been demonstrated to participate in the repair of DNA double-strand breaks under the control of BRCA1 (70). RAD50 also interacts with the telomeric-binding protein TERF2 (71), which has been demonstrated to recognize DNA double-strand breaks caused by ionizing radiation (72). Following oxidative or UV-induced DNA damage, APEX1, HMGB1, and PARP2 are the major effectors of DNA base excision repair, whereas BRCA1, PCNA and POLE2 play important roles in nucleotide excision repair (73–76). Thus, AP-4 may also regulate HDM2 transcription following DNA damage, perhaps by associating with DNA damage-responsive proteins.
Contribution of Q/P-rich Domain to Transcriptional Repression
Incremental truncation of AP-4 revealed that the Q/P-rich domain of AP-4 was, in part, responsible for transcriptional repression. Identification of a Gln/Pro-rich domain repressive activity contrasts with its previously established role in transcription activation (77). Our observations contribute to the increasing body of evidence that Pro- and/or Gln-rich domains mediate transcriptional repression, as exemplified by the Wilms tumor gene WT1 and Epstein-Barr virus nuclear antigen EBNA3C. The N-terminal P-rich domain of WT1 is shown to interact with the transcriptional corepressor BASP1 (78). Similarly, EBNA3C has a weak transcriptional repression domain enriched with proline and acidic residues that interacts with the corepressor CTBP1 (79). Interestingly, CTBP1 was also enriched in our quantitative proteomics study (Fig. 7, group D). Further studies will be required to determine whether other components in the AP-4 complex interacting with the Gln/Pro-rich domain of AP-4, such as EHMT and CTBP1, mediate the HDAC-independent HDM2 repression. In addition, our study revealed that several proteins specifically associate with the C-terminal domain of AP-4, such as CTCF, HDAC1, and SP-1. It is also of interest to determine their functional roles contributing to the HDM2 repression, allowing better understanding of AP-4-mediated HDAC-independent HDM2 repression.
Supplementary Material
Acknowledgments
We thank Professor Zee-Fen Chang at the Graduate Institute of Biochemistry and Molecular Biology, National Taiwan University; Dr. Chi-Meng Tzeng at the U-Vision Biotech Inc.; and Professor Jenn-Han Chen at the School of Dentistry, National Defense University for useful discussion and comments on the experimental design. We thank Professor Bert Vogelstein at Johns Hopkins University for generous gifts of HCT116 p53+/+ and HCT116 p53−/− cells lines. We also thank Mr. Chih-Chiang Tsou at the Institute of Information Science, Academia Sinica, for the assistance in the preparation of supplementary information.
Footnotes
* This work was supported by a grant from National Science Council, Taiwan (NSC96-2627-M-001-001).
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
1 The abbreviations used are:
- AP-4
- activating enhancer-binding protein 4
- APEX1
- DNA-(apurinic or apyrimidinic site) lyase
- bHLH
- basic-helix-loop-helix
- ChIP
- chromatin immunoprecipitation
- cICAT
- cleavable isotope-coded affinity tag
- CLOCK
- circadian locomotor output cycles protein kaput
- CTBP1
- C-terminal-binding protein 1
- FA
- formic acid
- CTCF
- CCCTC binding factor
- EHMT1
- euchromatic histone-lysine N-methyltransferase 1
- EHMT2
- euchromatic histone-lysine N-methyltransferase 2
- EMSA
- electrophoretic mobility shift assay
- HDAC
- histone deacetylase
- HDM2
- human homolog of murine double minute 2
- HDM2-P2
- HDM2 promoter P2
- HIV-1
- human immunodeficiency virus type 1
- HMG
- high mobility group protein
- iTRAQ
- isobaric tags for relative and absolute quantitation
- LC-ESI-MS/MS
- liquid chromatography electrospray ionization tandem mass spectrometry
- LR
- leucine repeat
- MU
- mutant
- NFAT
- nuclear factor of activated T cells
- NRSF
- neuron restrictive silencer factor, also known as REST
- p53 RE
- p53 responsive element
- PAHX-AP1
- phytanoyl-CoA α-hydroxylase-associated protein 1
- PARP2
- poly[ADP-ribose] polymerase 2
- PCNA
- proliferating cell nuclear antigen
- POLE2
- DNA polymerase epsilon subunit 2
- Q/P-rich
- glutamine/proline-rich
- SCX
- strong cation exchange
- SWI·SNF
- SWItch/Sucrose NonFermentable
- SMARC
- SWI·SNF-related matrix-associated actin-dependent regulator of chromatin
- SP1
- stimulatory protein 1
- TBP
- TATA-binding protein
- TERF2
- telomeric repeat-binding factor 2
- TOF
- time-of-flight
- TSA
- trichostatin A
- TTBS
- tris-tween buffered saline
- USF2
- upstream stimulatory factor 2
- WT
- wild-type
- TEMED
- N,N,N′,N′-tetramethylenediamine
- TEABC
- triethylammonium bicarbonate
- TCEP
- Tris (2-carboxyethyl)-phosphine.
REFERENCES
- 1.Hu Y. F., Lüscher B., Admon A., Mermod N., Tjian R. (1990) Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev 4, 1741–1752 [DOI] [PubMed] [Google Scholar]
- 2.Mermod N., Williams T. J., Tjian R. (1988) Enhancer binding factors AP-4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature 332, 557–561 [DOI] [PubMed] [Google Scholar]
- 3.Unk I., Kiss-Toth E., Boros I. (1994) Transcription factor AP-4 participates in activation of bovine leukemia virus long terminal repeat by p34 tax. Nucleic Acids Res 22, 4872–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou S. H., Garcia-Martínez L. F., Paulssen E. J., Gaynor R. B. (1994) Role of flanking E box motifs in human immunodeficiency virus type 1 TATA element function. J. Virol 68, 7188–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friez M., Hermansen R., Milavetz B. (1999) Chromatin structure of the simian virus 40 late promoter: a deletional analysis. J. Virol 73, 1990–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai K., Okamoto T. (2006) Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J. Biol. Chem 281, 12495–12505 [DOI] [PubMed] [Google Scholar]
- 7.Badinga L., Song S., Simmen R. C., Simmen F. A. (1998) A distal regulatory region of the insulin-like growth factor binding protein-2 (IGFBP-2) gene interacts with the basic helix-loop-helix transcription factor, AP-4. Endocrine 8, 281–289 [DOI] [PubMed] [Google Scholar]
- 8.Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. (1988) Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J 7, 3793–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fodor E., Weinrich S. L., Meister A., Mermod N., Rutter W. J. (1991) A pancreatic exocrine cell factor and AP4 bind overlapping sites in the amylase 2A enhancer. Biochemistry 30, 8102–8108 [DOI] [PubMed] [Google Scholar]
- 10.King-Jones K., Korge G., Lehmann M. (1999) The helix-loop-helix proteins dAP-4 and Daughterless bind both in vitro and in vivo to SEBP3 sites required for transcriptional activation of the drosophila gene Sgs-4. J. Mol. Biol 291, 71–82 [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto K., Ono T., Sato M., Nishida T., Oguma T., Tadakuma T. (2005) Regulation of the expression of caspase-9 by the transcription factor activator protein-4 in glucocorticoid-induced apoptosis. J. Biol. Chem 280, 27638–27644 [DOI] [PubMed] [Google Scholar]
- 12.Jung P., Menssen A., Mayr D., Hermeking H. (2008) AP4 encodes a c-MYC-inducible repressor of p21. Proc. Natl. Acad. Sci. U. S. A 105, 15046–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiu Y., Nakamura K., Abe M., Li N., Wen X. S., Jiang Y., Zhang D., Tsurui H., Matsuoka S., Hamano Y., Fujii H., Ono M., Takai T., Shimokawa T., Ra C., Shirai T., Hirose S. (2002) Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol 169, 4340–4346 [DOI] [PubMed] [Google Scholar]
- 14.Aranburu A., Carlsson R., Persson C., Leanderson T. (2001) Transcription factor AP-4 is a ligand for immunoglobulin-kappa promoter E-box elements. Biochem. J 354, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriamanalijaona R., Felisaz N., Kim S. J., King-Jones K., Lehmann M., Pujol J. P., Boumediene K. (2003) Mediation of interleukin-1beta-induced transforming growth factor beta1 expression by activator protein 4 transcription factor in primary cultures of bovine articular chondrocytes: possible cooperation with activator protein 1. Arthritis Rheum 48, 1569–1581 [DOI] [PubMed] [Google Scholar]
- 16.Cui Y., Narayanan C. S., Zhou J., Kumar A. (1998) Exon-I is involved in positive as well as negative regulation of human angiotensinogen gene expression. Gene 224, 97–107 [DOI] [PubMed] [Google Scholar]
- 17.Littlewood T. D., Evan G. I. (1995) Transcription factors 2: helix-loop-helix. Protein Profile 2, 621–702 [PubMed] [Google Scholar]
- 18.Kim M.-Y., Jeong B. C., Lee J. H., Kee H. J., Kook H., Kim N. S., Kim Y. H., Kim J. K., Ahn K. Y., Kim K. K. (2006) A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc. Natl. Acad. Sci. U. S. A 103, 13074–13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S. J., Choi H. J., Hong S., Huh Y., Chae H., Kim K. S. (2008) Transcription factor GATA-3 regulates the transcriptional activity of dopamine beta-hydroxylase by interacting with Sp1 and AP4. Neurochem. Res 33, 1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Wang H. (2000) MDM2 oncogene as a novel target for human cancer therapy. Curr. Pharm. Des 6, 393–416 [DOI] [PubMed] [Google Scholar]
- 21.Haupt Y., Maya R., Kazaz A., Oren M. (1997) Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- 22.Kubbutat M. H., Jones S. N., Vousden K. H. (1997) Regulation of p53 stability by Mdm2. Nature 387, 299–303 [DOI] [PubMed] [Google Scholar]
- 23.Wu X., Bayle J. H., Olson D., Levine A. J. (1993) The p53-mdm-2 autoregulatory feedback loop. Genes Dev 7, 1126–1132 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Li M., Wang H., Agrawal S., Zhang R. (2003) Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc. Natl. Acad. Sci. U. S. A 100, 11636–11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q., Raya A., DeJesus P., Chao S. H., Quon K. C., Caldwell J. S., Chanda S. K., Izpisua-Belmonte J. C., Schultz P. G. (2004) Identification of p53 regulators by genome-wide functional analysis. Proc. Natl. Acad. Sci. U. S. A 101, 3456–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gingras A. C., Aebersold R., Raught B. (2005) Advances in protein complex analysis using mass spectrometry. J. Physiol 563, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerrigan L. A., Kadonaga J. T. (1993) Purification of sequence-specific DNA-binding proteins by affinity chromatography. In Current Protocols in Molecular Biology ( Ausubel F. M., Brent B., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. eds), pp. 12.10.11–12.10.18, John Wiley and Sons, New York: [DOI] [PubMed] [Google Scholar]
- 28.Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. (1993) Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362, 722–728 [DOI] [PubMed] [Google Scholar]
- 29.Gygi S. P., Rist B., Gerber S. A., Turecek F., Gelb M. H., Aebersold R. (1999) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol 17, 994–999 [DOI] [PubMed] [Google Scholar]
- 30.Ross P. L., Huang Y. N., Marchese J. N., Williamson B., Parker K., Hattan S., Khainovski N., Pillai S., Dey S., Daniels S., Purkayastha S., Juhasz P., Martin S., Bartlet-Jones M., He F., Jacobson A., Pappin D. J. (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 [DOI] [PubMed] [Google Scholar]
- 31.Brand M., Ranish J. A., Kummer N. T., Hamilton J., Igarashi K., Francastel C., Chi T. H., Crabtree G. R., Aebersold R., Groudine M. (2004) Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat. Struct. Mol. Biol 11, 73–80 [DOI] [PubMed] [Google Scholar]
- 32.Kim B., Nesvizhskii A. I., Rani P. G., Hahn S., Aebersold R., Ranish J. A. (2007) The transcription elongation factor TFIIS is a component of RNA polymerase II preinitiation complexes. Proc. Natl. Acad. Sci. U. S. A 104, 16068–16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranish J. A., Yi E. C., Leslie D. M., Purvine S. O., Goodlett D. R., Eng J., Aebersold R. (2003) The study of macromolecular complexes by quantitative proteomics. Nat. Genet 33, 349–355 [DOI] [PubMed] [Google Scholar]
- 34.Mousson F., Kolkman A., Pijnappel W. W., Timmers H. T., Heck A. J. (2008) Quantitative proteomics reveals regulation of dynamic components within TATA-binding protein (TBP) transcription complexes. Mol. Cell. Proteomics 7, 845–852 [DOI] [PubMed] [Google Scholar]
- 35.Himeda C. L., Ranish J. A., Angello J. C., Maire P., Aebersold R., Hauschka S. D. (2004) Quantitative proteomic identification of Six4 as the trex-binding factor in the muscle creatine kinase enhancer. Mol. Cell. Biol 24, 2132–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Himeda C. L., Ranish J. A., Hauschka S. D. (2008) Quantitative proteomic identification of MAZ as a transcriptional regulator of muscle-specific genes in skeletal and cardiac myocytes. Mol. Cell. Biol 28, 6521–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelps M., Darley M., Primrose J. N., Blaydes J. P. (2003) p53-independent activation of the hdm2-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor alpha-positive breast cancer cells. Cancer Res 63, 2616–2623 [PubMed] [Google Scholar]
- 38.Urban A., Neukirchen S., Jaeger K. E. (1997) A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res 25, 2227–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933–2942 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y. R., Juan H. F., Huang H. C., Huang H. H., Lee Y. J., Liao M. Y., Tseng C. W., Lin L. L., Chen J. Y., Wang M. J., Chen J. H., Chen Y. J. (2006) Quantitative proteomic and genomic profiling reveals metastasis-related protein expression patterns in gastric cancer cells. J. Proteome Res 5, 2727–2742 [DOI] [PubMed] [Google Scholar]
- 41.Han C. L., Chien C. W., Chen W. C., Chen Y. R., Wu C. P., Li H., Chen Y. J. (2008) A multiplexed quantitative strategy for membrane proteomics: opportunities for mining therapeutic targets for autosomal-dominant polycystic kidney disease. Mol. Cell. Proteomics 7, 1983–1997 [DOI] [PubMed] [Google Scholar]
- 42.Li X. J., Zhang H., Ranish J. A., Aebersold R. (2003) Automated statistical analysis of protein abundance ratios from data generated by stable-isotope dilution and tandem mass spectrometry. Anal. Chem 75, 6648–6657 [DOI] [PubMed] [Google Scholar]
- 43.Lin W. T., Hung W. N., Yian Y. H., Wu K. P., Han C. L., Chen Y. R., Chen Y. J., Sung T. Y., Hsu W. L. (2006) Multi-Q: a fully automated tool for multiplexed protein quantitation. J. Proteome Res 5, 2328–2338 [DOI] [PubMed] [Google Scholar]
- 44.Zauberman A., Flusberg D., Haupt Y., Barak Y., Oren M. (1995) A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic Acids Res 23, 2584–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng X., Keller D., Wu L., Lu H. (2000) UV but not gamma irradiation accelerates p53-induced apoptosis of teratocarcinoma cells by repressing MDM2 transcription. Cancer Res 60, 6184–6188 [PubMed] [Google Scholar]
- 46.Wu L., Levine A. J. (1997) Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol. Med 3, 441–451 [PMC free article] [PubMed] [Google Scholar]
- 47.Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J. P., Sedivy J. M., Kinzler K. W., Vogelstein B. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501 [DOI] [PubMed] [Google Scholar]
- 48.Wu W. W., Wang G., Baek S. J., Shen R. F. (2006) Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J. Proteome Res 5, 651–658 [DOI] [PubMed] [Google Scholar]
- 49.DeSouza L., Diehl G., Rodrigues M. J., Guo J., Romaschin A. D., Colgan T. J., Siu K. W. (2005) Search for cancer markers from endometrial tissues using differentially labeled tags iTRAQ and cICAT with multidimensional liquid chromatography and tandem mass spectrometry. J. Proteome Res 4, 377–386 [DOI] [PubMed] [Google Scholar]
- 50.Kim L. J., Seto A. G., Nguyen T. N., Goodrich J. A. (2001) Human TAFII130 is a coactivator for NFATp. Mol. Cell. Biol 21, 3503–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiel G., Lietz M., Hohl M. (2004) How mammalian transcriptional repressors work. Eur. J. Biochem 271, 2855–2862 [DOI] [PubMed] [Google Scholar]
- 52.Medina V., Edmonds B., Young G. P., James R., Appleton S., Zalewski P. D. (1997) Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res 57, 3697–3707 [PubMed] [Google Scholar]
- 53.Patton W. F. (2002) Detection technologies in proteome analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 771, 3–31 [DOI] [PubMed] [Google Scholar]
- 54.Julka S., Regnier F. (2004) Quantification in proteomics through stable isotope coding: a review. J. Proteome Res 3, 350–363 [DOI] [PubMed] [Google Scholar]
- 55.Tan H. T., Tan S., Lin Q., Lim T. K., Hew C. L., Chung M. C. M. (2008) Quantitative and temporal proteome analysis of butyrate-treated colorectal cancer cells. Mol. Cell. Proteomics 7, 1174–1185 [DOI] [PubMed] [Google Scholar]
- 56.Gadgil H., Jurado L. A., Jarrett H. W. (2001) DNA affinity chromatography of transcription factors. Anal. Biochem 290, 147–178 [DOI] [PubMed] [Google Scholar]
- 57.Righetti P. G., Castagna A., Antonioli P., Boschetti E. (2005) Prefractionation techniques in proteome analysis: the mining tools of the third millennium. Electrophoresis 26, 297–319 [DOI] [PubMed] [Google Scholar]
- 58.Yaneva M., Tempst P. (2003) Affinity capture of specific DNA-binding proteins for mass spectrometric identification. Anal. Chem 75, 6437–6448 [DOI] [PubMed] [Google Scholar]
- 59.Petti F., Thomson S., Haley J. D. (2006) Peptide, domain, and DNA affinity selection in the identification and quantitation of proteins from complex biological samples. Anal. Biochem 356, 1–11 [DOI] [PubMed] [Google Scholar]
- 60.Mittler G., Butter F., Mann M. (2009) A SILAC-based DNA protein interaction screen that identifies candidate binding proteins to functional DNA elements. Genome Res 19, 284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motohashi H., Katsuoka F., Shavit J. A., Engel J. D., Yamamoto M. (2000) Positive or negative MARE-dependent transcriptional regulation is determined by the abundance of small Maf proteins. Cell 103, 865–875 [DOI] [PubMed] [Google Scholar]
- 62.Nagai T., Igarashi K., Akasaka J., Furuyama K., Fujita H., Hayashi N., Yamamoto M., Sassa S. (1998) Regulation of NF-E2 activity in erythroleukemia cell differentiation. J. Biol. Chem 273, 5358–5365 [DOI] [PubMed] [Google Scholar]
- 63.Kim J. W., Monila H., Pandey A., Lane M. D. (2007) Upstream stimulatory factors regulate the C/EBPα gene during differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun 354, 517–521 [DOI] [PubMed] [Google Scholar]
- 64.Li M., Zhang Z., Hill D. L., Chen X., Wang H., Zhang R. (2005) Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res 65, 8200–8208 [DOI] [PubMed] [Google Scholar]
- 65.Lee D. K., Suh D., Edenberg H. J., Hur M. W. (2002) POZ domain transcription factor, FBI-1, represses transcription of ADH5/FDH by interacting with the zinc finger and interfering with DNA binding activity of Sp1. J. Biol. Chem 277, 26761–26768 [DOI] [PubMed] [Google Scholar]
- 66.Motohashi H., Katsuoka F., Miyoshi C., Uchimura Y., Saitoh H., Francastel C., Engel J. D., Yamamoto M. (2006) MafG sumoylation is required for active transcriptional repression. Mol. Cell. Biol 26, 4652–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garriga-Canut M., Roopra A., Buckley N. J. (2001) The basic helix-loop-helix protein, SHARP-1, represses transcription by a histone deacetylase-dependent and histone deacetylase-independent mechanism. J. Biol. Chem 276, 14821–14828 [DOI] [PubMed] [Google Scholar]
- 68.Naruse Y., Aoki T., Kojima T., Mori N. (1999) Neural restrictive silencer factor recruits mSin3 and histone deacetylase complex to repress neuron-specific target genes. Proc. Natl. Acad. Sci. U. S. A 96, 13691–13696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battaglioli E., Andrés M. E., Rose D. W., Chenoweth J. G., Rosenfeld M. G., Anderson M. E., Mandel G. (2002) REST repression of neuronal genes requires components of the hSWI.SNF complex. J. Biol. Chem 277, 41038–41045 [DOI] [PubMed] [Google Scholar]
- 70.Zhong Q., Chen C. F., Li S., Chen Y., Wang C. C., Xiao J., Chen P. L., Sharp Z. D., Lee W. H. (1999) Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285, 747–750 [DOI] [PubMed] [Google Scholar]
- 71.Zhu X. D., Küster B., Mann M., Petrini J. H., de, Lange T. (2000) Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet 25, 347–352 [DOI] [PubMed] [Google Scholar]
- 72.Bradshaw P. S., Stavropoulos D. J., Meyn M. S. (2005) Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat. Genet 37, 193–197 [DOI] [PubMed] [Google Scholar]
- 73.Wood R. D. (1996) DNA repair in eukaryotes. Annu. Rev. Biochem 65, 135–167 [DOI] [PubMed] [Google Scholar]
- 74.Schreiber V., Amé J. C., Dollé P., Schultz I., Rinaldi B., Fraulob V., Ménissier-de Murcia J., de, Murcia G. (2002) Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem 277, 23028–23036 [DOI] [PubMed] [Google Scholar]
- 75.Hartman A. R., Ford J. M. (2002) BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat. Genet 32, 180–184 [DOI] [PubMed] [Google Scholar]
- 76.Prasad R., Liu Y., Deterding L. J., Poltoratsky V. P., Kedar P. S., Horton J. K., Kanno S., Asagoshi K., Hou E. W., Khodyreva S. N., Lavrik O. I., Tomer K. B., Yasui A., Wilson S. H. (2007) HMGB1 is a cofactor in mammalian base excision repair. Mol. Cell 27, 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choy B., Green M. R. (1993) Eukaryotic activators function during multiple steps of preinitiation complex assembly. Nature 366, 531–536 [DOI] [PubMed] [Google Scholar]
- 78.Carpenter B., Hill K. J., Charalambous M., Wagner K. J., Lahiri D., James D. I., Andersen J. S., Schumacher V., Royer-Pokora B., Mann M., Ward A., Roberts S. G. E. (2004) BASP1 is a transcriptional cosuppressor for the Wilms' tumor suppressor protein WT1. Mol. Cell. Biol 24, 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Touitou R., Hickabottom M., Parker G., Crook T., Allday M. J. (2001) Physical and functional interactions between the corepressor CtBP and the Epstein-Barr virus nuclear antigen EBNA3C. J. Virol 75, 7749–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.