Abstract
Tandem mass spectrometry was used to identify naturally processed peptides bound to major histocompatibility complex (MHC) I and MHC II molecules in central nervous system (CNS) of eight patients with multiple sclerosis (MS). MHC molecules were purified from autopsy CNS material by immunoaffinity chromatography with monoclonal antibody directed against HLA-A, -B, -C, and -DR. Subsequently peptides were separated by reversed-phase HPLC and analyzed by mass spectrometry. Database searches revealed 118 amino acid sequences from self-proteins eluted from MHC I molecules and 191 from MHC II molecules, corresponding to 174 identified source proteins. These sequences define previously known and potentially novel autoantigens in MS possibly involved in disease induction and antigen spreading. Taken together, we have initiated the characterization of the CNS-expressed MHC ligandome in CNS diseases and were able to demonstrate the presentation of naturally processed myelin basic protein peptides in the brain of MS patients.
T cells recognize antigen bound to MHC1 molecules (1). CD4 as well as CD8 T cells have been shown to play a pathogenic role in various autoimmune diseases (2). Pathogenic T cells infiltrate the target organs and locally secrete proinflammatory cytokines and chemokines leading to tissue inflammation and possibly subsequent tissue destruction (3–5). Local presentation of autoantigens by MHC molecules in the target tissue of the autoimmune attack, i.e. the central nervous system (CNS) in multiple sclerosis (MS) or the pancreas in diabetes, is therefore a prerequisite for local immune amplification (6). MS is an inflammatory and neurodegenerative disease of the CNS leading to myelin and axonal loss (7). There are different disease courses, i.e. relapsing-remitting, secondary chronic progressive, and primary progressive disease. Potential autoantigens in MS include myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG). It is thought that T cells enter the CNS from the systemic circulation and that they are subsequently reactivated in the CNS on MHC I and MHC II molecules expressed on local antigen-presenting cells (APC) (8).
To date, naturally presented HLA-bound peptides from patients with MS thus far have not been isolated and identified. So far, only circumstantial evidence exists for the local presentation of autoantigens such as MBP on MHC molecules in CNS (9). The aim of this study consisted of the characterization of the MHC-bound peptide repertoire derived from brains of patients with MS. Cutting edge technology combining HPLC and tandem mass spectrometry has recently allowed us to define peptides presented on APC from bronchoalveolar lavage from lungs of sarcoidosis patients (10). Applying a similar method on autopsy material of MS patients, for the first time we demonstrated local presentation of previously known and potential novel autoantigens in MS.
EXPERIMENTAL PROCEDURES
Patient Characterization
Brain autopsy samples from eight patients with different clinical subtypes of MS were provided by the UK Multiple Sclerosis Tissue Bank (supplemental Table 1).
Elution of HLA-presented Peptides
HLA-associated peptides were obtained by immunoprecipitation of HLA molecules from brain tissue of MS patients according to standard protocols (11, 12) using the HLA-A-, HLA-B-, and HLA-C-specific antibody W6/32 (13) and the HLA-DR-specific antibody L243 (14). In brief, about 50 g of snap frozen brain tissue was thawed at 4 °C in PBS containing 1.2% (w/v) CHAPS in the presence of protease inhibitors (Complete Protease Inhibitor Mixture Tablet, Roche Applied Science) and homogenized in a Dounce homogenizer. Subsequently HLA molecules and bound peptides were isolated with the solid-phase bound monoclonal antibody by immunoaffinity chromatography. Precipitates were eluted with 0.2% trifluoroacetic acid, ultrafiltrated by a 10-kDa ultrafilter, and lyophilized.
Liquid Chromatography-Mass Spectrometry
Lyophilized samples were resuspended in 150 μl of solvent A (0.1% (v/v) trifluoroacetic acid water) and loaded onto a C18 precolumn (Dionex) for concentration and desalting with a flow rate of 50 μl/min. The precolumn was placed in line for separation by a 75-μm-inner diameter fused silica microcapillary column packed with C18 reversed-phase material (Dionex). A binary gradient of 10–55% solvent B (0.1% formic acid in 80% (v/v) acetonitrile water) within 90 min was performed, applying a flow rate of 200–300 nl/min.
The peptides were analyzed by ESI mass spectrometry on a Q-TOF tandem mass spectrometer. A gold-coated glass capillary (PicoTip) was used for introduction into the ESI source. A potential of 2.2 kV was applied to the capillaries, resulting in sample flow rates of 20–30 nl/min. The cone voltage was 60 V. A quadrupole analyzer was used to select precursor ions for fragmentation in the collision cell. The collision gas was argon used at collision energies of 24–50 eV. MHC I-bound peptides were detected with an m/z range of 400–800 and double charge, whereas MHC II-bound peptides were measured in an m/z range of 500–1200 and double and triple charge.
Database Searches
In tandem mass spectrometry experiments, sequence information was obtained by manual interpretation of fragment spectra using computer-assisted database (NCBInr, non-redundant protein database) searching tools (Mascot search version 2.2, released February 28, 2007, Matrix Science) (15). For peak list generation MassLynx 4.0 with Service Pack 2 was used. Mascot searches were performed using Homo sapiens as taxonomy, no enzymatic specificity, ±0.3 Da for mass spectrometry tolerance, and ±0.3 Da for tandem mass spectrometry tolerance. These were done with database NCBInr 20070926 (5,519,594 sequences, 1,911,975,371 residues), taxonomy H. sapiens (human) (194,674 sequences); database NCBInr 20080110 (5,828,094 sequences; 2,009,144,511 residues), taxonomy H. sapiens (human) (199,003 residues); and database NCBInr 20080610 (6,573,034 sequences, 2,244,863,856 residues), taxonomy H. sapiens (human) (205,031 sequences). The evaluations were performed over a longer time period, therefore all versions were used. Fragment spectra from synthetic peptides were recorded and compared with spectra from the corresponding eluted natural peptides to prove their identity.
Epitope Prediction
For every peptide a prediction of the binding strength to the HLA type of the patients was performed using the matrix-based SYFPEITHI algorithm. The prediction score is explained in detail in the information section of SYFPEITHI.
HLA Genotyping
DNA for HLA genotyping was extracted from brain tissue using the QIAamp DNA Blood Mini kit (Qiagen). Sequence-specific oligonucleotide typing was performed using the Innogenetics Line Probe Assay (INNO-LiPA) test (Innogenetics).
RESULTS
Proteomics Analysis of the MHC I and MHC II Ligandome in the CNS
Brain autopsy samples from patients with different clinical subtypes of MS (supplemental Table 1) and different HLA haplotypes (supplemental Table 2) were analyzed using liquid chromatography-tandem mass spectrometry. We isolated HLA-A, -B, -C, and -DR molecules by affinity purification and then eluted the bound peptides by acid elution. Subsequently peptides were separated by reversed-phase HPLC and analyzed by mass spectrometry.
The tandem mass spectrometry-based peptide sequencing of HLA ligands extracted from brain yielded ∼34 different ligands per patient. Table I shows the HLA class I- and class II-associated ligands isolated from MS patient MS1. Sequence analysis by tandem mass spectrometry allowed the identification of 11 MHC I- and 23 MHC II-bound peptides. We adopted the SYFPEITHI database to calculate the ligand binding affinity to the respective MHC I and II alleles according to the haplotype of patient MS1 (HLA-A*01, -A*03, -B*08, -B*51, -Cw*07, or -Cw*15 for MHC I and HLA-DRB1*03 or -DRB1*04 for MHC II) (Table I). For eight of 11 MHC I ligands an assignment to the presenting HLA I molecule was carried out according to relatively high scores between 22 and 37 for binding to HLA-A*01, -A*03, or -B*51. (A SYFPEITHI score above 20 predicts sufficient affinity to bind to the respective HLA molecule. The SYFPEITHI scoring system evaluates every amino acid within a peptide. Optimal anchor residues are given the value 15; amino acids that are only slightly preferred in the respective position may be given the arbitrary value 1. Any value between 1 and 15 is possible, and negative values are also possible for amino acids that are disadvantageous for the peptide binding capacity at a certain sequence position.) HLA I molecules were not assigned to three ligands lacking the correct anchoring amino acids. By contrast, we assigned HLA-DRB1*03 or -DRB1*04 to all MHC II ligands according to the scores of SYFPEITHI, which were generally lower than those of MHC I ligands.
Table I. MHC class I and II ligands identified from one individual MS patient (MS1).
MS1 HLA typing is as follows: HLA class I: A*01, A*03, B*08, B*51, Cw*07, Cw*15; HLA class II: DRB1*03, DRB1*04, DQB1*0302, DQB1*0201 (DQB1*0204). Identified peptides from patients MS2–MS8 are shown in supplemental tables. SPARC, secreted protein acid rich in cysteine.
| No. | Entrez gene ID | Source protein | m/z | Charge | Mascot score | Sequence coverage | Peptide sequence | HLA assignment (SYFPEITHI)a |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| MHC class I restriction | ||||||||
| 1 | 118 | α-Adducin | 584.25 | 2 | 48 | 6 | LTDRELEEY | A*01 (33) |
| 2 | 147463 | Ankyrin repeat domain-containing protein 2 9 | 506.78 | 2 | 53 | 3 | VIRLLLASGA | NA |
| 3 | 256949 | Ankyrin repeat domain-containing protein 4 7 | 598.88 | 2 | 43 | 4 | ALMLAISHGRQ | NA |
| 4 | 311 | Annexin A1 1 | 677.25 | 2 | 71 | 7 | ETDLLDIRSEY | A*01 (34) |
| 5 | 3481 | Insulin-like growth factor II precursor | 488.26 | 2 | 37 | 5 | IPMGKSMLV | B*5101 (22) |
| 6 | 8543 | LIM domain-only protein 4 | 569.82 | 2 | 42 | 5 | KIADRFLLY | A*03 (24) |
| 7 | 51604 | Phosphatidylinositol glycan | 615.22 | 2 | 52 | 3 | DTDHYFLRY | A*01 (37) |
| 8 | 4735 | Septin 2 | 631.77 | 2 | 58 | 5 | YIDEQFERY | A*01 (30) |
| 9 | 9201 | Serine/threonine-protein kinase DCLK1 | 653.21 | 2 | 71 | 1 | YTERDASGMLY | A*01 (31) |
| 10 | 85358 | SH3 and multiple ankyrin repeat domains protein 3 | 515.77 | 2 | 41 | <1 | CPLSLAAQLD | NA |
| 11 | 7401 | USH3A (clarin 1) | 564.26 | 2 | 46 | 33 | TTGILSILFY | A*01 (24) |
| MHC class II restriction | ||||||||
| 1 | 60 | Actin | 677.71 | 3 | 113 | 10 | WISKQEYDESGPSIVHR | DRB1*0401 (18) |
| 2 | 3040 | α2-Globin | 506.30 | 4 | 77 | 13 | AAHLPAEFTPAVHASLDKF | DRB1*0401 (28) |
| 3 | 347 | Apolipoprotein D | 822.45 | 2 | 73 | 7 | NQELRADGTVNQIEG | DRB1*0401 (26) |
| 4 | 3043 | β-Globin | 623.70 | 3 | 107 | 65 | YQKVVAGVANALAHKYH | DRB1*0401 (26) |
| 5 | 22883 | Calsyntenin 1 | 767.48 | 2 | 60 | 1 | DPPLIALDKDAPLR | DRB1*0301 (31) |
| 6 | 1363 | Carboxypeptidase E | 649.99 | 3 | 78 | 3 | EPGEPEFKYIGNMHGNE | DRB1*0401 (22) |
| 7 | 83692 | CD99 antigen-like 2 | 635.89 | 2 | 66 | 6 | AEPPPPPPEPARI | DRB1*0301 (9) |
| 8 | 8760 | CDP-diacylglycerol synthase 2 | 613.41 | 2 | 44 | 2 | PEVLNRALSNL | DRB1*0301 (21) |
| 9 | 3336 | Early pregnancy factor (EPF) (chaperonin 10) | 699.41 | 2 | 61 | 44 | FRDGDILGKYVD | DRB1*0301 (19) |
| 10 | 2752 | Glutamine synthetase | 777.89 | 2 | 71 | 3 | LNETGDEPFQYKN | DRB1*0301 (20) |
| 11 | 2597 | Glyceraldehyde-3-phosphate dehydrogenase | 845.95 | 2 | 85 | 4 | YDNEFGYSNRVVDL | DRB1*0401 (22) |
| 12 | 3040 | Hemoglobin α-2 | 586.37 | 2 | 79 | 35 | VLSPADKTNVK | DRB1*0401 (20) |
| 13 | 3903 | Leukocyte-associated Ig-like receptor (LAIR) | 626.34 | 2 | 62 | 4 | FRIDSVSEGNAG | DRB1*0301 (20) |
| 14 | 3916 | Lysosome-associated membrane glycoprotein-1 | 786.00 | 2 | 44 | 3 | LNTILPDARDPAFK | DRB1*0301 (29) |
| 15 | 3107 | MHC class I protein HLA-C heavy chain | 642.67 | 3 | 54 | 22 | VDDTQFVRFDSDAASPR | DRB1*0401 (28) |
| 16 | 4155 | MBP-(84–94) | 673.39 | 2 | 57 | 6 | DENPVVHFFKN | DRB1*0401 (18) |
| 17 | 4155 | MBP-(95–106) | 625.38 | 2 | 32 | 7 | IVTPRTPPPSQG | DRB1*0401 (14) |
| 18 | 9378 | Neurexin 1 | 857.98 | 2 | 43 | 9 | ESNAIINDGKYHVVR | DRB1*0301 (28) |
| 19 | 9379 | Neurexin 2 | 562.00 | 3 | 57 | 2 | EPNAIVSDGKYHVVR | DRB1*0301 (29) |
| 20 | 113791 | Phosphoinositide 3-kinase | 764.41 | 2 | 52 | 5 | GHLYREDQTSPAPG | DRB1*0401 (28) |
| 21 | 84898 | Plexin domain-containing 2 | 725.45 | 2 | 64 | 2 | LDFLKAVDTNRAS | DRB1*0401 (26) |
| 22 | 8404 | SPARC-like protein 1 | 573.06 | 3 | 52 | 2 | KVKKIYLDEKRLLA | DRB1*0301 (37) |
| 23 | 6386 | Syntenin | 709.92 | 2 | 71 | 4 | ITSIVKDSSAARNG | DRB1*0301 (24) |
a The scores of individual peptides calculated using the SYFPEITHI epitope prediction program are shown in parentheses. NA, not assigned.
Using mass spectrometric analysis of eight investigated brains of MS patients, we altogether identified 309 peptides of which 118 were eluted from MHC I and 191 were eluted from MHC II molecules (supplemental Table 3). As already shown for patient MS1, higher binding scores were obtained for peptides restricted to MHC I molecules as compared with MHC II molecules.
Verification of the Identified Peptides
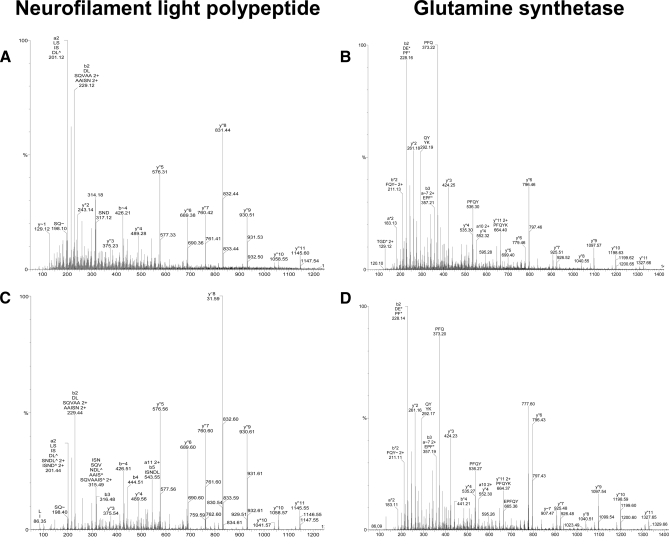
For confirmation of identified ligands, a subset of these peptides was synthesized to prove their identity. Mass spectrometric analysis was performed on the synthetic peptides, and obtained fragment spectra were compared with the corresponding spectra of the naturally presented peptides eluted from brain tissue. Fig. 1 shows an example of the fragmentation spectra of two naturally occurring peptides, neurofilament light chain NEFL-(72–84) (DLSQVAAISNDLK) (Fig. 1A) eluted from MHC I molecules in samples of patients MS3 and MS6 and glutamine synthetase (LNETGDEPFQYKN) (Fig. 1B) obtained from MHC II molecules in MS samples of patients MS1, MS4, MS5, MS7, and MS8. In all cases, spectra of the synthetic peptides matched the spectra of their eluted natural counterparts (Fig. 1, C and D), proving the correct interpretation of the recorded mass spectrometric data.
Fig. 1.
Examples of fragmentation-induced mass spectra. Mass spectra of the naturally eluted peptides neurofilament light polypeptide (DLSQVAAISNDLK) (A) and glutamine synthetase (LNETGDEPFQYKN) (B) in comparison with the corresponding synthetic peptides (C and D) recorded with a Q-TOF mass spectrometer are shown. The corresponding y and b series are marked.
Database Mining
The analysis of the constitutive HLA peptide repertoire presented by various HLA allotypes in brains of patients with MS allowed us to identify 239 non-redundant peptides derived from 174 non-redundant source proteins. Among these, we were able to identify several HLA ligands from 40 proteins that have been reported previously to be associated with MS or its animal model, experimental autoimmune encephalomyelitis (EAE) (Table II). These proteins could be categorized regarding their function and tissue localization into proteins related to apoptosis, cytoskeleton, enzymatic reactions, immune responses, CNS, serum, and others (Table II).
Table II. MS-associated proteins.
According to the literature, some of the source proteins have been shown previously to be associated with MS. These could be categorized in regard to the function and tissue localization. IGF, insulin-like growth factor; RR, relapsing-remitting; SPARC, secreted protein acid rich in cysteine; IFN, interferon.
| Entrez ID | Protein | Patients | Association with MS |
|---|---|---|---|
| Apoptosis | |||
| 311 | Annexin A1 | MS1, MS2, MS4, MS7 | Identified as potential new marker of active MS disease (49) |
| 9774 | Bcl-2 associated transcription factor 1 | MS4, MS6 | Antiapoptotic molecule increased in MS cortical neurons (50) |
| 332 | Survivin | MS2 | Heightened expression in activated T cells from MS patients (51) |
| Cytoskeleton | |||
| 60 | Actin | MS1, MS2, MS4, MS5, MS6, MS7, MS8 | Biomarker of axonal and neuronal damage (47) |
| 10376 | α-Tubulin | MS4, MS5, MS6, MS7 | Biomarker of axonal and neuronal damage (47) |
| 2934 | Gelsolin | MS5 | Lower gelsolin concentration in CSF of MS patients, indicating the possible utility for diagnostic purposes (32) |
| 4131 | Microtubule-associated protein 1B (MAP1B) | MS3 | MAP1B is a marker for actively myelinating oligodendrocytes in adult rat brain (52) |
| 3925 | Stathmin 1 | MS4 | Increased stathmin expression in the brains of MS patients (53) |
| Enzymes | |||
| 801 | Calmodulin 1 | MS5 | Calmodulin binds MBP in Ca2+-dependent manner (54) |
| 805 | Calmodulin 2 (phosphorylase kinase) | MS4, MS6 | Involved in migration of γ/δ T cells to the site of lesion in MS (55) |
| 2747 | Glutamate dehydrogenase (GDH) | MS3, MS5, MS3, MS5 | Glutamate excitotoxicity, evoked by altered glutamate homeostasis, was demonstrated in an animal model of MS; GS and GDH are present in oligodendrocytes in normal and non-MS white matter but are absent from active and chronic silent MS lesions, suggesting lasting metabolic impediments (30) |
| 2752 | Glutamine synthetase (GS) | MS1, MS3, MS4, MS5, MS7, MS8 | Glutamate excitotoxicity, evoked by altered glutamate homeostasis, was demonstrated in an animal model of MS; GS and GDH are present in oligodendrocytes in normal and non-MS white matter but are absent from active and chronic silent MS lesions, suggesting lasting metabolic impediments (30) |
| 2597 | Glyceraldehyde-3-phosphate dehydrogenase | MS1, MS3, MS4, MS6, MS7 | Autoantibodies in CSF of patients with MS (31) |
| 51564 | Histone deacetylase 7a | MS6 | Histone deacetylase inhibitors are discussed as a dual therapeutic modality in MS (56) |
| 4696 | NADH dehydrogenase | MS4, MS5 | There is a trend for impaired NADH dehydrogenase activity in association with oxidative damage to mitochondrial DNA in chronic active plaques in MS (57) |
| 113791 | Phosphoinositide 3-kinase (PI3K) | MS1 | Patients with RR-MS display an increase in peripheral Vδ2 γ/δ T cells; they transmigrate across endothelial cells by activation of the PI3K pathway (58) |
| Immune response | |||
| 336 | Early pregnancy factor (EPF) | MS1, MS3, MS4, MS5, MS8 | EPF has a protective effect on EAE in LEW rats (59) |
| 3577 | Interleukin 8 receptor, CXCR1 | MS8 | Treatment with methylprednisolone and mitoxantrone modulates the expression of CXCR1 in blood mononuclear cells (60); CXCR1 is constitutively expressed on oligodendrocytes and up-regulated around active and silent lesions in MS patients (61) |
| 3594 | Interleukin 12 receptor | MS12 | Suggested as a biomarker that can differentiate between relapsing-remitting and secondary progressive MS stages (47) |
| 4282 | Macrophage migration-inhibitory factor | MS4 | A therapeutic target in inflammatory diseases (62) |
| 3119 | MHC class antigen II HLA-DQβ1 | MS2 | HLA-DR and -DQ genes are the strongest genetic risk factors in MS (20, 45) |
| CNS | |||
| 6622 | α-Synuclein | MS4 | Up-regulated in neurons and glia (63) |
| 2670 | GFAP | MS2, MS5, MS6, MS8 | Elevated in CSF of MS patients (27); biomarker of gliosis (47) |
| 4155 | MBP | MS1, MS3, MS6, MS7, MS8 | Anti-MBP antibodies suggested for prediction of development of MS; biomarker of demyelination (47) |
| 4741 | Neurofilament-3, NF-M | MS7, MS8 | Biomarker of axonal and neuronal damage (47); autoantibodies in serum and CSF (64); transgenic MOG-(35–55)-reactive T cells respond to an NF-M epitope in MOG-deficient mice (29) |
| 4747 | Neurofilament light polypeptide, NF-L | MS3, MS6 | Biomarker of axonal and neuronal damage (47); elevated in CSF of MS patients (27); immunization with NF-L induces neurological disease and axonal degeneration in mice (28); autoantibodies in serum and CSF (65) |
| 6285 | S100 calcium-binding protein | MS2 | Raised levels in CSF in RR-MS patients (66) |
| 8404 | SPARC-like protein 1 | MS1, MS2, MS3, MS5 | Present in the CSF of MS patients (67) |
| 3925 | Stathmin 1 | MS4 | Increased expression of stathmin in CNS of MS patients (53) |
| Serum proteins | |||
| 2 | α2-Macroglobulin | MS2, MS8 | Significantly increased fractions of transformed to total α2-macroglobulin in plasma from patients with MS (68, 69) |
| 347 | Apolipoprotein D (apoD) | MS2 | Intrathecal apoD production is increased in MS, CSF apoD traces correlate with disease duration, corticosteroid treatment results in elevated CSF apoD levels (70) |
| 348 | Apolipoprotein E (apoE) | MS4 | ApoE-derived peptides ameliorate clinical disability and inflammatory infiltrates in the spinal cord in EAE (71) |
| 2512 | Ferritin | MS2, MS8 | Elevated ferritin levels in MS patients with chronic progressive active disease (72); elevated levels of ferritin are found in a certain percentage of MS patients (73) |
| 7018 | Transferrin | MS2 | There is evidence for iron dysregulation in the pathogenesis of MS; opposite to the ferritin levels, transferrin levels in patients are in a normal range (72) |
| 7450 | von Willebrand factor | MS7 | CXCR3−/− mice with EAE show more von Willebrand factor-immunoreactive vessels within inflamed spinal cords as compared with CXCR3+/+ mice (74) |
| Others | |||
| 2784 | Guanine nucleotide-binding protein (GNB) | MS4 | Trend to an increased GNB polymorphism in primary chronic progressive MS patients (75) |
| 4600 | Interferon-regulated resistance GTP-binding protein (MX1) | MS3 | Biomarker to measure IFN-β activity in mice following gene-based delivery (76) |
| 10226 | Mannose 6-phosphate receptor (IGF-II) | MS4 | No expression in plaques of MS patients that were characterized by a dense network of astrocytes, suggesting that IGF-II receptors in human brain are not involved in astrogliosis (77) |
| 7157 | p53 tumor suppressor | MS2 | Defects in the ATM-CHK2-p53 pathway in MS patients likely alter the regulation of the immune population of cells in MS and may contribute to the development or progression of the disease (78); increased p53 expression in oligodendrocytes in active MS lesion, feature in oligodendrocyte apoptosis and cell loss (79) |
| 7082 | Tight junction protein ZO-1 | MS4 | Abnormal tight junctions are most frequent in active white matter lesions but persist in inactive lesions of MS patients (80) |
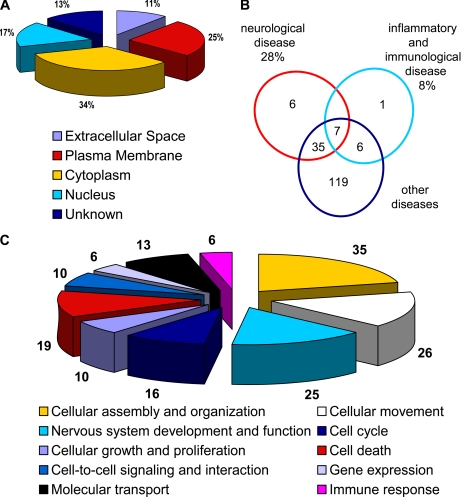
Ingenuity Pathways Analysis® of these 174 source proteins revealed that the largest quantity of eluted peptides was derived from proteins present in the cytoplasm (34%) followed by proteins derived from plasma membranes (25%), nucleus (17%), unknown origin (13%), and extracellular space (11%) (Fig. 2A). 68% of the eluted peptides derived from extracellular proteins were presented on MHC II molecules; 77% of the peptides derived from nuclear proteins were presented on MHC I molecules. In contrast plasma membrane- and cytoplasm-derived peptides were equally presented on MHC I and II molecules.
Fig. 2.
Subcellular, functional, and disease-associated categorization of source proteins by Ingenuity Pathways Analysis. A, a pie chart shows the percentages of proteins according to their subcellular localization. B, a Venn diagram depict the association of the proteins to neurological, inflammatory, immunological, and other diseases. C, a pie chart illustrates the functional association of the proteins.
In addition, the program was used to link the proteins with different diseases. Particularly relevant for this study were proteins involved in neurological diseases (28%) and also proteins associated with inflammatory and immunological diseases (8%). Moreover the analysis showed a high number of proteins involved in other diseases as well as a considerable number of proteins present in more than one group (Fig. 2B).
With regard to their biological functions, the source proteins of HLA ligands could be categorized into 10 partially overlapping functional groups (Fig. 2C). 35 of the source proteins participate in cellular assembly and organization, 26 are involved in cellular movement, 25 are involved in nervous system development and function, 19 are associated with cell death, 16 are associated with the cell cycle, 13 are associated with molecular transport, 10 are associated with cellular growth and proliferation, 10 are associated with cell-to-cell signaling and interaction, six are associated with gene expression, and six are associated with immune responses.
MHC-bound MBP Peptides
MBP is one of the most abundant myelin proteins. It is the best studied myelin protein in MS due in part to the finding that MBP is immunogenic and that MBP-specific T lymphocytes have encephalitogenic activity in experimental animals (16). It is known that T cells recognize a large number of MBP epitopes restricted by different HLA molecules (17–19). This view is supported by our finding of MBP epitopes presented by different MHC molecules in seven of the eight analyzed patients (Table III). We identified two HLA class I-restricted and 11 HLA class II-restricted amino acid sequences derived from MBP. MBP-derived HLA class I peptides were identified in two of eight MS patients, whereas MBP-derived MHC class II peptides could be identified in five of eight MS patients (Table III).
Table III. Multiple peptides identified from MBP sequence.
Diverse MBP peptides eluted in seven out of eight MS patients were presented on MHC I and II molecules.
| Protein (position) | Peptide sequence | Patient |
|---|---|---|
| MHC class I | ||
| MBP-(35–45) | DTGILDSIGRF | MS6, MS7 |
| MBP-(36–45) | TGILDSIGRF | MS7 |
| MHC class II | ||
| MBP-(10–19) | RHGSKYLATA | MS3 |
| MBP-(10–27) | RHGSKYLATASTMDHARH | MS3 |
| MBP-(11–25) | HGSKYLATASTMDHA | MS8 |
| MBP-(21–30) | TMDHARHGFL | MS3 |
| MBP-(77–91) | SHGRTQDENPVVHF | MS8 |
| MBP-(84–94) | DENPVVHFFKN | MS1, MS3 |
| MBP-(95–106) | IVTPRTPPPSQG | MS1, MS4 |
| MBP-(95–110) | IVTPRTPPPSQGKGRG | MS3 |
| MBP-(95–112) | IVTPRTPPPSQGKGRGLS | MS3 |
| MBP-(96–111) | VTPRTPPPSQGKGRGL | MS5 |
| MBP-(139–153) | HKGFKGVDAQGTLS | MS3 |
DISCUSSION
In this study, we identified naturally processed peptides presented on MHC I and II molecules eluted from brains of patients with MS. To our knowledge, it is the first time that this approach has been successfully applied to a CNS disease. MS is an inflammatory disease that leads to neurodegeneration (7). Early inflammatory events trigger subsequent events leading to demyelination, axonal and neuronal loss, and finally scar formation. So far, the autoimmune response has been considered to be mainly directed against myelin antigens like MBP (20). There are some indications that also axonal and neural components or astrocytic proteins could be autoantigens in MS (21). In this study we clearly demonstrate that self-antigens are presented in the course of MS that have been known for a long time, such as MBP peptides (19, 22–26). We found peptides derived from glial fibrillary acidic protein (GFAP) and neurofilament that have been shown to be enhanced in cerebrospinal fluid (CSF) of MS patients (27). Furthermore immunization with neurofilament can cause neurological disease and axonal damage in mice (28). The first example of immunological self-mimicry has been demonstrated recently for neurofilament (29). MOG-deficient mice that express a MOG-(35–55)-specific transgenic T cell receptor (TCR) recognize an epitope of the medium sized neurofilament NF-M and develop disease. The authors propose that the combined immune response to the two target structures by encephalitogenic T cells might be a reason to overcome resistance to EAE in C57BL/6 mice (29). Immunological self-mimicry could be of paramount importance in the pathogenesis of MS and induction of neurodegeneration.
In Table II we give an overview of the proteins that have been demonstrated to be of potential importance in MS. For example, these proteins are known to be biomarkers in MS, like actin (27); to be targets for autoantibodies, like glyceraldehyde-3-phosphate dehydrogenase (30); to have an effect in animal models of MS, e.g. early pregnancy factor (31); or to possibly be of diagnostic value, like gelsolin (32). Further analysis is necessary to clarify whether these proteins, and especially the identified peptides, are disease-relevant in MS. In addition we define many novel potential autoantigens derived from several cell types that have not been described in the context of MS. The recent description of double self-reactive T cells in EAE implicates that our identified peptides could be as well targets of such double reactive T cells in MS (29). Therefore they should be closely examined regarding shared TCR contact positions with known immunodominant epitopes, e.g. MBP-(85–99).
Various studies suggest that MBP is a candidate autoantigen in MS. The MBP-(85–99)·MHC complex has been demonstrated to be present in the CNS of MS patients using a monoclonal antibody (9). Evidence for the functional importance comes from the observation that transgenic mice expressing both the HLA-DR1*1501 molecule and an MBP-(84–102)-specific TCR derived from an MS patient spontaneously develop EAE (33). Extensive epitope mapping studies have been conducted in animals as well as in humans to identify potentially encephalitogenic MBP sequences. Many studies have identified MBP-(83–99) as immunodominant in EAE as well as in MS (34). This peptide promiscuously binds to various MS-associated HLA-DR molecules. However, it has been suggested that the unusually high binding affinity of this peptide to HLA-DR2 molecules might lead to the deletion of high avidity MBP-(83–99)-specific T cells from the peripheral T cell repertoire. Although the role of MBP-(84–102) in MS has been widely investigated, other MBP-derived epitopes have equally been shown to be immunodominant and might be more discriminatory between patients and healthy controls (35, 36). These peptides include MBP-(14–33) and the HLA-DR1*0401-associated peptide MBP-(113–131).
HLA class I-restricted MBP peptides are less well characterized because the strongest genetic association of MS is with HLA class II alleles and only to a lesser extent with HLA class I alleles (37). Therefore, MS is generally considered to be a CD4+ T cell-driven disease. Nevertheless CD8+ T cells have recently moved to the focus of attention in MS research because it was shown that CD8+ T cells are prominently present in MS lesions and are more clonally expanded in these lesions than CD4+ T cells (38). There is some indication that HLA-A2 may be protective and HLA-A3 may be disease-promoting in MS (39–41). Immunodominant HLA-A2-restricted MBP peptides include MBP-(88–96) and MBP-(112–120) (42). The two different HLA class I-restricted MBP sequences are overlapping (34–44 and 35–44) and consist of MBP sequences that have not previously been associated with pathogenic CD8+ T cell responses in MS.
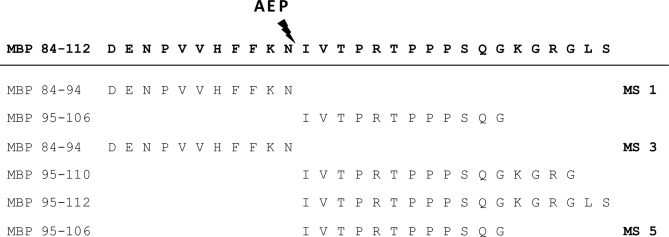
As far as the HLA-DR-restricted MBP epitopes are concerned, most MBP naturally processed peptides originate from regions that have previously been implicated in MBP immunodominance (34). Thus, four MBP peptides (MBP-(10–19), MBP-(10–27), MBP-(11–25), and MBP-(21–30)) are partially overlapping with the MBP-(14–33) immunodominant epitope. Several naturally processed MBP peptides partially overlap with the MBP-(84–102) region (Table III). Interestingly none of the peptides identified in this region completely corresponded to the very well characterized MBP-(83–99) epitope. As a matter of fact, for two of the patients, the MBP epitopes represented contiguous sequences (MS1: MBP-(84–94) and MBP-(95–106); MS2: MBP-(84–94) and MBP-(95–110)/(95–112) within this region that were cleaved between Asn-94 and Ile-95. Interestingly it has been shown previously that this site in MBP represents a cleavage site for asparagine endopeptidase, an enzyme important for early stage proteolysis during antigen processing (43) (Table IV). Thus, our results strongly support a biological role for asparagine endopeptidase in MBP processing in the brain of MS patients. Studies in SJL/J mice have shown that induction of immunological tolerance with MBP-(89–101) is ineffective because in these mice this treatment does not target the disease-relevant T cells that recognize either MBP-(89–94) or MBP-(95–101) (44). These observations made in an EAE model in conjunction with our data strengthen the notion that relevant antigen-specific MS therapies should be designed in such a way that they target T cells responsive to naturally processed epitopes of the autoantigen.
Table IV. Eluted peptides and asparagine endopeptidase (AEP) cleavage site.
We did not detect peptides derived from other well known myelin proteins, such as peptides from PLP and MOG. This is possibly because of the density of the presented peptides on MHC molecules. PLP is the most abundant myelin protein (50%) followed by MBP (40%). In contrast, MOG only makes up 0.01–0.05% of myelin. The reason why no PLP peptides or MOG peptides were identified might be 2-fold. As far as MOG peptides are concerned, the low abundance of MOG in the CNS might hamper the detection of MOG-derived peptides. In contrast, lack of detection of PLP-derived peptides might be due to the fact that PLP is generally highly palmitoylated, and current methodologies are still limited in their identification of such modified peptides.
As shown, we analyzed only CNS tissue from patients with MS and not from healthy controls. There are several reasons for this. First, the availability of CNS tissue especially from healthy and young individuals is very limited. Second, the comparison between healthy and control tissue would only allow conclusions in MHC haplotype-matched controls. Interestingly this point is underscored by the finding that common peptides could be eluted from APC in lungs of patients with sarcoidosis and brain tissue from MS patients that expressed HLA-DRB1*0301 (10) (Table V). Third, MHC II is mainly expressed on professional APC, and expression is strong under pathological conditions in MS (45). In contrast in healthy CNS, MHC expression is low. This argument is strongly supported in mice immunized with either MOG in complete Freund's adjuvant or PBS in complete Freund's adjuvant in which the number of isolated peptides out of the same amount of brain tissue is decreased more than 70% in PBS-immunized mice.2 The minimal number of cells allowing the analysis of HLA-bound peptides is ∼109 cells. To reach this amount of MHC II-expressing cells from CNS of healthy controls it would be necessary to greatly increase the amount of tissue.
Table V. Common peptides eluted from CNS and lung of HLA-DRB1*0301-positive MS and sarcoidosis patients, respectively.
Common peptides were mainly observed from patients carrying the same HLA-DR allele, HLA-DR*0301. The peptides are not identical but overlapping and share a minimum of 10 amino acids.
| Protein | Peptide sequence | Patients | Allele | Sarcoidosis patients |
|---|---|---|---|---|
| α2-Macroglobulin | SSKFQVDNNNRL | MS2, MS8 | DRB1*0301, DRB1*1501 | SSKFQVDNNNRL (10) |
| α2-Macroglobulin | GNRIAQWQSFQLEG | MS2 | DRB1*1501 | GNRIAQWQSFQLEG (10) |
| ATP synthase, H+-transporting, F0 complex | PKFEVIEKPQA | MS7 | DRB1*0301 | PTFKFEDPKFEVIEKPQA (10) |
| β-Globin | GKVNVDEVGGEALGRL | MS3, MS5, MS6 | DRB1*0301, DRB1*0101 | KVNVDEVGGEALGRL (10) |
| β-Globin | GKVNVDEVGGEALGRLL | MS5 | DRB1*0101 | KVNVDEVGGEALGRLL (10) |
| β-Globin | EVGGEALGRL | MS6 | DRB1*0301 | |
| β-Globin | EVGGEALGRLL | MS6 | DRB1*0301 | |
| β-Globin | VGGEALGRLL | MS7 | DRB1*0301 | |
| β-Globin | VHLTPEEKSAVTALWGKVNVDEVGGEALGRL | MS8 | DRB1*1501 | |
| Syntenin | ITSIVKDSSAARNG | MS1, MS2 | DRB1*0301 | ITSIVKDSSAARNGI (10) |
| Transferrin | YAVAVVKKDSG | MS2 | DRB1*0301 | DPQTFYYAVAVVKKDSG (10) |
Although many peptides presented by MHC I and II molecules have a significant binding affinity score, we observed HLA ligands with low scores. This is unexpected for peptides presented in MHC I molecules because of the distinct peptide motif of these proteins. A possible explanation is that the SYFPEITHI program does not cover all different HLA alleles, like e.g. HLA-C and HLA-DRB1*13.
Unlike traditional proteomics approaches (46), our approach has the clear advantage to be able to define stretches of proteins that are visible to the immune system and are therefore more likely to be involved in an immune response than peptides defined by classical epitope mapping studies. In addition, the presented MHC ligandome mirrors the proteome that is involved in the disease process. We identified peptides that mirror all the different features of the MS disease process like neurodegeneration, apoptosis, and remyelination. GFAP, tubulin, and actin are biomarkers for neurodegeneration; annexin, Bcl-2-associated transcription factor 1, and survivin are apoptosis-related proteins; and microtubule-associated protein 1B is a marker for actively myelinating oligodendrocytes. Therefore, we believe that disease-relevant information can be generated based on these data by looking at B and T cell responses to peptides and the proteins from which they are derived in the course of MS. Data generated accordingly might allow the disease-driving autoantigens in MS to be better defined. In addition such data can be used for definition of novel biomarkers (47). The eluted peptides are highly interesting in regard to antigen spreading that has been involved in chronicity of autoimmune diseases. It will be a major challenge to define the hierarchy and timing of spreading of the eluted peptides toward T cell epitopes in MS patients. It has been shown that induction of tolerance against autoantigens presented on MHC molecules has a great therapeutic potential in experimental autoimmune diseases (20, 48). Therefore the eluted sequences are also of great interest for the design of tolerogens capable of preventing or treating MS.
Supplementary Material
Footnotes
* This work was supported by German Research Foundation (Deutsche Forschungsgemeinschaft) Grants We 1947/4-1/2 and We 1947/5-1/2 (to R. W. and H. G. R.).
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
2 S. Haag, unpublished data.
1 The abbreviations used are:
- MHC
- major histocompatibility complex
- APC
- antigen-presenting cells
- CNS
- central nervous system
- MBP
- myelin basic protein
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- PLP
- proteolipid protein
- TCR
- T cell receptor
- HLA
- histocompatibility leukocyte antigen
- NCBI
- National Center for Biotechnology Information
- EAE
- experimental autoimmune encephalomyelitis
- GFAP
- glial fibrillary acidic protein
- CSF
- cerebrospinal fluid
- NF
- neurofilament.
REFERENCES
- 1.Rudolph M. G., Stanfield R. L., Wilson I. A. (2006) How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol 24, 419–466 [DOI] [PubMed] [Google Scholar]
- 2.Kamradt T., Mitchison N. A. (2001) Tolerance and autoimmunity. N. Engl. J. Med 344, 655–664 [DOI] [PubMed] [Google Scholar]
- 3.Dittel B. N. (2008) CD4 T cells: Balancing the coming and going of autoimmune-mediated inflammation in the CNS. Brain Behav. Immun 22, 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss H. A., Millward J. M., Owens T. (2007) CD8+ T cells in inflammatory demyelinating disease. J. Neuroimmunol 191, 79–85 [DOI] [PubMed] [Google Scholar]
- 5.Martino G., Furlan R., Brambilla E., Bergami A., Ruffini F., Gironi M., Poliani P. L., Grimaldi L. M., Comi G. (2000) Cytokines and immunity in multiple sclerosis: the dual signal hypothesis. J. Neuroimmunol 109, 3–9 [DOI] [PubMed] [Google Scholar]
- 6.von Herrath M., Sanda S., Herold K. (2007) Type 1 diabetes as a relapsing-remitting disease? Nat. Rev. Immunol 7, 988–994 [DOI] [PubMed] [Google Scholar]
- 7.Trapp B. D., Nave K. A. (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci 31, 247–269 [DOI] [PubMed] [Google Scholar]
- 8.Weissert R., Svenningsson A., Lobell A., de Graaf K. L., Andersson R., Olsson T. (1998) Molecular and genetic requirements for preferential recruitment of TCRBV8S2+ T cells in Lewis rat experimental autoimmune encephalomyelitis. J. Immunol 160, 681–690 [PubMed] [Google Scholar]
- 9.Krogsgaard M., Wucherpfennig K. W., Cannella B., Hansen B. E., Svejgaard A., Pyrdol J., Ditzel H., Raine C., Engberg J., Fugger L., Canella B. (2000) Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85–99 complex. J. Exp. Med 191, 1395–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahlström J., Dengjel J., Persson B., Duyar H., Rammensee H. G., Stevanoviæ S., Eklund A., Weissert R., Grunewald J. (2007) Identification of HLA-DR-bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J. Clin. Investig 117, 3576–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk K., Rötzschke O., Stevanoviæ S., Jung G., Rammensee H. G. (1991) Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351, 290–296 [DOI] [PubMed] [Google Scholar]
- 12.Seeger F. H., Schirle M., Gatfield J., Arnold D., Keilholz W., Nickolaus P., Rammensee H. G., Stevanoviæ S. (1999) The HLA-A*6601 peptide motif: prediction by pocket structure and verification by peptide analysis. Immunogenetics 49, 571–576 [DOI] [PubMed] [Google Scholar]
- 13.Brodsky F. M., Parham P. (1982) Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J. Immunol 128, 129–135 [PubMed] [Google Scholar]
- 14.Lampson L. A., Levy R. (1980) Two populations of Ia-like molecules on a human B cell line. J. Immunol 125, 293–299 [PubMed] [Google Scholar]
- 15.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 16.de Graaf K. L., Weissert R., Kjellén P., Holmdahl R., Olsson T. (1999) Allelic variations in rat MHC class II binding of myelin basic protein peptides correlate with encephalitogenicity. Int. Immunol 11, 1981–1988 [DOI] [PubMed] [Google Scholar]
- 17.Meinl E., Weber F., Drexler K., Morelle C., Ott M., Saruhan-Direskeneli G., Goebels N., Ertl B., Jechart G., Giegerich G. (1993) Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis. Complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J. Clin. Investig 92, 2633–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. (1991) A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J. Exp. Med 173, 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valli A., Sette A., Kappos L., Oseroff C., Sidney J., Miescher G., Hochberger M., Albert E. D., Adorini L. (1993) Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J. Clin. Investig 91, 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sospedra M., Martin R. (2005) Immunology of multiple sclerosis. Annu. Rev. Immunol 23, 683–747 [DOI] [PubMed] [Google Scholar]
- 21.Mathey E. K., Derfuss T., Storch M. K., Williams K. R., Hales K., Woolley D. R., Al-Hayani A., Davies S. N., Rasband M. N., Olsson T., Moldenhauer A., Velhin S., Hohlfeld R., Meinl E., Linington C. (2007) Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med 204, 2363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wucherpfennig K. W., Sette A., Southwood S., Oseroff C., Matsui M., Strominger J. L., Hafler D. A. (1994) Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J. Exp. Med 179, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt A. B., Kropshofer H., Kalbacher H., Kalbus M., Rammensee H. G., Coligan J. E., Martin R. (1994) Ligand motifs of HLA-DRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J. Immunol 153, 1665–1673 [PubMed] [Google Scholar]
- 24.Vergelli M., Kalbus M., Rojo S. C., Hemmer B., Kalbacher H., Tranquill L., Beck H., McFarland H. F., De Mars R., Long E. O., Martin R. (1997) T cell response to myelin basic protein in the context of the multiple sclerosis-associated HLA-DR15 haplotype: peptide binding, immunodominance and effector functions of T cells. J. Neuroimmunol 77, 195–203 [DOI] [PubMed] [Google Scholar]
- 25.Richert J. R., Robinson E. D., Deibler G. E., Martenson R. E., Dragovic L. J., Kies M. W. (1989) Human cytotoxic T-cell recognition of a synthetic peptide of myelin basic protein. Ann. Neurol 26, 342–346 [DOI] [PubMed] [Google Scholar]
- 26.Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. (1990) T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346, 183–187 [DOI] [PubMed] [Google Scholar]
- 27.Malmeström C., Haghighi S., Rosengren L., Andersen O., Lycke J. (2003) Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 28.Huizinga R., Heijmans N., Schubert P., Gschmeissner S., 't Hart B. A., Herrmann H., Amor S. (2007) Immunization with neurofilament light protein induces spastic paresis and axonal degeneration in Biozzi ABH mice. J. Neuropathol. Exp. Neurol 66, 295–304 [DOI] [PubMed] [Google Scholar]
- 29.Krishnamoorthy G., Saxena A., Mars L. T., Domingues H. S., Mentele R., Ben-Nun A., Lassmann H., Dornmair K., Kurschus F. C., Liblau R. S., Wekerle H. (2009) Myelin-specific T cells also recognize neuronal autoantigen in transgenic mouse model of multiple sclerosis. Nat. Med 15, 626–632 [DOI] [PubMed] [Google Scholar]
- 30.Werner P., Pitt D., Raine C. S. (2001) Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann. Neurol 50, 169–180 [DOI] [PubMed] [Google Scholar]
- 31.Kolln J., Ren H. M., Da R. R., Zhang Y., Spillner E., Olek M., Hermanowicz N., Hilgenberg L. G., Smith M. A., van den Noort S., Qin Y. (2006) Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol 177, 5652–5658 [DOI] [PubMed] [Google Scholar]
- 32.Kulakowska A., Drozdowski W., Sadzynski A., Bucki R., Janmey P. A. (2008) Gelsolin concentration in cerebrospinal fluid from patients with multiple sclerosis and other neurological disorders. Eur. J. Neurol 15, 584–588 [DOI] [PubMed] [Google Scholar]
- 33.Madsen L. S., Andersson E. C., Jansson L., krogsgaard M., Andersen C. B., Engberg J., Strominger J. L., Svejgaard A., Hjorth J. P., Holmdahl R., Wucherpfennig K. W., Fugger L. (1999) A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat. Genet 23, 343–347 [DOI] [PubMed] [Google Scholar]
- 34.Martin R., McFarland H. F., McFarlin D. E. (1992) Immunological aspects of demyelinating diseases. Annu. Rev. Immunol 10, 153–187 [DOI] [PubMed] [Google Scholar]
- 35.Muraro P. A., Vergelli M., Kalbus M., Banks D. E., Nagle J. W., Tranquill L. R., Nepom G. T., Biddison W. E., McFarland H. F., Martin R. (1997) Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA-DR4 (B1*0401) subjects is associated with a restricted T cell receptor repertoire. J. Clin. Investig 100, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielekova B., Sung M. H., Kadom N., Simon R., McFarland H., Martin R. (2004) Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol 172, 3893–3904 [DOI] [PubMed] [Google Scholar]
- 37.Yeo T. W., De Jager P. L., Gregory S. G., Barcellos L. F., Walton A., Goris A., Fenoglio C., Ban M., Taylor C. J., Goodman R. S., Walsh E., Wolfish C. S., Horton R., Traherne J., Beck S., Trowsdale J., Caillier S. J., Ivinson A. J., Green T., Pobywajlo S., Lander E. S., Pericak-Vance M. A., Haines J. L., Daly M. J., Oksenberg J. R., Hauser S. L., Compston A., Hafler D. A., Rioux J. D., Sawcer S. (2007) A second major histocompatibility complex susceptibility locus for multiple sclerosis. Ann. Neurol 61, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babbe H., Roers A., Waisman A., Lassmann H., Goebels N., Hohlfeld R., Friese M., Schröder R., Deckert M., Schmidt S., Ravid R., Rajewsky K. (2000) Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med 192, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jersild C., Fog T. (1972) Histocompatibility (HL-A) antigens associated with multiple sclerosis. Acta Neurol. Scand. Suppl 51, 377. [PubMed] [Google Scholar]
- 40.Fogdell-Hahn A., Ligers A., Grønning M., Hillert J., Olerup O. (2000) Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens 55, 140–148 [DOI] [PubMed] [Google Scholar]
- 41.Brynedal B., Duvefelt K., Jonasdottir G., Roos I. M., Akesson E., Palmgren J., Hillert J. (2007) HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS ONE 2, e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang Y. C., Li S., Rivera V. M., Hong J., Robinson R. R., Breitbach W. T., Killian J., Zhang J. Z. (2004) Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J. Immunol 172, 5120–5127 [DOI] [PubMed] [Google Scholar]
- 43.Manoury B., Mazzeo D., Fugger L., Viner N., Ponsford M., Streeter H., Mazza G., Wraith D. C., Watts C. (2002) Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat. Immunol 3, 169–174 [DOI] [PubMed] [Google Scholar]
- 44.Anderton S. M., Viner N. J., Matharu P., Lowrey P. A., Wraith D. C. (2002) Influence of a dominant cryptic epitope on autoimmune T cell tolerance. Nat. Immunol 3, 175–181 [DOI] [PubMed] [Google Scholar]
- 45.Höftberger R., Aboul-Enein F., Brueck W., Lucchinetti C., Rodriguez M., Schmidbauer M., Jellinger K., Lassmann H. (2004) Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 14, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han M. H., Hwang S. I., Roy D. B., Lundgren D. H., Price J. V., Ousman S. S., Fernald G. H., Gerlitz B., Robinson W. H., Baranzini S. E., Grinnell B. W., Raine C. S., Sobel R. A., Han D. K., Steinman L. (2008) Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 [DOI] [PubMed] [Google Scholar]
- 47.Bielekova B., Martin R. (2004) Development of biomarkers in multiple sclerosis. Brain 127, 1463–1478 [DOI] [PubMed] [Google Scholar]
- 48.Harrison L. C., Honeyman M. C., Morahan G., Wentworth J. M., Elkassaby S., Colman P. G., Fourlanos S. (2008) Type 1 diabetes: Lessons for other autoimmune diseases? J. Autoimmun 31, 306–310 [DOI] [PubMed] [Google Scholar]
- 49.Alexander J. S., Minagar A., Harper M., Robinson-Jackson S., Jennings M., Smith S. J. (2007) Proteomic analysis of human cerebral endothelial cells activated by multiple sclerosis serum and IFNbeta-1b. J. Mol. Neurosci 32, 169–178 [DOI] [PubMed] [Google Scholar]
- 50.Dutta R., McDonough J., Chang A., Swamy L., Siu A., Kidd G. J., Rudick R., Mirnics K., Trapp B. D. (2007) Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain 130, 2566–2576 [DOI] [PubMed] [Google Scholar]
- 51.Sharief M. K., Semra Y. K. (2001) Heightened expression of survivin in activated T lymphocytes from patients with multiple sclerosis. J. Neuroimmunol 119, 358–364 [DOI] [PubMed] [Google Scholar]
- 52.Wu H. Y., Dawson M. R., Reynolds R., Hardy R. J. (2001) Expression of QKI proteins and MAP1B identifies actively myelinating oligodendrocytes in adult rat brain. Mol. Cell. Neurosci 17, 292–302 [DOI] [PubMed] [Google Scholar]
- 53.Liu A., Stadelmann C., Moscarello M., Bruck W., Sobel A., Mastronardi F. G., Casaccia-Bonnefil P. (2005) Expression of stathmin, a developmentally controlled cytoskeleton-regulating molecule, in demyelinating disorders. J. Neurosci 25, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libich D. S., Hill C. M., Bates I. R., Hallett F. R., Armstrong S., Siemiarczuk A., Harauz G. (2003) Interaction of the 18.5-kD isoform of myelin basic protein with Ca2+-calmodulin: effects of deimination assessed by intrinsic Trp fluorescence spectroscopy, dynamic light scattering, and circular dichroism. Protein Sci 12, 1507–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poggi A., Zocchi M. R., Carosio R., Ferrero E., Angelini D. F., Galgani S., Caramia M. D., Bernardi G., Borsellino G., Battistini L. (2002) Transendothelial migratory pathways of V delta 1+TCR gamma delta+ and V delta 2+TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients: involvement of phosphatidylinositol 3 kinase and calcium calmodulin-dependent kinase II. J. Immunol 168, 6071–6077 [DOI] [PubMed] [Google Scholar]
- 56.Gray S. G., Dangond F. (2006) Rationale for the use of histone deacetylase inhibitors as a dual therapeutic modality in multiple sclerosis. Epigenetics 1, 67–75 [DOI] [PubMed] [Google Scholar]
- 57.Lu F., Selak M., O'Connor J., Croul S., Lorenzana C., Butunoi C., Kalman B. (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci 177, 95–103 [DOI] [PubMed] [Google Scholar]
- 58.Poggi A., Catellani S., Fenoglio D., Borsellino G., Battistini L., Zocchi M. R. (2007) Adhesion molecules and kinases involved in gammadelta T cells migratory pathways: implications for viral and autoimmune diseases. Curr. Med. Chem 14, 3166–3170 [DOI] [PubMed] [Google Scholar]
- 59.Harness J., Cavanagh A., Morton H., McCombe P. (2003) A protective effect of early pregnancy factor on experimental autoimmune encephalomyelitis induced in Lewis rats by inoculation with myelin basic protein. J. Neurol. Sci 216, 33–41 [DOI] [PubMed] [Google Scholar]
- 60.Bielecki B., Mazurek A., Wolinski P., Glabinski A. (2008) Treatment of multiple sclerosis with methylprednisolone and mitoxantrone modulates the expression of CXC chemokine receptors in PBMC. J. Clin. Immunol 28, 122–130 [DOI] [PubMed] [Google Scholar]
- 61.Omari K. M., John G. R., Sealfon S. C., Raine C. S. (2005) CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain 128, 1003–1015 [DOI] [PubMed] [Google Scholar]
- 62.Hoi A. Y., Iskander M. N., Morand E. F. (2007) Macrophage migration inhibitory factor: a therapeutic target across inflammatory diseases. Inflamm. Allergy Drug Targets 6, 183–190 [DOI] [PubMed] [Google Scholar]
- 63.Papadopoulos D., Ewans L., Pham-Dinh D., Knott J., Reynolds R. (2006) Upregulation of alpha-synuclein in neurons and glia in inflammatory demyelinating disease. Mol. Cell. Neurosci 31, 597–612 [DOI] [PubMed] [Google Scholar]
- 64.Bartos A., Fialová L., Soukupová J., Kukal J., Malbohan I., Pit'ha J. (2007) Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J. Neurol 254, 20–25 [DOI] [PubMed] [Google Scholar]
- 65.Bartos A., Fialová L., Soukupová J., Kukal J., Malbohan I., Pitha J. (2007) Antibodies against light neurofilaments in multiple sclerosis patients. Acta Neurol. Scand 116, 100–107 [DOI] [PubMed] [Google Scholar]
- 66.Rejdak K., Petzold A., Stelmasiak Z., Giovannoni G. (2008) Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult. Scler 14, 59–66 [DOI] [PubMed] [Google Scholar]
- 67.Hammack B. N., Fung K. Y., Hunsucker S. W., Duncan M. W., Burgoon M. P., Owens G. P., Gilden D. H. (2004) Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult. Scler 10, 245–260 [DOI] [PubMed] [Google Scholar]
- 68.Jensen P. E., Humle Jørgensen S., Datta P., Sørensen P. S. (2004) Significantly increased fractions of transformed to total alpha2-macroglobulin concentrations in plasma from patients with multiple sclerosis. Biochim. Biophys. Acta 1690, 203–207 [DOI] [PubMed] [Google Scholar]
- 69.Gunnarsson M., Sundström P., Stigbrand T., Jensen P. E. (2003) Native and transformed alpha2-macroglobulin in plasma from patients with multiple sclerosis. Acta Neurol. Scand 108, 16–21 [DOI] [PubMed] [Google Scholar]
- 70.Reindl M., Knipping G., Wicher I., Dilitz E., Egg R., Deisenhammer F., Berger T. (2001) Increased intrathecal production of apolipoprotein D in multiple sclerosis. J. Neuroimmunol 119, 327–332 [DOI] [PubMed] [Google Scholar]
- 71.Li F. Q., Sempowski G. D., McKenna S. E., Laskowitz D. T., Colton C. A., Vitek M. P. (2006) Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther 318, 956–965 [DOI] [PubMed] [Google Scholar]
- 72.Sfagos C., Makis A. C., Chaidos A., Hatzimichael E. C., Dalamaga A., Kosma K., Bourantas K. L. (2005) Serum ferritin, transferrin and soluble transferrin receptor levels in multiple sclerosis patients. Mult. Scler 11, 272–275 [DOI] [PubMed] [Google Scholar]
- 73.Orbach H., Zandman-Goddard G., Amital H., Barak V., Szekanecz Z., Szucs G., Danko K., Nagy E., Csepany T., Carvalho J. F., Doria A., Shoenfeld Y. (2007) Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann. N.Y. Acad. Sci 1109, 385–400 [DOI] [PubMed] [Google Scholar]
- 74.Liu L., Huang D., Matsui M., He T. T., Hu T., Demartino J., Lu B., Gerard C., Ransohoff R. M. (2006) Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J. Immunol 176, 4399–4409 [DOI] [PubMed] [Google Scholar]
- 75.Haase C. G., Schmidt S., Faustmann P. M. (2002) Frequencies of the G-protein beta3 subunit C825T polymorphism and the delta 32 mutation of the chemokine receptor-5 in patients with multiple sclerosis. Neurosci. Lett 330, 293–295 [DOI] [PubMed] [Google Scholar]
- 76.Petry H., Cashion L., Szymanski P., Ast O., Orme A., Gross C., Bauzon M., Brooks A., Schaefer C., Gibson H., Qian H., Rubanyi G. M., Harkins R. N. (2006) Mx1 and IP-10: biomarkers to measure IFN-beta activity in mice following gene-based delivery. J. Interferon Cytokine Res 26, 699–705 [DOI] [PubMed] [Google Scholar]
- 77.Wilczak N., De Bleser P., Luiten P., Geerts A., Teelken A., De Keyser J. (2000) Insulin-like growth factor II receptors in human brain and their absence in astrogliotic plaques in multiple sclerosis. Brain Res 863, 282–288 [DOI] [PubMed] [Google Scholar]
- 78.Deng X., Ljunggren-Rose A., Maas K., Sriram S. (2005) Defective ATM-p53-mediated apoptotic pathway in multiple sclerosis. Ann. Neurol 58, 577–584 [DOI] [PubMed] [Google Scholar]
- 79.Wosik K., Antel J., Kuhlmann T., Brück W., Massie B., Nalbantoglu J. (2003) Oligodendrocyte injury in multiple sclerosis: a role for p53. J. Neurochem 85, 635–644 [DOI] [PubMed] [Google Scholar]
- 80.Leech S., Kirk J., Plumb J., McQuaid S. (2007) Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol 33, 86–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.