Abstract
Context:
Isolated lumbar paraspinal muscle fatigue causes lower extremity and postural control deficits.
Objective:
To describe the change in body position during gait after fatiguing lumbar extension exercises in persons with recurrent episodes of low back pain compared with healthy controls.
Design:
Case-control study.
Setting:
Motion analysis laboratory.
Patients or Other Participants:
Twenty-five recreationally active participants with a history of recurrent episodes of low back pain, matched by sex, height, and mass with 25 healthy controls.
Intervention(s):
We measured 3-dimensional lower extremity and trunk kinematics before and after fatiguing isometric lumbar paraspinal exercise.
Main Outcome Measure(s):
Measurements were taken while participants jogged on a custom-built treadmill surrounded by a 10-camera motion analysis system.
Results:
Group-by-time interactions were observed for lumbar lordosis and trunk angles (P < .05). A reduced lumbar spine extension angle was noted, reflecting a loss of lordosis and an increase in trunk flexion angle, indicating increased forward trunk lean, in healthy controls after fatiguing lumbar extension exercise. In contrast, persons with a history of recurrent low back pain exhibited a slight increase in spine extension, indicating a slightly more lordotic position of the lumbar spine, and a decrease in trunk flexion angles after fatiguing exercise. Regardless of group, participants experienced, on average, greater peak hip extension after lumbar paraspinal fatigue.
Conclusions:
Small differences in response may represent a necessary adaptation used by persons with recurrent low back pain to preserve gait function by stabilizing the spine and preventing inappropriate trunk and lumbar spine positioning.
Keywords: gait analysis, spine
Key Points
In healthy participants, isolated lumbar paraspinal muscle fatiguing exercise caused a more forward-flexed trunk and less lordotic and more laterally bent spine position during jogging gait.
Participants with low back pain exhibited fewer postural adjustments in response to isolated paraspinal muscle fatiguing exercise. This may be a necessary mechanism to avoid potentially detrimental spine positions.
Low back pain (LBP) remains a significant health care issue, with more than 70% of people in the United States experiencing at least 1 episode during their lifetime.1 The annual incidence of LBP has been estimated to involve up to 45% of the population, resulting in more than $40 billion in economic costs in the form of medical treatment and lost wages.2,3 Individuals who want to maintain a healthy lifestyle may be restricted because of recurring and disabling nonspecific LBP.4 Those who must continue with normal and necessary activities of daily living may choose an adaptive mechanism to preserve function. Some may use an unfavorable adaptive strategy, possibly exposing muscles and joints to injury or long-term degenerative processes. Kinematic changes during activities and gait may help to explain the recurrent nature of LBP.
Poor lumbar extension endurance, measured as the duration of sustained isometric contraction of the lumbar paraspinals muscles (ie, the Biering-Sorensen test), has been identified as a risk factor for developing LBP.5–7 Similarly, persons with current LBP8 or a history of LBP9 exhibit poor lumbar extension endurance compared with controls. Thus, the rate of fatigue in these muscles may be different during exercise in persons with lumbar paraspinal weakness and poor endurance. Research10–13 using models of isolated lumbar paraspinal fatigue recreates a condition of poor core stability in order to study potential adaptations during exercise in controlled settings. The core describes the active and passive structures comprising and providing stability to the lumbo-pelvic-hip complex.14
Isolated lumbar paraspinal fatiguing exercise has been used to compare postural control and neuromuscular response in healthy persons and in those with recurrent LBP.10–13 In healthy persons, similar localized lumbar paraspinal fatigue resulted in deteriorated postural sway and caused participants to exhibit a forward-flexed position while standing.10,13 Recreationally active persons with recurrent LBP and healthy controls experienced reduced quadriceps voluntary activation11,12 after isolated lumbar paraspinal fatiguing exercise. Isolated lumbar paraspinal fatigue may cause kinematic adaptations during jogging gait that may affect persons with recurrent LBP differently than uninjured individuals. Difficulty maintaining appropriate positioning and stability of the trunk as a result of excessive fatigue may affect lower extremity joints during activities and may help to describe lower extremity injury risk in persons with poor core and trunk stability. Therefore, the purpose of our study was to compare 3-dimensional trunk and lower extremity joint kinematics during jogging gait before and after lumbar paraspinal fatiguing exercise in persons with a history of recurrent episodes of LBP and controls.
METHODS
A 2 × 2 repeated-measures, time-series design (pretest, posttest) with static group comparison was used to compare lower extremity and trunk kinematics during jogging gait after fatiguing isometric lumbar extension exercise. The independent variables were group (history of LBP, control) and time (baseline, postfatiguing exercise set). The dependent variables were peak sagittal-plane, frontal-plane, and transverse-plane angles of the knee, hip, lumbar spine, and trunk during the loading phase of jogging.
Twenty-five participants with a history of recurrent LBP, including 12 females (age = 22.3 ± 2.7 years, height = 169.2 ± 6.5 cm, mass = 64.5 ± 6.2 kg) and 13 males (age = 22.9 ± 3.5 years, height = 183.4 ± 7.8 cm, mass = 83.5 ± 11.8 kg), were matched for sex, height, and mass with 12 healthy females (age = 20.8 ± 1.0 years, height = 169.5 ± 7.2 cm, mass = 64.6 ± 7.3 kg) and 13 healthy males (age = 24.5 ± 4.5 years, height = 182.4 ± 6.1 cm, mass = 81.8 ± 11.0 kg). All volunteers provided informed consent before participating. This study was approved by our university's institutional review board.
All participants were recreationally active, had healthy knees (no current pain, no history of knee injury or surgery), and denied history of lower extremity surgery or recent injury (within 6 months). Volunteers were excluded from participating if they reported a history of intervertebral disc injury, cancer, neurologic injury, or radicular symptoms in the lower extremity; vertebral fracture; or spine surgery. The group with recurrent LBP group consisted of persons who met the above inclusion criteria but reported at least 3 LBP episodes within the past 3 years or at least 5 LBP episodes in their lifetime. An episode was defined as LBP sufficient to impose limitations or modifications to daily activities.11,12 Persons in the control group reported never having had LBP.
Instruments
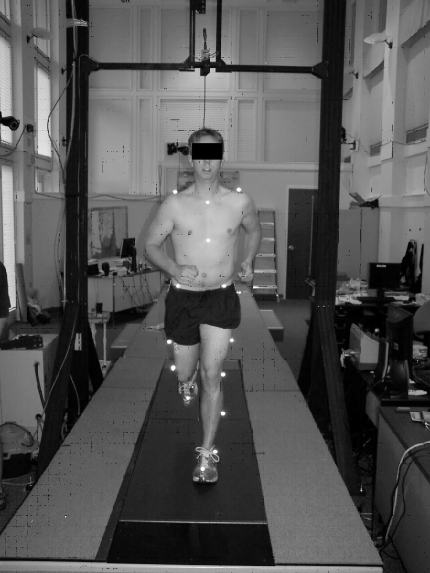
Joint angles were calculated during jogging gait with a 10-camera motion analysis system (model 624; Vicon Motion Systems Inc, Lake Forest, CA) in conjunction with a multiaxis strain gauge force plate embedded beneath the moving belt of a custom-built treadmill (AMTI; Watertown, MA) (Figure 1). Force and video were sampled at 120 Hz.
Figure 1.
Experimental setup for recording 3-dimensional kinematics during jogging gait.
A lumbar hyperextension chair (Figure 2) was used to allow participants to comfortably perform isometric lumbar paraspinal muscle contractions. The chair's footpads provided leverage, so the torso was unsupported by any part of the chair. Handlebars helped to support upper body weight and relieve the lumbar paraspinal muscles during rest periods.
Figure 2.
Testing position for lumbar paraspinal muscle fatiguing exercise.
To quantify paraspinal fatigue, we used lumbar paraspinal muscle surface electromyography (EMG). Signals were amplified with a high-gain, differential-input, biopotential amplifier (model EMG100C; Biopac Systems, Inc, Goleta, CA) with a gain of 1000 and digitized with a 16-bit data acquisition system (model MP150; Biopac Systems) at 2000 Hz with a common-mode rejection ratio of 110 dB, an input impedance of 1.0 MΩ, and a noise voltage of 0.2 µV.
Procedures
Before data collection, a licensed and certified athletic trainer (J.M.H.) performed a physical examination on all participants. Volunteers were excluded if they displayed any lower quarter neurologic bilateral asymmetry, pain (greater than 3/10 on a 10-point scale) with standing lumbar extension, the inability to extend the spine at least 15° (measured with a standard goniometer), or a positive straight-leg test.11,12
Participant Preparation
Two round, 35-mm–diameter, pre-gelled Ag-AgCl surface electrodes were placed on the skin over the lumbar paraspinal muscle group after the skin was shaved, debrided, and cleaned with isopropyl alcohol. Electrodes were placed about 2 cm apart, parallel to muscle fiber orientation at approximately the L4–L5 spinal level, and over muscle tissue, as verified by palpation and visual inspection of surface EMG signal during an active contraction. A ground electrode was placed on the anterior mid-tibia. Participants were then fit with 22 (16 lower body and 6 upper body) 14-mm–diameter, retroreflective markers according to the plug-in-gait15,16 guidelines for marker placement on the torso, pelvis, thigh, shank, and foot. Markers were placed bilaterally on the second metatarsal head, posterior heel, lateral malleolus, shank, lateral epicondyle of the knee, thigh, anterior superior iliac spine, posterior superior iliac spine, and acromion process; on the xyphoid process and jugular notch of the sternum; and over the spinous processes of C7 and T10.
Testing Protocol
After a 5-minute warm-up and familiarization period on the treadmill (Figure 1), participants jogged at a self-selected, comfortable speed for approximately 60 seconds while we recorded baseline 3-dimensional kinematic data. Participants then positioned themselves in the lumbar extension exercise chair (Figure 2) and performed 1 set of fatiguing, isometric lumbar extension exercise. Once they experienced mild lumbar extensor fatigue, they returned immediately (within seconds) to the treadmill and jogged for approximately 60 seconds while we recorded postexercise kinematic data. Treadmill speed during postfatigue data collection was matched exactly to baseline speed. Jogging kinematics and kinetics are reliable17,18 and comparable with over-ground jogging when data are recorded on a treadmill.19
Fatiguing Exercise
The fatiguing isometric lumbar extension exercise set consisted of repeated cycles of 10-second, gravity-resisted isometric contractions followed by a 10-second rest. During each contraction, participants were verbally encouraged and provided with verbal feedback in order to ensure they maintained a trunk position parallel to the floor. A 1-second clip of surface EMG during each isometric contraction was recorded from the right-sided lumbar paraspinal muscles and processed to quantify the amount of local muscle fatigue during the exercise set by calculating the median frequency (MedF) from each contraction. We calculated the MedF in the time it took to perform each exercise repetition in order to provide near “real-time” feedback regarding muscle fatigue. The procedure for calculating MedF from each repetition is described in detail elswhere.11,12 Fatigue is a continuous process, during which the proportion of motor unit recruitment shifts from higher-frequency (more fatigable) to lower-frequency (less fatigable) motor units.20 In order to monitor lumbar paraspinal fatigue, we continuously monitored the downward shift in MedF while the volunteers exercised. When the downward shift in MedF exceeded 10%12 (compared with the MedF from the first repetition), the participant was instructed to stop the exercise set and return to the treadmill for postexercise data collection.
Data Analysis
We recorded peak joint angles in 3 planes for the trunk, spine, hip, and knee. The peak joint angles during the loading phase of stance were defined as the highest angle measured in each plane during the first 50% of the stance phase. The stance phase included the time the limbs were in contact with the force plate from ipsilateral heel contact to toe-off. The loading phase was considered the portion of the stance phase during which the muscles were eccentrically contracting to attenuate impact forces. We averaged the peak joint angle for 5 consecutive loading phases for analysis. Marker data were filtered with a Woltring filter and interpreted with Vicon Workstation software (version 5.0; Los Angeles, CA). Joint angles were calculated as previously described.15,16 Specifically, trunk and pelvis segment angles were determined based on the internally fixed laboratory coordinate system; the spine angle was calculated as a relative angle between the trunk and pelvis segments.
We performed a fixed-model, repeated-measures analysis of variance to compare peak joint angles (within participants) between baseline and postfatiguing exercise and between groups (recurrent LBP and control). We used t tests for post hoc analysis, where appropriate. For analysis, we used SPSS (version 13.0; SPSS Inc, Chicago, IL). We defined statistical significance as P < .05.
RESULTS
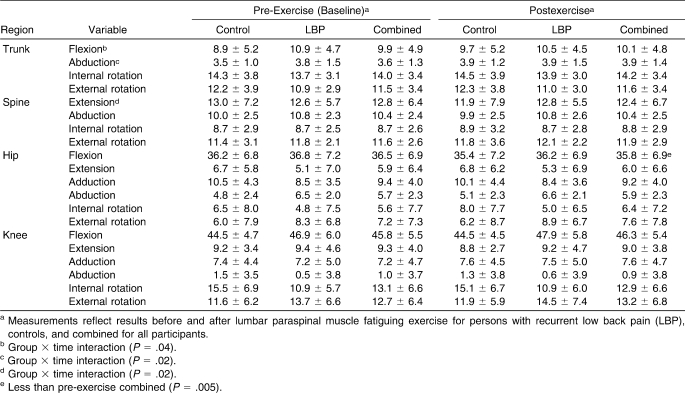
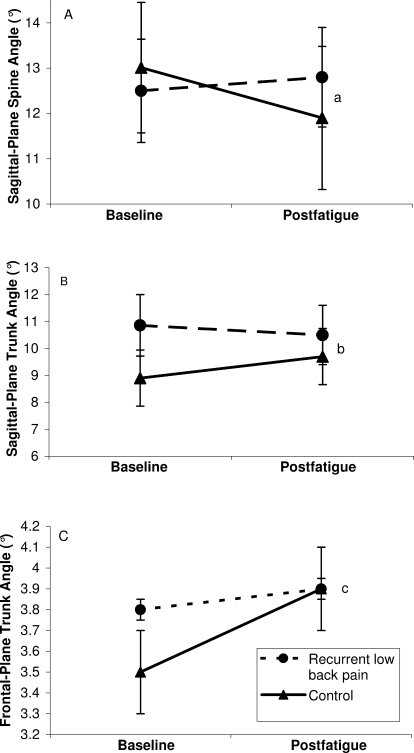
Group-by-time interactions were observed for peak spine extension angles (F1,36 = 4.7, P = .04), peak trunk flexion angles (F1,36 = 5.8, P = .02), and peak trunk abduction angles (lateral flexion) (F1,36 = 6.0, P = .02) (Table 1). After fatiguing exercise, control volunteers experienced, on average, a 1.1° reduction (P = .02) in peak spine angle (an estimate of lumbar lordosis by which higher values indicate greater lumbar lordosis), whereas persons with recurrent LBP experienced, on average, a 0.2° increase (P = .62) in peak spine extension angle (Figure 3). In control participants, peak trunk flexion angle increased by 0.8° (P = .04) after fatiguing exercise, but this value was reduced by 0.4° (P = .62) in persons with recurrent LBP (Figure 3). Finally, control volunteers experienced an increase in peak trunk abduction (lateral flexion) of 0.4° (P = .05), and persons with LBP experienced an increase of 0.1° (P = .22) after paraspinal exercise.
Table 1.
Selected Kinematic Variables (Mean ± SD, °)
Figure 3.
Peak sagittal-plane spine (A) and trunk (B) and frontal-plane trunk (C) angles (mean ± SEM) measured during the loading phase of jogging gait before (baseline) and after fatiguing isometric lumbar extension exercise. The control group exhibited reduced sagittal-plane spine (a P = .02) and trunk (b P = .04) angles and frontal-plane trunk (c P = .05) angles postexercise, whereas the group with low back pain did not (P = .62, P = .62, and P = .22, respectively). Greater spine angle values indicate greater lumbar lordosis, and greater trunk angles indicate greater trunk flexion and lateral flexion in the sagittal plane and frontal plane, respectively.
On average, persons with recurrent LBP exhibited greater peak hip abduction angles (F1,36 = 5.3, P = .03) but less peak knee internal rotation (F1,36 = 4.7, P = .04) during jogging gait compared with the control group. Although average peak knee flexion angles were higher in patients with LBP during jogging gait, the finding was nonsignificant (F1,36 = 3.1, P = .09, 1−β = 0.40).
A main effect for time was noted. On average, volunteers experienced a reduction in peak hip flexion angles after lumbar paraspinal fatigue (F1,36 = 8.8, P = .005; Table 1).
DISCUSSION
Persons with a history of recurrent LBP position their trunk and spine differently than do controls in response to fatiguing isometric lumbar extension exercise. Overall, isolated lumbar paraspinal fatiguing exercise caused a more forward-flexed trunk and a less lordotic and more laterally bent spine position in healthy participants during jogging gait. Persons with LBP exhibited consistently fewer postural adjustments at the spine in response to isolated paraspinal fatigue, which may illustrate an adaptation to simulated core instability in healthy volunteers. Persons with a history of recurring LBP may be exhibiting a coping mechanism to avoid potentially detrimental spine positioning. However, greater lumbar muscle activation has been reported with more lordotic lumbar positioning versus more kyphotic positioning21 and in more flexed positions of the trunk,22 indicating that these positions may provide better dynamic stability but may increase the risk for fatigue in these muscles during prolonged tasks. Because the force-producing capacity of a muscle is reduced as it fatigues, persons with recurrent LBP may use different positioning strategies while coping with fatigued muscles in an attempt to stabilize the spine. This adaptation may explain why persons with a history of LBP experience excessive fatigue in the muscles that support the lumbar spine, hips, and pelvis.23
Similar to the fatiguing intervention in the current study, isolated lumbar paraspinal fatigue has been used by previous researchers10,13 investigating postural control. Although a variety of methods are available for inducing localized muscular fatigue, these protocols are all similar in that the localized fatigue in essence creates an artificial condition of core instability in healthy volunteers. High levels of fatigability in the lumbar paraspinal muscles have been reported as a risk factor for LBP.6,7,23 In healthy persons without a history of LBP, lumbar paraspinal fatigue resulted in an anteriorly displaced center of pressure and center of mass, indicating a forward-leaning posture13,24 and an altered postural control strategy in response to a balance perturbation after isolated lumbar paraspinal fatigue.24 In addition, lumbar fatigue resulted in a more forward-flexed posture and impaired postural control during quiet standing.13 Our findings are consistent with those of these previous authors in suggesting that a more forward-flexed posture results from localized lumbar paraspinal fatigue in healthy individuals who have never experienced LBP. However, in persons who have experienced recurrent LBP, the opposite occurred. Finally, isolated lumbar fatigue24,25 and a forward-leaning posture26 have been associated with an increase in antagonist muscle activity in the rectus abdominis muscle in healthy persons. We did not record EMG during jogging gait; however, we speculate that this response occurred in the control group, as they experienced forward trunk posture during jogging gait. In persons with recurrent LBP, a lack of abdominal muscle strength and endurance, common in this population, may help to explain why they did not experience a similar adaptive postural mechanism during jogging gait.
Altered trunk and lumbar spine positioning during activity may change compressive loading of the intervertebral facet joints, increasing compressive loading at the intervertebral disc and pressure in the nucleus pulposus.27 This may result in abnormal compression or tension stresses on the anterior and posterior aspects of the intervertebral disc, respectively, potentially increasing the likelihood of LBP.27,28 In addition, a forward-flexed posture of the trunk causes greater intervertebral fluid loss and less nutrient diffusion into the disc.29 In our study, persons with LBP may have been adapting to isolated lumbar fatigue by avoiding potentially injurious trunk and spine positions.
During gait, a more forward-leaning position of the trunk causes a forward excursion of the body's center of mass,30,31 which is characterized by sustained knee flexion during the stance phase in addition to faster ground reaction force loading rates31 and increased metabolic demand.32 These changes may also alter lower extremity joint moments. For example, greater lateral trunk sway has been associated with reduced ipsilateral knee adduction moments.33 Similarly, it is reasonable to expect a change in the sagittal-plane knee moment with a more forward-flexed posture of the spine and trunk. Previously, lumbar paraspinal fatigue resulted in reduced quadriceps activation11,12 and reduction in the external knee flexion moment,34 indicating a coping response that may include quadriceps avoidance during gait.35 In the current study, we observed slightly greater knee flexion angles (however, they were not statistically significant and were of suboptimal statistical power) during the first half of the stance phase of gait. This finding is similar to the finding of compensatory mechanisms for maintaining a more upright posture of the trunk during gait.36 Crouch gait, characterized by excessive knee flexion during terminal swing and the initial phase of stance36 (ie, loading phase), has been observed as an adaptive mechanism to excessive forward lean during gait.31 This lower extremity compensatory mechanism has been observed during gait in persons with postsurgical flatback deformity whose gait patterns resemble those of persons with advanced knee joint osteoarthritis.37 This compensation orients the trunk in a more vertical position but may place abnormal stresses on the lower extremity joints during the stance phase of gait. Based on the current data, we cannot comment on the potential influence of altered trunk posture during gait on lower extremity injury risk.
Persons with chronic LBP typically have poor endurance in the muscles that support the lumbo-pelvic-hip complex; in particular, poor lumbar paraspinal endurance has been linked with risk for developing LBP.7 We used the model of core instability in healthy persons and observed a postural response during jogging gait. Persons in the LBP group were recreationally active and reported recurring episodes of LBP. Through our screening history and physical examination, we attempted to isolate those with muscle-related LBP by excluding volunteers who may have had other conditions contributing to recurring LBP, such as disc or bone injury, tumor, or nerve involvement. Therefore, we hypothesize that the participants in the recurrent LBP group might have been exhibiting a familiar postural coping mechanism that the control group was not able to use. This may represent a shunting response to the core as a global protective mechanism to avoid unnecessary movements of the trunk that may predispose the lower extremity and spine joints to inappropriate or excessive forces. Persons in the LBP group, who were recreationally active, may have experienced altered spine positioning previously and so were using a mechanism to avoid such positions in order to preserve function during jogging gait.
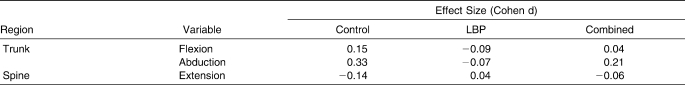
The kinematic changes we observed are small and raise an important distinction between statistical significance and clinical importance. The effect sizes for the control group from prefatigue to postfatigue ranged from small to medium (Table 2); however, kinematic movements were recorded during a self-selected jogging task at a comfortable pace and not during a provocative maneuver, such as a drop landing or other simulated perturbation. Tasks that mimic common joint injury mechanisms may magnify subtle changes over time or between groups. However, the effects of very small changes in joint positions observed during jogging gait in the current study may represent changes that would potentially have a greater, cumulative effect on an athlete over the course of an entire game, season, career, or lifetime. Although we did not observe large effect sizes, small fluctuations in spine kinematics may be of considerable clinical importance with regard to the long-term joint health of persons with recurring LBP. Zazulak et al38 measured transverse-plane trunk proprioception in athletes and followed them prospectively for 3 years. A mean, statistically significant difference of 0.7° was seen in transverse-plane trunk active reposition error in females who experienced knee joint injuries during the follow-up period compared with females who did not sustain knee injuries. This small difference resulted in an odds ratio that increased 2.9-fold (in terms of the likelihood of experiencing a knee injury) for every degree in increased transverse-plane active position error.38 In addition, small differences in maximum flexion, extension, and lateral trunk displacement in response to a sudden perturbation were predictors for knee ligament injury, with 91% sensitivity and 68% specificity, indicating that greater trunk displacement predicted knee ligament injury.39 For men and women, a history of LBP predicted risk for knee ligament inury.39 Therefore, small differences in core proprioception and neuromuscular control and LBP history may have profound effects on lower extremity injury risk in active populations.
Table 2.
Effect Sizes for Selected Pre-Exercise to Postexercise Changes in Jogging Gait Kinematics Between Persons with Recurrent Low Back Pain (LBP) and Controls
To conclude, in response to fatiguing isometric lumbar extension exercise, persons with a history of recurrent LBP position their trunk and spine differently than do controls. The observed differences are very small; however, they may represent a necessary adaptation used by persons with recurrent LBP to preserve gait function by stabilizing the spine and preventing inappropriate trunk and lumbar spine positioning. These changes may be of clinical importance in the development of recurrent LBP.
Acknowledgments
We thank Karen Raphaels, Gabriele Paolini, Jen Boxer, and Pat Riley for contributing to this research through assistance with data collection and analysis.
Footnotes
Joseph M. Hart, PhD, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. D. Casey Kerrigan, MD, MS; Julie M. Fritz, PhD, PT, ATC; and Christopher D. Ingersoll, PhD, ATC, FNATA, FACSM, contributed to conception and design; analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
REFERENCES
- 1.Papageorgiou A. C., Croft P. R., Ferry S., Jayson M. I., Silman A. J. Estimating the prevalence of low back pain in the general population: evidence from the South Manchester Back Pain Survey. Spine. 1995;20(17):1889–1894. doi: 10.1097/00007632-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Andersson G. The epidemiology of spinal disorders. In: Frymoyer J., editor. The Adult Spine. New York, NY: Raven Press; 1997. pp. 93–141. [Google Scholar]
- 3.Andersson G. B. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 4.Verbunt J. A., Sieben J. M., Seelen H. A., et al. Decline in physical activity, disability and pain-related fear in sub-acute low back pain. Eur J Pain. 2005;9(4):417–425. doi: 10.1016/j.ejpain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Biering-Sorensen F. A one-year prospective study of low back trouble in a general population: the prognostic value of low back history and physical measurements. Dan Med Bull. 1984;31(5):362–375. [PubMed] [Google Scholar]
- 6.Biering-Sorensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine. 1984;9(2):106–119. doi: 10.1097/00007632-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sorensen F., Thomsen C. E., Hilden J. Risk indicators for low back trouble. Scand J Rehabil Med. 1989;21(3):151–157. [PubMed] [Google Scholar]
- 8.Latimer J., Maher C. G., Refshauge K., Colaco I. The reliability and validity of the Biering-Sorensen test in asymptomatic subjects and subjects reporting current or previous nonspecific low back pain. Spine. 1999;24(20):2085–2090. doi: 10.1097/00007632-199910150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds M. J., Olson S. L., Jones S., et al. Psychometric characteristics and clinical usefulness of physical performance tests in patients with low back pain. Spine. 1998;23(22):2412–2421. doi: 10.1097/00007632-199811150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Davidson B. S., Madigan M. L., Nussbaum M. A. Effects of lumbar extensor fatigue and fatigue rate on postural sway. Eur J Appl Physiol. 2004;93(1–2):183–189. doi: 10.1007/s00421-004-1195-1. [DOI] [PubMed] [Google Scholar]
- 11.Hart J. M., Fritz J. M., Kerrigan D. C., Saliba E. N., Gansneder B. M., Ingersoll C. D. Quadriceps inhibition after repetitive lumbar extension exercise in persons with a history of low back pain. J Athl Train. 2006;41(3):264–269. [PMC free article] [PubMed] [Google Scholar]
- 12.Hart J. M., Fritz J. M., Kerrigan D. C., Saliba E. N., Gansneder B. M., Ingersoll C. D. Reduced quadriceps activation after lumbar paraspinal fatiguing exercise. J Athl Train. 2006;41(1):79–86. [PMC free article] [PubMed] [Google Scholar]
- 13.Madigan M. L., Davidson B. S., Nussbaum M. A. Postural sway and joint kinematics during quiet standing are affected by lumbar extensor fatigue. Hum Mov Sci. 2006;25(6):788–799. doi: 10.1016/j.humov.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Hammill R. R., Beazell J. R., Hart J. M. Neuromuscular consequences of low back pain and core dysfunction. Clin Sports Med. 2008;27(3):449–462,ix. doi: 10.1016/j.csm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Davis R. B., III, Õunpuu S., Tyburski D., Gage J. R. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10(5):575–587. [Google Scholar]
- 16.Kadaba M. P., Ramakrishnan H. K., Wootten M. E. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 17.Diss C. E. The reliability of kinetic and kinematic variables used to analyse normal running gait. Gait Posture. 2001;14(2):98–103. doi: 10.1016/s0966-6362(01)00125-4. [DOI] [PubMed] [Google Scholar]
- 18.Ferber R., McClay Davis I., Williams D. S., III, Laughton C. A comparison of within- and between-day reliability of discrete 3D lower extremity variables in runners. J Orthop Res. 2002;20(6):1139–1145. doi: 10.1016/S0736-0266(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 19.Riley P. O., Dicharry J., Franz J., Croce U. D., Wilder R. P., Kerrigan D. C. A kinematics and kinetic comparison of overground and treadmill running. Med Sci Sports Exerc. 2008;40(6):1093–1100. doi: 10.1249/MSS.0b013e3181677530. [DOI] [PubMed] [Google Scholar]
- 20.Basmajian J. V., De Luca C. J. Muscles Alive: Their Functions Revealed by Electromyography. Baltimore, MD: Williams & Wilkins; 1985. [Google Scholar]
- 21.Arjmand N., Shirazi-Adl A. Biomechanics of changes in lumbar posture in static lifting. Spine. 2005;30(23):2637–2648. doi: 10.1097/01.brs.0000187907.02910.4f. [DOI] [PubMed] [Google Scholar]
- 22.Farrokhi S., Pollard C. D., Souza R. B., Chen Y. J., Reischl S., Powers C. M. Trunk position influences the kinematics, kinetics, and muscle activity of the lead lower extremity during the forward lunge exercise. J Orthop Sports Phys Ther. 2008;38(7):403–409. doi: 10.2519/jospt.2008.2634. [DOI] [PubMed] [Google Scholar]
- 23.Kankaanpää M., Taimela S., Laaksonen D., Hanninen O., Airaksinen O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil. 1998;79(4):412–417. doi: 10.1016/s0003-9993(98)90142-3. [DOI] [PubMed] [Google Scholar]
- 24.Wilson E. L., Madigan M. L., Davidson B. S., Nussbaum M. A. Postural strategy changes with fatigue of the lumbar extensor muscles. Gait Posture. 2006;23(3):348–354. doi: 10.1016/j.gaitpost.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Granata K. P., Orishimo K. F., Sanford A. H. Trunk muscle coactivation in preparation for sudden load. J Electromyogr Kinesiol. 2001;11(4):247–254. doi: 10.1016/s1050-6411(01)00003-7. [DOI] [PubMed] [Google Scholar]
- 26.Horak F. B., Moore S. P. The effect of prior leaning on human postural responses. Gait Posture. 1993;1(4):203–210. [Google Scholar]
- 27.Adams M. A., Hutton W. C. The effect of posture on the lumbar spine. J Bone Joint Surg Br. 1985;67(4):625–629. doi: 10.1302/0301-620X.67B4.4030863. [DOI] [PubMed] [Google Scholar]
- 28.Adams M. A., Hutton W. C. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br. 1980;62(3):358–362. doi: 10.1302/0301-620X.62B3.6447702. [DOI] [PubMed] [Google Scholar]
- 29.Adams M. A., Hutton W. C. The effect of posture on the fluid content of lumbar intervertebral discs. Spine. 1983;8(6):665–671. doi: 10.1097/00007632-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Grasso R., Zago M., Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. J Neurophysiol. 2000;83(1):288–300. doi: 10.1152/jn.2000.83.1.288. [DOI] [PubMed] [Google Scholar]
- 31.Saha D., Gard S., Fatone S. The effect of trunk flexion on able-bodied gait. Gait Posture. 2008;27(4):653–660. doi: 10.1016/j.gaitpost.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Saha D., Gard S., Fatone S., Ondra S. The effect of trunk-flexed postures on balance and metabolic energy expenditure during standing. Spine. 2007;32(15):1605–1611. doi: 10.1097/BRS.0b013e318074d515. [DOI] [PubMed] [Google Scholar]
- 33.Briem K., Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27(1):78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- 34.Hart J. M., Kerrigan D. C., Fritz J. M., Saliba E. N., Gansneder B., Ingersoll C. D. Jogging gait kinetics following fatiguing lumbar paraspinal exercise. J Electromyograph Kinesiol. In press. [DOI] [PubMed]
- 35.Berchuck M., Andriacchi T. P., Bach B. R., Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871–877. [PubMed] [Google Scholar]
- 36.van der Krogt M. M., Doorenbosch C. A., Harlaar J. Muscle length and lengthening velocity in voluntary crouch gait. Gait Posture. 2007;26(4):532–538. doi: 10.1016/j.gaitpost.2006.11.208. [DOI] [PubMed] [Google Scholar]
- 37.Sarwahi V., Boachie-Adjei O., Backus S. I., Taira G. Characterization of gait function in patients with postsurgical sagittal (flatback) deformity: a prospective study of 21 patients. Spine. 2002;27(21):2328–2337. doi: 10.1097/00007632-200211010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Zazulak B. T., Hewett T. E., Reeves N. P., Goldberg B., Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35(3):368–373. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]
- 39.Zazulak B. T., Hewett T. E., Reeves N. P., Goldberg B., Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35(7):1123–1130. doi: 10.1177/0363546507301585. [DOI] [PubMed] [Google Scholar]