Introduction
The Olympic Games is the largest sport event in the world. In Beijing, 10500 athletes competed, selected from a large group of elite athletes in 204 countries. Sports participation on the elite level, aside from winning medals, fame and other rewards, is also important from a health perspective. There is no longer any doubt that regular physical activity reduces the risk of premature mortality in general, and of coronary heart disease, hypertension, colon cancer, obesity, and diabetes mellitus in particular. The question is whether the health benefits of sports participation outweigh the risk of injury and long-term disability, especially in high-level athletes. Sarna et al (2000) have studied the incidence of chronic disease and life expectancy of former male world-class athletes from Finland in endurance sports, power sports and team sports. The overall life expectancy was longer in the high-level athlete compared to a reference group (75.6 versus 69.9 years). The same group also showed that the rate of hospitalization later in life was lower for endurance sports and power sports compared to the reference group (Kujala 1996). This resulted from a lower rate of hospital care for heart disease, respiratory disease and cancer. However, the athletes were more likely to have been hospitalized for musculoskeletal disorders. Thus, the evidence suggests that although there is a general health benefit from sports participation, injuries represent a significant side effect.
One priority of the International Olympic Committee (IOC) is to protect the health of the athlete. During recent years, prevention of injuries and illnesses has been high on the IOC agenda. During the Athens Games an injury surveillance system was applied for all team sports (Junge et al 2006). During the Beijing Games, the IOC ran, for the first time, an injury surveillance system covering all the athletes, showing a 10% incidence of injuries (Junge et al 2008). In Vancouver and London the surveillance system will include disease conditions as well. The surveillance studies are prerequisites for providing evidence for health development in sports as well as for developing prevention programs. Another method to decrease injuries and diseases in the elite athlete is to perform a pre-participation examination (PPE) or periodic health evaluation (PHE) of all elite athletes (Junge at al 2009). PHE in various forms have been available for many years, but a recent analysis (Wingfield et al 2004) has questioned the efficacy of PHEs in detecting serious problems in the elite athlete.
In March 2009, the IOC assembled an expert group to discuss the current state of health evaluations for athletes, aiming to provide recommendations for a practical PHE for the elite athlete, as well as to outline the need for further research. The task of the group was to review the benefits as well as potential negative effects of PHE at the elite sport level. The group did not take any position as to whether PHE should be recommended as compulsory for participation in sport. That is for the relevant sports authorities to decide.
The PHE can serve many purposes. It includes a comprehensive assessment of the athlete's current health status and risk of future injury or disease and, typically, is the entry point for medical care of the athlete. The PHE also serves as a tool for continuous health monitoring in athletes. Recent advances in this field relate to: (i) data on sudden cardiac death and other noncardiac medical problems, and the detection of risk factors and groups; (ii) a consensus conference on concussion; (iii) data on eating disorders and (iv) data on risk factors for musculoskeletal injuries. This paper addresses each of these advances in more detail after a discussion on the purpose of a PHE and the evidence we have supporting the different components of the PHE.
Purposes of the medical evaluation
In a narrow sense, the main purpose of the PHE is to screen for injuries or medical conditions that may place an athlete at risk for safe participation. Athletes may be affected by conditions that do not have overt symptoms and that can only be detected by periodic health evaluations. One example is cardiovascular abnormalities, such as hypertrophic cardiomyopathy, arrythmogenic right ventricular cardiomyopathy or congenital coronary arteries anomalies. These are typically silent until a potentially fatal arrhythmia occurs, but may in some cases be detected through a careful cardiovascular examination.
Screening is a strategy used in a population to detect a disease in individuals without signs or symptoms of that disease. The intention is to identify pathologic conditions early, thus enabling earlier intervention and management in the hope of reducing future morbidity and mortality. Although screening may lead to an earlier diagnosis, not all screening programs have been shown to benefit the person being screened.
To ensure that screening programs confer the intended benefit, the World Health Organisation published what have become known as the Wilson-Jungner criteria for appraising a screening programme (Wilson & Jungner 1968). The main criteria are that the condition being screened for is an important health problem (depends not just on how serious the condition is, but also how common it is), that there is a detectable early stage, that treatment at an early stage is of more benefit than at a later stage and that a suitable test is available to detect disease in the early stage.
From a public health perspective, there is insufficient evidence to date to mandate any specific screening tests for elite athletes apart from those recommended for the general population. This is mainly the consequence of the low risk of serious conditions in this population. An important limitation is also the lack of suitable screening tests; such tests must be reliable (repeatable, good inter-observer agreement), sensitive (detects all those with increased risk), specific (detects only those with increased risk), affordable (ideally cheap, easy to perform, widely available), acceptable to the screening population and subject to quality assurance.
However, the PHE may serve other purposes than just screening athletes for future health problems. One obvious goal is to ensure that current health problems are managed appropriately and, ultimately, to determine whether an athlete is medically suitable to engage in a particular sport or event. Even elite athletes with easy access to medical care do not always seek medical attention for injuries or disease, despite having significant symptoms.
Some silent conditions are common and, although not severe from a health perspective, may influence sports performance. An example of this is mild iron deficiency, which is common in female athletes. Periodic health evaluations and ongoing monitoring represent an opportunity to diagnose and manage such conditions. They also provide an opportunity to identify conditions that are barriers to performance. An example is astigmatism, which can be detected on a simple test of visual acuity. Another important function of periodic health evaluations is that they allow the athlete an opportunity to establish a relationship with the health personnel who will be involved in providing continuing care.
Finally, the PHE also represents an opportunity to look for characteristics which may put the elite athlete at risk for future injury or disease. However, as mentioned above, there is limited direct evidence to suggest that it is possible to predict future outcomes based on the PHE. Nevertheless, there is evidence in some areas, such as injury risk factor assessment (Bahr & Engebretsen 2009), that holds future promise and warrants investigation related to the PHE. Depending on the sport and the age, ethnic origin and gender of the athlete, it may be prudent to include an assessment of specific risk factors in the PHE.
General requirements of a PHE
It is important to address and balance the ethical and legal aspects of the PHE in order to help protect the rights and responsibilities of athletes, physicians, sporting organizations and other persons concerned. In the context of designing and implementing a PHE, the following considerations need to be taken into account:
PHE should be based on sound scientific and medical criteria.
PHE should be performed in the primary interest of the athlete, that is, assessing his/her health in relation to his/her practice of a given sport.
PHE should be performed under the responsibility of a physician trained in sports medicine, preferably by the physician responsible for providing ongoing medical care for the athlete, e.g. the team physician.
The decision concerning the nature and scope of the PHE should take into account individual factors, such as the geographical region, the sport discipline, the level of competition, age and gender of the athlete.
The setting of the evaluation should be chosen to optimize the accuracy of the examination and respect the privacy of the athlete. The PHE should preferably be carried out in the physician's office, which assures privacy, access to prior medical records, and an appropriate patient-physician relationship.
A physician can only perform a PHE with the free and informed consent of the athlete and, if applicable, his/her legal guardian.
If PHE evidences that an athlete is at serious medical risk, the physician must strongly discourage the athlete from continuing training or competing until the necessary medical measures have been taken.
Based on such advice, it is the responsibility of the athlete to decide whether to continue training or competing.
If a physician is requested to issue a medical certificate, he or she must have explained in advance to the athlete the reason for the PHE and its outcome, as well as the nature of information provided to the third parties. In principle, the medical certificate may only indicate the athlete's fitness or unfitness to participate in training or competition and should minimize disclosure of confidential medical information.
In many settings, the PHE is used to offer medical clearance to participate in sport and is seen as a one-time certification for future involvement in elite sport. However, the evaluation of the athlete's health should ideally be seen as a dynamic, ongoing process.
While many aspects of the PHE will be common to all elite athletes, it should be tailored to be gender, age, race, culture and sport specific when appropriate. If any injury or medical condition is identified, it should be managed in a manner consistent with the existing standards of medical care. If warranted, this may involve referral to the appropriate specialists for further evaluation and management. It should be noted that the PHE is also the time that medications or nutritional products in use or prescribed should be reviewed to determine if a Therapeutic Use Exemption (TUE) application to the World Anti Doping Association (WADA) is needed.
The timing of the PHE should ideally allow for sufficient time for management of any injuries or medical problems well before major competitions. For example, it is preferable to conduct a PHE during the off-season so that rehabilitation or other treatment can restore the athlete to optimal health before facing maximal physical stress.
As the PHE is the only contact that many elite athletes will have with medical personnel, it should be seen as an opportunity for education regarding other health risks and health-related behavior.
The following document is laid out in sections that correspond to the various areas of evaluation appropriate to the elite athlete.
1. Cardiology
1.1 Introduction
The scope of the cardiovascular PHE is to detect potentially lethal cardiovascular disease in elite athletes and start appropriate management to reduce the risk for sudden cardiac death and/or disease progression in a timely fashion.
1.2 Evidence base
Cardiovascular (CV) risk of competitive sport participation
Regular participation in training and athletic competition is associated with an increased risk for sudden cardiac death (SCD), with an average relative risk for athletes of 2.8 times compared to their nonathletic counterpart (Corrado et al 2003). It is worthy to note, however, that sport is not per se the cause for greater incidence of SCD. It is the combination of intensive physical exercise in athletes with underlying cardiovascular disease, which can trigger ominous arrhythmias leading to cardiac arrest. The relative risk of sport participation is different according to the underlying disease, and it is greatest in case of cardiomyopathies (such as hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy) or congenital coronary arteries anomalies (Corrado et al 2003).
Rationale for CV evaluation in elite competitive athletes
The vast majority of the athletes dying suddenly do not experience premonitory symptoms (Maron 2003); therefore, the PHE represents the only strategy capable to identify athletes with silent cardiac disease, and allow appropriate management to reduce the risk of SCD and disease progression. Identifying asymptomatic athletes with underlying cardiovascular disease through the PHE is important because SCD could be prevented by lifestyle modification, including (when necessary) restriction from competitive sports activity, but also prophylactic treatment by drugs, implantable cardioverter defibrillator (ICD) or other therapeutic options. Athletes carrying an increased cardiac risk may have a favourable long-term outcome thanks to timely identification and appropriate clinical management (Corrado et al 1998).
Rationale for including the 12-lead Electrocardiogram (ECG) in the PHE
Recent scientific evidence supports the role of ECG in reducing mortality in screened athletes (Corrado et al 2006). This concept is based on the recognition that ECG is abnormal in most individuals with hypertrophic cardiomyopathy (up to 90%) and arrhythmogenic right ventricular cardiomyopathy (up to 80%). The ECG can also identify athletes with WPW syndrome and ion channel diseases, such as Lènegre conduction disease, long or short QT syndromes, and Brugada syndrome (Corrado et al 2007, Lawless and Best 2008). However, criticism has been voiced related to available data on the use of ECG in elite athletes due to the lack of an unscreened athletic control group. A comparison of athletes screened with ECG vs. athletes non-screened will require two matched large athlete populations (several thousand athletes, in consideration of the low incidence of cardiomyopathies) undergoing long-term follow-up (at least two decades, due to the young age of athletes at initial evaluation).
It has been demonstrated that adding a 12-lead ECG examination to history and physical examination results in a substantial increase in the ability to identify potentially lethal heart disorders (Corrado et al 2007, Lawless and Best 2008) and this strategy has been endorsed in “The Lausanne Recommendations” (Bille et al 2006) and the European Society of Cardiology recommendations (Corrado et al 2005). However, it is not currently recommended by the American Heart Association (Chaitman 2007, Myerburg and Vetter 2007).
1.3 Proposal for PHE
The following questions regarding cardiovascular abnormalities should be included:
Family history
Family history of one or more relatives with disability or death of heart disease (sudden/unexpected) before age 50
Family history of cardiomyopathy, coronary artery disease, Marfan syndrome, long QT syndrome, severe arrhythmias, or other disabling cardiovascular disease
Personal history
Syncope or near-syncope
Exertional chest pain or discomfort
Shortness of breath or fatigue out of proportion to the degree of physical effort
Palpitations or irregular heartbeat
Physical examination should be performed according to the best clinical care and should investigate the presence of:
Musculoskeletal and ocular features suggestive of Marfan syndrome
Diminished and delayed femoral artery pulses
Mid- or end-systolic clicks
Abnormal second heart sound (single or widely split and fixed with respiration)
Heart murmurs (systolic grade >2/6 and any diastolic)
Irregular heart rhythm
Brachial, bilateral blood pressure >140/90 mmHg on more than one reading
The 12-lead ECG
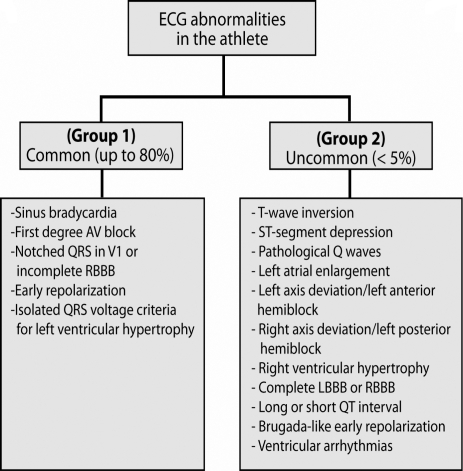
The 12-lead ECG should be recorded on a non-training day, during rest, according to best clinical practice. Interpretation of the ECG abnormalities can be categorized according to the criteria defined by Corrado et al (2008) into two groups: 1) the most common in trained athletes (sinus bradycardia, first degree AV block, notched QRS in V1 or incomplete right bundle branch block, isolated QRS voltage criteria for LV hypertrophy) consistent with athlete's age, ethnical origin and level of athletic conditioning, and that do not require additional testing; 2) all other less common ECG abnormalities should be further evaluated to exclude cardiovascular disease (Fig 1).
Figure 1.
12-Lead electrocardiographic abnormalities in the athlete. Abbreviations: AV, atrioventricular block; RBBB, right bundle branch block; LBBB, left bundle branch block.
Further investigations
At present, there is no agreement regarding the need for routine use of echocardiography in the PHE. Neither is there a role for routine use of other imaging or invasive testing. However, in the presence of abnormal findings either at history, physical examination or 12-lead ECG, additional testing should be performed in order to confirm (or exclude) cardiovascular disease. In most instances, echocardiography is the first-line test, but other imaging modalities (such as cardiac magnetic resonance) or invasive testing, when necessary, may be pursued. In adult athletes (>35 years) exercise ECG testing in the context of PHE is efficient to detect otherwise unsuspected cardiac abnormalities (Sofi 2008) and is currently recommended for elite athletes with increased cardiovascular risk profile (Thompson 2007).
1.4 Management of athletes with CV abnormalities
The IOC PHE Consensus Group recommends that any athlete identified with a CV abnormality should be managed according to the current, widely accepted clinical recommendations, i.e., Bethesda Conference #36 and ESC recommendations (Maron and Zipes 2005, Pelliccia et al 2005). The group acknowledges that identification of cardiac disease in an athlete represents a challenging question regarding the ethical, medical and legal consequence with particular regard to the need for disqualification from competition. However, there is scientific evidence that preventing athletes with specific cardiovascular abnormality from regular training and competition is an efficient strategy for preventing SCD (Corrado et al 1998, Biffi et al 2004). Unnecessary exclusion from participation of competitive athletes with non lethal diseases is a problem. Therefore, there is a need for a common agreement of sports eligibility guidelines and management of competitive athletes with cardiovascular diseases in the future (Pelliccia et al 2008). The main goal should be to reduce the number of unnecessary disqualifications and to adapt (rather than restrict) sports activity in relation to the specific cardiovascular risk.
Finally, we recognize that young competitive athletes (<18 years) require specific expertise in the evaluation, interpretation of findings and management.
1.5 Educational programmes
The sport organizations together with scientific sport societies should encourage and support educational activities intended to enhance the knowledge and skill of physicians involved in the cardiology part of the PHE process.
1.6 Research
Although there are issues of debate regarding wide-scale mandatory use of the ECG for athlete screening (Chaitman 2007, Pelliccia 2008), there is sufficient evidence to justify a staged implementation with evaluation to assess the properties of the test (sensitivity, specificity, predictive value) in a variety of sporting populations. Staged implementation would provide a natural control group to measure differences in outcome between ECG screened and unscreened groups. Finally, the mortality effects of a screening program documented in Italy need to be replicated in other ethnic populations where the underlying disease conditions may differ from those seen in Italy.
The sport organizations and scientific sport societies should encourage research that could expand our current knowledge and data base regarding the mechanisms and strategies to prevent SCD in competitive athletes.
2. Non-Cardiac Medical Conditions
2.1 Introduction
To date, the main elements of the PHE have been to screen elite athletes for possible risk for sudden cardiovascular death (Beckerman et al 2004, Corrado et al 2005), musculoskeletal injury (Garrick 2004), and head injury (McCrory 2004). Furthermore, elements of the PHE that focus on non-cardiac medical conditions have to date been confined to hematological conditions (Fallon 2004), lung disease, particularly exercise-induced bronchoconstriction (Holzer and Brukner 2004), and specific medical concerns of the female athlete (Rumball and Lebrun 2004).
However, sports physicians who regularly perform medical assessments on elite athletes, as well as members of the medical team that accompany athletes to the Olympic Games and other international sports events, commonly encounter medical conditions that are non-injury related, and are of a non-cardiac nature (Derman 2003, Derman 2004, Grissom et al 2006).
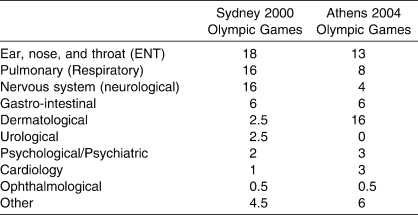
In one study, it was reported that 50% of the 1804 athletes seen at the multipurpose medical facility at the 1996 Olympic Games were treated for non-injury related illnesses (Wetterhall et al 1998). In another study conducted in the athlete medical clinic during the 2002 Winter Olympic Games, medical diagnoses, notably respiratory conditions, were more commonly reported than traumatic conditions (Grissom et al 2002). Furthermore, in two other studies, over 50% of the medical consultations in a participating team during two Olympic Games were non-injury related (Derman 2003, Derman 2004). It is important to note that the frequency of cardiac-related medical consultations reported in these two studies was very low (Derman 2003, Derman 2004).
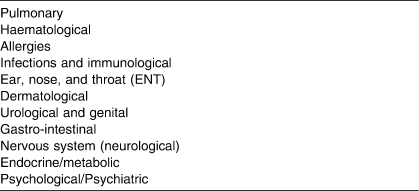
Therefore, medical conditions in systems other than the cardiovascular system are very common in elite athletes. These conditions can occur immediately before competitions, during periods of training in preparation for competitions, and after competitions. In two reports, the frequency of medical conditions reported in athletes during the Olympic Games has been documented (Table 1). These data indicate that medical conditions, other than cardiovascular conditions, are common in elite athletes, yet these conditions have not received much attention in a PHE. A spectrum of medical conditions can occur in athletes across a number of medical systems (Table 2) and these can be identified during a PHE (Rifat et al 1995, Lively 1999). Finally, a number of these conditions are transient and can be treated. Therefore, clearance for sports participation when athletes suffer from these conditions is an ongoing process and requires ongoing monitoring and assessment.
Table 1.
Frequency (% of All Formal Medical Consultations) of Medical Consultations at the Olympic Games in a Team
Table 2.
Non-Cardiac Systems That Should Be Considered in a Periodic Health Evaluation
The purpose of this section is to 1) briefly review the evidence base for including elements in the PHE that focus on non-cardiac medical conditions, 2) recommend elements in the medical history, physical examination and special investigations that could be included in a PHE to identify significant non-cardiac medical conditions, and 3) suggest future directions for research in this area.
2.2 Evidence base: Non-cardiac medical conditions
There is very little data available on the inclusion of assessment for non-cardiac medical conditions in a PHE. Evidence for the inclusion of screening tests to identify non-cardiac medical conditions in a PHE is therefore largely limited to expert opinion and case series. However, the identification of some non-cardiac medical conditions is frequently included in the medical history, physical examination and profile of special investigations of existing PHE recommendations (Joy et al 2004, Batt et al 2004, Brukner et al 2004, Fuller et al 2007, Constantini and Mann 2005, Nichols et al 1995). The evidence base for including screening to identify non-cardiac medical conditions in a PPE will be briefly reviewed below.
2.2.1 Pulmonary system
The rationale for including an assessment of the pulmonary system in a PHE is that respiratory symptoms that are suggestive of asthma are common in athletes (Fitch et al 2008). At the time of a PHE, these symptoms can be identified, and the clinical examination, together with objective special tests can be used to confirm the diagnosis of asthma (Fitch et al 2008). The prevalence of asthma in athletes is high and varies from 3–23% in summer sports to 12–50% in winter sports (Carlsen et al 2008, Cummiskey et al 2008). Furthermore, during a PHE, respiratory tract conditions other than asthma that can also give rise to respiratory symptoms in athletes can be identified (Cummiskey et al 2008).
2.2.2 Hematological
The main rationale for including routine hematological assessment during a PHE is based on the higher than expected prevalence of decreased iron stores in athletes, particularly female athletes (Fallon 2004, Gropper et al 2006, Sinclair and Hinton 2005, Fallon 2007, Eliakim et al 2002, Rietjens et al 2002, Di Santolo et al 2008). An additional rationale is to determine if the athlete has anemia (iron deficiency or other), and to identify other illnesses such as infections (Fallon 2004). It is noteworthy that hematological testing has been suggested as a screening/monitoring tool for blood doping (hematological passport) as well (Schumacher et al 2002). The likelihood of a positive result on routine hematological screening is higher in physically active females compared with male athletes (Fallon 2004, Fallon 2007, Dubnov et al 2006).
2.2.3 Allergies
The rationale for including assessments in the PHE to identify allergies, particularly allergic rhinoconjunctivitis, in elite athletes is based on the fact that 1) significantly higher than expected prevalence of allergic conditions has been observed in elite athletes (Katelaris et al 2006, Katelaris et al 2000, Katelaris 2001, Hawarden et al 2002); 2) travelling athletes could be exposed to a variety of allergens at different venues where international competitions take place (Katelaris et al 2000); and 3) acute and chronic allergies could result in morbidity and also reduce athletic performance (Katelaris et al 2003).
2.2.4 Infections and immunological
The rationale to consider infective disease in a PHE is based on a number of important considerations. Firstly, it is established that during intense training and immediately following competitions, there is evidence of immune suppression in athletes that could predispose them to infective disease (Gleeson 2006, Ekblom et al 2006). Secondly, acute systemic infective illness is a contra-indication to participation in sports because of the risk of viral myocarditis, organ injury (splenomegaly) and in some cases increased risk of transmission of the infective illness to fellow athletes (Schwellnus et al 2008, Luke and d'Hemecourt 2007, Pirozzolo and MeMay 2007). Thirdly, the PHE provides an opportunity to assess whether an athlete has been immunized against infective conditions, including those that may be associated with international travel to specific regions. There are a number of infective illnesses that could be considered when performing a PHE and these have been reviewed recently (Schwellnus et al 2008, Luke and d'Hemecourt 2007).
2.2.5 Ear, nose and throat (ENT)
The rationale for including the ear, nose and throat (ENT) assessment in a PHE is based on the high incidence medical consultations during international competitions that are related to this system in elite athletes (Table 1). Furthermore, the common illnesses encountered in the ENT system of athletes are allergies (Katelaris et al 2006, Katelaris et al 2003) and upper respiratory tract infections (Grissom et al 2002, Ekblom et al 2006, Schwellnus et al 2008, Malm 2006). The basis for including this spectrum of conditions in PHE has already been discussed.
2.2.6 Dermatological
The rationale for including a dermatological assessment in the PHE is that skin disorders are very common in athletes (Adams 2008, Adams 2002, Adams 2003). Furthermore, participation in sports may predispose athletes to certain skin conditions and there is a risk of transmission of certain skin conditions during sports (Adams 2008). Therefore, clearance to compete may have to be withheld temporarily if athletes suffer from some skin infections (Adams 2008).
2.2.7 Urological
The rationale for including an assessment of the urological system in the PHE is not based on strong evidence. However, it is known that 1) renal and bladder disease can be asymptomatic; and 2) conditions such as asymptomatic haematuria, proteinuria and pyuria are often encountered when screening in athletes is conducted (Rayner and Schwellnus 2008). Although these conditions may not be clinically significant, they do however require further evaluation to exclude underlying urological disease.
2.2.8 Gastro-intestinal (GIT)
The rationale for including an assessment of the gastro-intestinal (GIT) system in the PHE is that GIT symptoms are very common in athletes (particularly endurance athletes) during sports participation (Schwellnus and Wright 2008). Exclusion of significant underlying GIT disease is therefore important in athletes, particularly those that regularly suffer from GIT symptoms during exercise (Schwellnus and Wright 2008). Furthermore, GIT conditions are also frequently encountered when travelling with athletes to international competitions (Derman 2003, Derman 2004).
2.2.9 Nervous system (neurological)
The rationale for including assessment of neurological conditions in the PHE of athletes is that neurological conditions are common (McCrory 2008) and can include a variety of different conditions such as headaches and epilepsy. Furthermore, although uncommon, stroke can occur in younger adults, including athletes. It has been suggested that the PHE should include screening for the risk factors of stroke in young athletes (McCrory 2008).
2.2.10 Endocrine/metabolic
The rationale for routine enquiry to determine if elite athletes have underlying endocrine and metabolic disease is 1) that these conditions do occur in elite athletes; 2) one of the more common endocrine conditions in elite athletes is diabetes mellitus - nine Olympic athletes required therapeutic use exemption for the use of insulin in the 2004 Summer Olympic Games (Tsitsimpikou et al 2009); and 3) elite athletes with existing endocrine and metabolic disease may require counseling and advice because medication they may use could contravene doping control regulations (Anderson et al 2008).
2.2.11 Ophthalmology
The principle rationale for including ophthalmological assessment in the PHE is that ophthalmic conditions, particularly reduced visual acuity, have been reported in 4.5–25% of college athletes undergoing a PHE (Rifat et al 1995, Lively 1999, Carek and Mainous 2003). Other less common ophthalmological conditions can also be identified.
2.3 Proposal for content of the PHE
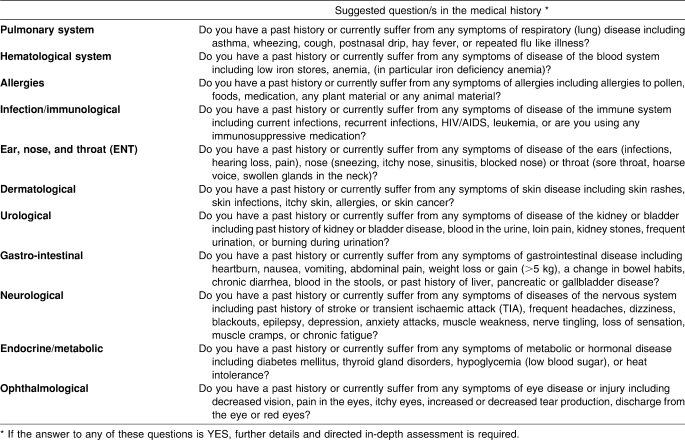
Assessment of non-cardiac medical conditions during a PHE would include an appropriate systematic medical history (Table 3). A directed physical examination and selected special investigations (Table 4) should follow. Routine investigations that are recommended are 1) urinalysis (males and females) and 2) tests for iron stores (female athletes). According to best medical practice guidelines, these elements should be included in the PHE to assess elite athletes for the presence of non-cardiac medical conditions.
Table 3.
A List Of Suggested Questions in the Medical History to Identify Non-Cardiac Conditions in a Periodic Health Evaluation
Table 4.
Key Elements in the Physical Examination for Non-Cardiac Conditions in a Periodic Health Evaluation
2.4 Possible future directions in research
Broad research areas:
Defining the scope of the problem in each system (prevalence of non-cardiac medical conditions in elite athletes)
Determining the impact of these medical conditions on performance, short term risk, and longer term outcome in elite athletes
Investigate which diagnostic tests have the highest sensitivity, specificity and predictive value for each condition
Determine if identification and management of these conditions reduces morbidity and improves performance in elite athletes
3. Concussion in Sport
3.1 Introduction
Concussion in sport is defined as a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces (Aubry et al 2002). Estimates in the United States range from 1.6 to 3.8 million concussions per year related to sport and recreation (Langois 2006 and Centers for Disease Control and Prevention 2007). There is evidence that concussions are particularly prevalent in collision sport (Browne 2000, Guskiewicz 2006).
3.2 Evidence base
The IOC and several members of the present consensus group participated in the recent Zurich Consensus Statement on Concussion in Sport (McCrory et al 2009) and endorse its concepts and principles. A structured consensus-development conference format was used, modelled after the protocol of the National Institutes of Health, including development of evidence-based recommendations. The specific evidence and rationale are provided in detail in the manuscript by McCrory et al 2009.
3.3 Proposal for content of the PHE
It is recognized that a structured concussion history is an important component of the PHE, and should include specific questions regarding:
previous symptoms of a concussion; not just the perceived number of past concussions; appreciating the fact that many athletes will not recognize all the concussions they may have suffered in the past
all previous head, orofacial or cervical spine injuries
whether repeated concussions produce disproportionate severity of symptoms given the amount of impact; alerting the clinician to a progressively increasing vulnerability to injury
use of protective equipment such as helmets, facial protection and mouth guards; including their age and state of repair
the ability of the athlete to adopt protective behavior such as avoiding overly aggressive or high risk situations
The purpose in obtaining such a history may identify athletes that fit into a high risk category and provides an opportunity for the healthcare provider to educate the athlete in regard to the significance of concussive injury.
3.4 Possible future directions for concussion in sport
The Concussion in Sport Group (McCrory et al 2009) had extensive discussion and general agreement on the principle of doing baseline assessment (neuropsychological, balance, etc) during the PHE in high risk sports. The intent is to provide a comparison point for possible future post injury testing. While a specific recommendation for baseline examination in the PHE was not included in the concussion consensus document, one of the suggested future directions in research was the “clinical assessment where no baseline assessment has been performed” (McCrory et al 2009).
4. Dental Injuries
4.1 Introduction
Oral health evaluation has significant relevance to the establishment of an improvement in oral health. Good oral health will ensure good function and the ability of the athlete to compete at an optimal level without being compromised by dental disease or an otherwise preventable emergency.
4.2 Evidence base
Statistics collected by the IOC at recent summer games (Fasel 2008) have highlighted the level of dental disease in many participants. The use of a DMF Index (“decayed-missing-filled” a dental screening tool) and appropriate radiographs to measure dental disease may also identify oral health behavior and the extent of poor oral health prevalent in our elite athlete group (Levin et al 2004, WHO 4th ed. 1997; 40–7). The identification of erosion, the prevalence of which is estimated to be 25.4–37.4% in athletes, may be an indicator of excessive use of sports beverages, which are acidic in nature (Sirimaharaj et al 2008, Vasan 1998). In addition, erosion may be caused by acidic reflux, which may be indicative of an underlying eating disorder (Milosevic 1999).
The presence of wisdom teeth (Fuselier et al 2002, Yamada et al 1998) and certain malocclusions are risk factors for future injury (Kvittem et al 1998, Burden 1995). The presence or absence of wisdom teeth may affect the risk profile for mandibular fracture in combative sports (Andrade et al 2007, Ma'aita et al 2000, Schwimmer et al 1983). Additionally, associated pericoronitis and periodontal infection may affect athlete performance (Kerr 1983).
4.3 Proposal for content of the PHE
The physical examination should include the following elements:
DMF; which is an acronym for decayed, missing and filled teeth. These three elements together with recent dental history can be used as an indicator for underlying oral health.
Other risk factors for orofacial injury that can be targeted for prevention are:
Malocclusion where the overjet (overlap of upper over lower incisors) is greater than 6 mm (Kvittem et al 1998, Burden 1995).
Presence of braces or orthodontic appliances (Croll et al 1996).
4.4 Possible future directions in oral health
Efforts should be made to educate athletes and authorities about the considerable benefits in preventing dental injuries by providing custom made mouthguards for those taking part in “at risk” sports (e.g. collision and contact sports)(Andrade et al 2008), and to continue to measure or quantify the benefits. Additionally, further studies are required to assess more accurately the oral health of the athlete population and educational programs should be expanded and targeted to those sports where the risks identified above influence athlete health (Cornwell 2005, Badel et al 2007). The IOC group encourages athletes to be provided with regular dental examinations.
5. Musculoskeletal Injuries
5.1 Introduction
Musculoskeletal injuries are common in sports. Acute injuries are most common in sports in which the speed is high and the risk of falling is great (e.g. downhill skiing) and in team sports where there is significant contact between players (e.g. ice hockey and soccer). Overuse injuries make up the large portion of injuries in aerobic sports that require long training sessions with repetitive motion (e.g. long-distance running, cycling or cross-country skiing). However, a large number of overuse injuries also occur in technical sports, in which the same movement is repeated numerous times (e.g. tennis, javelin throwing, weightlifting and high jumping). The injury profile also varies from sport to sport; each sport has its distinctive injury pattern. This pattern must be considered carefully when designing the musculoskeletal component of the PHE. The practitioner must be familiar with the most common injury types associated with the sport in question and the examination should be targeted on these injury types and their risk factors.
The main objective of the musculoskeletal PHE is to detect current injuries and ensure that these are managed appropriately. While an injured athlete may have gone through rehabilitation in the middle of the season, focus is often on return to play, sometimes using taping and bracing to protect from further injury. The off-season should be used as an opportunity to get the athlete back to full fitness. Therefore, the best timing of the PHE may be the immediate post-season (while there is still time to work on problems that are identified) rather than the pre-season.
A previous injury is the most consistent risk factor for new recurrent injuries. This has been demonstrated for a number of different injury types, such as ankle sprains, muscle strains and knee ligament injuries. A previous injury could compromise joint function through reduced mechanical stability or neuromuscular control, or muscle function through scar tissue formation, reduced strength, or more subtle changes in the length-tension relationship. It follows then, that training programs to restore strength and neuromuscular control can help prevent recurrent injuries. One important aim of the musculoskeletal PHE is therefore to identify sequelae or deficits resulting from previous injuries.
Ideally, the musculoskeletal PHE should be used to identify athletes at risk for injury. However, although a number of risk factors have been identified which makes an athlete susceptible to different injuries, the examination needs to be focused on the characteristics and requirements of the sport in question. A complete review of risk factors associated with injuries to the different body regions can be found in the IOC Handbook on Sports Injury Prevention (Bahr & Engebretsen 2009).
5.2 Evidence base
The evidence base for designing a musculoskeletal PHE to detect risk factors for future injury is limited. Players with a history of previous injury or symptoms indicating reduced function are a group with an increased injury risk that should be targeted for soft tissue examinations and with specific prevention programs addressing their deficits. However, in the asymptomatic athlete with no history of previous injury, there is limited evidence to prescribe specific tests – even using more advanced functional tests – to identify athletes at risk. One limitation is that the reproducibility of such tests is often low. Another is that the predictive value of such tests is for the most part unknown.
5.3 Proposal for the content of the PHE
The fundamental element of the musculoskeletal PHE is obtaining a thorough history of current and previous musculoskeletal injuries. To improve the history, one can use self-report forms; these should go in detail for the regions and injury types associated with the sport in question to ensure that no injuries and symptoms are missed. The clinical examination should follow up on any symptoms or injuries reported, consisting of inspection, palpation, range of motion, strength and laxity exams, effusions, muscle testing and relevant functional exams. Additional imaging (e.g. ultrasound, MRI) or more advanced functional tests (e.g. strength tests, balance tests) may be indicated based on history and physical examination.
5.4 Possible future research directions
Large-scale population-based studies are needed to evaluate the components of history and examination that can be used to identify athletes at risk, intervene and change outcome.
6. PHE Issues Specific to Women
6.1 Introduction
Female athlete participation in sport has increased tremendously with 42% of the athletes at the Beijing Olympic Games being female (International Olympic Committee 2008). Despite the well-documented health benefits of exercise, there are two medical conditions unique to the female athlete that may have long-term consequences that can be avoided through early detection and treatment. Low energy availability from caloric intake inadequate to meet the energy requirements of exercise can lead to secondary menstrual disorders and low bone density. This is referred to as the female athlete triad (Nattiv et al 2007). In addition, iron deficiency anemia is more common in the female athlete (Peeling et al 2008). The health and performance consequences of these two conditions can be prevented through early detection and treatment. The PHE provides a unique opportunity for the team physician to detect and treat low energy availability, menstrual disorders, low bone density and iron deficiency anemia.
6.2 Evidence base
The prevalence of eating disorders (anorexia nervosa (AN), bulimia nervosa (BN), anorexia athletica (AA), and eating disorders not otherwise specified (ED-NOS)) in the sport population was found to be 15–31% in comparison with 5–13% in the general population. The prevalence in males is generally lower than in females (Sundgot-Borgen and Torstveit 2004, Byrne and McLean 2002). The sports at highest risks are the aesthetic sports that emphasize thinness, endurance sports and weight class sports (Sundgot-Borgen and Torstveit 2004). The prevalence of secondary amenorrhea varies widely depending on the type of sport and is reported as high as 65% in long distance runners (Dusek 2001) in comparison to 2–5% in the general population (Bachmann and Kemmann 1982). The incidence of secondary amenorrhea increases with weekly mileage (Sanborn et al 1982), with athletes in sports emphasizing leanness (Torstveit and Sundgot-Borgen 2005) and in athletes less than 15 years of age (Baker et al 1981). The incidence of primary amenorrhea is 22% in cheerleading, diving and gymnastics (Beals and Manore 2002) in comparison to <1% in the general population (Chumlea et al 2003). Likewise, the frequency of low bone mineral density in athletes is two to four times greater than the general population (Khan et al 2002). Stress fractures occur more frequently in athletes with abnormal menstrual cycles and low bone mineral density (Bennell et al 1999). Short and long term consequences of the female athlete triad are well documented in the literature and effective treatment exists (Nattiv et al 2007).
For the detection of eating disorders and disordered eating, several validated screening tools exist: the Eating Disorder Examination Questionnaire (EDE-Q) (Carter et al 2001, Passi et al 2003, Wolk et al 2005), the SCOFF Questionnaire (Luck et al 2002, Morgan et al 1999) and the Eating Disorder Screen for Primary Care (ESP) (Cotton et al 2003). A comparative study of the SCOFF and ESP screening tools by Cotton et al. identified four questions that could serve as positive predictors and two as negative predictors of disordered eating (see table included in Appendix 1 PHE Form) (Cotton et al 2003). A population of high school endurance runners with low bone mineral density was found to score higher on the EDE-Q subscale of Dietary Restraint in comparison with other subscales. The validated questions correlating caloric restriction and low bone mineral density are shown in Table 5 (Barrack et al 2008).
Table 5.
Dietary Restraint Questions (EDE-Q) Correlated With Low Bone Mineral Density in High School Endurance Runners
6.3 Proposal for content for the PHE
The history and physical examination of the female athlete should address each component of the female athlete triad. Validated questions to detect eating disorders and disordered eating should be utilized (Table 5). Assessment of menstrual pattern and a history of stress fractures should be included. A systems review may identify body systems affected by low energy availability. If so, completion of a nutritional analysis identifies the athlete at risk for energy imbalance and iron deficiency.
A physical examination should include a body mass index. The following physical signs can be found in advanced cases of eating disorders but are likely to be absent in early detection; lanugo, petechiae, subconjunctival haemorrhages, swelling of the parotid glands, erosion of tooth enamel, bradycardia, peripheral edema. A complete gynecological examination is recommended in the athlete with primary or secondary amenorrhea.
The laboratory examination should include a CBC for all female athletes and a serum ferritin for those athletes in endurance sports. For those athletes identified at risk for the female athlete triad based on a screening questionnaire, physical examination abnormalities and initial blood work, measurement of body composition is recommended. For these athletes, laboratory tests include a hormonal screen (TSH, LH, FSH, estradiol, prolactin, Beta HCG, Testosterone, 17-OH -Progesterone, Sex Hormone Binding Capacity, Cortisol, DHEA-S, Androstenadione), CBC, biochemistry screen, bone mineral density (iDXA scan) to assess bone health, ECG to rule out electro conductive abnormalities from electrolyte disturbance in eating disorders and a nutritional analysis to assess energy balance.
6.4 Future direction
6.4.1 Research
Further research should be directed toward ascertaining the cause–effect relationship between the female athlete triad and sports participation. Validating the questions for detecting the triad is essential for accurate diagnosis and for earlier identification of athletes who would benefit from intervention. Further research into the relationship between low ferritin as a premonitor of iron deficiency anemia is important for both medical and performance reasons.
6.4.2 Education
Health care professionals working with female athletes should be qualified and experienced in the detection, diagnosis and treatment of the female athlete triad and iron deficiency anemia.
Educational programs for athletes and coaches focused on prevention of the female athlete triad and iron deficiency anemia are recommended to decrease the incidence of these health concerns, to maximize athletic performance and to ensure that female athletes enjoy the benefits of sport participation.
7. Technology
7.1 Community of practice/training
The Internet represents an ideal platform for the establishment of a portal/community where medical and scientific experts can share information for the purpose of enhancing best clinical practice and advancing science related to PHE. It can also act as a forum for ongoing education and training of the medical, administrative and athlete participants.
7.2 Data collection
Medical/research community portal
The PHE research project may contain a Web Portal where the sports medicine and scientific community with an interest in PHE can access, review and comment on the latest information, results and progress of this PHE research project.
Data collection tools
The questionnaire components of the PHE facilitate the standardization of the PHE and permits analysis of the sensitivity and specificity of the questions asked. Where practical, the questionnaire components can be completed via the Internet provided any such Internet based programs conform to the recognized technology industry standards for network security and authentication, and that they are in compliance with the local guidelines and/or standards for the collection and storage of Personal Health Information. An internet based version of the PHE Questionnaire would be time-and cost-efficient incremental step in the evolution of the PHE, enabling the participation of athletes, medical professionals and administrators from jurisdictions with limited facilities and budgets. An Internet based Questionnaire application provides an efficient means to enforce data collection standards across multiple input points, therefore improving the accuracy of any research outcomes.
7.3 Data mining
Web based data repositories present an ideal environment within which de-identified aggregate PHE data can be mined and analyzed, thereby facilitating the ongoing improvement of best practices related to the PHE. In all cases the security and privacy of this personal health data is of the utmost importance.
Where software tools are employed to facilitate the input and or collection of athlete data the following principles should be considered:
Data security and privacy
Data processing should be in keeping with good faith efforts
Data processing should be proportional (only collect the data that is needed)
Data should be processed only for the purposes indicated and agreed to by the athlete and or his/her legal representative
The Data should be precise
The Data should be protected against any form of unauthorized processing and provide full and complete audit trail of all transactions related to the Data
The Data should be legitimate
The Data should be transparent to the owner (the athlete maintains access)
Furthermore any organization implementing PHE should adopt best practices with respect to human behavior and the safeguarding of all personal medical data.
8. PHE Form
A final objective was to provide a practical PHE Form that could be used by various groups and form a starting point for further evaluation. Testing and standardization in medicine is not possible if we do not begin somewhere. The first step in creating such a form was to collect existing forms, including those in widespread use. These included the FIFA Pre Competition Medical Assessment (PCMA), the Pre-Participation Physical Evaluation form (Matheson 2005), the electronic Pre-Participation Evaluation (Meeuwisse 2003), the National Hockey League Pre-Participation Medical Evaluation form, plus specialized forms including the American Heart Association recommendations (Maron, 2007), the Lausanne Recommendations (Bille 2006), plus the items outlined in the text of this manuscript. The second step was to combine these into a master set of questions/items that was inclusive. This was circulated to the authors for review.
The third step will be to go through refinement in the future. This should be done on the basis of scientific evidence, which will come through evaluation with prospective data collection in a variety of sporting populations.
9. Scientific Advisors
The IOC now has high-level scientific advisors who are capable of monitoring new developments in the field of PHE, and of advising the IOC in relation to the use and abuse of PHE. These advisors will help ensure that athletes and coaches receive the benefits of these developments in improving their ability to prevent injury, and to enhance therapy if needed. The IOC will follow this field and hold a new conference in 2011.
10. Future Directions
It is recommended that PHEs be set up and conducted as research projects. Presently, the decision to implement a compulsory PHE must be taken by the International Federations. It must be in compliance with ethical and legal requirements applicable to biomedical research involving human beings. Project findings should be shared and used by the medical and scientific community to further develop and improve best practices. In particular, such research should be conducted “in accordance with the recognized principles of research ethics, in particular the Helsinki Declaration adopted by the World Medical Association (Edinburgh, 2000), and the applicable laws” (Article 7.5 of the Olympic Movement Medical Code).
Governing sports bodies (National Olympic Committees and International Federations) are encouraged to support research activities to provide sport discipline specific scientific and medical evidence as related to the improvement and application of PHE.
This article has been co-published in the following journals: American Journal of Sports Medicine, British Journal of Sports Medicine, Clinical Journal of Sport Medicine, International Sports Medicine Journal (FIMS), Journal of Athletic Training, Journal of Science and Medicine in Sports, Scandinavian Journal of Medicine and Science in Sports, South African Journal of Sports Medicine.
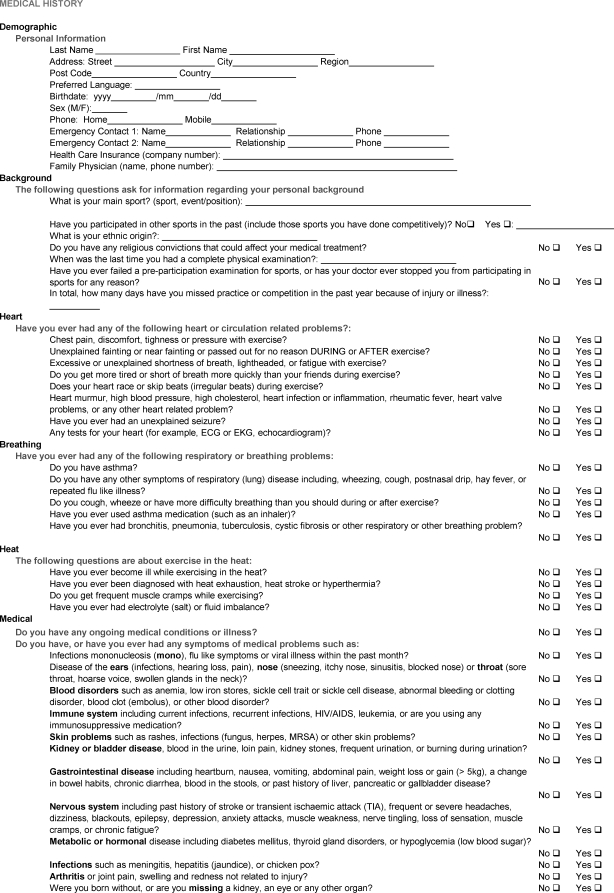
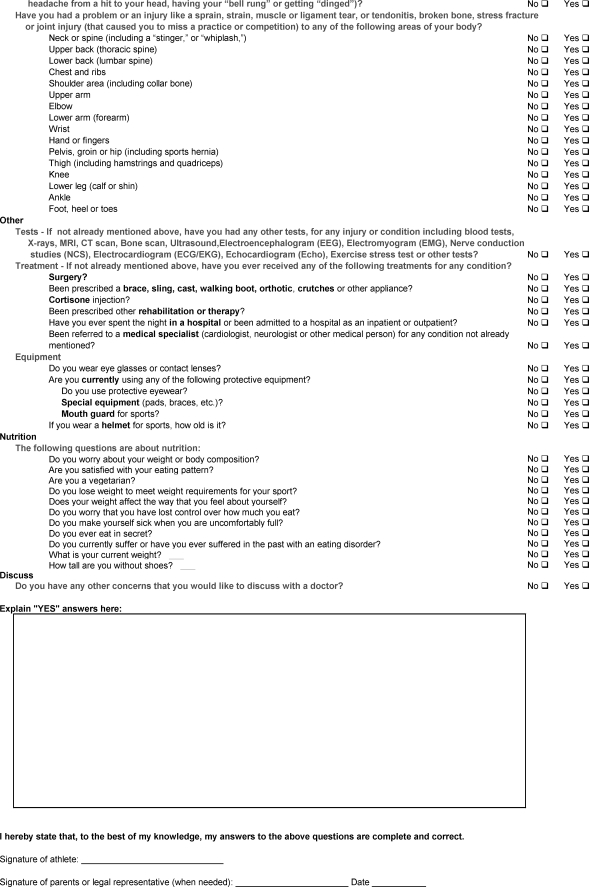
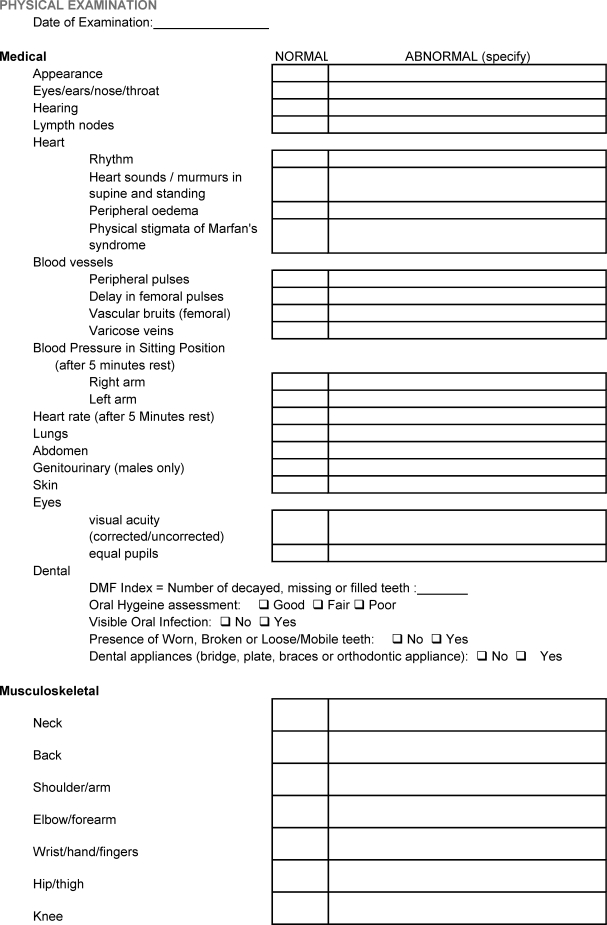
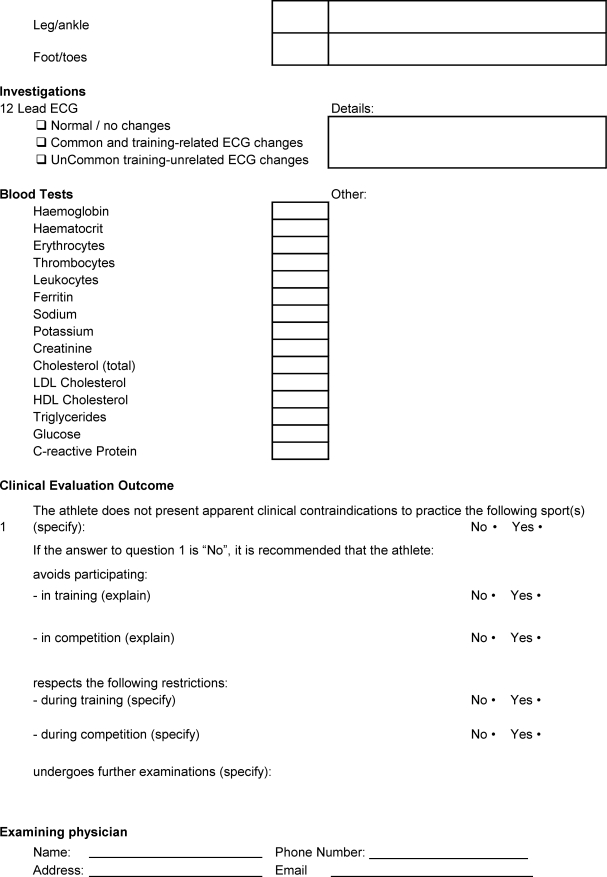
APPENDIX 1. ATHLETE PERIODIC HEALTH EVALUATION (PHE) FORM.
APPENDIX 2.
CONSENSUS STATEMENT CONTRIBUTORS.
Footnotes
This statement is also being published in the American Journal of Sports Medicine, British Journal of Sports Medicine, Clinical Journal of Sport Medicine, International Sports Medicine Journal (FIMS), Journal of Science and Medicine in Sport, Scandinavian Journal of Medicine and Science in Sports, and South African Journal of Sports Medicine. The manuscript was prepared by the authors and is printed here without editing. The contents are those of the International Olympic Committee and do not constitute the official position of the National Athletic Trainers' Association.
Reference List
Introduction
- Bahr R., Engebretsen L., editors. IOC Handbook of Sports Medicine and Science –Sports Injury Prevention. Oxford, United Kingdom: Wiley-Blackwell; 2009. [Google Scholar]
- Junge A., Langevoort G., Pipe A., et al. Injuries in team sport tournaments during the 2004 Olympic Games. Am J Sports Med. 2006;34(4):565–576. doi: 10.1177/0363546505281807. [DOI] [PubMed] [Google Scholar]
- Junge A., Engebretsen L., Alonso J. M., et al. Injury surveillance in multi-sport events: the International Olympic Committee approach. Br J Sports Med. 2008;42(6):413–421. doi: 10.1136/bjsm.2008.046631. [DOI] [PubMed] [Google Scholar]
- Wingfield K., Matheson G. O., Meeuwisse W. H. Preparticipation evaluation: an evidence-based review. Clin J Sport Med. 2004;14(3):109–122. doi: 10.1097/00042752-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Wilson J. M. G., Jungner G. Principles and Practice of Screening for Disease. Public Health Papers No. 34, Geneva, Switzerland: World Health Organisation; 1968. [Google Scholar]
- Sarna S., Sahi T., Koskenvuo M., Kaprio J. Increased life expectancy of world class male athletes. Med Sci Sports Exerc. 1993;25(2):237–244. [PubMed] [Google Scholar]
- Kujala U. M., Sarna S., Kaprio J., Koskenvuo M. Hospital care in later life among former world-class Finnish athletes. JAMA. 1996;276(3):216–220. [PubMed] [Google Scholar]
Cardiology
- Corrado D., Basso C., Rizzoli G., Schiavon M., Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42(11):1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Maron B. J. Sudden death in young athletes. New Engl J Med. 2003;349(11):1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- Corrado D., Basso C., Schiavon M., Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. New Engl J Med. 1998;339(6):364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- Corrado D., McKenna W. J. Appropriate interpretation of the athlete's electrocardiogram saves lives as well as money. Eur Heart J. 2007;28(16):1920–1922. doi: 10.1093/eurheartj/ehm275. [DOI] [PubMed] [Google Scholar]
- Lawless C. E., Best T. M. Electrocardiograms in athletes: interpretation diagnostic accuracy. Med Sci Sport Exerc. 2008;40(5):787–798. doi: 10.1249/MSS.0b013e318164dd18. [DOI] [PubMed] [Google Scholar]
- Bille K., Figueiras D., Schamasch P., et al. Sudden Cardiac Death in Athletes: the Lausanne Recommendations. Eur J Cardiovasc Prev Rehabil. 2006;13(6):859–875. doi: 10.1097/01.hjr.0000238397.50341.4a. [DOI] [PubMed] [Google Scholar]
- Corrado D., Pelliccia A., Bjørnstad H. H., et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516–524. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- Chaitman B. R. An electrocardiogram should not be included in routine preparticipation screening of young athletes. Circulation. 2007;116(22):2610–2615. doi: 10.1161/CIRCULATIONAHA.107.711465. [DOI] [PubMed] [Google Scholar]
- Myerburg R. J., Vetter V. L. Electrocardiograms should be included in preparticipation screening of athletes. Circulation. 2007;116(22):2616–2626. doi: 10.1161/CIRCULATIONAHA.107.733519. [DOI] [PubMed] [Google Scholar]
- Corrado D., Basso C., Pavei A., Michieli P., Schiavon M., Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296(13):1593–1601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- Corrado D., Basso C., Schiavon M., Pelliccia A., Thiene G. Pre-participation screening of young competitive athletes for prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52(24):1981–1989. doi: 10.1016/j.jacc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Zipes D. P. Introduction: eligibility recommendations for competitive athletes with cardiovascular abnormalities. General considerations. J Am Coll Cardiol. 2005;45(8):1318–1321. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Pelliccia A., Fagard R., Bjørnstad H. H., et al. Recommendations for competitive sports participation in athletes with cardiovascular disease. A Consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(14):1422–1445. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- Biffi A., Maron B. J., Verdile L., et al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44(5):1053–1058. doi: 10.1016/j.jacc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- Pelliccia A., Zipes D. P., Maron B. J. Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52(24):1990–1996. doi: 10.1016/j.jacc.2008.08.055. [DOI] [PubMed] [Google Scholar]
- Wilson J. M. G., Jungner G. Principles and Practice of Screening for Disease. Geneva, Switzerland: World Health Organisation; 1968. Public Health Paper 34. [Google Scholar]
Non-Cardiac Medical Conditions
- Beckerman J., Wang P., Hlatky M. Cardiovascular screening of athletes. Clin J Sport Med. 2004;14(3):127–133. doi: 10.1097/00042752-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Corrado D., Pelliccia A., Bjornstad H. H., et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol: Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516–524. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- Garrick J. G. Preparticipation orthopedic screening evaluation. Clin J Sport Med. 2004;14:123–6. doi: 10.1097/00042752-200405000-00003. [DOI] [PubMed] [Google Scholar]
- McCrory P. Preparticipation assessment for head injury. Clin J Sport Med. 2004;14(3):139–144. doi: 10.1097/00042752-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Fallon K. E. Utility of hematological and iron-related screening in elite athletes. Clin J Sport Med. 2004;14(3):145–152. doi: 10.1097/00042752-200405000-00007. [DOI] [PubMed] [Google Scholar]
- Holzer K., Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14(3):134–138. doi: 10.1097/00042752-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Rumball J. S., Lebrun C. M. Preparticipation physical examination: selected issues for the female athlete. Clin J Sport Med. 2004;14(3):153–160. doi: 10.1097/00042752-200405000-00008. [DOI] [PubMed] [Google Scholar]
- Derman W. E. Medical care of the South African Olympic team: the Sydney 2000 experience. South Afr J Sports Med. pp. 22–25. 2003;Dec.
- Derman W. E. Profile of medical and injury consultations of Team South Africa during the XXVIIIth Olympiad, Athens 2004. South Afr J Sports Med. 2008;20(3):72–76. [Google Scholar]
- Grissom C. K., Finnoff J. T., Murdock D. C., Culberson J. T. Nordic venue medical services during the 2002 Winter Olympics. J Emerg Med. 2006;30(2):203–210. doi: 10.1016/j.jemermed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wetterhall S. F., Coulombier D. M., Herndon J. M., Zaza S., Cantwell J. D. Medical care delivery at the 1996 Olympic Games. Centers for Disease Control and Prevention Olympics Surveillance Unit. JAMA. 1998;279(18):1463–1468. doi: 10.1001/jama.279.18.1463. [DOI] [PubMed] [Google Scholar]
- Rifat S. F., Ruffin M. T., 4th, Gorenflo D. W. Disqualifying criteria in a preparticipation sports evaluation. J Fam Pract. 1995;41(1):42–50. [PubMed] [Google Scholar]
- Lively M. W. Preparticipation physical examinations: a collegiate experience. Clin J Sport Med. 1999;9(1):3–8. doi: 10.1097/00042752-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Joy E. A., Paisley T. S., Price R., Jr, Rassner L., Thiese S. M. Optimizing the collegiate preparticipation physical evaluation. Clin J Sport Med. 2004;14(3):183–187. doi: 10.1097/00042752-200405000-00012. [DOI] [PubMed] [Google Scholar]
- Batt M. E., Jaques R., Stone M. Preparticipation examination (screening): practical issues as determined by sport: a United Kingdom perspective. Clin J Sport Med. 2004;14(3):178–182. doi: 10.1097/00042752-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Brukner P., White S., Shawdon A., Holzer K. Screening of athletes: Australian experience. Clin J Sport Med. 2004;14(3):169–177. doi: 10.1097/00042752-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Fuller C. W., Ojelade E. O., Taylor A. Preparticipation medical evaluation in professional sport in the UK: theory or practice? Br J Sports Med. 2007;41(12):890–896. doi: 10.1136/bjsm.2007.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantini N., Mann G. Preparticipation examination: the Israeli perspective. Clin J Sport Med. 2005;15(2):111–112. doi: 10.1097/01.jsm.0000152717.14506.c5. [DOI] [PubMed] [Google Scholar]
- Nichols A. W., Buxton B. P., Ho K. W. Pre-participation examination: a new form for Hawaii. Hawaii Med J. 1995;54(3):434–438. [PubMed] [Google Scholar]
- Fitch K. D., Sue-Chu M., Anderson S. D., Boulet L. P., Hancox R. J., McKenzie D. C., et al. Asthma and the elite athlete: summary of the International Olympic Committee's consensus conference, Lausanne, Switzerland, January 22–24, 2008. J Allergy Clin Immunol. 2008;122(2):254–260. doi: 10.1016/j.jaci.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Carlsen K. H., Anderson S. D., Bjermer L., et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387–403. doi: 10.1111/j.1398-9995.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Cummiskey J., Carlsen K., Kim K., et al. Sports pulmonology. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 268–301. [Google Scholar]
- Gropper S. S., Blessing D., Dunham K., Barksdale J. M. Iron status of female collegiate athletes involved in different sports. Biol Trace Elem Res. 2006;109(1):1–14. doi: 10.1385/BTER:109:1:001. [DOI] [PubMed] [Google Scholar]
- Sinclair L. M., Hinton P. S. Prevalence of iron deficiency with and without anemia in recreationally active men and women. J Am Diet Assoc. 2005;105(6):975–978. doi: 10.1016/j.jada.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Fallon K. E. Screening for haematological and iron-related abnormalities in elite athletes-Analysis of 576 cases. J Sci Med Sport. 2008;11(3):329–336. doi: 10.1016/j.jsams.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Eliakim A., Nemet D., Constantini N. Screening blood tests in members of the Israeli National Olympic team. J Sports Med Phys Fitness. 2002;42(2):250–255. [PubMed] [Google Scholar]
- Rietjens G. J., Kuipers H., Hartgens F., Keizer H. A. Red blood cell profile of elite Olympic distance triathletes: a three-year follow-up. Int J Sports Med. 2002;23(6):391–396. doi: 10.1055/s-2002-33736. [DOI] [PubMed] [Google Scholar]
- Di Santolo M., Stel G., Banfi G., Gonano F., Cauci S. Anemia and iron status in young fertile non-professional female athletes. Eur J Appl Physiol. 2008;102(6):703–709. doi: 10.1007/s00421-007-0647-9. [DOI] [PubMed] [Google Scholar]
- Schumacher Y. O., Jankovits R., Bultermann D., Schmid A., Berg A. Hematological indices in elite cyclists. Scand J Med Sci Sports. 2002;12(5):301–308. doi: 10.1034/j.1600-0838.2002.10112.x. [DOI] [PubMed] [Google Scholar]
- Dubnov G., Foldes A. J., Mann G., Magazanik A., Siderer M., Constantini N. High prevalence of iron deficiency and anemia in female military recruits. Mil Med. 2006;171(9):866–869. doi: 10.7205/milmed.171.9.866. [DOI] [PubMed] [Google Scholar]
- Katelaris C. H., Carrozzi F. M., Burke T. V., Byth K. Patterns of allergic reactivity and disease in Olympic athletes. Clin J Sport Med. 2006;16(5):401–405. doi: 10.1097/01.jsm.0000244606.56935.59. [DOI] [PubMed] [Google Scholar]
- Katelaris C. H., Carrozzi F. M., Burke T. V., Byth K. A springtime Olympics demands special consideration for allergic athletes. J Allergy Clin Immunol. 2000;106(2):260–266. doi: 10.1067/mai.2000.108603. [DOI] [PubMed] [Google Scholar]
- Katelaris C. H. Allergy and athletes. Curr Allergy Asthma Rep. 2001;1(5):397–398. doi: 10.1007/s11882-001-0022-6. [DOI] [PubMed] [Google Scholar]
- Hawarden D., Baker S., Toerien A., et al. Aero-allergy in South African Olympic athletes. S Afr Med J. 2002;92(5):355–356. [PubMed] [Google Scholar]
- Katelaris C. H., Carrozzi F. M., Burke T. V. Allergic rhinoconjunctivitis in elite athletes: optimal management for quality of life and performance. Sports Med. 2003;33(6):401–406. doi: 10.2165/00007256-200333060-00002. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Immune system adaptation in elite athletes. Curr Opin Clin Nutr Metab Care. 2006;9(6):659–665. doi: 10.1097/01.mco.0000247476.02650.18. [DOI] [PubMed] [Google Scholar]
- Ekblom B., Ekblom O., Malm C. Infectious episodes before and after a marathon race. Scand J Med Sci Sports. 2006;16(4):287–293. doi: 10.1111/j.1600-0838.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- Schwellnus M. P., Jeans A., Motaung S., Swart J. Exercise and infections. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 344–364. [Google Scholar]
- Luke A., d'Hemecourt P. Prevention of infectious diseases in athletes. Clin Sports Med. 2007;26(3):321–344. doi: 10.1016/j.csm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzolo J. J., LeMay D. C. Blood-borne infections. Clin Sports Med. 2007;26(3):425–431. doi: 10.1016/j.csm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Malm C. Susceptibility to infections in elite athletes: the S-curve. Scand J Med Sci Sports. 2006;16(1):4–6. doi: 10.1111/j.1600-0838.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- Adams B. B. Dermatology. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 326–343. [Google Scholar]
- Adams B. B. Dermatologic disorders of the athlete. Sports Med. 2002;32(5):309–21. doi: 10.2165/00007256-200232050-00003. [DOI] [PubMed] [Google Scholar]
- Adams B. B. Skin infections in sport. Intl Sports Med J. 2003;4 http://www.ismj.com. [Google Scholar]
- Rayner B., Schwellnus M. P. Exercise and the kidney. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 375–389. [Google Scholar]
- Schwellnus M. P., Wright J. Gastrointestinal system and exercise: a clinical approach to gastrointestinal problems in athletes. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 365–374. [Google Scholar]
- McCrory P. Neurologic problems in sport. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 412–428. [Google Scholar]
- Tsitsimpikou C., Tsiokanos A., Tsarouhas K., et al. Medication use by athletes at the Athens 2004 Summer Olympic Games. Clin J Sport Med. 2009;19(1):33–38. doi: 10.1097/JSM.0b013e31818f169e. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Trojian T., Kraemer W. J. Endocrinology. In: Schwellnus M. P., editor. The Olympic Textbook of Medicine in Sport. Oxford, United Kingdom: Wiley-Blackwell; 2008. pp. 302–325. [Google Scholar]
- Carek P. J., Mainous A. G., III A thorough yet efficient exam identifies most problems in school athletes. J Fam Pract. 2003;52(2):127–134. [PubMed] [Google Scholar]
Head Injuries
- Aubry M., Cantu R., Dvorak J., et al. Summary and agreement statement of the 1st International Symposium on Concussion in Sport, Vienna 2001. Clin J Sport Med. 2002;12(1):6–11. doi: 10.1097/00042752-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Langlois J. A., Rutland-Brown W., Wald M. M. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities – United States, 2001–2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733–737. [PubMed] [Google Scholar]
- Browne G. J., Lam L. T. Concussive head injury in children and adolescents related to sports and other leisure physical activities. Br J Sports Med. 2006;40(2):163–168. doi: 10.1136/bjsm.2005.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K. M., Weaver N. L., Padua D. A., Garrett W. E., Jr Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W., Johnston K., et al. Consensus statement on concussion in sport: 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185–200. doi: 10.1097/JSM.0b013e3181a501db. [DOI] [PubMed] [Google Scholar]
Dental Injuries
- Andrade R. A., Evans P. L. S., Almeida A. L. S., Rodrigues-Da Silva J. J. Prevalence of dental trauma in Pan American Games athletes. Presented at: International Association for Dental Research 86th Annual Meeting; Toronto, Ontario, Canada; July 1–5, 2008.
- Badel T., Jerolimov V., Panduric J., Carek K. [Custom made mouthguards and the prevention of orofacial injuries in sports] Act Med Croatica. 2007;61(suppl 1):9–14. [PubMed] [Google Scholar]
- Burden D. J. An investigation of the association between overjet size, lip coverage, and traumatic injury to maxillary incisors. Eur J Orthod. 1995;17(6):513–517. doi: 10.1093/ejo/17.6.513. [DOI] [PubMed] [Google Scholar]
- Croll T. P., Castaldi C. R. Customised protective mouthguards for orthodontic patients. J Clin Orthod. 1996;30(1):15–19. [PubMed] [Google Scholar]
- Cornwell H. Dental trauma due to sport in the paediatric patient. J Calif Dent Assoc. 2005;33(6):457–461. [PubMed] [Google Scholar]
- Fasel R. Analysis of Pathological and Therapeutic Services at the Olympic Games with Special Attention to the Oral Health of Athletes [dissertation] Spain: University of Barcelona; 2008. [Google Scholar]
- Fuselier J. C., Ellis E. E., 3rd, Dodson T. B. Do mandibular third molars alter the risk of angle fracture? J Oral Maxillofac Surg. 2002;60(5):514–518. doi: 10.1053/joms.2002.31847. [DOI] [PubMed] [Google Scholar]
- Kerr L. Dental problems in athletes. Clin Sports Med. 1983;2(1):115–122. [PubMed] [Google Scholar]
- Kvittem B., Hardie N., Roettger M., Conroy J. Incidence of orofacial injuries in high school sport. J Public Health Dent. 1998;58(4):288–293. doi: 10.1111/j.1752-7325.1998.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Levin L., Samorodnitzky G. R. Self assessed dental status, oral behavior, DMF, and dental anxiety. J Dent Educ. 2005;69(12):1385–1389. [PubMed] [Google Scholar]
- Ma'aita J., Alkwrikat A. Is the mandibular third molar a risk factor for mandibular angle fracture? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(2):143–146. doi: 10.1067/moe.2000.103527. [DOI] [PubMed] [Google Scholar]
- Milosevic A. Sports drinks hazard to teeth. Br J Sports Med. 1997;31(1):28–30. doi: 10.1136/bjsm.31.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic A. Eating disorders and the dentist. Br Dent J. 1999;186(3):109–113. doi: 10.1038/sj.bdj.4800036. [DOI] [PubMed] [Google Scholar]
- Schwimmer A., Stern R., Kritchman D. Impacted third molars: a contributing factor in mandibular fractures in contact sports. Am J Sports Med. 1983;11(4):262–266. doi: 10.1177/036354658301100415. [DOI] [PubMed] [Google Scholar]
- Sirimaharaj V., Brearley Messer L., Morgan M. V. Acidic diet and dental erosion among athletes. Aust Dent J. 2002;47(3):228–236. doi: 10.1111/j.1834-7819.2002.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Standards Australia. Guidelines for the Fabrication, Use and Maintenance of Sports Mouthguards. Sydney, Australia: Standards Australia; 2003; 1–7. Document HB209-2003. [Google Scholar]
- Vasan N. An In Vitro Examination of the Erosive Potential of 3 Popular Sports Drinks on Human Dental Enamel [master's thesis] Australia: University of Melbourne; 1998. [Google Scholar]
- World Health Organisation. Oral Health Surveys. 4th ed. Geneva, Switzerland: World Health Organisation; 1997. Dentition status and treatment needs; pp. 40–47. [Google Scholar]
- Yamada T., Sawaki Y., Tomida S., Tohani I., Veda M. Oral injury and mouthguard use by athletes in Japan. Endod Dent Traumatol. 1998;14(2):84–87. doi: 10.1111/j.1600-9657.1998.tb00816.x. [DOI] [PubMed] [Google Scholar]
Musculoskeletal
- Bahr R., Engebretsen L., editors. Sports Injury Prevention: Olympic Handbook of Sports Medicine. Vol. 2009 Oxford, United Kingdom: Wiley-Blackwell; [Google Scholar]
- Maffulli N., Wong J., Almekinders L. C. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Rompe J. D., Nafe B., Furia J. P., Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374–383. doi: 10.1177/0363546506295940. [DOI] [PubMed] [Google Scholar]
Issues Specific to Women
- Women and sport in the Olympic Games. International Olympic Committee. http://www.olympic.org/uk/organisation/missions/women/activities/women_uk.asp. Published 2009. Accessed July 2, 2009.
- Nattiv A., Loucks A., Manore M. M., Sanborn C., Sundgot-Borgen J., Warren M. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- Peeling P., Dawson B., Goodman C., Landers G., Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103(4):381–391. doi: 10.1007/s00421-008-0726-6. [DOI] [PubMed] [Google Scholar]
- Sundgot-Borgen J., Torstveit M. K. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14(1):25–32. doi: 10.1097/00042752-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Byrne S., McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5(2):80–94. doi: 10.1016/s1440-2440(02)80029-9. [DOI] [PubMed] [Google Scholar]
- Dusek T. Influence of high intensity training on menstrual cycle disorders in athletes. Croat Med J. 2001;42(1):79–82. [PubMed] [Google Scholar]
- Bachmann G. A., Kemmann E. Prevalence of oligomenorrhea and amenorrhea in a college population. Am J Obstet Gynecol. 1982;144(1):98–102. doi: 10.1016/0002-9378(82)90402-1. [DOI] [PubMed] [Google Scholar]
- Sanborn C. F., Martin B. J., Wagner W. W., Jr Is athletic amenorrhea specific to runners? Am J Obstet Gynecol. 1982;143(8):859–861. doi: 10.1016/0002-9378(82)90463-x. [DOI] [PubMed] [Google Scholar]
- Torstveit M. K., Sundgot-Borgen J. Participation in leanness sports but not training volume is associated with menstrual dysfunction: a national survey of 1276 elite athletes and controls. Br J Sports Med. 2005;39(3):141–147. doi: 10.1136/bjsm.2003.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. R., Mathur R. S., Kirk R. F., Williamson H. O. Female runners and secondary amenorrhea: correlation with age, parity, mileage, and plasma hormonal and sex-hormone binding globulin concentrations. Fertil Steril. 1981;36(2):183–187. [PubMed] [Google Scholar]
- Beals K. A., Manore M. M. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12(3):281–293. doi: 10.1123/ijsnem.12.3.281. [DOI] [PubMed] [Google Scholar]
- Chumlea W. C., Schubert C. M., Roche A. F., et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- Khan K. M., Liu-Ambrose T., Sran M. M., Ashe M. C., Donaldson M. G., Wark J. D. New criteria for female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36(1):10–13. doi: 10.1136/bjsm.36.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennell K., Matheson G., Meeuwisse W., Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91–122. doi: 10.2165/00007256-199928020-00004. [DOI] [PubMed] [Google Scholar]
- Carter J. C., Stewart D. A., Fairburn C. G. Eating disorder examination questionnaire: norms for young adolescent girls. Behav Res Ther. 2001;39(5):625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Passi V. A., Bryson S. W., Lock J. Assessment of eating disorders in adolescents with anorexia nervosa: self-report questionnaire versus interview. Int J Eat Disord. 2003;33(1):45–54. doi: 10.1002/eat.10113. [DOI] [PubMed] [Google Scholar]
- Wolk S. L., Loeb K. L., Walsh B. T. Assessment of patients with anorexia nervosa: interview versus self-report. Int J Eat Disord. 2005;37(2):92–99. doi: 10.1002/eat.20076. [DOI] [PubMed] [Google Scholar]
- Luck A. J., Morgan J. F., Reid F., et al. The SCOFF questionnaire and clinical interview for eating disorders in general practice: comparative study. BMJ. 2002;325(7273):755–756. doi: 10.1136/bmj.325.7367.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. F., Reid F., Lacey J. H. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467–1468. doi: 10.1136/bmj.319.7223.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M. A., Ball C., Robinson P. Four simple questions can help screen for eating disorders. J Gen Intern Med. 2003;18(1):53–56. doi: 10.1046/j.1525-1497.2003.20374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack M. T., Rauh M. J., Barkai H. S., Nichol J. F. Dietary restraint and low bone mass in female adolescent endurance runners. Am J Clin Nutr. 2008;87(1):36–43. doi: 10.1093/ajcn/87.1.36. [DOI] [PubMed] [Google Scholar]
PHE Form
- Matheson G. O., Boyajian-O'Neill L. A., Cardone D., et al. Preparticipation Physical Evaluation. 3rd ed. Minneaplis MN: McGraw Hill; 2005. pp. 93–94. [Google Scholar]
- Meeuwisse W. H., Matheson G. O., Wroble R. Prevalence of positive responses on sports preparticipation screening in Ohio students. Clin J Sport Med. 2003;13(6):381. [Google Scholar]
- Maron B. J., Thompson P. D., Ackerman M. J., et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 Update. A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–1655. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- Bille K., Figueiras D., Schamasch P., et al. Sudden cardiac death in athletes: the Lausanne recommendations. Eur J Cardiovasc Prev Rehabil. 2006;13(6):859–875. doi: 10.1097/01.hjr.0000238397.50341.4a. [DOI] [PubMed] [Google Scholar]