Abstract
Engineering complex tissues requires a precisely formulated combination of cells, spatiotemporally released bioactive factors, and a specialized scaffold support system. Injectable materials, particularly those delivered in aqueous solution, are considered ideal delivery vehicles for cells and bioactive factors and can also be delivered through minimally invasive methods and fill complex 3D shapes. In this review, we examine injectable materials that form scaffolds or networks capable of both replacing tissue function early after delivery and supporting tissue regeneration over a time period of weeks to months. The use of these materials for tissue engineering within the craniofacial complex is challenging but ideal as many highly specialized and functional tissues reside within a small volume in the craniofacial structures and the need for minimally invasive interventions is desirable due to aesthetic considerations. Current biomaterials and strategies used to treat craniofacial defects are examined, followed by a review of craniofacial tissue engineering, and finally an examination of current technologies used for injectable scaffold development and drug and cell delivery using these materials.
Keywords: biomedical materials, tissue engineering, drug delivery, hydrogels, polymeric materials
1. Introduction
Reconstruction of the oral and maxillofacial complex following traumatic insult, tumor ablation, or congenital deformities remains a formidable challenge for clinicians. A myriad of tissue types and morphologically complex structures are present in a relatively small area, with the consequence that defects often involve multiple tissue types including the facial skeleton, special sense organs, soft tissues (i.e. muscle, subcutaneous fat, skin, mucosa), salivary glands, cartilage, nerves, vessels, and teeth. Current clinical strategies designed to address such composite defects need to restore both the functional and aesthetic characteristics of the affected region. Additional considerations include the routine presence of bacterial contamination from the oral and sinus cavities, the ability to withstand the mechanical stresses imposed by masticatory function, and the special aesthetic challenges posed by the restoration of symmetric facial structures.
The morbidity and limitations associated with current surgical techniques and materials for oral and maxillofacial reconstruction has spurred the development of tissue engineering (TE) strategies to address these shortcomings. Injectable TE methods (with the ability to deliver both a therapeutic cell population and bioactive factors) are particularly attractive examples of how TE can be combined with minimally invasive techniques to reduce morbidity. The purpose of this review is to outline contemporary methods and materials in oral and maxillofacial reconstruction, describe currently available injectable tissue engineering systems, and discuss the use of such systems for the delivery of cells and bioactive factors to regenerate complex tissues within the oral and maxillofacial region.
2. Reconstructive materials commonly used in the oral and maxillofacial region
Advances in surgical techniques, biomaterials science, and cell biology have established several approaches to the reconstruction of the oral and maxillofacial region depending upon certain characteristics of the defect such as size, shape, and vascularity. Autologous tissue remains the “gold standard” material, although associated harvesting procedures may result in donor site morbidity and require additional surgical time. Allograft or xenograft tissues do not require additional operative time and expense but may suffer from batch-to-batch variability and carry the risk of potential viral or bacterial transmission and immune-mediated regenerative compromise. Synthetic materials offer the ability to precisely control biologically important characteristics such as porosity or hydrophilicity/hydrophobicity through the manufacturing process, but they usually require the addition of bioactive factors or cells to promote tissue regeneration.
When complex, composite defects are encountered following treatment for neoplastic or cystic pathology and with high-velocity ballistic injuries, autologous tissue reconstruction using pre-vascularized hard and soft tissue grafts usually represents the technique of choice. However, for single tissue defects (i.e. solely bone or soft tissue regeneration) which are much more common, the surgeon can choose from a wide range of autograft, allograft, xenograft, and synthetic materials currently available (Table 1).
Table 1.
Summary table of materials and methods currently used for oral and maxillofacial reconstruction.
| Tissue Type | Source | Clinically Available Methods & Materials |
|---|---|---|
| Hard tissue | Autologous | Non-vascularized bone grafts from iliac crest, tibia, skull, and mandible. |
| Allogeneic | Mineralized allogeneic bone available from tissue banks. | |
| Demineralized bone matrix combined with various carriers: | ||
| w/hyaluronic acid (i.e. DBX®) | ||
| w/glycerol (i.e. Grafton®) | ||
| w/gelatin (i.e. Regenafil®) | ||
| w/poloxamer (i.e. Dynagraft®) | ||
| w/calcium sulfate (i.e. Allomatrix®) | ||
| Xenogeneic | Crosslinked bovine collagen I coated in hydroxyapatite (i.e. Healos®). | |
| Deproteinized bovine bone (i.e. Bio-Oss®). | ||
| Porcine collagen I and III resorbable membrane (i.e. Bio-Gide®). | ||
| 60% hydroxyapatite + 40% tricalcium phosphate ceramics in bovine fibrillar collagen carrier (i.e. Collagraft®). | ||
| Synthetic | Ceramics | |
| Hydroxyapatite of natural origin | ||
| Coral sources (i.e. Pro Osteon® and Biocoral®). | ||
| Bovine bone (i.e. Bio-Oss®). | ||
| Synthetic hydroxyapatite. | ||
| Synthetic unsintered calcium deficient apatite. | ||
| Synthetic β-tricalcium phosphate. | ||
| Synthetic biphasic calcium phosphate. | ||
| Calcium phosphate cements (i.e. Norian®, BoneSource®, Mimix®). | ||
| Polymers | ||
| Poly(α-hydroxy esters) such as poly(lactic acid), poly(glycolic acid), and poly(lactic- co-glycolic acid). Applications include resorbable fixation plates and screws, and resorbable membranes. | ||
| Porous high density polyethylene implants (i.e. MedPor®). | ||
| Poly(methyl methacrylate) implants. | ||
| Recombinant growth factors | ||
| Bone morphogenetic protein-2 in an absorbable collagen sponge (i.e. Infuse®). | ||
| Platelet-derived growth factor in a β-tricalcium phosphate carrier (i.e. GEM 21S®). | ||
| Soft Tissue | Autologous | Soft tissue grafts |
| Full-thickness and partial-thickness skin grafts. | ||
| Oral mucosa grafts (i.e from the free gingiva, buccal mucosa, palate, etc.). | ||
| Microfat grafting. | ||
| Dermal fibroblast harvest, ex vivo culture and re-implantation (i.e. Isolagen®). | ||
| Soft tissue flaps | ||
| Local flaps (i.e. buccal fat pad). | ||
| Regional flaps (i.e. from pectoralis major, deltopectoral, temporalis). | ||
| Vascularized free flaps (i.e. from the radial forearm, anterolateral thigh, etc.). | ||
| Allogeneic | Short-term skin allografts. | |
| Long-term face transplants. | ||
| Freeze-dried de-epithelialized acellular dermal graft (i.e. Alloderm®). | ||
| Xenogeneic | Dermal fillers | |
| Fibrillar bovine collagen I and III (i.e. Zyderm®). | ||
| Crosslinked fibrillar bovine collagen I and III (i.e. Zyplast®). | ||
| Crosslinked hyaluronic acid derivatives (i.e. Hylaform®, Restylane®, Captique®). | ||
| Synthetic | Dermal fillers | |
| Liquid silicone. | ||
| Poly(methyl methacrylate) microspheres in collagen solution (i.e. ArteFill®). | ||
| Poly(L-lactic acid) microparticles in carboxymethylcellulose gel (i.e. Sculptra®). | ||
| Composite tissue | Autologous | Vascularized osseo-fascio-cutaneous free flaps (i.e. from the radial forearm, fibula, iliac crest, deltoid, and scapula). |
| Non-vascularized osteochondral grafts (i.e. from the ribs). | ||
| Synthetic | Polymeric prosthesis materials | |
| Poly(methyl methacrylate) | ||
| Polyurethane elastomers | ||
| Silicone elastomers (i.e. MDX 4-4210®) | ||
| Tissue engineering approaches | ||
| Titanium mesh scaffold filled with bone marrow and Bio-Oss® blocks coated with recombinant BMP-7. Construct placed in muscle for 7 weeks, then transplanted to mandible using microvascular techniques. |
2.1. Osseous reconstruction
2.1.1. Autologous tissue
Many clinicians consider harvested autologous bone (i.e. taken from the same individual) as the “gold standard” material for the reconstruction of osseous defects. Autologous bone grafts by their very nature are able to deliver a physiologically optimized combination of osteogenic cells and growth factors in a mineralized scaffold. In 1956, Axhausen[1] described two “osteogenetic phases” of bone regeneration when using bone grafts. The first phase (i.e. osteogenesis) begins several days after the grafting procedure and is attributed to the activities of surviving, pre-existing osteogenic cells, which form osteoid within the transplanted bone. The second phase (i.e. osteoinduction) occurs several weeks later, particularly in response to the resorption and remodeling of the bone graft by osteoclasts resulting in exposure of invading host-site stem cells to osteoinductive factors such as bone morphogenetic proteins contained within the mineralized matrix of the original graft. An autologous graft is also capable of initiating bone formation through an osteoconductive mechanism, if it is placed in proximity to a well vascularized bed and bone forming cells. With autologous bone grafts, immunologic rejection and disease transmission are absent.
Depending on the amount of bone graft required, the iliac crest, tibia, skull, and mandible[2, 3] are common areas in which particulate bone or blocks can be harvested. However, the supply of donor tissue is limited and morbidity increases as larger amounts of bone are harvested. While the incidence is low, complications related to iliac crest bone harvesting such as persistent post-operative pain, nerve injury, arterial injury, scarring, hemorrhage, hematoma, infection, and gait disturbance have been reported.[4]
Non-vascularized autogenous bone grafts offer a relatively predictable means of filling osseous defects and inducing new bone growth. Following graft remodeling, complete integration into the host site occurs. Several parameters govern the success of such grafts including prevailing conditions within the host site and stability of the graft. Sufficient soft tissue bed vascularity and coverage of the non-vascularized bone graft are typically required to prevent healing complications such as wound dehiscence or infection[5] and allow for survival of the transplanted osteogenic cells within the graft. Rigid fixation of the graft allows rapid neovascularization of newly formed bone and survival of transplanted cells. In some cases, soft tissue bed vascularity may be compromised, as in patients who have undergone radiation therapy for malignant disease. A course of hyperbaric oxygen treatments can be undertaken to promote soft tissue oxygenation and neovascularization, optimizing the quality of the recipient tissue bed prior to receiving the non-vascularized bone graft.
Vascularized bone grafts are less dependent on the presence of an optimized soft tissue recipient bed, although their size and shape are largely dictated by the morphology of the donor site. Since vascularized bone must be initially transferred with a peri-osseous cuff of soft tissue containing its blood supply, a second operation is frequently required to remove the excess soft tissue associated with the graft. Additional procedures may also be required to augment the volume of grafted bone to allow for dental implant rehabilitation. Nonetheless, despite the more technically demanding nature of microvascular free tissue transfer, vascularized bone and soft tissue grafts are now commonly used by experienced surgeons in the reconstruction of large composite tissue defects.
2.1.2. Allogeneic tissue
Allograft bone (i.e. harvested from an individual of the same species as the recipient) is typically derived from human cadavers and is available from bone banks and other commercial vendors. Donor site morbidity and limitations as to the quantity of graft are no longer considerations. Both cortical and cortico-cancellous allograft bone is available in the form of particulate, chips, and blocks. Large cortical allografts undergo minimal revascularization and remodeling leading to the accumulation of microfractures over time and the persistence of a non-vital graft incapable of physiological adaptation to functional loads.[6]
There is also a potential risk of bacterial, viral, or prion transmission with allograft bone, as well as immunologic rejection depending on the method adopted for bone preservation. To address these concerns, donor selection and screening (i.e. for human immunodeficiency viruses 1 and 2, and hepatitis B and C in the United States) combined with tissue processing (i.e. washing to ensure blood component removal,[7] freeze-drying, or gamma irradiation[8]) are used by bone banks and vendors to increase the safety of allograft bone products. As a result of these measures, the risk of disease transmission has been calculated to be quite small (i.e. the risk of receiving allograft bone from an HIV-infected donor is approximately 1 in 1.6 million[9]).
Ideally, allograft processing should produce safe yet biologically active products for use in osseous reconstruction. However, some processing methods have been associated with detrimental effects on the mechanical and biological performance of allograft bone. Examples include the promotion of microcracks in cortical bone grafts with freeze-drying, increased brittleness of cortical bone with gamma irradiation, and a decrease in the osteoinductivity of demineralized bone allograft with higher levels of gamma irradiation or ethylene oxide sterilization.[10] As a result, current processing methods render mineralized allograft bone a predominantly osteoconductive material with minimal osteoinductivity and no osteogenicity.
The biological activity of allograft bone can be augmented by the addition of autogenous bone or platelet rich plasma.[11] Alternatively, treatment of mineralized allograft bone with hydrochloric acid (0.5 – 0.6 M) or a 1:1 formic acid-citric acid mixture yields demineralized bone matrix (DBM) which is less immunogenic[12] and possesses both osteoconductive and osteoinductive properties (through the exposure of bone morphogenetic proteins previously contained within the mineral component of the allograft).[10] The biological activity of DBM is not consistent. Variations in growth factor content from lot-to-lot and between different commercial formulations of DBM have been reported[13] and confirmed by variations in osteoinductive potential between various products seen in animal studies.[14] To enhance intra-operative handling properties, DBM has been combined with carriers such as hyaluronate (i.e. DBX® from Synthes, USA), glycerol (Grafton® from Osteotech, USA), gelatin (Regenafil® from Regeneration Technologies, USA), poloxamer (Dynagraft® from GenSci Regeneration Sciences, Canada) and calcium sulfate (Allomatrix® from Wright Medical Technology, USA) by various commercial vendors.[15]
2.1.3. Xenogeneic tissue
Xenogeneic bone grafts (i.e. harvested from a different species) have the same potential advantages as allograft bone, in that virtually unlimited amounts can be procured without donor-site morbidity. As with the use of allografts, there is a small risk of pathogen transmission, although the risk of bovine spongiform encephalopathy (BSE) or the transmission of porcine endogenous retroviruses (PERVs) from xenogeneic products is low,[16] because unlike central nervous system tissues (i.e. brain and spinal cord), bone, skin, or skeletal muscle are not believed to contain infectious levels of transmissible spongiform encephalopathy agents.[17] The chemical and heat treatment of bovine bone to denature and remove proteins has also proven to be effective in the inactivation of prions.[18]
Although immunologic rejection of transplanted xenogeneic tissues is a possibility considering the large histocompatibility mismatch between animal and human tissues, processed xenograft bone has been used (either alone or in combination with autograft bone) in numerous dental applications such as implantology,[19] maxillary sinus floor augmentation,[20] alveolar ridge preservation,[21] and periodontal regeneration without significant reaction.[22] Several commercial products available for dental and orthopedic bone regenerative applications include cross-linked bovine collagen I fibers coated in hydroxyapatite (Healos® Bone Graft replacement from DePuy Spine, USA), deproteinized bovine bone (Bio-Oss® from Osteohealth, USA), porcine collagen I and III resorbable membrane (Bio-Gide® from Osteohealth, USA), and a composite of 60% hydroxyapatite + 40% tricalcium phosphate ceramics in a bovine fibrillar collagen carrier (Collagraft® from Angiotech Pharmaceuticals, USA).[16]
2.1.4. Synthetic biomaterials Ceramics
The extracellular matrix of bone has been described as a composite material composed of collagen type I fibrils mineralized with nanocrystals of hydroxyapatite.[23] Approximately 70% of bone by weight is composed of calcium salts, with hydroxyapatite (Ca10(PO4)6(OH)2) as the primary mineral constituent. Strictly speaking, bone mineral is not purely hydroxyapatite, and the presence of ion impurities has actually led to the consensus that a more accurate term for the inorganic component is carbonatehydroxyapatite with the formula (Ca,Mg,Na)10(PO4HPO4CO3)6(OH)2.[24] Devoid of an organic component, calcium salts such as hydroxyapatite are biocompatible, non-immunogenic components of bone and are considered to be osteoconductive. Consequently, there has been much interest in designing synthetic osteoconductive grafting materials based on these naturally occurring calcium salts.
LeGeros[24] has characterized commercially available calcium phosphate (CaP) biomaterials as either hydroxyapatite of natural origin or synthetically produced CaP. The hydroxyapatite of natural origin is either obtained from bovine bone (as discussed above) or from certain species of coral. These naturally derived sources of hydroxyapatite are processed so that their macroporous, interconnected structure is maintained, allowing for in-growth of host tissue upon implantation, as well as the diffusion of nutrients throughout the graft material.
Coralline-derived ceramics are typically prepared in two ways. The first method involves a solid-state hydrothermal exchange reaction called the Replamineform process, in which the calcium carbonate-based coral skeleton is converted to a calcium phosphate-based material (while still maintaining its architecture), which is predominantly in the form of hydroxyapatite.[25] This material is marketed under the name Pro Osteon® (Interpore Cross International, USA). Since Replamineform grafting materials are nearly non-resorbable,[26] newer coralline ceramics that have undergone a partial Replamineform process have been developed, resulting in a material which is composed mainly of calcium carbonate with calcium phosphate present only on the internal and external surfaces. This material is marketed as Biocoral® (Biocoral, USA) and has been shown to be resorbed and replaced by bone over time.[26]
Numerous synthetic CaP biomaterials are commercially available and have been classified according to their composition by LeGeros[24] as hydroxyapatite, unsintered calcium deficient apatite, beta-tricalcium phosphate, or biphasic calcium phosphate. Hydroxyapatite can be produced with either a dense or macroporous morphology, and is typically sintered at temperatures above 1000 °C in granular or block forms. The high heat of sintering produces a material that cannot be reshaped to fit into a bone defect (i.e. if in block form) and is non-resorbable.[27] Beta-tricalcium phosphate (β-TCP) has the formula Ca3(PO4)2 and like hydroxyapatite is a brittle material with low fracture resistance. Both hydroxyapatite and β-TCP are biocompatible, osteoconductive, and bioactive (i.e. they develop a direct, adherent bond with bone).[24]
Under physiological conditions, hydroxyapatite is essentially a non-resorbable material, while on the other hand β-TCP has been shown to degrade within 6 weeks after implantation.[28] The dissolution of CaP biomaterials is dependent on composition (hydroxyapatite vs. β-TCP ratio), surface area of the implant (particulate vs. block form), porosity, and crystallinity (sintering creates larger, slower dissolving crystals).[24] Biphasic CaP products which contain hydroxyapatite and β-TCP in various ratios (the higher the β-TCP content, the greater the resorbability[29]) are aimed at the provision of a bone grafting material which is able to degrade within a physiologically optimized time frame, while providing some measure of mechanical stability until sufficient bone in-growth has occurred.[28]
CaP cements are also available, and these combine a dry powder (CaP) and a liquid component (i.e. an inorganic or organic acid, or sodium phosphate solutions) in a setting reaction that occurs under physiologic pH and temperatures.[24] Examples include Norian® (Synthes Craniomaxillofacial, USA), BoneSource® (Stryker Leibinger, Germany), and Mimix® (Walter Lorenz Surgical, USA). A variety of CaP compounds have been used for the solid phase such as dicalcium phosphate, dicalcium phosphate dihydrate, calcium-deficient hydroxyapatite, and amorphous calcium phosphate.[27] These cements are injectable and able to be molded for variable periods before hardening. They are also described as resorbable, though clinical experience has demonstrated retention of the material over extended periods. While CaP cements have been successfully used for clinical applications such as vertebroplasty[30] and cranial defect repair,[31] they are brittle and contraindicated for use in areas of mobility, active infection, or in situations where they directly contact the sinuses or dura.[27]
Synthetic polymers
The long and successful history of synthetic polymers in medicine combined with the ability to control their material properties has generated much interest in their use for bone regeneration strategies. Polymers currently used for oral and maxillofacial osseous reconstruction/augmentation include silicones, poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(ethylene), poly(caprolactone) (PCL) and poly(methyl methacrylate) (PMMA). These materials are biologically inert and do not possess osteogenic, osteoconductive, or osteoinductive properties. Hence, none of these materials have been incorporated into commercial bone grafting products as of yet. However, the biocompatibility of many synthetic polymers, combined with the ability to reproducibly control their composition, rate of degradation, pore size, porosity, interconnectivity, hydrophobicity/hydrophilicity, ability for cell attachment, morphology, and handling properties has made them attractive materials for investigation as scaffolds and delivery vehicles of cells, drugs, and growth factors in tissue engineering.
Currently, the most common synthetic absorbable polymers available for oral and maxillofacial applications include the poly(alpha-hydroxy esters) PGA, PLA, and their copolymer, PLGA. Once implanted, these materials slowly degrade through hydrolysis and the by-products (lactic acid from PLA and glycolic acid from PGA) are incorporated into the Krebs cycle and eventually eliminated as carbon dioxide and water.[32] They have been used as resorbable sutures for the past 40 years, and recently as degradable plates and screws for bone fixation following craniofacial surgery,[33] orthognathic surgery,[34] and trauma.[35, 36] Advantages of these devices over traditional titanium plates and screws include elimination of long-term palpable devices and continued skull growth in the pediatric population once they have degraded.[33] Resorbable membranes made of PLA and PLGA have also been successfully used as barriers for use in guided tissue regeneration procedures to treat periodontal defects.[37]
The biocompatibility of non-degradable synthetic polymers has led to their commercialization as permanent implants for craniofacial augmentation or reconstruction. Solid facial implants made from silicone elastomer have been used for almost 50 years, and are available for skeletal augmentation of the malar eminence, zygomatic arch, and chin.[38] These implants are available in various pre-contoured forms and can be carved intra-operatively to customize the shape for implantation in a particular area. Once implanted, the smooth-surfaced silicone implants are encapsulated by an avascular fibrous capsule, although initial fixation of the implant is important to prevent displacement or subsequent mobility which can lead to complications.[38] Porous high-density polyethylene (HDPE) (MedPor®, from Porex Industries, USA) is also available as a customizable pre-fabricated porous implant and has found applications as a skeletal augmentation material, a space maintainer following globe exenteration, and as a structural support for orbital reconstruction following trauma and tumor resection.[39]
Another non-degradable polymer commonly used in craniofacial osseous reconstruction is PMMA. The biocompatibility of PMMA, established over a 50 year history of clinical use, has led to it being the most frequently used synthetic material for skull reconstruction in the world.[40] In situ curing PMMA cement is available as a two-phase system, in which a powder (consisting of PMMA polymer particles and a polymerization catalyst) is mixed with a liquid (consisting of MMA monomer). The combination produces a moldable, putty-like material that polymerizes into a rigid, high strength solid within 10–15 minutes. The exothermic nature of the setting reaction and the leaching of unreacted monomer from the implanted PMMA has been shown to cause bone necrosis and inflammation.[41] Consequently, PMMA cement is usually polymerized extra-corporeally before insertion into the defect. PMMA can also be obtained as a pre-formed implant, whose shape is customized to fit a patient’s bone defect through the use of computed tomography stereolithographic models.[42]
Since PMMA does not bond directly to the surrounding hard and soft tissues, techniques have been developed to allow better fixation of the material, such as the incorporation of titanium mesh scaffolding in cranioplasty[43] or by combining PMMA with carboxymethylcellulose gel to generate surface porosity.[44] PMMA is considered to be the alloplastic material of choice for cranioplasty in adults with good soft tissue quality and no frontal sinus exposure or previous history of infection. It should be used with caution in children since this essentially “permanent” and rigid material cannot adapt to a growing craniofacial skeleton.[40]
Recombinant growth factors
In view of the biological limitations associated individually with autograft, allograft, and synthetic materials, surgeons have attempted to augment the activity and physical properties with composite grafts combining several different materials. For example, particulate allograft bone can be used as an “expander” for autograft bone, resulting in less bone having to be harvested from a donor site, but still allowing for a grafting material which is osteoinductive, osteoconductive, and osteogenic. Recently, the commercial availability of recombinant growth factor products has given oral and maxillofacial surgeons an additional option for the reconstruction of bony defects. In the United States, recombinant human bone morphogenetic protein-2 (BMP-2) in an absorbable collagen sponge carrier (Infuse® from Medtronic, USA) has been approved for maxillary sinus augmentation and localized alveolar ridge augmentation in the oral and maxillofacial region. Orthopedic procedures approved by the United States Food and Drug Administration (FDA) for the use of Infuse® bone graft include spinal fusion and open tibia fractures. Platelet-derived growth factor (PDGF) in a β-TCP carrier (GEM 21S® from Osteohealth, USA) has been approved in the United States as well for the treatment of bone defects and gingival tissue recession associated with advanced periodontal disease.
The use of recombinant growth factor products for bone regeneration is appealing since they reduce the need for bone harvesting, are readily available, and contain high concentrations of a purified biological agent involved in the bone healing process. The implantation of such factors into a bone defect and the controlled release of the factor over time should promote the proliferation and differentiation of osteogenic stem cells within the wound, accelerating the healing process. Thus, products such as Infuse® contain the osteoinductivity of autograft bone, combined with the convenience of “off the shelf” demineralized bone matrix, without the concerns of pathogen transmission or batch-to-batch variations in potency. These advantages have already spurred surgeons to find “off-label” uses of BMP-2 such as the reconstruction of mandibular continuity defects following tumor resection, the grafting of maxillary clefts, and the reconstruction of hard tissue avulsion defects following trauma.[45]
Since our clinical experience with such technology is relatively new, issues such as: 1) the long-term effects of implanting materials containing supra-physiologic doses of growth factors and, 2) the use of growth factors for the reconstruction of defects associated with neoplasms, remain unresolved. In addition, the potential efficacy of these materials and their ability to reduce operating time and donor site morbidity will have to be weighed against their relatively high cost.
2.2. Soft tissue reconstruction
2.2.1. Autologous tissue
Similar to osseous reconstruction, autologous tissue is the standard for reconstruction of oral and maxillofacial soft tissue defects. Small to medium-sized superficial defects can be repaired with skin grafts, which can be harvested as either “full-thickness” or “partial-thickness”. Both types of skin graft contain the entire epidermis, but full-thickness grafts incorporate the entire dermal component (including dermal appendages such as hair follicles or sweat glands if present), while partial-thickness grafts are harvested at the level of the more superficial papillary dermis, leaving the deeper reticular dermis in place. Autologous grafts can also be harvested from various regions of the oral cavity, including the free gingiva, buccal mucosa, and palate. During the first 2–3 days of transplantation, nutrient exchange to the graft occurs through serum imbibition, after which the graft becomes revascularized through anastomoses between the host and donor vessels. Thus, the survival of both types of skin grafts relies on a recipient site that is well vascularized and immobility of the graft against its nutrient bed.
Unlike free grafts, soft tissue flaps are prepared in such a way that their blood supply is maintained following transfer to the recipient site. The donor tissue for local flaps is located close to the recipient site so that the tissue can be advanced or rotated into position while retaining a nerve and blood supply through its pedicle. A number of local flaps have been used for reconstruction of oral and facial defects including those involving the lip (i.e. Abbe flap)[46] and oral cavity (i.e. palatal flaps and tongue flaps).[47] Larger oral and maxillofacial soft tissue defects require more tissue and can be reconstructed with regional flaps. Like local flaps, regional flaps rely on an intact vascular pedicle for their blood supply, although the donor site is more distant. Examples of regional flaps include the pectoralis major, deltopectoral, and temporalis flaps. The pectoralis major flap can be used to transfer both muscle and skin (hence its classification as a “myocutaneous” flap) to large oral and maxillofacial defects.[48] The temporalis muscle flap is another regional flap which can be used for soft tissue reconstruction of oral defects. It is elevated from the temple and rotated into the orbit or oral cavity. Regional flaps for closure of palatal defects are reliable and versatile, but create cosmetic defects at the donor site and often require secondary procedures to remove excess tissue.[49] Such flaps are also unable to transfer bone along with the soft tissue and cannot address the comprehensive needs of a composite defect.
The advent of microvascular surgical techniques in the 1980s allowed the development of a new way to transfer soft and hard tissue together in the form of vascularized free flaps. These flaps are harvested from distant sites along a dominant arterial supply and venous system and re-anastomosed to vessels at the recipient site, providing an instantaneous vascular system. A variety of free flaps have been described for oral and maxillofacial reconstruction, and they can be harvested with soft tissue alone (i.e. fascio-cutaneous or fascial flaps) or with a combination of hard and soft tissue (i.e. osseo-fascio-cutaneous flaps). A review by Gonzalez-Garcia et al.[50] lists numerous vascularized free flaps available for such purposes, with donor sites such as the radius, fibula, iliac crest, deltoid, anterolateral thigh (ALT), and scapula. The authors also describe the versatility of the radial forearm free flap (RFFF) in particular, which can be used to cover defects involving any location within the oral cavity including the floor of the mouth, tongue, gingiva, buccal mucosa, soft palate, and retromolar area. In skilled hands, the overall failure rates of vascularized free flaps for soft tissue reconstruction in the head and neck region are relatively low, ranging from 5.5–8.8%,[50] indicating that this versatile technique is predictable and an important technique for the reconstruction of composite defects or those where vascular compromise of the recipient bed is an issue.
Aside from the reconstruction of soft tissue defects, autologous soft tissue has also been used for cosmetic facial augmentation. Composite grafts such as dermofat or adipofascial grafts from the ALT region have been used for this purpose,[51] as well as local flaps such as the buccal fat pad flap.[52] “Microfat” grafting has also been described,[53] in which adipose tissue is harvested atraumatically by suctioning or direct excision and then injected into the subcutaneous or intramuscular layers of the deficient site. While the supply of donor site adipose tissue is generally abundant, substantial overfilling of a defect is required due to the unpredictable stability and longevity of the injected fat over time.
The ex vivo expansion of harvested autologous cells for dermal augmentation has also been attempted commercially. Isolagen® (from Isolagen Technologies, USA) is a product currently in Phase 3 clinical trials within the United States where it is being investigated for the treatment of wrinkles.[54] The process involves harvesting skin from the post-auricular region using a 3 mm biopsy punch, and sending the specimen to the manufacturer to isolate and culture dermal fibroblasts. 4–6 weeks later, an autologous expanded explant is ready for use as an injectable dermal filler. Two to four treatments are typically required to obscure a wrinkle. As growth factors are used during the culturing process, this product is considered a “medical device” and requires additional safety testing prior to approval by the United States FDA.
2.2.2. Allogeneic tissue
Autologous skin grafts are the preferred method for the treatment of burns. Patients with extensive burns, however, often lack sufficient donor tissue. The temporary use of allograft skin for third-degree facial burn coverage has been reported,[55] where it was used to promote initial vascularization of the wound bed following debridement, then removed 6 days later prior to the placement of a split-thickness skin autograft. Allograft skin usually undergoes rejection within 2 weeks, although there are reports of skin allografts persisting in non-immunosuppressed burn patients for up to 7 weeks, possibly due to the repopulation of the allograft by host cells.[56] Skin grafting involving the entire face is associated with poor aesthetic and functional results, since multiple grafts are required, producing a patchwork appearance.[57]
One of the most spectacular and controversial solutions to the problem of total facial reconstruction is the use of allograft transplantation of a composite flap containing all the soft tissue structures: skin, fat, muscle, and nerves. The first human partial face transplant was completed in 2005.[58] Apart from difficult ethical and psychological issues involved with such a procedure, patients must be placed on immunosuppressive drugs for life.[59] The initial outcome of such work appears promising, and satisfactory functional and aesthetic results have been observed 18 months post-transplantation.[60]
A more biocompatible allogeneic grafting material for soft tissue reconstruction is freeze-dried, de-epithelialized, acellular dermal graft (Alloderm® from LifeCell, USA). The removal of all cellular components from the graft reduces the potential for pathogen transmission while also decreasing the immunogenicity of the material. The resulting product is an acellular dermal matrix which can be used as sheets or as an injectable particulate. Both formulations undergo rapid vascularization and repopulation of the graft material with host cells derived from the wound site.[61, 62] Alloderm® sheets have been used for the treatment of acute burns[63] and the reconstruction of eyelids[64] and buccal mucosal defects,[65] while the injectable material Cymetra® has been described for use in lip augmentation procedures.[66]
2.2.3. Xenogeneic tissue
One of the most commonly used dermal fillers is bovine collagen. Commercial preparations such as Zyderm® (from Allergan, USA) are composed of purified, fibrillar collagen type I and III and are approved for cosmetic procedures for the treatment of wrinkles, frown lines, crow’s feet, and acne scars. A related product called Zyplast® (from Allergan, USA) is composed of cross-linked bovine collagen which is less prone to enzymatic degradation after injection, but is recommended for injection into deeper defects because it may result in a beaded appearance.[67] While these materials have variable rates of degradation depending on the area of injection, collagen fillers typically last 2–4 months in duration. One of the main drawbacks to the use of bovine collagen is the risk of a severe allergic reaction, thus a skin test should be performed on all patients prior to treatment. Approximately 3–10% of patients will display a positive response such as redness, itching, tenderness, or firmness at the test site, and should not undergo grafting with this material.[67]
Hyaluronic acid is a glycosaminoglycan that forms a major non-structural component of the connective tissue extracellular matrix and aids the skin in maintaining its turgor through its hydrophilicity. Since the hyaluronic acid moiety is identical across all species, xenogeneic hyaluronic acid is not immunogenic in humans.[68] Cross-linked hyaluronic acid derivatives are commercially available as soft tissue fillers, including Hylaform® (from Biomatrix, USA) which is derived from rooster combs, and Restylane® (from Medicis Aesthetics, USA) or Juvéderm® (from Allergan, USA) which are produced through bacterial fermentation. Like the bovine collagen fillers, these hyaluronic acid-derived products are approved by the FDA as injectable materials for soft tissue augmentation. While allergic skin testing is not necessary prior to treatment, rare allergic reactions have been known to occur to the residual avian/bacterial proteins in these materials.[68] The dermal augmentation achieved with hyaluronic acid-derived products has been reported to persist for longer periods compared with bovine collagen (up to 6 months) and longevity can be extended up to 9 months with the concomitant use of botulinum toxin to reduce recipient site mobility around the filler material.[69]
2.2.4. Synthetic biomaterials
Up to this point, the injectable materials discussed for soft tissue augmentation have all been “temporary” in nature, with the results generally lasting less than one year. Although some permanent injectable dermal fillers are available, Homicz and Watson[70] caution that changes in facial form or adverse reactions to injected materials may actually warrant the use of temporary fillers.
One of the most controversial permanent soft tissue fillers is liquid silicone, which has been used by physicians for more than 50 years. Currently the FDA approves medical-grade liquid silicone injections solely for ophthalmologic use to tamponade retinal detachments. However, some physicians have also used it off-label for soft tissue augmentation using a “microdroplet” technique, in which the silicone is injected in 0.01 ml increments, 1 mm apart in the sub-dermal layer.[71] Over several weeks, the injected silicone droplets produce a granulomatous reaction in the host tissues, and are encapsulated as foreign bodies within fibrous tissue. While some clinicians prefer the more natural feeling augmentation which can be achieved with silicone, the injection of large volumes has sometimes led to severe local and system reactions.[70]
Another permanent injectable dermal filler material is ArteFill® (from Arte Medical, USA) which is a FDA-approved combination of PMMA microspheres suspended in a solution of 3.5% ultrapurified bovine collagen and 0.3% lidocaine.[72] The manufacturer recommends the injection of small quantities of ArteFill® every 1–3 months to minimize the chances of severe inflammatory reactions. Following injection, the bovine collagen is degraded and each microsphere is encapsulated in a fibrous sheath with minimal foreign body reaction, although some authors have reported the induction of foreign body granulomas following ArteFill® injections for lip augmentation.[73]
In an effort to address the need for a temporary material with longer lasting results, a new product called Sculptra® (from Dermik Laboratories, USA) has recently been approved by the FDA for the treatment of HIV-associated facial lipatrophy. Sculptra® is an injectable filler composed of freeze-dried, crystalline, irregularly sized microparticles of poly-L-lactic acid (PLLA) combined with sodium carboxymethylcellulose as a delivery vehicle.[74] PLLA is a well-known biocompatible and biodegradable polymer which has been used in numerous medical technologies ranging from resorbable sutures and plates and screws to drug delivery vehicles. To minimize the risk of complications from aggressive use of Sculptra® injections (such as the formation of nodules and granulomas), recommendations include limiting the volume of each injection, placing the material subcutaneously and not intradermally, post-injection massage of the area, and a delay of 6 weeks between treatment sessions.[74] Some authors have reported results lasting up to 18–24 months, fulfilling its promise as a longer-lasting yet non-permanent dermal filler material.
2.3. Composite tissue reconstruction
Severe traumatic insults and the surgical treatment of extensive oral and maxillofacial pathology can involve a considerable loss of facial tissues. Local control of disease requires complete removal without regard for aesthetically sensitive areas and may produce defects which are disfiguring and impose significant emotional stress on the patient. While tissue engineering holds promise for the future, the current mainstay of reconstruction and rehabilitation in patients with large composite tissue deformities remains with a combination of microvascular free tissue transfers and maxillofacial prosthetics.
Since vascularized free flaps have already been discussed, this section will focus on the use of maxillofacial prosthetics for the restoration of complex defects involving the loss of multiple tissues.
2.3.1. Commonly used polymeric materials for maxillofacial prostheses Acrylic resins
Polymethylmethacrylate is the most widely used acrylic polymer in health care. Variations in the molecular structure produces hard polymethacrylates used for dentures and orthopedic bone cement while various soft polyacrylates (i.e. ethyl or butyl acrylates) are used in contact lenses.[75]
Methyl methacrylate resins are readily available and the durability and color stability of PMMA make it an excellent material for facial prostheses. The strength of PMMA enables the clinician to thin the exposed margins of the prosthesis, improving the aesthetic result. In addition, benefits to the patient include compatibility with most adhesive systems and easily cleaning.[76]
Acrylic resins are most successfully employed in specific types of facial defects, namely those in which minimal movement of the underlying tissue bed occurs during function (i.e. prosthetic eyes). If placed in an inappropriate location, rigid PMMA facial prostheses can be uncomfortable and erosion may occur through the soft tissue.
Polyetherurethane elastomers
Polyetherurethanes have a variety of commercial uses and have also become popular for biomedical applications. In general, polyetherurethanes possess a number of favorable characteristics making them suitable for restoring defects with mobile tissue beds.[77] Polyurethanes can be made flexible without compromising edge strength, allowing the clinician to thin the margins giving the prosthesis a lifelike appearance and feel. In addition, when processed properly these elastomers are chemically inert, abrasion-resistant, and do not require the use of plasticizers to attain their flexibility.
A serious drawback to the use of polyurethanes in maxillofacial prosthetics is the difficulty in processing these materials consistently. A precise, stoichiometric admixing of all the components is necessary, with little margin for error. Furthermore, the toxic and hazardous diisocyanate component is moisture sensitive, as water contamination will cause gas bubble formation resulting in poor curing of the material with defects. As a consequence, either specially prefabricated metal molds must be utilized for the polymerization reaction or if stone molds are employed, they must be thoroughly dehydrated prior to use. In addition, facial prostheses fabricated from polyurethane are not color-stable, possibly due to the effects of ultraviolet light and surface oxidation.[76] From the patient perspective, additional problems with polyurethane prostheses are their poor compatibility with adhesive systems and difficulty to clean.[78]
Silicone elastomers
Technically called polydimethylsiloxane (PDMS), the silicones are probably the most widely used materials in maxillofacial prostheses.[76] Silicone elastomers are formed by cross-linking the PDMS chains into a network, a process which is also referred to as vulcanization. Compounding the material with silica fillers typically provides additional strength.
The numerous silicone elastomers used for maxillofacial prostheses have been classified into two general categories based on the type of cross-linking reaction used to form the final shape of the device: room-temperature vulcanizing (RTV) and heat-vulcanizing (HTV). Thus, vulcanization can occur both with and without the application of heat, and depends on the specific catalysts and cross-linking agents utilized by the two general types of silicone elastomers.
Although HTV silicone elastomers have been shown to have excellent thermal stability, color stability upon ultraviolet light exposure, and biologic inertness, they do not possess sufficient flexibility to function well on moveable tissue beds. Clinically, the aesthetics of this material have been criticized for their opacity and lifeless appearance.[76] Nonetheless, when compared to their RTV silicone counterparts, HTV silicone elastomers exhibit better physical and mechanical properties, partly by overcoming the problem of hand-mixing pigments as typically used for fabricating prostheses from viscous RTV silicones [75].
Designed for cross-linking at room temperature, RTV silicone elastomers are composed of relatively short-chain silicone polymers which are partially end-blocked with hydroxyl groups.[77] In general, some limiting aspects of RTV silicone elastomers include air entrapment from mixing the various components prior to cross-linking.[75] These voids persist in the finished prosthesis, which may lower tear resistance and help accumulate skin exudates. Silica fillers are used to enhance tensile strength as well as mask discoloration of the material, although a considerable amount of translucency is lost, thus compromising the ability to achieve optimal intrinsic coloration of the material through the incorporation of pigments.
An improved alternative is MDX 4-4210® (from Dow Corning, USA), a Medical Grade RTV silicone elastomer which is the most commonly used material in clinical practice for the fabrication of maxillofacial prostheses.[79] This material has a chloroplatinic acid catalyst and a hydro-methylsiloxane cross-linking agent, allowing for curing to take place through an addition-type reaction, and hence a lack of reaction byproducts.[76] MDX 4-4210® has been reported to address the general limitations of RTV silicone elastomers with superior coloration qualities and edge strength,[80] reducing the need for tear repair which typically requires the skilled application of additional PDMS or reinforcement of the edge with fabric. Although MDX 4-4210® does not possess all the characteristics of an ideal maxillofacial polymer, it has many desirable properties as discussed. Nonetheless, efforts continue to improve the material properties of this popular maxillofacial prosthetic material by increasing tear strength[81] and surface wettability.[82]
2.4. Tissue engineering approaches for composite tissue regeneration
Up until 2004, autologous tissue remained the only source for transferring viable hard and soft tissue simultaneously. From vascularized osseo-fascio-cutaneous free flaps for craniofacial reconstruction, to simple, non-vascularized costo-chondral grafts used for the reconstruction of the mandibular condyle, an allogeneic, xenogeneic, or alloplastic material does not exist which can match the characteristics of composite tissue grafts/flaps.
Recently however, a tissue engineering approach for mandibular regeneration in a patient was reported by Warnke et al.,[83] in which a custom, vascularized bone graft was used to restore masticatory function and aesthetic form to a patient who had undergone subtotal mandibulectomy 8 years prior. The patient had received post-surgical radiation treatment, decreasing the probability for a successful non-vascularized bone graft. He was also on anti-coagulation therapy for an aortic valve replacement which increased his risk of severe post-operative bleeding following a large bone harvest.
Thus, a tissue engineering strategy was selected, in which a titanium mesh tray was custom designed for the mandibular defect and then filled with blocks of Bio-Oss® (deproteinized bovine bone) coated with recombinant BMP-7 in a bovine collagen type 1 carrier. In addition, 20 mL of bone marrow was aspirated from the patient’s right iliac crest and mixed with Bio-Oss® particulate as a “grout” between the Bio-Oss® blocks of the construct. This approach utilized all the components of a tissue engineering strategy: cells (from the bone marrow), growth factors (the recombinant BMP-7 and endogenous growth factors in the marrow aspirate), and a scaffold (both the titanium mesh for the overall morphology of the implant and Bio-Oss® blocks providing an osteoconductive material).
To overcome the problem of transplanting this construct into the poorly vascularized tissue bed of the residual mandible, it was instead placed within the latissmus dorsi muscle of the patient for 7 weeks to allow for revascluarization of the construct. In this way, a vascularized free flap transfer of the TE construct and its accompanying soft tissue envelope was possible, whereby the thoracodorsal artery and vein of the latissmus dorsi were anastomosed to vessels of the neck. Warnke et al. were able to follow the patient for 15 months until he passed away from cardiac arrest, but during that time the patient’s quality of life improved dramatically as his ability to eat and speak had improved.[84]
This brief overview of conventional treatments for disfiguring and large composite tissue defects using vascularized free flaps and prostheses illustrates the fact that all of the materials and methods currently in clinical use fall short of providing complete aesthetic and functional regeneration of lost tissues. The following sections will provide an overview of current research in craniofacial tissue engineering which has the potential to revolutionize the clinical methods of reconstruction we know today.
3. Engineering multiple craniofacial tissues
Tissue engineering strategies rely on the use of cells, bioactive factors, and scaffolds or combinations thereof. The scaffold serves the purpose of a delivery vehicle, a space-filling and structurally supportive agent, and can be designed to be biointeractive, i.e. to guide tissue regeneration. The field of tissue engineering has made significant advances over the past 15 years. Interdisciplinary research spanning basic cell biology to nanotechnology has deepened our understanding of nature and enabled methods of biomimicry to augment or replace tissue or even organ function. Research on the regeneration of virtually all types of tissues is being conducted, and products for cartilage, bone, and skin regeneration are already approved for commercial use by the United States FDA. The engineering of more complex tissues remains a challenge, but encouraging advances in the form of a tissue engineered bladder[85] and an increased focus on issues specific to engineering complex tissues[86] have recently appeared in the literature.
The coordinated regeneration of multiple tissues in the complex craniofacial environment requires a deep understanding of their physiology and remodeling characteristics. Complex tissues must be engineered with the structural and functional characteristics of native tissue in a process that is not only biocompatible but also interactive and integrative with neighboring tissues simultaneously. Another challenge lies in that one type of tissue can be found in various structures that serve different functions and have therefore different properties. For example, the cartilaginous structures found in the craniofacial region have very distinct characteristics. A specifically tailored approach may be required to regenerate the weight-bearing, dense, and bilaminar cartilage found in the temporomandibular joint (TMJ) and a quite different approach required to create the delicate elastic cartilage found in the ears or nose.[87]
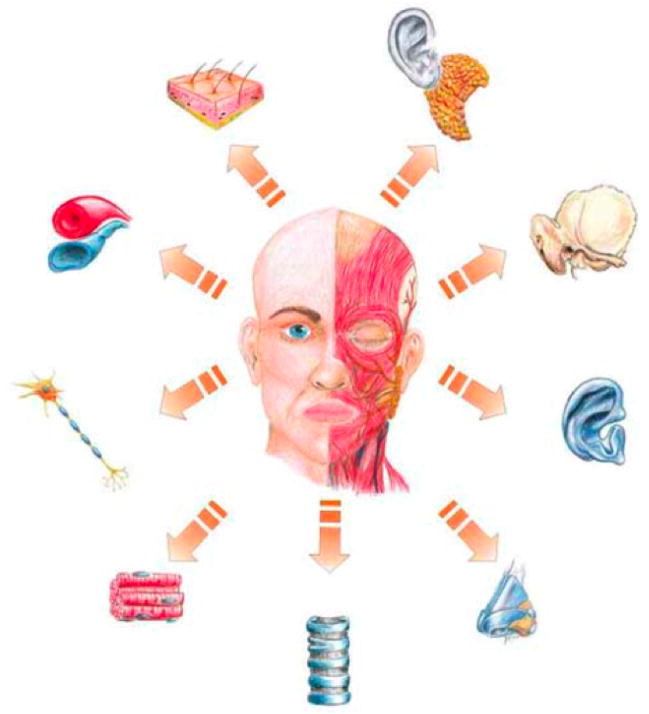
Tissues of the craniofacial region include skin, bone, muscle, cartilage, adipose tissue, tendons, ligaments, salivary glands, blood vessels, nerves, and teeth (Figure 1). Extensive research is conducted on each of these tissues and the need for them well established,[88] but few studies focus on regenerating multiple tissues in tandem. Recent advances in craniofacial tissue engineering, as summarized by Mao et al.,[89] include integrated bone and cartilage layers for the TMJ condyle, various elements of the periodontium, craniofacial bone, cranial suture-like structures as well as adipose tissue. Tissue engineering of skin is not always reported in articles reviewing craniofacial tissue regeneration; however, skin regeneration is an important aspect to consider, as trauma is one of the major causes of tissue loss. Trauma affects both hard and soft tissues, damaging the skin and severely compromising its protective barrier function.
Figure 1.
Craniofacial tissues needed in reconstruction. Reproduced with permission from [88], Springer Science+Business Media: European Archives of Otorhinolaryngology, Tissue Engineering in head and neck reconstructive surgery: what type of tissue do we need?, 264, 2007, 1344, Ulrich Reinhart Goessler, Jens Stern-Straeter, Katrin Riedel, Gregor M. Bran, Karl Hörmann, Frank Riedel, Figure 1.
The next sections will focus on briefly reviewing the distinct anatomical and physiological properties of craniofacial and oral components, as well as progress towards engineering these tissues. Finally, the parameters that will allow for tissue engineering of complex structures will be discussed.
3.1. Bone
Tissue engineering of the cranial and facial bones holds great potential towards the functional and aesthetic restoration of this tissue. Craniofacial bone serves as a protective barrier to the intracranial structures and maintains the shape of the head and face. Bone loss results in severe functional and aesthetic consequences such as problems in mastication and compromised head and facial contour with collapse of the surrounding soft tissues. Bone is a highly vascularized and cellular tissue. The inorganic mineral component of bone extracellular matrix (ECM) provides the mechanical strength of the matrix.[90] Approaches towards bone tissue engineering are numerous, and much progress has been reported towards that goal. Desirable bone tissue engineering constructs are osteoconductive and osteoinductive. Review articles on bone tissue engineering considerations have been extensively published.[91–95] Our group and other researchers have been using synthetic biomaterials in conjunction with growth factors and/or cellular delivery to regenerate bone. Synthetic polymers, ceramics, or composites thereof are biomaterials commonly investigated for bone tissue engineering; many of these systems are injectable as well and will be discussed in subsequent sections.
Osteogenesis is likely very strongly dependent on angiogenesis.[86] Besides the facilitated transport of nutrients, oxygen, and minerals, blood vessels stimulate bone morphogenesis due to the osteogenic effects of vascular cells.[96] This association has led many researchers to investigate the incorporation of angiogenic growth factors into bone tissue engineering models.[97–99] Potent osteogenic factors such as BMP-2 have been shown to induce ectopic bone formation, i.e. in sites where bone would not normally grow.[100, 101] As our understanding of bone biology and development deepens, potential frontiers open within tissue engineering. One such breakthrough was the isolation and identification of mesenchymal stem cells (MSCs), a class of bone tissue progenitor cells. Biomechanical and biochemical factors that enhance the bone-forming capacity of these cells are currently heavily examined.[102–105]
3.2. Skin
The skin is the largest organ in the body. Its barrier function protects the internal organs from the external environment, maintains water and temperature homeostasis and provides communication with the immune system. Skin consists of two main layers, the epidermis and the dermis, the latter being vascularized. Bell et al.[106] and Burke et al.[107] reported some of the first attempts to create a full thickness, tissue engineered skin graft. Natural polymers such as collagen gels have been widely used as matrices for skin tissue engineering.[106–108] Vascularization is critical for success of engineered skin, and room for improvement in this area exists for current tissue engineered skin[109]. This has been addressed by various techniques such as seeding skin cells together on a scaffold with endothelial cells that can then form new vessels.[108, 110] The potential role of growth factors in skin tissue engineering is reviewed elsewhere.[109] Skin tissue engineering has been largely successful relative to other tissues and must now be performed in combination with tissue engineering of other less superficial tissues such as bone and muscle for the treatment of deep wounds or for regeneration following aggressive tumor resections.
3.3. Cartilaginous structures
Cartilage regeneration in vivo seldom occurs due to the avascular nature and relatively sparse cellularity of native cartilage. Therefore, tissue engineering strategies employing scaffolds, cells and bioactive factors represent one of the only methods to regenerate or repair cartilage following injury. In craniofacial structures, cartilage is mainly found in the ear, the nasal tip, and the TMJ. As mentioned before, the cartilage in these structures has distinct characteristics and serves different functions which are reflected by differences in the composition and architecture of the various cartilage types. In the first two organs, cartilage provides shape and flexibility but has no load-bearing properties. In order to increase ECM production and considering the relatively low number of chondrocytes in cartilage, cell transplantation is one of the most common approaches in cartilage regeneration. Much research has been devoted in identifying the right cell source and culture conditions for cartilage tissue engineering and for auricular and nasal tissues in particular.[111, 112] Kamil et al.[113] were able to engineer full-sized human cartilaginous skeletons for the external ear and the nasal tip in vitro by seeding chondrocytes on prefabricated, biodegradable scaffolds. Even though injectable hydrogels which mimic the cartilaginous matrix have been used extensively as models for articular cartilage regeneration, there has been limited use of these systems in nasal and auricular cartilage tissue engineering. The TMJ is another distinct, cartilaginous structure within the joint connecting the mandible to the temporal bone; TMJ cartilage can be found on the surface of the mandibular condyle, as a layer lining the temporal bone, and as the TMJ disc positioned between the two bone surfaces.[114, 115] These load-bearing structures lubricate the surface between the bones and may have shock-absorbing and load-distributing properties.[114] The TMJ disc is composed of fibrocartilage, a type of cartilage characterized by high collagen fiber content in its matrix.
Review articles summarizing TMJ properties and design parameters using the tissue engineering paradigm of cells, scaffolds and stimuli, are available.[114–116] Tissue engineering of the mandibular condyle needs to account for its distinct architecture, consisting of a cartilage layer and the underlying subchondral bone. Successful attempts towards regeneration of that complex structure have been performed using pre-differentiated, osteogenic and chondrogenic, cell populations,[117] although no consistently successful tissue engineering solution is clinically available.
3.4. Oral and dental tissues: periodontium and teeth
The oral cavity represents a unique environment for tissue regeneration as there is widespread need to develop functional tooth replacements with proper attachment, and healing within the oral cavity must occur in the presence of the oral flora, which creates a “contaminated” environment.[86] Engineering a whole tooth is a difficult task due to the complexity of the teeth, which are mineralized, multi-layered matrices. Enamel, dentin, and cementum layers form the hard tissue part of teeth, whereas dental pulp forms the soft tissue in the central core. An additional hurdle in tooth tissue engineering is the creation of appropriate innervation and vasculature.[118] Other common dental conditions include diseases of the periodontium, which can lead to tooth loss. The periodontium is comprised of cementum, the periodontal ligament, which is the fibrous connective tissue surrounding the root of a tooth, and the attached alveolar bone. Approaches towards the regeneration of the periodontal ligament and surrounding osseous defects with growth factors, gene and cellular delivery have been reviewed elsewhere.[89]
3.5. Muscle and adipose tissue
The treatment of craniofacial injuries or anomalies often requires the regeneration of soft tissues such as skeletal muscle and adipose tissue. Skeletal muscle directly attaches to bone and, in the craniofacial region, allows for mastication, respiration, eye movement, and facial expression.[119] Skeletal muscle tissue engineering is still at an early stage. Results from in vitro studies have shed light on the extracellular matrix remodeling of muscle[120–122] and identified conditions for increased skeletal muscle differentiation and growth.[123, 124] Shah et al.[119] have investigated the potential of a three-dimensional phosphate glass fiber scaffold for craniofacial muscle engineering. Human muscle-derived cells seeded on these scaffolds and cultured in vitro with insulin-like growth factor 1 (IGF-1) were able to proliferate and produce prototypical muscle fibers.
Adipose tissue regeneration is needed for the reconstruction of craniofacial structures such as the cheek, chin and jaw.[125, 126] There have been several studies that demonstrated the potential for in vivo adipose tissue regeneration. MSCs isolated from the bone marrow[127–129] or adipose tissue[128] have been shown to be promising candidates for adipose tissue engineering. Conditions that promote differentiation of these uncommitted progenitor cells to adipose cells, such as adipogenic culture media[127, 128, 130] and growth factor regimes,[129] have been reported. An interesting approach towards the creation of vascularized adipose tissue was recently suggested by Marra et al.[131] Particles of the small intestinal submucosa were used as a carrier for the delivery of progenitor fat cells. The cells attached and proliferated on these particles, and cell survival in vivo was noted for a period of 14 days. This injectable vehicle allowed also for the incorporation of PLGA microspheres loaded with fibroblast growth factor 2 (FGF-2), which enhanced vascularization.
3.6. Future directions and considerations
Tissue engineering of complex tissues such as these found in the craniofacial region is a demanding task. One needs to account for multiple cellular phenotypes and find ways to enhance cellular interactions towards tissue repair, possibly stimulating their behavior by supplying bioactive factors. Furthermore, the problem of insufficient vascularization must be overcome since most tissues are strongly dependent on blood supply for growth. Creating stratified tissue architectures and recreating the physiological structure-function properties of the native tissues is the ultimate goal. The choice of a tissue engineering scaffold can significantly aid in this process, not only by serving as a delivery vehicle for cells and bioactive factors but also by providing the ability to interact with and guide tissue growth. Cell-material interactions and mass transport are only some of the important parameters that need to be incorporated into the design. Additionally, one needs to consider that the location and form of craniofacial tissues requires special treatment. For this delicate region, aesthetic considerations are important and there should ideally be minimal scar formation. All these parameters will be further examined in the next sections where the use of injectable systems for multiple tissue regeneration is discussed.
4. Injectable systems in tissue engineering
As highlighted in the previous sections, technologies and strategies in tissue engineering, and in particular those designed for engineering multiple functional tissues, utilize the delivery of cells and bioactive factors in combination with the placement of a support structure or scaffold. Injectable systems, particularly aqueous systems, hold great promise in tissue engineering applications as they can potentially deliver water soluble drugs and growth factors in combination with cells to a tissue defect in a manner that provides an adequate environment for long term cell survival, proliferation, and differentiation.
In the clinical setting, particularly when considering surgical repair, revisions, and/or subsequent reconstruction/regeneration of tissue defects, injectable materials at present hold the most immediate promise in treating defects for which minimally invasive strategies already exist. The delivery of cells, bioactive factors, and support materials via an injectable system within the context of an endoscopic, arthroscopic, laparoscopic, or radiologically guided procedure is feasible given the current state of the art. Within the craniofacial complex (CFC), however, most current surgical techniques towards the treatment of traumatic injury, tumor resection, or congenital deformity are somewhat more invasive than those previously mentioned. Injectable systems are attractive in this setting due to the ability of these systems to conform to complex craniofacial shapes, contours, and defects without the need for extensive presurgical modeling. Additionally, due to the close proximity of multiple tissue types within the CFC, injectable systems containing multi- or totipotent cell populations and growth factors will potentially allow for more natural remodeling and regeneration of damaged, diseased, and excised tissues. In the case of a staged surgical intervention, the use of injectable systems to aid tissue regeneration may avoid the need for multiple invasive operations, thus reducing the morbidity and negative aesthetic effects associated with these repeated procedures. Finally, and as mentioned previously, there are many aesthetic cases where the use of injectable materials is already widespread due to the requirement in these cases for no incision as to minimize potential scarring. As described before, these materials are however not used for tissue engineering purposes but are rather volume fillers with biological activity that is often limited to being encapsulated as part of the foreign body response.
While the ability to deliver cells and bioactive factors make injectable systems attractive alternatives to the preformed implant materials currently used, the success of any injectable system will largely be determined by the support system or framework the system provides. This scaffold must provide early mechanical support commensurate to that of the tissue it replaces, allow cells to survive, proliferate, and differentiate, and it must provide for the controlled release of any drugs or growth factors delivered simultaneously. Above all, this matrix must be biocompatible and ideally will be biodegradable such that with time regenerated tissue will replace the biomaterial component of the system, resulting in functional, healthy tissues approximating those of the premorbid individual and avoiding long term implant failure requiring subsequent retrieval (Figure 2).[132] A large number of materials have been developed and applied in vitro and in animal models towards this end; we will highlight a number of those materials in the next section to introduce promising materials and fabrication/synthetic strategies for creating injectable tissue engineering scaffolds.
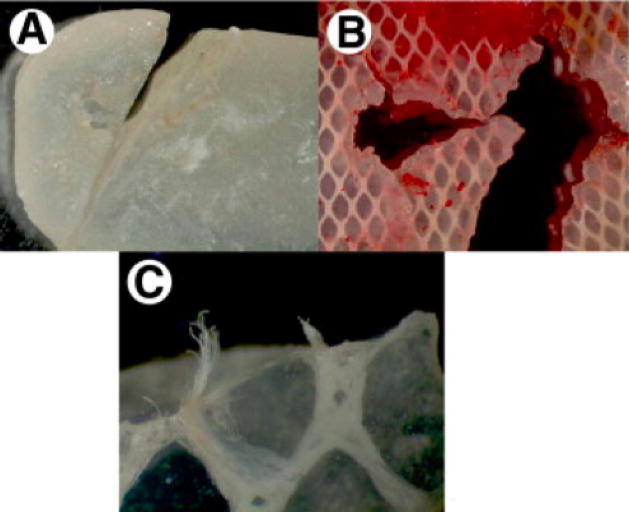
Figure 2.
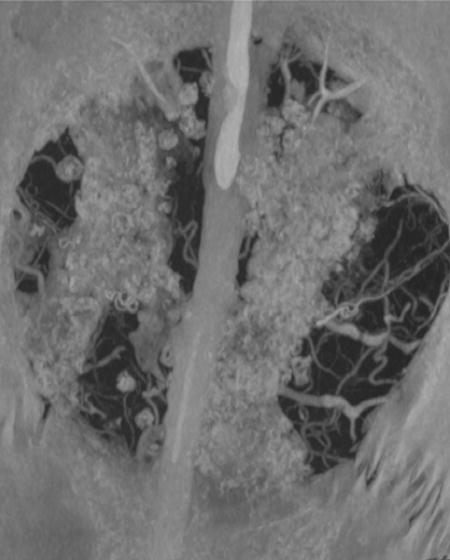
These images show Silastic® implant fragments surgically retrieved from patients. The primary reason for retrieval was patient pain, likely secondary to implant failure. A and B show fracture lines within the implant, while C shows fraying and exposure of Dacron fibers that are intended to reinforce the implant. Reproduced with permission from [132], Journal of Oral and Maxillfacial Surgery, 66, J. N. A. R. Ferreira, C.-C. Ko, S. Myers, J. Swift, J. R. Fricton, Evaluation of Surgically Retrieved Temporomandibular Joint Alloplastic Implants: Pilot Study, 1112–1124, Copyright (2008), with permission from Elsevier
5. Injectable scaffolds
5.1. Requirements
In addition to the basic requirements for any biomaterial to be considered in clinical applications (biocompatibility of the material and subsequent degradation products, handling characteristics allowing achievement of any necessary processing and end functions, etc.), a number of unique requirements exist for complex tissue engineering within the CFC. Three fundamental requirements for biomaterial scaffolds used in the CFC include the ability to fit complex anatomical defects, provide mechanical support for regenerating and surrounding tissues, and deliver bioactive factors to aid tissue regeneration.[133]
The following sections of this paper will demonstrate the ability of injectable biomaterial scaffolds to fit complex defects, given that the defects have well defined borders or given the incorporation of a rigid template to define the shape as well as the well documented delivery of bioactive factors and cells with injectable material scaffolds. Mechanical requirements for biomaterials in the CFC vary widely based on tissue type and location. Within a human mandible, for example, significant regional variation has been found for cortical bone thickness and the direction of maximum stiffness, with maximum elastic moduli exceeding 30 GPa.[134, 135] Similarly, the cranial vault and zygoma exhibit high levels of anisotropy and elastic properties based on regional and functional character.[136] Facial muscles have wide variations in tensile properties as well,[137] thus making it apparent that a single material without variable properties will not likely be successful for applications in which multiple tissues or large portions of one structure are to be regenerated. For such applications, combinations of materials or materials with varying mechanical and biological properties must be investigated. Therefore, in addition to those requirements laid out previously, any injectable biomaterial to be used for complex tissue engineering in the CFC must either have tunable mechanical properties or be able to be integrated into a delivery system or scheme that involves the use of other biomaterials. An ideal system will also degrade in a manner so that the degradation rate is proportional to the rate with which tissue ingrowth or differentiation occurs into or within the scaffold and the extent of mechanical support needed by that specific tissue.
The following section provides examples of injectable materials. These materials, selected from amongst a multitude of injectable materials currently being studied as biomaterials for tissue engineering and other applications, have been chosen to highlight different classes of materials and different methods that are commonly used or promising in their potential for future use and development in making the transition from an injectable material to a tissue engineering scaffold.
5.2. Injectable scaffold materials
5.2.1. Calcium phosphate cements
As described earlier, there are a number of CaP cement-based injectable biomaterials that are currently used and regulated in clinical applications. While these materials have achieved widespread success for bone defect repair and regeneration, concerns about degradation and the brittle mechanical profile of these materials limits their successful usage in many applications.[31] Most commonly used for bone related applications, CaP composites have also been used as soft tissue fillers, demonstrating the potential versatility of these materials.[138]
CaP composites have been studied to alleviate some of the negative properties of materials based solely on CaP cement. Macroporous CaP materials are more favorable for tissue engineering applications than nonporous implants.[139] PLGA microparticles incorporated within an injectable CaP formulation can, upon degradation of the PLGA, yield macroporosity for tissue ingrowth and, possibly through a lowered local pH upon degradation of the PLGA, can accelerate degradation of the CaP phase.[140–143] The incorporation of other degradable particles such as poly(trimethyl carbonate) and gelatin microparticles has yielded similar favorable results within injectable formulations,[144, 145] and the potential for drug or growth factor release from these systems has been well demonstrated.[146–150] Including a water soluble porogen such as mannitol can both improve the injectability of CaP cement while also improving the flexural strength and toughness of the resulting scaffold.[151] Although not part of an injectable system, the incorporation of an absorbable polymer mesh within a CaP cement can also significantly increase the flexural strength and toughness of the material while resulting in macroporosity upon degradation of the network.[152, 153] CaP composite systems that utilize an injectable CaP cement with a porogen have distinct advantages over CaP cements alone and will likely, once gaining final regulatory approval, be counted among the preferred choices of clinicians for bone regeneration and tissue engineering applications.
A second class of composite scaffolds uses CaP granules within water soluble carriers. These materials, which among others can use modified[154, 155] or unmodified[156–158] hydroxy-propyl-methyl-cellulose as the polymer carrier, have resulted in faster initial osteoconduction in vivo when compared to other macroporous CaP cements. If achieved rapidly enough, this early bony apposition could potentially ameliorate any mechanical deficiencies in the material, facilitating its use in load bearing tissue applications. Smaller, nanosized CaP crystals have been incorporated into other injectable biomaterials,[159] and coating of mesenchymal stem cells with CaP nanorods enhances osteoblastic differentiation and extracellular matrix production of the cells,[160] indicating that CaP materials, at least in some forms or as composites, may be suitable carriers for the injection and induction of stem cells.
5.2.2. In situ polymerizable and crosslinkable materials
Polymeric biomaterials are the most widely studied class of biomaterials investigated for use as injectable scaffolds. In situ solidification of polymeric systems is typically achieved either through phase separation or via polymerization or crosslinking of reactive monomers and macromer chains. Systems that utilize phase separation to solidify or harden in situ will be considered along with other self-assembling systems in a subsequent section.
Both in situ polymerization and crosslinking often use a radical initiator that, through interactions with reactive functional groups such as the double bond within a vinyl or acrylic moiety, results in enhanced mechanical properties and shape definition of the scaffold. Photopolymerization using ultraviolet light-activated initiators is one method by which biomaterials can be crosslinked in situ. One such radical initiator is bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO), a molecule that is activated by long wavelength ultraviolet light and has been successfully used to crosslink networks of poly(propylene fumarate) (PPF).[161, 162] Photopolymerizable interpenetrating networks of poly(ethylene glycol) (PEG) and poly(ethylene glycol) diacrylate (PEGDA) have been used to encapsulate human MSCs, and the network mechanical properties can influence extracellular matrix (ECM) deposition and cellular differentiation.[163] A similar PEG network used photoinitiation to pattern the hydrogel surface.[164] Successful encapsulation of and differentiation of MSCs into osteoblast-like cells on poly(D,L lactide-co-ε caprolactone) photocrosslinked injectable scaffolds has also been demonstrated.[165, 166]
Transdermal photopolymerization has been successfully performed using ultraviolet light;[167, 168] however, most clinical applications using ultraviolet-activated photocrosslinkable or photopolymerizable systems will require an open defect to allow for penetration of light to the material, a potential drawback in the clinical setting. Using a near IR light source would however allow deeper tissue and material penetration such that larger material volumes could be polymerized/crosslinked, and the use of such a light source also allows for three-dimensional patterning of the material.[169] Similar to three-dimensional patterning, Sun et al. demonstrated microstructural control of polymer nanocomposites with tunable physical characteristics based on crosslinking density as influenced through crosslinker and photoinitiator concentrations.[170] Similarly, Burdick et al. utilized a microfluidic approach to create surface peptide gradient hydrogels via photopolymerization.[171]
Thermal initiators are perhaps more amenable to minimally invasive delivery and in situ formation of an injectable scaffold. Systems that are activated near or at body temperature (37 °C) are ideal as they utilize normal in vivo conditions to initiate scaffold or network formation. Ammonium persulfate/N,N,N′,N′-tetramethylethyldiamine (APS/TEMED) is a water soluble thermal initiator system with demonstrated cytocompatibility that has been studied as part of an in situ crosslinkable oligo(poly(ethylene glycol) fumarate) (OPF) hydrogel.[172] One of the main problems with radically initiated systems is that, although tolerated at low concentrations, high initiator concentrations can be cytotoxic to encapsulated cells,[173] thus limiting the amount of initiator that can be included for in situ forming systems and subsequently limiting the range of control over important parameters such as formation kinetics and material mechanical properties.
In addition to free radical initiation, a variety of chemical crosslinking or polymerization strategies for the in situ formation of biomaterial scaffolds exist. In situ crosslinkable PEGDA gels were fabricated using a reverse emulsion and Michael-type addition, resulting in materials with an ultimate compressive strength of nearly 7 MPa and making them potential scaffolds for load bearing soft tissue regeneration.[174] Nano-and microscale control of scaffold architecture has been demonstrated for in situ prepared nanocomposites and microspheres using a condensation reaction and interfacial polymerization, respectively.[175, 176]
More biologically motivated systems for in situ scaffold formation also exist. Enzymatic methods for chemically crosslinking polymer chains in situ have been used to fabricate matrices composed of peptide modified synthetic polymers[177, 178] and natural polymers.[179–181] Transglutaminases, which naturally crosslink blood clots and other biological structures, are commonly used to crosslink protein scaffolds or peptide modified scaffolds as the enzyme facilitates an acyl transfer reaction between a free amine group and a γ-carboxyamide.[182, 183]
5.2.3. Stimulus responsive systems
Materials that undergo physical gelation rather than chemical crosslinking are also being explored for use as injectable scaffold materials for tissue engineering. These materials can undergo physical gelation in response to one or multiple changes in their surrounding environment including changes in temperature, pH, ions, and pressure or the presence of electrical and/or magnetic fields.[184–186]
Thermogelling materials, which undergo entropically driven phase separation above their lower critical solution temperature (LCST), are widely investigated for use in drug delivery and tissue engineering applications.[187] For in situ gel formation following injection, water-soluble materials with LCSTs at or below normal body temperature are desirable as they rapidly gel following injection and can then encapsulate cells and bioactive factors within a well-hydrated network. Poly(N-isopropylacrylamide) (PNIPAAm) has a LCST of 32 °C; however, when copolymerized with hydrophilic molecules, PNIPAAm containing materials with LCSTs closer to physiological temperature and thus undergoing less syneresis at 37 °C have been synthesized.[188–191] Other synthetic thermogelling polymers that have been studied as tissue engineering scaffolds and cell delivery vehicles include block copolymers of poly(ethylene oxide) and poly(propylene oxide)[192–194] as well as copolymers of PEG with PPF,[195] poly(organophosphazenes),[196] and PLA.[197] Natural polymers and associated composites also demonstrate thermogelling character and have been explored as injectable tissue engineering matrices.[198–200] The use of tandem gelation combining physical and chemical gelation techniques has led to materials that undergo rapid thermogelation and subsequent chemical crosslinking, yielding injectable materials that combine the favorable kinetics associated with physical gelation with the mechanical characteristics and stability of covalently crosslinked materials.[201–204]
5.2.4. Self assembling materials
Similar to stimulus responsive materials, injectable self-assembling materials undergo gelation or precipitation, often with the ability to form precise nano- or microscale structures without the need for chemical crosslinking or initiating agents. Many such systems use hydrophobicity, either of the bulk material for phase segregation or of certain molecular domains for amphiphiles, as the key means by which self-assembly occurs.
Injectable materials that self assemble in situ via phase segregation are often delivered in the form of a water insoluble polymer injected in solution with a water miscible solvent. Following injection, the solvent diffuses into the tissue space, allowing the polymer component to precipitate within the aqueous environment of the injection site. The solvent must thus be biocompatible to surrounding tissues and cytocompatible if cellular delivery is to be achieved; dimethyl sulfoxide (DMSO) and N-methyl-2-pyrrolidone (NMP) are two such solvents. Phase separating systems based on PLGA have been studied for nearly 20 years,[205] and continuing recent research has been directed at optimizing PLGA and other polymeric systems by better controlling the solvent removal rate and drug release kinetics in vivo.[206, 207]
Tisseel® (Baxter Biosciences, USA), one of the earliest developed and clinically most successful of these phase separation systems uses a dual injection of fibrinogen and thrombin to form a fibrin clot or scaffold. Thrombin cleaves soluble fibrinogen into insoluble fibrin that then self assembles into fibrils resulting in, in combination with platelets, a clot. These fibrin clots, formed from the same biomolecules as used for natural clotting in vivo but at higher relative concentrations, are widely used as an adhesive sealant to achieve hemostasis during surgical procedures. Although Tisseel® as packaged for current clinical applications is not an ideal scaffold material because the high crosslinking density of the fibrin network prevents cell migration into and throughout the clot,[208] more dilute fibrinogen and thrombin solutions can support stem cell proliferation[209, 210] and with appropriate mechanical properties can also induce stem cell differentiation.[211] Fibrin-based systems have also been modified, resulting in promising materals for engineering specific tissue types. Research has yielded engineered BMP-2 fusion proteins that incorporate into fibrin matrices and enhance bone regeneration[212] and fibrin gels with incorporated ECM peptides that enhance key aspects of nerve regeneration (Figure 3).[213] Although not truly self assembling systems, as mentioned previously biomaterials such as PEG can also be modified with peptides so that they can be crosslinked using similar thrombin/clotting factor systems, eliminating the need for soluble synthetic initiators that may be cytotoxic to encapsulated cells or surrounding host tissue.[178]
Figure 3.
Cross sectional images of nerves regenerated in self assembling fibrin tubes. A 4 mm segmental defect was created in the dorsal root nerve of a rat and then bridged with a polymer tube implant. The gap was either left empty (A, B), filled with unmodified fibrin (C, D), or filled with fibrin modified with four peptides from the laminin family of adhesion molecules (E, F). The homogeneity of the nerve and alignment of the neuritis can be appreciated in the samples receiving peptide-modified fibrin bridges (A, C, E bar= 50 μm, B, D, F bar = 25 μm). Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology (213), copyright (2000).
Self-assembling amphiphiles are another promising class of injectable materials for tissue engineering. A recently developed self-assembling peptide hydrogel undergoes shear thinning, such that when an appropriate shear stress is applied, the material thins into a low viscosity gel allowing for injection.[214] After injection, the gel recovers its initial mechanical rigidity, making it a promising candidate for injectable applications. Peptide amphiphiles that self assemble with nanostructural organization in aqueous solution can be modified with peptide sequences to influence cell behavior, leading to increased cellular adhesion (Figure 4)[215, 216] or guided axonal regeneration within an injured spinal cord.[217] Kirkham et al. have investigated similarly functional self assembling peptide amphiphiles that nucleate mineralization in physiological conditions, an effect that has applications in dental and other hard tissue engineering applications.[218, 219] Self-assembling peptide amphiphiles modified with heparin have been used to stimulate angiogenesis[220] and, within a titanium scaffold, to aid in bone regeneration;[221] these applications demonstrate the ability of modified peptide amphiphiles to aid in regeneration of multiple tissue types, making them ideal candidates for complex tissue engineering.
Figure 4.
Focal adhesion formation of cells expressing green fluorescent protein labeled integrin (A–F) or yellow fluorescent protein labeled signal transduction protein (paxillin, G–L) in response to peptide amphiphiles expressing RGDS sequence(s). (A, G) branched peptide amphiphile with one cyclic RGDS, (B, H) branched peptide amphiphile with two RGDS, (C, I) branched peptide amphiphile with one RGDS, (D, J) branched peptide amphiphile with one d-RGDS, (E, K) linear peptide amphiphile with one RGDS, and (F, L) show cells on linear peptide amphiphile with one RGDS. Focal adhesions are seen as brightly fluorescent spots and demonstrate the presence of cellular organization during adhesion and migration in response to the presence of RGDS. This represents an example of how at the cellular level behavior can be regulated via substrate modification, a potentially powerful tool for regenerating of complex tissues. Reprinted from [216], Biomaterials, 28, H. Storrie, M. O. Guler, S. N. Abu-Amara, T. Volberg, M. Rao, B. Geiger, S. I. Stupp, Supramolecular crafting of cell adhesion, 4608-18, Copyright (2007), with permission from Elsevier.
Other promising self-assembly strategies for injectable scaffold fabrication exist. Micro- and nanosphere injection for use as drug delivery vehicles and scaffolds is possible;[222, 223] however, simple injection of the uncrosslinked particles offers little control over bulk mechanical properties or material behavior in vivo as the individual particles are bound only by space limitations and may migrate. Self-assembly techniques that result in crosslinking of the particles allow for augmented mechanical characteristics and control over scaffold architecture even as tissue remodeling commences. Ionically crosslinked networks of positively and negatively charged nano-[224] and microspheres[225] exhibit tunable mechanical properties and hold potential as both drug and cell delivery vehicles. Salem et al. crosslinked biotinylated PLA-PEG microparticles using avidin in the presence of cells to create injectable cell-containing matrices with mechanical strength suitable to support bone regeneration in vivo (Figure 5).[226]
Figure 5.
Schematic representation of avidin induced self assembly of biotinylated PEG-PLA microparticles. Reproduced from [226].
6. Drug delivery via injectable scaffolds
Most of the materials and techniques used in the development of injectable scaffolds for tissue engineering were first or have concurrently been investigated as injectable drug delivery systems. Many of the requirements for injectable drug delivery systems and injectable scaffolds are the same as any injectable biomaterial in that they must be biocompatible, and precise control of drug release kinetics will be of great benefit in both areas. In tissue engineering, there are, particularly for applications such as bone and dental regeneration, more demands upon a material and scaffold to be mechanically similar to the tissue being replaced since at the time of delivery the material must not only support regeneration but also largely perform the structural function of the native tissue. Because of this, not all materials developed for drug delivery are suitable for tissue engineering purposes. Detailed reviews of injectable materials for drug delivery[227] and drug delivery from injectable tissue engineering matrices[228] are available; the following sections will briefly examine delivery of antibiotics and growth factors, two agents critical to successful tissue regeneration within the CFC, within injectable tissue engineering scaffolds.
6.1. Antibiotic delivery
The presence of infection is an important parameter that must be considered for nearly any reconstructive technique, be it the currently used surgical techniques utilizing implants and flaps or proposed tissue engineering solutions. Infections following traumatic craniofacial injuries are a common occurrence, particularly when the injury involves wound contamination through either penetration of a foreign object or loss of skin.[229] Additonally, open communication with the oral cavity can lead to infection from oral flora, and in many cases latent infection that may not lead to clinical signs of infection but may hinder wound healing and tissue regeneration is present.[230–232] Antibiotic delivery may thus be an important aspect of tissue engineering strategies in the CFC, both for curing and preventing latent or active posttraumatic and postsurgical infections.
Although antibiotics can and have been incorporated into many commercially available bone cements, poor release kinetics and the sensitivity of many antibiotics to the high curing temperatures associated with cements such as PMMA make incorporation into the bulk material an inefficient and in some cases ineffective strategy.[233–235] Many drug delivery systems for antibiotic and other bioactive factors utilize drug-loaded microspheres or microparticles. At small particle or sphere sizes, these systems are easily injectable, have well characterized and tunable release kinetics, and can be fabricated from biocompatible, biodegradable materials such as PLGA[236, 237] or gelatin.[238]
Tobramycin loaded PLGA-PEG blend microparticles have been shown to have well-controlled release profiles and can maintain tissue tobramycin concentrations over the minimum inhibitory concentration of Staphyloccous aureus for over one month,[239] resulting in more effective treatment of osteomyelitis when compared to tobramycin released from a bulk bone cement.[240] PLGA microspheres injected with chemically crosslinkable PPF exhibited continuous drug release over one month, and the degradation of the microspheres yielded scaffold porosity to facilitate tissue ingrowth without compromising the compressive strength of the scaffold.[241] In the absence of microspheres, greater control of release kinetics can be achieved by including a hydrogel component or coating along with a bulk CaP cement.[242, 243] Osteoblasts cultured in the presence of antibiotic-loaded microspheres made of nanohydroxyapatite attached to and proliferated well in the material;[244] however, there is some concern regarding stem cell proliferation and differentiation capacity in the presence of antibiotics,[245] meaning combined delivery of stem cells with antibiotics must be carefully considered and studied prior to implementation.
6.2. Growth factor delivery via injection for engineering multiple tissues
Growth factors are extracellular signal proteins that mediate the growth, proliferation and differentiation of cells. All these processes are crucial for tissue growth and repair in vivo, often determining the success of an engineered tissue. Growth factors act by binding to specific receptors on the same cell that has secreted the factors (autocrine signaling), neighboring cells (paracrine signaling), or distant cells (endocrine signaling). Upon binding, a cascade of cellular events is initiated, leading to cell proliferation, differentiation and maturation, as well as to the production of extracellular matrix and other growth factors.[246] Growth factors can act on multiple cell types and are generally not specific for one type of tissue, making them especially useful for complex tissue engineering. The following paragraphs aim to categorize the use of growth factors towards regeneration of certain tissues, and review the injectable carriers available for their delivery.
6.2.1. Angiogenic growth factors
The formation of blood vessels has been identified as a key factor towards tissue regeneration and growth. Angiogenesis is the formation of new blood vessels from existing ones and is driven by endothelial cell proliferation and migration. This is opposed to vasculogenesis which is the formation of blood vessels de novo.[247] The two main growth factor families that have been shown to directly stimulate angiogenesis are vascular endothelial growth factor (VEGF) and FGF. Moreover, the well-characterized transforming growth factor-β (TGF-β) superfamily has an indirect effect on angiogenesis in vivo, as does the PDGF family[248]. Growth factors inducing angiogenesis contribute largely to the wound healing process, as for example the TGF-β superfamily.[249]
6.2.2. Osteogenic growth factors
Growth factors that encourage the formation of new bone have been identified and applied to heal bone defects around medical and dental implants and without implant placement.[250] Review articles on the properties and delivery of osteogenic growth factors are available.[251, 252] BMPs, which are part of the TGF-β superfamily, have osteo-and chondroinductive properties.[253] The BMP family consists of at least 20 different proteins, among which two (BMP-2 and BMP-7) are commercially available for clinical applications.[254] Other factors include the insulin-like growth factors (IGF), which have been shown to have an effect on tissue formation, especially bone, FGF, which are synthesized by skeletal cells,[246] and PDGF, which is known for enhancing protein synthesis in bone.[255]
6.2.3. Chondrogenic growth factors
Members of the growth factor families listed above have been also shown to stimulate the formation of new cartilage, including TGF-β, BMP and IGF proteins.[256–258] The regulatory effects of growth factors on articular chondrogenesis are summarized elsewhere.[259, 260]
6.3. Injectable growth factor delivery
Tissue engineering of complex craniofacial tissues, as has been stressed in previous sections, requires thorough understanding of these tissues’ biology as well as their environment. In order to mimic physiological processes and regenerate tissue in vivo, growth factor delivery will be an important aspect to consider. The expression of different growth factors during the healing cascade within complex tissue defects has been the object of much research, and the challenge facing tissue engineers is to direct the release of multiple growth factors at various time intervals from a scaffold as to simulate and enhance the actual healing process.[86] Many studies so far have used growth factors at concentrations much higher than physiological concentration in order to increase their bioavailability, although improving efficiency in growth factor loading and delivery is the focus of some new delivery vehicles.[261] Delivery of higher concentrations of growth factor than physiologically encountered may not, however, have the desired effects and also increases costs tremendously. Controlled release of growth factors is therefore a crucial variable in achieving a safe and effective dosage, and the scaffold can play a role in achieving appropriate temporal and spatial delivery. The next paragraphs will review the recent advancements in growth factor delivery from injectable tissue engineering carriers.
6.3.1. Polymeric carrier materials for multiple tissue regeneration
Polymers are probably the most widely used class of injectable materials for achieving controlled growth factor release. The importance of controlled release kinetics was highlighted in a study conducted by Woo et al.[262] Using a carboxymethylcellulose hydrogel with PLGA microparticles loaded with recombinant human BMP-2, they showed that sustained release of the growth factor promoted bone healing significantly better than a burst release system. Similar to antibiotic delivery, many other systems incorporating microparticles for growth factor delivery have been developed. The encapsulation of growth-factor loaded gelatin microparticles in OPF hydrogels minimizes the burst release often associated with this type of material.[257] Growth factor loading on gelatin microparticles is achieved by polyionic complexation, and the in vivo release is governed by the enzymatic degradation of gelatin.[263] A design for multiple tissue regeneration was suggested with such a composite. A bilayered OPF hydrogel system with incorporated TGF-β1-loaded gelatin microparticles, consisting of a bone forming and a cartilage forming layer, was used with promising results for osteochondral repair (Figure 6).[264] The same scaffold was later used for dual delivery of TGF-β1 and IGF-1. It was designed to release TGF-β1, a chemoattractant and morphogen, rapidly, followed by release of IGF-1, a stimulator of extracellular matrix formation, in a more sustained manner.[265] The in vivo findings, however, did not suggest a synergy between these factors as expected from in vitro observations and did not result in significant improvement of new tissue quality. Dual growth factor delivery has been also proposed for bone repair. Patel et al.[99] have investigated the effect of dual delivery of an angiogenic (VEGF) and an osteogenic (BMP-2) growth factor in a rat critical size cranial defect. The growth factors were incorporated in gelatin microparticles and were injected into porous PPF scaffolds. After four weeks, the dual release system resulted in much higher bone formation than either growth factor alone, indicating a synergistic effect in this time interval. At twelve weeks, BMP-2 and dual release groups showed increased bone formation over VEGF alone and control groups but were not significantly different from each other (Figure 7). In addition to growth factors released from polymer matrices to induce cellular differentiation, an alternate strategy utilizes growth factors to encourage cell migration into or throughout a hydrogel in a similar manner to the previously mentioned adhesion molecule-modified peptide amphiphiles and fibrin gels.[266]
Figure 6.
Osteochondral tissue repair in rabbit knees 14 weeks after implantation with a bilayered scaffold (A, D, G – TGF-β1 releasing porous chondral and porous subchondral layers; B, E, H - TGF-β1 releasing porous chondral and nonporous subchondral layers; C, F, I porous chondral layer and nonporous subchondral layer). The boxed regions in (A–C) (2.5× magnification) are shown at higher magnifications in (D–F) (10×), and (G–I) (20×). Arrows point towards the joint surface, while columnar arrangements of chondrocytes, cell clusters, and cartilage fissures are respectively indicated by CO, CL, and FI. The images demonstrate the ability of a bilayered scaffold along with regionally specific growth factor release to regenerate multiple tissues within the same construct. Reproduced from [264].
Figure 7.
Microcomputed tomography images of rat cranial bone defects treated with angiogenic, osteogenic or both growth factors. Figures A-D represent an untreated control group, angiogenic VEGF-treated group, osteogenic BMP-2-treated group, and a dual delivery group at 4 weeks. Blood vessels and bone are visible. Figures E–H represent control, VEGF, BMP-2 and dual groups at 12 weeks. Blood vessel formation was not evaluated at this time point. Bar represents 200 μm. Reprinted from [99], Bone, 43, Z S. Patel, S. Young, Y. Tabata, J.A. Jansen, M. Wong, A.G. Mikos, Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model, 931-40, Copyright (2008), with permission from Elsevier
Thermoresponsive hydrogels have been also used for growth factor delivery. These hydrogels are typically liquid at ambient temperature and solidify at close to physiological temperatures. A poly(NIPAAm-co-acrylic acid) hydrogel was blended with hyaluronic acid and used for TGF-β3, dexamethasone, and cell delivery.[267] Gao et al.[268] were able to conjugate rhBMP-2 to thermoreversible polymers while maintaining the osteoinductive properties of the growth factor. Thermoresponsive Tetronic® was copolymerized with e-caprolactone and subsequently conjugated with heparin, resulting in polymeric micelles. Basic FGF, a heparin-binding growth factor, could be released from these micelles in a controlled manner over two months.[269] Moreover, the amphiphilic nature of the micelles allows for the dual delivery of a hydrophilic molecule such as a growth factor together with a more hydrophobic compound.[270]
Heparin has been also used in combination with a modified chitosan hydrogel for sustained release of FGF-2. Significant angiogenesis and fibrous tissue formation was observed in animals following injection of the loaded hydrogel.[271] The angiogenic effects of VEGF alone[272] or in sequential release with PDGF-BB[273] were investigated using different molecular weight alginate polymers. Good spatiotemporal control of the release kinetics was achieved with these hydrogels, resulting in a promising vehicle for stimulating angiogenesis. Hosseinkhani et al. developed another interesting carrier applied towards the same goal. They synthesized an injectable, self-assembling peptide-amphiphile that allows for simple mixing of an aqueous solution with basic FGF suspension and can be injected and self-assemble in vivo. This system proved advantageous for angiogenesis as compared to basic FGF or amphiphile injection alone and holds promise as a carrier for therapeutic proteins.[274] Hiemstra et al. have also investigated a dextran-based system for FGF delivery that forms in situ via a Michael-type addition.[275]
6.3.2. Injectable ceramic materials and their composites for bone tissue regeneration
Calcium phosphate (CaP) cements have been used with good results in bone tissue engineering. Their advantages include easy handling and injectability as well as osteoconductivity.[276] In order to further improve their potential for bone regeneration, these carriers have been delivered together with growth factors, such as recombinant human TGF-β1.[277] Using calcium phosphate cement as a delivery vehicle for recombinant human BMP-2, Kroese-Deutman et al. observed bone growth in an ectopic, subcutaneous, animal model after ten weeks of implantation.[101] This and other studies have revealed the potency of rhBMP-2 as an osteoinductive factor, and further attempts have been made towards achieving a more controlled release profile. The use of CaP with embedded PLGA microspheres resulted in an injectable delivery system that exhibited linear release profiles without an initial burst in vivo[146] and showed that the controlled release of a low BMP-2 dose was able to induce bone growth in an experimental animal model.[149]
7. Cell delivery for engineering complex tissues
The delivery of cells to a defect is a means to accelerate the healing process as the body would not have to rely on host cells being recruited to that site. Cellular delivery vehicles may also allow for regeneration of native tissues before fibrosis and scarring occur. The cell source and type is a topic of much discussion and research over the past decades, and approaches using xenogeneic, allogeneic, or autologous cells have been studied. There are advantages and disadvantages involved in all of these approaches, and the decision has to be made with careful consideration of the application and the necessary steps to translate from research to a final tissue engineered product. The other big question facing researchers is the choice between differentiated cells and either embryonic or adult stem cells. Stem cells have the ability to proliferate in an undifferentiated state or, with the application of certain stimuli, to differentiate into one or more cell lineages. Investigators have recognized the tremendous potential of stem cells in regenerative medicine, and given their versatility, they can offer a valuable solution for engineering complex tissues. Embryonic stem cells are isolated from embryonic or fetal tissues, and are capable of giving rise to all types of tissues.[278] Adult stem cells can be mainly categorized into hematopoietic and mesenchymal stem cells. Hematopoietic stem cells (HSCs) are derived from the bone marrow and are blood cell progenitors. MSCs can be found in a variety of tissues, including bone marrow, periosteum, adipose, and possibly the umbilical cord matrix and can differentiate into bone, cartilage, muscle, fat, and other tissues.[279–282] Most craniofacial tissues derive from MSCs, and there is evidence that for some craniofacial structures such as the TMJ, MSCs are better suited for tissue regeneration than differentiated cells (Figure 8).[283] A recent review article by Mao et al.[89] outlines advances in the field of craniofacial tissue engineering using stem cells.
Figure 8.
Safranin O/fast green stained (left) and chondroitin-4-sulfate immunohistochemically stained cultures of human umbilical cord matrix (HUCM) derived stem cells and TMJ cartilage cells. HUCM were cultured in control (top left) and chondrogenically supplemented (top right) media for 4 weeks following initial culture in chondrogenic media. HUMCs produced more glycosaminoglycans as shown by both staining modalities than already differentiated TMJ chondrocytes, indicating the utility of stem cells in craniofacial tissue engineering compared to differentiated cells. Reproduced with permission from [283]. Copyright (2007) Mary Ann Liebert.
Once in the body, stem cells receive signals that govern their fate. These signals can be chemical or mechanical in nature, and are provided either by the physiologic environment or can be incorporated in the cell delivery vehicle. The mechanisms by which these signals affect stem cells are not yet fully understood. The field of tissue engineering is greatly advanced by stem cell biology findings, and tissue engineering scaffolds have been used with promising results for stem cell delivery for various tissues. The nature of injectable materials, which allows for simultaneous cell and bioactive agent encapsulation, makes them particularly attractive for this application, as does the ability to control scaffold mechanical properties, which can provide the appropriate mechanobiological environment for cell proliferation and differentiation.
7.1. Cell delivery systems
Hydrogels, due to their high water content, are excellent ECM analogues, and are used extensively for cell delivery. Injectable hydrogel systems such as OPF, with or without the addition of growth factors, were shown to promote mesenchymal stem cell differentiation, with possible applications in bone and cartilage regeneration (Figure 9).[284, 285] By controlling the scaffold swelling and mechanical characteristics, appropriate signals were transduced to the cells. Also the addition of a functional group to the polymer, for example a group that will enhance biomineralization, has been shown to induce cell differentiation, helping MSCs turn into osteoblast-like cells.[286] The importance of growth factors in enhancing cell function for tissue engineering applications has been outlined in the previous section. Yamada et al. utilized the abundance of growth factors released by platelets by delivering MSCs in a platelet-rich plasma gel, observing bone formation and neovascularization in vivo.[287] Morphogenic factors are often co-injected with cells but can also be applied to the cells to pre-differentiate them in vitro prior to administration. An example for engineering complex tissues using the latter strategy is the work of Alhadlaq et al.[288] Rat MSCs were treated for three to four days with either chondrogenic or osteogenic supplements and loaded in two hydrogel layers formed by photopolymerization. After four weeks in vivo, stratified layers of chondrogenesis and osteogenesis were observed. The authors concluded that this approach could offer a solution for tissue engineering complex tissues using a single adult mesenchymal stem cell population.
Figure 9.
Cumulative secretion of osteopontin, a marker of osteogenic differentiation, from OPF hydrogel matrices with encapsulated MSCs. (A) MSCs encapsulated in OPF formulated from PEG with MW 10,000 Da and 3,000 Da were cultured in dexamethasone containing (+) and non-supplemented (−) culture media for 28 days. (B) Two types of OPF/MSC formulations maintained for 28 days in culture media without dexamethasone. The samples with higher molecular weight PEG (10K) underwent greater swelling than the less hydrophilic samples (PEG MW 3K), which led to enhanced osteogenic differentiation of encapsulated MSCs. Reproduced from [285].
7.2. Co-culture models and future considerations
Another exciting option for stimulating cell differentiation and proliferation is the co-culture of multiple cell types. The signals exchanged between cells help augment tissue regeneration by guiding differentiation and matrix production down the desired pathway. Co-cultures essentially mimic the physiological environment, where different cells act cooperatively and bioactive factors secreted by one cell type provide cues for the action of another cell type. It is known, for example, that in endochondral bone formation, cartilage precedes bone development. Alsberg et al.[289] co-transplanted chondrocytes and osteoblasts embedded in hydrogels and observed formation of distinct tissue types. Gerstenfeld et al.[290] investigated the effect of chondrocytes on mesenchymal stem cell osteogenesis. They found that MSCs co-cultured with chondrocytes underwent osteogenic differentiation, but did not observe this when MSCs were cultured with fibroblasts or osteoblasts. In a parallel study, treatment of MSCs with BMP-7 induced both chondrogenesis and osteogenesis. Osteogenic differentiation alone was observed in the MSCs co-cultured with chondrocytes.[290] Interestingly, co-culture of MSCs with cartilage, separated by a membrane and without any external growth factors, was found to increase markers of chondrogenic differentiation. Therefore, it seems that the secretion of soluble factors from the whole cartilaginous matrix controls chondrogenesis of mesenchymal stem cells, and that the MSCs in turn influence the already formed cartilage, possibly preventing its hypertrophy or ossification.[291]
One of the important hurdles to overcome in tissue engineering is the blood supply to the growing tissue. Cell co-cultures could potentially play a role in this area by delivering cell populations that would form new blood vessels and also initiate tissue development. Early studies by Sun et al. showed promise in bone regeneration and angiogenesis using a vascular endothelial cell/bone marrow MSC co-culture model.[292] Using a different approach, the formation of microvascular networks in vivo was demonstrated with neural progenitor and endothelial cells embedded in a macroporous hydrogel.[293]
Research in the area of co-culture systems is crucial to our understanding of cell communication with soluble signaling molecules. Models for establishing these relations exist; however, it must be noted that precautions should be taken until there is a clear understanding of the mechanisms that govern cellular interactions, especially involving stem cells. In a direct co-culture setup, there may be danger of cell fusion and endocytosis.[294] Therefore, many researchers use membrane-separated cell culture chambers where cells are not in direct contact, but soluble factors secreted by one population can freely float in the culture medium and act on the other cell type. Once it is determined whether direct cell contact is necessary or paracrine mechanisms are involved, new directions for cell co-culture and possibly the delivery of multiple cell types together with stem cells will be developed. Facing the problem of complex tissue regeneration, the option of cell therapy with multiple populations seems appealing, particularly considering the multipotency of stem cells. We can anticipate great progress towards that goal in the years to come.
8. Injectable materials for engineering complex tissues
The last 15 years has seen a remarkable influx of ideas and technologies to the areas of biomaterials and tissue engineering. While this research has resulted in an expansion of knowledge and in select cases an impact on clinical medicine, regenerating complex tissues remains a largely unaddressed challenge. Advances specifically in injectable biomaterials are frequently presented; however, tissue regeneration in humans using injectable materials remains an unmet goal. As has been shown, regenerating complex tissues is a complicated endeavor that, particularly in the CFC where a number of structures reside in limited space, will require a synergistic approach drawing on knowledge from many areas of science and engineering.
8.1. Goals and future directions
Continued advances in biomaterials and tissue engineering will be required to realize the goal of regenerating complex craniofacial tissues using injectable biomaterials. First, a more complete understanding of the function and utility of stem cells within the context of tissue engineering will be necessary for regenerating any complex tissue. Adult and embryonic stem cells are a powerful tool for regenerative medicine applications, but to tailor complex tissues, precise control over differentiation and subsequently morphogenesis must be achieved. Advances in these areas may result from better understanding of development and natural tissue regeneration, such as more precise knowledge of growth factor and cell signaling cascades. At the materials level, new materials are continually introduced, and much current work focuses on promising materials tailored for engineering specific tissues. The integration of these materials with one another or the development of materials with multiple patterned, micro- and nanoscale domains for specific tissue and organ patterning will be critical to complex tissue engineering.[295, 296] The interaction of these domains with stem cells and the effect of bioactive factor release kinetics must be well characterized and utilized. The creation of surface[297, 298]and 3D[299] patterning or pore gradients and orientation,[300, 301] for example, and in particular mechanical gradients via any of the methods for solidification discussed can be of great benefit to tissue engineers, as such patterning can influence cell behavior including differentiation.[302] For complex tissue engineering, an injectable material that can encapsulate cells and bioactive factors and deliver them to a multi-tissue defect so that different regions of the defect regenerate into anatomically oriented and functionally capable tissues is the end goal; however, many hurdles must be overcome in the path to developing such a system.
9. Conclusions
This review paper aims to examine recent advances in injectable biomaterials for tissue engineering within the challenging but illustrative context of complex tissue engineering within the craniofacial complex. A region with great need for better biomaterials and tissue engineering strategies, currently used techniques and biomaterials for craniofacial augmentation and repair were described to establish the current end-stage state of the art. The challenge of engineering complex tissues and advances in tissue engineering the broad scope of craniofacial tissues were described followed by an overview of injectable material technologies and drug and cellular delivery via injectable materials. Finally, the notable challenges and perceived future needs and directions in addressing the challenges described were mentioned. Many promising injectable biomaterials exist, and forays into in vivo testing using animal models have been largely successful. The goal, however, of regenerating complex tissues remains unmet in the clinic and will require continued commitment and advancement within the field.
Abbreviations
- ALT
anterolateral thigh
- APS
ammonium persulfate
- β-TCP
beta-tricalcium phosphate
- BAPO
bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide
- BMP
bone morphogenetic protein
- BSE
bovine spongiform encephalopathy
- CaP
calcium phosphate
- CFC
craniofacial complex
- DBM
demineralized bone matrix
- DMAP
2,2-dimethoxy-2-phenyl-acetophenone
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- HDPE
high-density polyethylene
- HIV
human immunodeficiency virus
- HSC
hematopoietic stem cell
- HTV
heat-vulcanizing
- HUCM
human umbilical cord matrix stem cell
- IGF
insulin-like growth factor
- LCST
lower critical solution temperature
- MSC
mesenchymal stem cell
- NMP
N-methyl-2-pyrrolidone
- OHNC
oral, head, and neck cancer
- OPF
oligo(poly(ethylene glycol) fumarate)
- PCL
poly(ε-caprolactone)
- PDGF
platelet-derived growth factor
- PDMS
polydimethylsiloxane
- PEG
poly(ethylene glycol)
- PEGDA
poly(ethylene glycol) diacrylate
- PERVs
porcine endogenous retroviruses
- PGA
poly(glycolic acid)
- PLA
poly(lactic acid)
- PLLA
poly(L-lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PMMA
poly(methyl methacrylate)
- PNIPAAm
poly(N-isopropyl acrylamide)
- PPF
poly(propylene fumarate)
- PTFE
poly(tetrafluoroethylene)
- RFFF
radial forearm free flap
- RTV
room-temperature vulcanizing
- TE
tissue engineering
- TEMED
N,N,N′,N′-tetramethylethyldiamine
- TGF-β
transforming growth factor-β
- TMJ
temporomandibular joint
- VEGF
vascular endothelial growth factor
Biography
Antonios G. Mikos is the Louis Calder Professor of Bioengineering and Chemical and Biomolecular Engineering at Rice University. His research focuses on the synthesis, processing, and evaluation of new biomaterials for use as scaffolds for tissue engineering, as carriers for controlled drug delivery, and as non-viral vectors for gene therapy. He is the author of over 350 publications and 23 patents. He is the editor of 10 books and the author of one textbook (Biomaterials: The Intersection of Biology and Materials Science, Pearson Prentice Hall, 2008). Mikos is a Fellow of the International Union of Societies for Biomaterials Science and Engineering and a Fellow of the American Institute for Medical and Biological Engineering. He has been recognized by various awards including the Alpha Chi Sigma Award for Chemical Engineering Research of the American Institute of Chemical Engineers, the Robert A. Pritzker Distinguished Lecturer Award of the Biomedical Engineering Society, the Edith and Peter O’Donnell Award in Engineering of The Academy of Medicine, Engineering and Science of Texas, the Marshall R. Urist Award for Excellence in Tissue Regeneration Research of the Orthopaedic Research Society, and the Clemson Award for Contributions to the Literature of the Society for Biomaterials.

Footnotes
This review is a tribute to Robert Langer’s 60th birthday and his contributions to the field of materials science. The corresponding author was a postdoctoral fellow in Dr. Langer’s laboratory and owes, along with all past, present, and future members of the Mikos laboratory, a great deal of thanks and credit to Dr. Langer for his role as a pioneer in the field and as a mentor.
Work in the area of biomaterials science and tissue engineering is supported by the National Institutes of Health (R01 DE15164, R01 DE17441) (AGM). Work in the specific area of craniofacial tissue engineering is also supported by the Department of Defense through the Armed Forces Institute of Regenerative Medicine (AGM, MW). JDK acknowledges support from the Baylor College of Medicine Medical Scientist Training Program (NIH T32 GM07330), Rice Institute of Biosciences and Bioengineering’s Biotechnology Training Grant (NIH T32 GM008362), and is currently supported by a training fellowship from the Keck Center Nanobiology Training Program of the Gulf Coast Consortia (NIH Grant No. 5 T90 DK070121-04). SY gratefully acknowledges financial support from a Research Fellowship given by the Oral and Maxillofacial Surgery Foundation.
References
- 1.Axhausen W. J Bone Joint Surg Am. 1956;38-A:593. [PubMed] [Google Scholar]
- 2.Gutta R, Waite PD. The British journal of oral & maxillofacial surgery. 2007. [DOI] [PubMed] [Google Scholar]
- 3.Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. J Oral Maxillofac Surg. 2008;66:235. doi: 10.1016/j.joms.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Sen MK, Miclau T. Injury. 200738(Suppl 1):S75. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Smolka W, Iizuka T. J Craniomaxillofac Surg. 2005;33:1. doi: 10.1016/j.jcms.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O’Keefe RJ, Boyce BF, Schwarz EM. Nature medicine. 2005;11:291. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomford WW, Mankin HJ. The Orthopedic clinics of North America. 1999;30:565. doi: 10.1016/s0030-5898(05)70109-7. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen H, Morgan DA, Forwood MR. Cell Tissue Bank. 2007;8:93. doi: 10.1007/s10561-006-9020-1. [DOI] [PubMed] [Google Scholar]
- 9.Buck BE, Malinin TI. Clin Orthop Relat Res. 1994:8. [PubMed] [Google Scholar]
- 10.Boyce T, Edwards J, Scarborough N. The Orthopedic clinics of North America. 1999;30:571. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 11.Peleg M. Dent Implantol Update. 2004;15:89. [PubMed] [Google Scholar]
- 12.Sandhu HS, Khan SN, Suh DY, Boden SD. Eur Spine J. 2001;10(Suppl 2):S122. doi: 10.1007/s005860100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. J Biomed Mater Res A. 2007;81:437. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 14.Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. J Bone Joint Surg Am. 2004;86-A:2243. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Acarturk TO, Hollinger JO. Plast Reconstr Surg. 2006;118:862. doi: 10.1097/01.prs.0000232385.81219.87. [DOI] [PubMed] [Google Scholar]
- 16.Laurencin CT, El-Amin SF. J Am Acad Orthop Surg. 2008;16:4. doi: 10.5435/00124635-200801000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Dormont D. Infect Control Hosp Epidemiol. 1996;17:521. doi: 10.1086/647360. [DOI] [PubMed] [Google Scholar]
- 18.Wenz B, Oesch B, Horst M. Biomaterials. 2001;22:1599. doi: 10.1016/s0142-9612(00)00312-4. [DOI] [PubMed] [Google Scholar]
- 19.Mannai C. J Oral Maxillofac Surg. 2006;64:1420. doi: 10.1016/j.joms.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Garofalo GS. Minerva Stomatol. 2007;56:373. [PubMed] [Google Scholar]
- 21.Vance GS, Greenwell H, Miller RL, Hill M, Johnston H, Scheetz JP. Int J Oral Maxillofac Implants. 2004;19:491. [PubMed] [Google Scholar]
- 22.Scheyer ET, Velasquez-Plata D, Brunsvold MA, Lasho DJ, Mellonig JT. Journal of periodontology. 2002;73:423. doi: 10.1902/jop.2002.73.4.423. [DOI] [PubMed] [Google Scholar]
- 23.Bernhardt A, Lode A, Boxberger S, Pompe W, Gelinsky M. J Mater Sci Mater Med. 2008;19:269. doi: 10.1007/s10856-006-0059-0. [DOI] [PubMed] [Google Scholar]
- 24.LeGeros RZ. Clin Orthop Relat Res. 2002:81. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Shors EC. Orthop Clin North Am. 1999;30:599. doi: 10.1016/s0030-5898(05)70113-9. [DOI] [PubMed] [Google Scholar]
- 26.Friedman CD. Facial Plast Surg Clin North Am. 2002;10:175. doi: 10.1016/s1064-7406(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham LL. J Long Term Eff Med Implants. 2005;15:609. doi: 10.1615/jlongtermeffmedimplants.v15.i6.40. [DOI] [PubMed] [Google Scholar]
- 28.Spivak JM, Hasharoni A. Eur Spine J. 2001;10(Suppl 2):S197. doi: 10.1007/s005860100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daculsi G, Laboux O, Malard O, Weiss P. J Mater Sci Mater Med. 2003;14:195. doi: 10.1023/a:1022842404495. [DOI] [PubMed] [Google Scholar]
- 30.Oner FC, Dhert WJ, Verlaan JJ. Injury. 2005;36(Suppl 2):B82. doi: 10.1016/j.injury.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Friedman CD, Costantino PD, Takagi S, Chow LC. J Biomed Mater Res. 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Dunne M, Corrigan I, Ramtoola Z. Biomaterials. 2000;21:1659. doi: 10.1016/s0142-9612(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 33.Sanger C, Soto A, Mussa F, Sanzo M, Sardo L, Donati PA, Di Pietro G, Spacca B, Giordano F, Genitori L. The Journal of craniofacial surgery. 2007;18:926. doi: 10.1097/scs.0b013e3180a771e9. [DOI] [PubMed] [Google Scholar]
- 34.Fedorowicz Z, Nasser M, Newton JT, Oliver RJ. Cochrane Database Syst Rev. 2007:CD006204. doi: 10.1002/14651858.CD006204.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Bell RB, Kindsfater CS. J Oral Maxillofac Surg. 2006;64:31. doi: 10.1016/j.joms.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin RM, Block MS, Wilk R, Malloy RB, Kent JN. J Oral Maxillofac Surg. 2007;65:89. doi: 10.1016/j.joms.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 37.Christgau M, Bader N, Schmalz G, Hiller KA, Wenzel A. J Clin Periodontol. 1998;25:499. doi: 10.1111/j.1600-051x.1998.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 38.Quatela VC, Chow J. Facial Plast Surg Clin North Am. 2008;16:1. doi: 10.1016/j.fsc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Yaremchuk MJ. Plast Reconstr Surg. 2003;111:1818. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 40.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. The Journal of craniofacial surgery. 2003;14:144. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Santin M, Motta A, Borzachiello A, Nicolais L, Ambrosio L. Journal of materials science. 2004;15:1175. doi: 10.1007/s10856-004-5668-x. [DOI] [PubMed] [Google Scholar]
- 42.Groth MJ, Bhatnagar A, Clearihue WJ, Goldberg RA, Douglas RS. Arch Facial Plast Surg. 2006;8:381. doi: 10.1001/archfaci.8.6.381. [DOI] [PubMed] [Google Scholar]
- 43.Lara WC, Schweitzer J, Lewis RP, Odum BC, Edlich RF, Gampper TJ. Journal of long-term effects of medical implants. 1998;8:43. [PubMed] [Google Scholar]
- 44.Vaandrager JM, van Mullem PJ, de Wijn JR. Ann Plast Surg. 1988;21:583. doi: 10.1097/00000637-198812000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Herford AS, Boyne PJ, Williams RP. J Calif Dent Assoc. 2007;35:335. [PubMed] [Google Scholar]
- 46.Closmann JJ, Pogrel MA, Schmidt BL. J Oral Maxillofac Surg. 2006;64:367. doi: 10.1016/j.joms.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Rapidis AD, Alexandridis CA, Eleftheriadis E, Angelopoulos AP. J Oral Maxillofac Surg. 2000;58:158. doi: 10.1016/s0278-2391(00)90330-6. [DOI] [PubMed] [Google Scholar]
- 48.Marx RE, Smith BR. J Oral Maxillofac Surg. 1990;48:1168. doi: 10.1016/0278-2391(90)90533-8. [DOI] [PubMed] [Google Scholar]
- 49.Wong TY, Chung CH, Huang JS, Chen HA. J Oral Maxillofac Surg. 2004;62:667. doi: 10.1016/j.joms.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Garcia R, Rodriguez-Campo FJ, Naval-Gias L, Sastre-Perez J, Munoz-Guerra MF, Usandizaga JL, Diaz-Gonzalez FJ. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:29. doi: 10.1016/j.tripleo.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Wolff KD, Kesting M, Loffelbein D, Holzle F. Journal of reconstructive microsurgery. 2007;23:497. doi: 10.1055/s-2007-992349. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez OM. Ann Plast Surg. 1999;43:109. [PubMed] [Google Scholar]
- 53.Locke MB, de Chalain TM. Ann Plast Surg. 2008;60:98. doi: 10.1097/SAP.0b013e318038f74c. [DOI] [PubMed] [Google Scholar]
- 54.Ward BB, Feinberg SE, Friedman CD. Facial Plast Surg. 2002;18:3. doi: 10.1055/s-2002-19822. [DOI] [PubMed] [Google Scholar]
- 55.Bajnrauh R, Nguyen EV, Reifler DM, Wilcox RM. Ophthal Plast Reconstr Surg. 2007;23:409. doi: 10.1097/IOP.0b013e318137a1a3. [DOI] [PubMed] [Google Scholar]
- 56.Banks ND, Milner S. Plast Reconstr Surg. 2008;121:230e. doi: 10.1097/01.prs.0000305391.98958.95. [DOI] [PubMed] [Google Scholar]
- 57.Wendt JR, Ulich T, Rao PN. Plast Reconstr Surg. 2004;113:1347. doi: 10.1097/01.prs.0000112741.11726.91. [DOI] [PubMed] [Google Scholar]
- 58.Devauchelle B, Badet L, Lengele B, Morelon E, Testelin S, Michallet M, D’Hauthuille C, Dubernard JM. Lancet. 2006;368:203. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 59.Pomahac B, Aflaki P, Chandraker A, Pribaz JJ. Transplantation. 2008;85:1693. doi: 10.1097/TP.0b013e318176b29e. [DOI] [PubMed] [Google Scholar]
- 60.Dubernard JM, Lengele B, Morelon E, Testelin S, Badet L, Moure C, Beziat JL, Dakpe S, Kanitakis J, D’Hauthuille C, El Jaafari A, Petruzzo P, Lefrancois N, Taha F, Sirigu A, Di Marco G, Carmi E, Bachmann D, Cremades S, Giraux P, Burloux G, Hequet O, Parquet N, Frances C, Michallet M, Martin X, Devauchelle B. N Engl J Med. 2007;357:2451. doi: 10.1056/NEJMoa072828. [DOI] [PubMed] [Google Scholar]
- 61.Sclafani AP, Romo T, 3rd, Jacono AA, McCormick S, Cocker R, Parker A. Arch Facial Plast Surg. 2000;2:130. doi: 10.1001/archfaci.2.2.130. [DOI] [PubMed] [Google Scholar]
- 62.Sclafani AP, Romo T, 3rd, Jacono AA, McCormick SA, Cocker R, Parker A. Arch Facial Plast Surg. 2001;3:101. doi: 10.1001/archfaci.3.2.101. [DOI] [PubMed] [Google Scholar]
- 63.Callcut RA, Schurr MJ, Sloan M, Faucher LD. Burns. 2006;32:583. doi: 10.1016/j.burns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Taban M, Douglas R, Li T, Goldberg RA, Shorr N. Arch Facial Plast Surg. 2005;7:38. doi: 10.1001/archfaci.7.1.38. [DOI] [PubMed] [Google Scholar]
- 65.Kellner DS, Fracchia JA, Voigt E, Armenakas NA. Urology. 2007;69:372. doi: 10.1016/j.urology.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Sclafani AP, Romo T, 3rd, Jacono AA. Arch Facial Plast Surg. 2002;4:252. doi: 10.1001/archfaci.4.4.252. [DOI] [PubMed] [Google Scholar]
- 67.Douglas RS, Donsoff I, Cook T, Shorr N. Facial Plast Surg. 2004;20:117. doi: 10.1055/s-2004-861751. [DOI] [PubMed] [Google Scholar]
- 68.Monheit GD. Facial Plast Surg. 2004;20:153. doi: 10.1055/s-2004-861757. [DOI] [PubMed] [Google Scholar]
- 69.Rohrich RJ, Ghavami A, Crosby MA. Plast Reconstr Surg. 2007;120:41S. doi: 10.1097/01.prs.0000248794.63898.0f. [DOI] [PubMed] [Google Scholar]
- 70.Homicz MR, Watson D. Facial Plast Surg. 2004;20:21. doi: 10.1055/s-2004-822955. [DOI] [PubMed] [Google Scholar]
- 71.Humble G, Mest D. Facial Plast Surg. 2004;20:181. doi: 10.1055/s-2004-861762. [DOI] [PubMed] [Google Scholar]
- 72.Rullan PP. Facial Plast Surg. 2004;20:111. doi: 10.1055/s-2004-861750. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann C, Schuller-Petrovic S, Soyer HP, Kerl H. J Am Acad Dermatol. 1999;40:100. doi: 10.1016/s0190-9622(99)70536-0. [DOI] [PubMed] [Google Scholar]
- 74.Lam SM, Azizzadeh B, Graivier M. Plast Reconstr Surg. 2006;118:55S. doi: 10.1097/01.prs.0000234612.20611.5a. [DOI] [PubMed] [Google Scholar]
- 75.Lontz JF. Dent Clin North Am. 1990;34:307. [PubMed] [Google Scholar]
- 76.Beumer J, Curtis TA, Marunick MT. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. Ishiyaku EuroAmerica Inc; St. Louis: 1996. [Google Scholar]
- 77.Chalian VA. In: Biocompatibility of Dental Materials. Smith DC, Williams DF, editors. Vol. 4. CRC Press; Boca Raton: 1981. p. 247. [Google Scholar]
- 78.Gonzalez JB. J Prosthet Dent. 1978;39:179. doi: 10.1016/s0022-3913(78)80018-3. [DOI] [PubMed] [Google Scholar]
- 79.Andres CJ, Haug SP, Brown DT, Bernal G. J Prosthet Dent. 1992;68:519. doi: 10.1016/0022-3913(92)90422-7. [DOI] [PubMed] [Google Scholar]
- 80.Moore DJ, Glaser ZR, Tabacco MJ, Linebaugh MG. J Prosthet Dent. 1977;38:319. doi: 10.1016/0022-3913(77)90309-2. [DOI] [PubMed] [Google Scholar]
- 81.Aziz T, Waters M, Jagger R. J Biomed Mater Res B Appl Biomater. 2003;65:252. doi: 10.1002/jbm.b.10559. [DOI] [PubMed] [Google Scholar]
- 82.Aziz T, Waters M, Jagger R. J Dent. 2003;31:213. doi: 10.1016/s03005712(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 83.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, Russo PA, Bolte H, Sherry E, Behrens E, Terheyden H. Lancet. 2004;364:766. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 84.Warnke PH, Wiltfang J, Springer I, Acil Y, Bolte H, Kosmahl M, Russo PA, Sherry E, Lutzen U, Wolfart S, Terheyden H. Biomaterials. 2006;27:3163. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 85.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Lancet. 2006;367:1241. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 86.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan D, Vunjak-Novakovic G. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warren SM, Fong KD, Chen CM, Loboa EG, Cowan CM, Lorenz HP, Longaker MT. Tissue Eng. 2003;9:187. doi: 10.1089/107632703764664666. [DOI] [PubMed] [Google Scholar]
- 88.Goessler U, Stern-Straeter J, Riedel K, Bran G, Hörmann K, Riedel F. Eur Arch Otorhinolaryngol. 2007;264:1343. doi: 10.1007/s00405-007-0369-y. [DOI] [PubMed] [Google Scholar]
- 89.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. J Dent Res. 2006;85:966. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Downey PA, Siegel MI. Phys Ther. 2006;86:77. doi: 10.1093/ptj/86.1.77. [DOI] [PubMed] [Google Scholar]
- 91.Habibovic P, de Groot K. J Tissue Eng Regen Med. 2007;1:25. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 92.Holland TA, Mikos AG. Adv Biochem Eng Biotechnol. 2006;102:161. doi: 10.1007/b137205. [DOI] [PubMed] [Google Scholar]
- 93.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. J Bone Joint Surg Am. 2008;90(Suppl 1):36. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 94.Kretlow JD, Mikos AG. Tissue Eng. 2007;13:927. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 95.Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Curr Stem Cell Res Ther. 2006;1:345. doi: 10.2174/157488806778226803. [DOI] [PubMed] [Google Scholar]
- 96.Villanueva JE, Nimni ME. J Bone Miner Res. 1990;5:733. doi: 10.1002/jbmr.5650050710. [DOI] [PubMed] [Google Scholar]
- 97.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 98.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Biomaterials. 2006;27:3249. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 99.Patel SY, Tabata ZSY, Jansen JA, Wong M, Mikos AG. Bone. submitted. [Google Scholar]
- 100.Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Biomaterials. 2007;28:2763. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 101.Kroese-Deutman HC, Ruhe PQ, Spauwen PH, Jansen JA. Biomaterials. 2005;26:1131. doi: 10.1016/j.biomaterials.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 102.Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI. J Biomed Mater Res A. 2007;82:129. doi: 10.1002/jbm.a.31082. [DOI] [PubMed] [Google Scholar]
- 103.Dadsetan M, Hefferan TE, Szatkowski JP, Mishra PK, Macura SI, Lu L, Yaszemski MJ. Biomaterials. 2008;29:2193. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, Park KH. Biomaterials. 2007;28:2631. doi: 10.1016/j.biomaterials.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Sumanasinghe RD, Osborne JA, Loboa EG. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31913. [DOI] [PubMed] [Google Scholar]
- 106.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T. Science. 1981;211:1052. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 107.Burke JF, Yannas IV, Quinby WC, Jr, Bondoc CC, Jung WK. Ann Surg. 1981;194:413. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Am J Transplant. 2005;5:1002. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 109.Metcalfe AD, Ferguson MW. J R Soc Interface. 2007;4:413. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. Faseb J. 1998;12:1331. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 111.Farhadi J, Fulco I, Miot S, Wirz D, Haug M, Dickinson SC, Hollander AP, Daniels AU, Pierer G, Heberer M, Martin I. Ann Surg. 2006;244:978. doi: 10.1097/01.sla.0000247057.16710.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, Lowder E, Landis WJ. Tissue Eng. 2006;12:691. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 113.Kamil SH, Kojima K, Vacanti MP, Bonassar LJ, Vacanti CA, Eavey RD. Laryngoscope. 2003;113:90. doi: 10.1097/00005537-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 114.Allen KD, Athanasiou KA. Tissue Eng. 2006;12:1183. doi: 10.1089/ten.2006.12.1183. [DOI] [PubMed] [Google Scholar]
- 115.Detamore MS, Athanasiou KA. Tissue Eng. 2003;9:1065. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 116.Johns DE, Athanasiou KA. Proc Inst Mech Eng [H] 2007;221:509. doi: 10.1243/09544119JEIM158. [DOI] [PubMed] [Google Scholar]
- 117.Alhadlaq A, Mao JJ. J Dent Res. 2003;82:951. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 118.Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. Adv Drug Deliv Rev. 2007;59:308. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shah R, Sinanan AC, Knowles JC, Hunt NP, Lewis MP. Biomaterials. 2005;26:1497. doi: 10.1016/j.biomaterials.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 120.Kaar JL, Li Y, Blair HC, Asche G, Koepsel RR, Huard J, Russell AJ. Acta Biomater. 2008;4:1411. doi: 10.1016/j.actbio.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Lewis MP, Machell JR, Hunt NP, Sinanan AC, Tippett HL. Eur J Oral Sci. 2001;109:209. doi: 10.1034/j.1600-0722.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 122.Tippett HL, Dodgson LK, Hunt NP, Lewis MP. Eur J Orthod. 2008;30:217. doi: 10.1093/ejo/cjm105. [DOI] [PubMed] [Google Scholar]
- 123.Gawlitta D, Boonen KJ, Oomens CW, Baaijens FP, Bouten CV. Tissue Eng Part A. 2008;14:161. doi: 10.1089/ten.a.2007.0095. [DOI] [PubMed] [Google Scholar]
- 124.Stern-Straeter J, Bran G, Riedel F, Sauter A, Hormann K, Goessler UR. Int J Mol Med. 2008;21:49. [PubMed] [Google Scholar]
- 125.Powers MP, Bosker H. J Oral Maxillofac Surg. 1996;54:934. doi: 10.1016/s0278-2391(96)90386-9. [DOI] [PubMed] [Google Scholar]
- 126.Tzikas TL. Facial Plast Surg. 2004;20:135. doi: 10.1055/s-2004-861754. [DOI] [PubMed] [Google Scholar]
- 127.Alhadlaq A, Tang M, Mao JJ. Tissue Eng. 2005;11:556. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 128.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Biomaterials. 2007;28:5280. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Goepferich A, Blunk T. Tissue Eng. 2005;11:1840. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 130.Hong L, Peptan IA, Colpan A, Daw JL. Cells Tissues Organs. 2006;183:133. doi: 10.1159/000095987. [DOI] [PubMed] [Google Scholar]
- 131.Marra KG, Defail AJ, Clavijo-Alvarez JA, Badylak SF, Taieb A, Schipper B, Bennett J, Rubin JP. Plast Reconstr Surg. 2008;121:1153. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 132.Ferreira JNAR, Ko CC, Myers S, Swift J, Fricton JR. J Oral Maxillofac Surg. 2008;66:1112. doi: 10.1016/j.joms.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hollister SJ, Lin CY, Saito E, Lin CY, Schek RD, Taboas JM, Williams JM, Partee B, Flanagan CL, Diggs A, Wilke EN, Van Lenthe GH, Muller R, Wirtz T, Das S, Feinberg SE, Krebsbach PH. Orthod Craniofac Res. 2005;8:162. doi: 10.1111/j.1601-6343.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 134.Schwartz-Dabney CL, Dechow PC. J Dent Res. 2002;81:613. doi: 10.1177/154405910208100907. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz-Dabney CL, Dechow PC. Am J Phys Anthropol. 2003;120:252. doi: 10.1002/ajpa.10121. [DOI] [PubMed] [Google Scholar]
- 136.Peterson J, Dechow PC. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2003;274A:785. doi: 10.1002/ar.a.10096. [DOI] [PubMed] [Google Scholar]
- 137.Hannam AG, Stavness I, Lloyd JE, Fels S. J Biomech. 2008;41:1069. doi: 10.1016/j.jbiomech.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 138.Sipp JA, Ashland J, Hartnick CJ. Archives of Otolaryngology--Head & Neck Surgery. 2008;134:268. doi: 10.1001/archotol.134.3.268. [DOI] [PubMed] [Google Scholar]
- 139.Real RPd, Ooms E, Wolke JGC, Vallet-Regí M, Jansen JA. J Biomed Mater Res A. 2003;65A:30. doi: 10.1002/jbm.a.10432. [DOI] [PubMed] [Google Scholar]
- 140.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. J Biomed Mater Res A. 2005;74:533. doi: 10.1002/jbm.a.30341. [DOI] [PubMed] [Google Scholar]
- 141.Habraken WJEM, Wolfe JGC, Mikos AG, Jansen JA. J Biomater Sci Polym Ed. 2006;17:1057. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 142.Ruhe PQ, Hedberg-Dirk EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. Tissue Eng. 2006;12:789. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 143.Link DP, van den Dolder J, van den Beucken JJ, Cuijpers VM, Wolke JG, Mikos AG, Jansen JA. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 144.Habraken WJ, Zhang Z, Wolke JG, Grijpma DW, Mikos AG, Feijen J, Jansen JA. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 145.Habraken WJ, de Jonge LT, Wolke JG, Yubao L, Mikos AG, Jansen JA. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31703. [DOI] [PubMed] [Google Scholar]
- 146.Ruhe PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. J Control Release. 2005;106:162. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 147.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 148.Ruhe PQ, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. J Mater Sci Mater Med. 2006;17:919. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 149.Bodde EW, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31830. [DOI] [PubMed] [Google Scholar]
- 150.DiCicco M, Goldfinger A, Guirand F, Abdullah A, Jansen SA. J Biomed Mater Res B Appl Biomater. 2004;70:1. doi: 10.1002/jbm.b.30014. [DOI] [PubMed] [Google Scholar]
- 151.Xu HHK, Weir MD, Burguera EF, Fraser AM. Biomaterials. 2006;27:4279. doi: 10.1016/j.biomaterials.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 152.Xu HHK, Quinn JB, Takagi S, Chow LC. Biomaterials. 2004;25:1029. doi: 10.1016/s0142-9612(03)00608-2. [DOI] [PubMed] [Google Scholar]
- 153.Xu HHK, Carl J, Simon G. J Orthop Res. 2004;22:535. doi: 10.1016/j.orthres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 154.Fellah BH, Weiss P, Gauthier O, Rouillon T, Pilet P, Daculsi G, Layrolle P. J Orthop Res. 2006;24:628. doi: 10.1002/jor.20125. [DOI] [PubMed] [Google Scholar]
- 155.Trojani C, Boukhechba F, Scimeca JC, Vandenbos F, Michiels JF, Daculsi G, Boileau P, Weiss P, Carle GF, Rochet N. Biomaterials. 2006;27:3256. doi: 10.1016/j.biomaterials.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 156.Gauthier O, Goyenvalle E, Bouler JM, Guicheux J, Pilet P, Weiss P, Daculsi G. J Mater Sci Mater Med. 2001;12:385. doi: 10.1023/a:1011284517429. [DOI] [PubMed] [Google Scholar]
- 157.Gauthier O, Khairoun I, Bosco J, Obadia L, Bourges X, Rau C, Magne D, Bouler JM, Aguado E, Daculsi G, Weiss P. J Biomed Mater Res A. 2003;66:47. doi: 10.1002/jbm.a.10506. [DOI] [PubMed] [Google Scholar]
- 158.Gauthier O, Muller R, Stechow D, Lamy B, Weiss P, Bouler JM, Aguado E, Daculsi G. Biomaterials. 2005;26:5444. doi: 10.1016/j.biomaterials.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 159.Leeuwenburgh SC, Jansen JA, Mikos AG. J Biomater Sci Polym Ed. 2007;18:1547. [PubMed] [Google Scholar]
- 160.Gonzalez-McQuire R, Green DW, Partridge KA, Oreffo ROC, Mann S, Davis SA. Adv Mater. 2007;19:2236. [Google Scholar]
- 161.Fisher JP, Holland TA, Dean D, Engel PS, Mikos AG. J Biomater Sci Polym Ed. 2001;12:673. doi: 10.1163/156856201316883476. [DOI] [PubMed] [Google Scholar]
- 162.Fisher JP, Dean D, Mikos AG. Biomaterials. 2002;23:4333. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 163.Buxton AN, Zhu J, Marchant R, West JL, Yoo JU, Johnstone B. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 164.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Biomaterials. 2006;27:2519. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 165.Declercq HA, Gorski TL, Tielens SP, Schacht EH, Cornelissen MJ. Biomacromolecules. 2005;6:1608. doi: 10.1021/bm050031s. [DOI] [PubMed] [Google Scholar]
- 166.Declercq HA, Cornelissen MJ, Gorskiy TL, Schacht EH. J Mater Sci Mater Med. 2006;17:113. doi: 10.1007/s10856-006-6814-4. [DOI] [PubMed] [Google Scholar]
- 167.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Proc Natl Acad Sci U S A. 1999;96:3104. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Plast Reconstr Surg. 1999;104:1014. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 169.Hahn MS, Miller JS, West JL. Adv Mater. 2006;18:2679. [Google Scholar]
- 170.Sun ZB, Dong XZ, Chen WQ, Nakanishi S, Duan XM, Kawata S. Adv Mater. 2008;20:914. [Google Scholar]
- 171.Burdick JA, Khademhosseini A, Langer R. Langmuir. 2004;20:5153. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 172.Temenoff JS, Shin H, Conway DE, Engel PS, Mikos AG. Biomacromolecules. 2003;4:1605. doi: 10.1021/bm030056w. [DOI] [PubMed] [Google Scholar]
- 173.Shin H, Temenoff JS, Mikos AG. Biomacromolecules. 2003;4:552. doi: 10.1021/bm020121m. [DOI] [PubMed] [Google Scholar]
- 174.Vernon B, Tirelli N, Bachi T, Haldimann D, Hubbell JA. J Biomed Mater Res A. 2003;64:447. doi: 10.1002/jbm.a.10369. [DOI] [PubMed] [Google Scholar]
- 175.Gray DH, Hu SL, Juang E, Gin DL. Adv Mater. 1997;9:731. [Google Scholar]
- 176.Wang J, Liu C, Chi P. Int J Biol Macromol. 2008;42:450. doi: 10.1016/j.ijbiomac.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 177.Ehrbar M, Rizzi SC, Hlushchuk R, Djonov V, Zisch AH, Hubbell JA, Weber FE, Lutolf MP. Biomaterials. 2007;28:3856. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 178.Sanborn TJ, Messersmith PB, Barron AE. Biomaterials. 2002;23:2703. doi: 10.1016/s0142-9612(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 179.Jin R, Hiemstra C, Zhong Z, Feijen J. Biomaterials. 2007;28:2791. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 180.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. Biomaterials. 2008;29:438. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 181.Halloran DMO, Collighan RJ, Griffin M, Pandit AS. Tissue Eng. 2006;12:1467. doi: 10.1089/ten.2006.12.1467. [DOI] [PubMed] [Google Scholar]
- 182.Griffin M, Casadio R, Bergamini CM. Biochem J. 2002;368:377. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heath DJ, Christian P, Griffin M. Biomaterials. 2002;23:1519. doi: 10.1016/s0142-9612(01)00282-4. [DOI] [PubMed] [Google Scholar]
- 184.Galaev IY, Mattiasson B. Trends Biotechnol. 1999;17:335. doi: 10.1016/s0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- 185.Qiu Y, Park K. Adv Drug Deliv Rev. 2001;53:321. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 186.Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE, Ulijn RV. Adv Mater. 2006;18:611. [Google Scholar]
- 187.Klouda L, Mikos AG. Eur J Pharm Biopharm. 2008;68:34. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin HH, Cheng YL. Macromolecules. 2001;34:3710. [Google Scholar]
- 189.Ohya S, Nakayama Y, Matsuda T. Biomacromolecules. 2001;2:856. doi: 10.1021/bm010040a. [DOI] [PubMed] [Google Scholar]
- 190.Ohya S, Nakayama Y, Matsuda T. J Artif Organs. 2004;7:181. doi: 10.1007/s10047-004-0265-9. [DOI] [PubMed] [Google Scholar]
- 191.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Biomacromolecules. 2008;9:1558. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 192.Sosnik A, Cohn D. Biomaterials. 2004;25:2851. doi: 10.1016/j.biomaterials.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 193.Sosnik A, Cohn D. Biomaterials. 2005;26:349. doi: 10.1016/j.biomaterials.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 194.Cohn D, Sosnik A, Garty S. Biomacromolecules. 2005;6:1168. doi: 10.1021/bm0495250. [DOI] [PubMed] [Google Scholar]
- 195.Fisher JP, Jo S, Mikos AG, Reddi AH. J Biomed Mater Res A. 2004;71:268. doi: 10.1002/jbm.a.30148. [DOI] [PubMed] [Google Scholar]
- 196.Seong JY, Jun YJ, Jeong B, Sohn YS. Polymer. 2005;46:5075. [Google Scholar]
- 197.Jeong B, Lee KM, Gutowska A, An YH. Biomacromolecules. 2002;3:865. doi: 10.1021/bm025536m. [DOI] [PubMed] [Google Scholar]
- 198.Nair LS, Starnes T, Ko JWK, Laurencin CT. Biomacromolecules. 2007;8:3779. doi: 10.1021/bm7006967. [DOI] [PubMed] [Google Scholar]
- 199.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. J Control Release. 2005;103:609. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 200.Zan J, Chen H, Jiang G, Lin Y, Ding F. J Appl Polym Sci. 2006;101:1892. [Google Scholar]
- 201.Cellesi F, Tirelli N, Hubbell JA. Macromol Chem Phys. 2002;203:1466. [Google Scholar]
- 202.Cellesi F, Tirelli N, Hubbell JA. Biomaterials. 2004;25:5115. doi: 10.1016/j.biomaterials.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 203.Lee BH, West B, McLemore R, Pauken C, Vernon BL. Biomacromolecules. 2006;7:2059. doi: 10.1021/bm060211h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Robb SA, Lee BH, McLemore R, Vernon BL. Biomacromolecules. 2007;8:2294. doi: 10.1021/bm070267r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dunn R, English J, Cowsar D, Venderbelt D. Patent 4,938,763. United States Patent. :1990.
- 206.Bakhshi R, Vasheghani-Farahani E, Mobedi H, Jamshidi A, Khakpour M. Polym Adv Technol. 2006;17:354. [Google Scholar]
- 207.Tae G, Kornfield JA, Hubbell JA. Biomaterials. 2005;26:5259. doi: 10.1016/j.biomaterials.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 208.Brittberg M, Sjögren-Jansson E, Lindahl A, Peterson L. Biomaterials. 1997;18:235. doi: 10.1016/s0142-9612(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 209.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. Biomaterials. 2003;24:2497. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 210.Ho W, Tawil B, Dunn JCY, Wu BM. Tissue Eng. 2006;12:1587. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 211.Catelas I, Sese N, Wu BM, Dunn JCY, Helgerson S, Tawil B. Tissue Eng. 2006;12:2385. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 212.Schmoekel HG, Weber FE, Schense JC, Grätz KW, Schawalder P, Hubbell JA. Biotechnol Bioeng. 2005;89:253. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 213.Schense JC, Bloch J, Aebischer P, Hubbell JA. Nat Biotech. 2000;18:415. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 214.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Proc Natl Acad Sci U S A. 2007;104:7791. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Biomacromolecules. 2006;7:1855. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, Stupp SI. Biomaterials. 2007;28:4608. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 217.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. J Neurosci. 2008;28:3814. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, Brookes SJ, Aggeli A. J Dent Res. 2007;86:426. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 219.Firth A, Aggeli A, Burke JL, Yang X, Kirkham J. Nanomedicine. 2006;1:189. doi: 10.2217/17435889.1.2.189. [DOI] [PubMed] [Google Scholar]
- 220.Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, Stupp SI. Nano Lett. 2006;6:2086. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 221.Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, Stupp SI. Biomaterials. 2008;29:161. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 222.Kang SW, Jeon O, Kim BS. Tissue Eng. 2005;11:438. doi: 10.1089/ten.2005.11.438. [DOI] [PubMed] [Google Scholar]
- 223.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Nat Mater. 2008;7:248. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 224.Wang Q, Wang LM, Detamore MS, Berkland C. Adv Mater. 2008;20:236. [Google Scholar]
- 225.Van Tomme SR, Van Steenbergen MJ, De Smedt SC, Van Nostrum CF, Hennink WE. Biomaterials. 2005;26:2129. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 226.Salem AK, Rose FRAJ, Oreffo ROC, Yang X, Davies MC, Mitchell JR, Roberts CJ, Stolnik-Trenkic S, Tendler SJB, Williams PM, Shakesheff KM. Adv Mater. 2003;15:210. [Google Scholar]
- 227.Hatefi A, Amsden B. J Control Release. 2002;80:9. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 228.Kretlow JD, Klouda L, Mikos AG. Adv Drug Deliv Rev. 2007;59:263. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 229.Malanchuk VO, Kopchak AV. Journal of Cranio-Maxillofacial Surgery. 2007;35:57. doi: 10.1016/j.jcms.2006.07.865. [DOI] [PubMed] [Google Scholar]
- 230.Will MJ, Goksel T, Stone CG, Doherty MJ. Oral Maxillofacial Surg Clin N Am. 2005;17:331. doi: 10.1016/j.coms.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 231.Petersen K, Hayes DK, Blice JP, Hale RG. J Trauma. 2008;64:S265. doi: 10.1097/TA.0b013e318163d2a6. [DOI] [PubMed] [Google Scholar]
- 232.Wade AL, Dye JL, Mohrle C, Galarneau MR. J Trauma. 2007;63:836. doi: 10.1097/01.ta.0000251453.54663.66. [DOI] [PubMed] [Google Scholar]
- 233.Penner MJ, Duncan CP, Masri BA. J Arthroplasty. 1999;14:209. doi: 10.1016/s0883-5403(99)90128-6. [DOI] [PubMed] [Google Scholar]
- 234.Penner MJ, Masri BA, Duncan CP. J Arthroplasty. 1996;11:939. doi: 10.1016/s0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 235.Bayston R, Rodgers J. J Clin Pathol. 1990;43:866. doi: 10.1136/jcp.43.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jain RA. Biomaterials. 2000;21:2475. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 237.Virto MR, Elorza B, Torrado S, Elorza MdLA, Frutos G. Biomaterials. 2007;28:877. doi: 10.1016/j.biomaterials.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 238.Young S, Wong M, Tabata Y, Mikos AG. J Control Release. 2005;109:256. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 239.Ambrose CG, Gogola GR, Clyburn TA, Raymond AK, Peng AS, Mikos AG. Clin Orthop Relat Res. 2003:279. doi: 10.1097/01.blo.0000093920.26658.ae. [DOI] [PubMed] [Google Scholar]
- 240.Ambrose CG, Clyburn TA, Louden K, Joseph J, Wright J, Gulati P, Gogola GR, Mikos AG. Clin Orthop Relat Res. 2004:293. doi: 10.1097/01.blo.0000126303.41711.a2. [DOI] [PubMed] [Google Scholar]
- 241.Kempen DHR, Kim CW, Lu L, Dhert WJA, Currier BL, Yaszemski MJ. Controlled release from poly(lactic-co-glycolic acid) microspheres embedded in an injectable, biodegradable scaffold for bone tissue engineering; Leganes, Madrid, Spain. 2003. [Google Scholar]
- 242.Roman J, Cabanas MV, Pena J, Doadrio JC, Vallet-Regi M. J Biomed Mater Res A. 2008;84:99. doi: 10.1002/jbm.a.31394. [DOI] [PubMed] [Google Scholar]
- 243.Kim HW, Knowles JC, Kim HE. J Mater Sci Mater Med. 2005;16:189. doi: 10.1007/s10856-005-6679-y. [DOI] [PubMed] [Google Scholar]
- 244.Ferraz MP, Mateus AY, Sousa JC, Monteiro FJ. J Biomed Mater Res A. 2007;81A:994. doi: 10.1002/jbm.a.31151. [DOI] [PubMed] [Google Scholar]
- 245.Cohen S, Samadikuchaksaraei A, Polak JM, Bishop AE. Tissue Eng. 2006;12:2025. doi: 10.1089/ten.2006.12.2025. [DOI] [PubMed] [Google Scholar]
- 246.Taboas CKKJM, Huang GT-J, Tuan RS. In: Translational Approaches in Tissue Engineering and Regenerative Medicine. Mao GV-NJJ, Mikos AG, Atala A, editors. Artech House, Inc; Boston: 2008. p. 83. [Google Scholar]
- 247.Drake CJ. Birth Defects Res C Embryo Today. 2003;69:73. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- 248.Patel ZS, Mikos AG. J Biomater Sci Polym Ed. 2004;15:701. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 249.Lawrence WT, Diegelmann RF. Clin Dermatol. 1994;12:157. doi: 10.1016/0738-081x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 250.Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, Hedberg EL, Mikos AG. J Control Release. 2005;101:127. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 251.Luginbuehl V, Meinel L, Merkle HP, Gander B. Eur J Pharm Biopharm. 2004;58:197. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 252.Varkey M, Gittens SA, Uludag H. Expert Opin Drug Deliv. 2004;1:19. doi: 10.1517/17425247.1.1.19. [DOI] [PubMed] [Google Scholar]
- 253.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 254.Schmidmaier G, Schwabe P, Wildemann B, Haas NP. Injury. 2007;38(Suppl 4):S35. doi: 10.1016/s0020-1383(08)70007-x. [DOI] [PubMed] [Google Scholar]
- 255.Ross R, Raines EW, Bowen-Pope DF. Cell. 1986;46:155. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 256.Fan H, Zhang C, Li J, Bi L, Qin L, Wu H, Hu Y. Biomacromolecules. 2008;9:927. doi: 10.1021/bm7013203. [DOI] [PubMed] [Google Scholar]
- 257.Holland TA, Tabata Y, Mikos AG. J Control Release. 2003;91:299. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 258.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Biochem Biophys Res Commun. 2004;320:914. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 259.Darling EM, Athanasiou KA. Ann Biomed Eng. 2003;31:1114. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 260.Holland TA, Mikos AG. J Control Release. 2003;86:1. doi: 10.1016/s0168-3659(02)00373-5. [DOI] [PubMed] [Google Scholar]
- 261.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. FASEB J. 2008;22:2949. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Woo BH, Fink BF, Page R, Schrier JA, Jo YW, Jiang G, DeLuca M, Vasconez HC, DeLuca PP. Pharm Res. 2001;18:1747. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- 263.Tabata Y, Nagano A, Ikada Y. Tissue Eng. 1999;5:127. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 264.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. J Biomed Mater Res A. 2005;75:156. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 265.Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteoarthritis Cartilage. 2007;15:187. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 266.Gobin AS, West JL. Biotechnol Prog. 2003;19:1781. doi: 10.1021/bp0341390. [DOI] [PubMed] [Google Scholar]
- 267.Na K, Kim S, Woo DG, Sun BK, Yang HN, Chung HM, Park KH. J Biomed Mater Res A. 2007;83:779. doi: 10.1002/jbm.a.31374. [DOI] [PubMed] [Google Scholar]
- 268.Gao TJ, Kousinioris NA, Wozney JM, Winn S, Uludag H. Tissue Eng. 2002;8:429. doi: 10.1089/107632702760184691. [DOI] [PubMed] [Google Scholar]
- 269.Lee JS, Go DH, Bae JW, Lee SJ, Park KD. J Control Release. 2007;117:204. doi: 10.1016/j.jconrel.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 270.Lee JS, Bae JW, Joung YK, Lee SJ, Han DK, Park KD. Int J Pharm. 2008;346:57. doi: 10.1016/j.ijpharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 271.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Biomaterials. 2004;25:699. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 272.Silva EA, Mooney DJ. J Thromb Haemost. 2007;5:590. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 273.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Cardiovasc Res. 2007;75:178. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 274.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Biomaterials. 2006;27:5836. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 275.Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. J Control Release. 2007;122:71. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 276.Jupiter JB, Winters S, Sigman S, Lowe C, Pappas C, Ladd AL, Van Wagoner M, Smith ST. J Orthop Trauma. 1997;11:110. doi: 10.1097/00005131-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 277.Blom EJ, Klein-Nulend J, Wolke JG, van Waas MA, Driessens FC, Burger EH. J Biomed Mater Res. 2002;59:265. doi: 10.1002/jbm.1241. [DOI] [PubMed] [Google Scholar]
- 278.Vogel G. Science. 1999;283:1432. doi: 10.1126/science.283.5407.1432. [DOI] [PubMed] [Google Scholar]
- 279.Caplan AI. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 280.Caplan AI. Tissue Eng. 2005;11:1198. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 281.Romanov YA, Svintsitskaya VA, Smirnov VN. Stem Cells. 2003;21:105. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 282.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 283.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 284.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Biomaterials. 2007;28:3217. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. J Biomed Mater Res A. 2004;70:235. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 286.Wang DA, Williams CG, Yang F, Cher N, Lee H, Elisseeff JH. Tissue Eng. 2005;11:201. doi: 10.1089/ten.2005.11.201. [DOI] [PubMed] [Google Scholar]
- 287.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Tissue Eng. 2004;10:955. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 288.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Ann Biomed Eng. 2004;32:911. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 289.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Proc Natl Acad Sci U S A. 2002;99:12025. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gerstenfeld LC, Cruceta J, Shea CM, Sampath K, Barnes GL, Einhorn TA. J Bone Miner Res. 2002;17:221. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 291.Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Cell Physiol Biochem. 2007;20:665. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 292.Sun H, Qu Z, Guo Y, Zang G, Yang B. Biomed Eng Online. 2007;6:41. doi: 10.1186/1475-925X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. Proc Natl Acad Sci U S A. 2006;103:2512. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Popov BV, Serikov VB, Petrov NS, Izusova TV, Gupta N, Matthay MA. Tissue Eng. 2007;13:2441. doi: 10.1089/ten.2007.0001. [DOI] [PubMed] [Google Scholar]
- 295.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. Nat Mater. 2007;6:997. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 296.Sun W, Puzas E, Sheu TJ, Liu X, Fauchetl PM. Adv Mater. 2007;19:921. [Google Scholar]
- 297.Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, Wong JY. Biomaterials. 2008;29:2565. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Czarnecki JS, Lafdi K, Tsonis PA. Tissue Eng. 2008;14:255. doi: 10.1089/tea.2007.0169. [DOI] [PubMed] [Google Scholar]
- 299.Albrecht DR, Underbill GH, Mendelson A, Bhatia SN. Lab on a Chip. 2007;4:702. doi: 10.1039/b701306j. [DOI] [PubMed] [Google Scholar]
- 300.Woodfield TBF, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J. Tissue Eng. 2005;11:1297. doi: 10.1089/ten.2005.11.1297. [DOI] [PubMed] [Google Scholar]
- 301.Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Biomaterials. 2005;26:3187. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 302.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]