Abstract
Women’s sexual interest changes with hormonal fluctuations across the menstrual cycle. It is unclear how hormones modify women’s sexual behavior and desire, but one possibility is that they alter women’s positive appraisals of stimuli and thus their sexual interest. Using 3 T fMRI, we measured neural activation in women at two time points in their menstrual cycle (late follicular, luteal) while they evaluated photos of men presented as potential sexual partners. Participants were ten heterosexual women aged 23–28 none of who was using hormonal contraceptives or in a committed relationship. In an event-related design, the women were presented with as series of photos of male faces and asked questions to assess their degree of sexual interest in the men depicted. Results demonstrate an overall effect of menstrual cycle phase on neural activation. During their follicular versus luteal phase, women demonstrated increased activation in the right medial orbitofrontal cortex (OFC), suggesting increased positive appraisal. Activation in the OFC was positively correlated with women’s estradiol to progesterone ratios. There were no areas that demonstrated increased activation during the luteal versus follicular phase. The observed increase in activation in the OFC during the follicular phase may reflect a hormonally mediated increase in appetitive motivation and may prime women towards increased sexual interest and behavior around ovulation.
Keywords: Menstrual cycle, Hormones, Orbitofrontal cortex, Sexual behavior
Introduction
Many studies have found increased sexual desire, masturbation, and sexual initiation during the ovulatory phase of a woman’s menstrual cycle (e.g., Bullivant et al., 2004; Gangestad and Thornhill, 2008; Harvey 1987; Tarin and Gomez-Piquer, 2002; Wilcox et al., 2004), but the mechanisms by which hormones influence women’s sexual interest are poorly understood. Knowledge of cyclic changes in brain activation in systems related to mating and appetitive behavior in women may increase our understanding of how hormones mediate women’s sexual behavior across the menstrual cycle. Recent fMRI studies have shown that phase of the menstrual cycle influences how women process monetary risk and reward (Dreher et al., 2007). In anticipation of a monetary reward during a gambling task, women in the follicular phase showed higher levels of neural activation in the orbitofrontal cortex and amygdala than they did during the luteal phase (Dreher et al., 2007). Recent fMRI studies have also demonstrated increased limbic system activation in response to erotic videos during mid-cycle in women of reproductive age (Gizewski et al., 2006), and in postmenopausal women administered estradiol and testosterone (Archer et al., 2006). Together these studies suggest that hormones may be acting in a causal manner on neural processes that influence women’s responses to appetitive, including sexual, stimuli.
These increases in neural responses to appetitive stimuli such as money and erotic videos (Dreher et al., 2007, Gizewski et al., 2006) may be the result of a more generalized mid-cycle increase in positive appraisal. For example, work using event-related potential (ERP) methodology to compare neural responses to sexual versus neutral stimuli found that women showed an increase in the late positive component (LPC) in the response to sexual stimuli during the ovulatory phase of the menstrual cycle (Krug et al., 2000). Because the LPC is believed to be sensitive to valence and levels of emotional processing, this phase-related response to sexual stimuli was interpreted to reflect greater interest in, and positive appraisal of, sexual stimuli during the ovulatory phase. Recently, Mass et al. (2008) reported increases in the activity of the zygomatic muscle during the follicular versus luteal phase of women who were viewing pictures of nude men. The authors interpreted this finding to reflect increased positive appraisal of the male stimuli due to the involvement of the zygomatic muscle in smiling. As reported in nonhuman species, increases in estradiol during the follicular phase may increase dopamine signaling to limbic structures to enhance the reward value of positive reinforcers, including sexual reinforcers, and thereby increase sexual behavior with the increase in appetitive motivation and positive appraisal (reviewed in Pfaus et al., 2003, Pfaus, 2008).
The current study was designed to test the hypothesis that neural activation of the limbic system in response to potential sexual partners changes across the menstrual cycle. Because recent work suggests that the neural mechanisms underlying economic and social decision making overlap (Hayden et al., 2007; Izuma et al., 2008; Lee, 2008; Rupp et al., 2009a), we predicted that women would demonstrate phase-dependent differences in neural activation when evaluating men, resembling those cyclic differences previously observed during economic decision making (Dreher et al., 2007). To test this, we measured brain activation in women across two time points while they viewed male faces and evaluated the men depicted as potential sexual partners. Based on the hypothesis that hormones mediate the reward value and positive evaluation of sexually relevant stimuli, which were male faces in the current study, we expected to see differences in neural activation across women’s menstrual cycles in brain areas involved in risk and reward assessment. Brain regions that are recruited during decision making and reward processing with face stimuli include the orbitofrontal cortex, anterior cingulate, insula, nucleus accumbens, and amygdala (Harris et al., 2007; Kranz and Ishai, 2006; O’Doherty et al., 2003; Winston et al., 2007). These brain regions have also been shown to respond differently depending on hormonal state (Dreher et al., 2007; Gizewski et al., 2006). We predicted that this network of brain regions would be influenced by the phase of a woman’s menstrual cycle at the time of testing, specifically that limbic system activation would be greater during the follicular versus luteal phase.
Methods
Participants
A total of 16 heterosexual women were recruited for this study. The following inclusion criteria were applied: not using any form of hormonal birth control, not currently in an exclusive relationship, between ages 23 and 28, and reporting normal menstrual cycles with an average length of 28–32 days. Participants were also screened to make sure that they did not have any contraindications for MRI, including included magnetic life-support devices (e.g., pacemakers and aneurysm clips), metal prostheses or other metallic objects (e.g., cochlear implants, steel pins, metal fragments), recent tattoos, non-removable metal jewelry, and pregnancy. Recruitment was accomplished through emails and flyers. Twelve of the 16 women successfully completed both test sessions within a one-month period. Of the four incomplete participants, two did not complete testing due to scheduling difficulties, and two did not complete their second test session because they experienced discomfort with the fMRI procedures (body piercing removal, anxiety). Participants were on average 25.15 years old (SD = 1.91), none reported currently using hormonal contraceptives, 11 were Caucasian, one was African American, all but one reported some previous sexual experience (number of lifetime sexual partners, Mean ± SD = 5.25 ± 3.70), none reported currently using medication or being under treatment for any psychological disorders, all reported regular periods between 28 and 32 days, all women were single, and two women reported currently having a sexual partner (nonexclusive). All subjects gave informed consent and the Institutional Review Board of Indiana University approved all procedures.
Stimuli
Participants viewed 224 male face photos during imaging, which included 56 different male faces presented four times. The male face pictures were taken from public domain websites on the internet. All faces were edited to the same 640 × 480 pixel resolution with the same limited amount of background, and converted to black and white in Adobe Photoshop (Version 7.0.1, Adobe Systems Inc.). Photos were selected to be of the same general age range as participants, depicting a neutral expression, and from a variety of ethnic back-grounds. The pictures were of variable attractiveness based on pilot ratings from 15 heterosexual, naturally cycling women not involved in the fMRI study.
As part of a larger study, and to promote participants’ attention in the current study, attributes of the faces were varied across presentations so as to appear more or less masculine or be described as more or less risky as sexual partners. Faces were masculinized and feminized using computer morphing software (Psychomorph, Rowland and Perrett, 1995, Perception Lab, University of St. Andrews). This software can create both a masculinized and feminized version of the same male face to allow controlled comparison. Risk was varied by associating information about the men’s supposed number of previous sexual partners and their typical use of condoms with their photos and presenting that information to the subjects simultaneously with the photos. The entire study presentation design included, therefore, two presentations of the same male face from 56 originals across two risk conditions. Results specifically related to the masculinity and sexual risk manipulations are presented elsewhere (Rupp et al., 2009a,b). Due to large differences in subjective ratings for low and high risk potential sexual partners (Rupp et al., 2009a), only pictures from the low risk condition were included in the analysis of menstrual cycle phase. The current paper therefore includes data from both the masculinized and feminized exemplar of the same face presented as a relatively low risk sexual partner (Mosher et al., 2005), for a total of 112 photos. That is, we averaged woman’s responses across the two presentations of an individual male face within the low risk condition before we compared responses across phases of the menstrual cycle.
Participants were also presented 56 black and white photos of houses as a control condition. House stimuli were included as neutral stimuli to determine whether possible observed menstrual cycle effects on neural activation were specific to the male faces, or reflected a more generalizable cyclic pattern independent of the stimulus type.
Procedure
Women were tested at two phases of their menstrual cycle, the follicular and luteal. Determination of menstrual phase was done using a counting method and verified by later hormone assay. Target testing for the follicular phase session was between days 10–12 after the women reported menstruation began and testing for the luteal phase was days 19–23 following menstruation. These time windows were chosen based on a 28 day cycle and to reflect differences in sexual proceptivity (Bullivant et al., 2004; Harvey, 1987), the ratio of estradiol to progesterone (reviewed in Puts, 2006), and likelihood of conception (Lynch et al., 2006; Wilcox et al., 2001) across the two testing time points. Four participants started testing in their follicular phase and eight began testing during their luteal phase. For women who began testing in the luteal phase, the timing for the next follicular session occurred following the next menstrual period of the consecutive menstrual cycle. We used the progesterone assays, described in more detail below, to verify that we had classified participants correctly into the follicular and luteal phases, which are characterized by low and high progesterone, respectively, and to ensure ovulatory cycles (Israel et al., 1972).
Participants began the test session with a blood draw (5–6 mL) that was centrifuged following collection and the serum frozen at −80 °C. Serum was assayed for estradiol (range, 53.0–405.7 pg/mL; detection limit, 3.94 pg/mL; Inter-assay coefficients of variation averaged, 8.1%; Intra-assay coefficients of variation averaged, 8.0%), progesterone (range, 0.20–27.9 ng/mL; detection limit, 0.30 ng/mL; Inter-assay coefficients of variation averaged, 3.3%; Intra-assay coefficients of variation averaged, 4.0%), free testosterone (range, 0.29–3.18 pg/mL; detection limit, 0.12 pg/mL; Inter-assay coefficients of variation averaged, 7.3%; Intra-assay coefficients of variation averaged, 4.8%), and total testosterone (range, 0.10–0.80 ng/mL; detection limit, 0.006 ng/mL; Inter-assay coefficients of variation averaged, 8.4%; Intra-assay coefficients of variation averaged, 6.5%) using commercially available RIA kits (Diagnostic Systems Laboratories, Webster, TX).
Imaging took place at the Indiana University Imaging Research Facility. Participants were screened for MRI contraindications and then comfortably positioned in an fMRI scanner (3 T Siemens TRIO) to measure brain activation while evaluating the stimuli. During imaging, participants performed a task in which they evaluated men as potential sexual partners and houses as potential rentals from photos. During the four-second presentation of each male face, participants were asked to judge “How likely would you be to have sex with this person?” (1 = very unlikely, 2 = unlikely, 3 = likely, 4 = very likely). During the task, when presented with a picture of a male face, each woman was instructed to imagine herself in a scenario in which she was open to a sexual encounter (Appendix A). When presented with a house, she was asked to evaluate the house for how likely she would be to rent it (1–4), based on an imagined scenario in which the house was available and she was open to moving (Appendix B). As described above, participants viewed a total of 224 black and white pictures of male faces and 56 photos of houses, for a total 280 pictures. Pictures were presented in eight blocks. Each block consisted of 35 pictures inter-mixed with seven pictures of houses and 28 pictures of faces equally representing the four face conditions. Blocks were randomized for presentation order across and within subjects such that each participant saw a different order of blocks than other participants and also a different order between her follicular and luteal sessions. All stimuli were presented using MATLAB 5.2 (MATHWORKS Inc., Natick, MA) on a Macintosh computer in a rapid variable ISI event-related design. Stimuli were rear-projected using a Mitsubishi XL30U projector onto a frosted glass screen which was seen by the participant as a reflection in a mirror positioned in front of the participant’s line of view.
Imaging parameters
Imaging was carried out using a Siemens Magnetom Trio 3 T whole body MRI and collected on an eight-channel phased-array head coil. Each MRI session took about 1 h, during which the following scans were acquired: 1) 3-plane scout used for choosing slice planes for the remaining scans (10 s); 2) Gradient-echo T2* EPI scans for BOLD-based functional neuroimaging (duration ~5 min, 8 scans/session, ~40 min); and 3) T1 3-D turbo-flash structural scan of the entire brain at high resolution (1-mm isotropic voxels) (8 min).The functional pulse sequence had the following EPI parameters: TE = 25 ms, flip angle = 70°, FOV= 220 × 220 mm, matrix 64 × 64, in-plane resolution = 3.4 × 3.4 mm, slice thickness = 3.4 mm, gap thickness= 0 mm. A typical volume was 33 EPI slices acquired at a time of 60 ms per slice for a total volume acquisition time of 2 s (TR = 2). Slices were acquired parallel to the ACPC plane to efficiently cover the entire brain. High resolution T1-weighted anatomical volumes were acquired using Turbo-flash 3-D (T1 = 1100 ms, TE = 3.93 ms, TR = 14.375 ms, Flip Angle= 12°) with 160 sagittal slices with a thickness of 1 mm and a field of view of 224 × 256 (voxel size = 1 × 1 × 1).
Data analysis
Imaging data were preprocessed and analyzed using Brainvoyager™ software. Functional data preprocessing included 3-D motion correction, slice scan-time correction, spatial smoothing (3-D Gaussian FWHM 6 mm), and linear trend removal. Functional slice data were co-registered to high-resolution structural volumes for each individual and normalized to Talairach space. A random-effects general linear model (GLM) procedure was used to calculate average statistical parametric maps (SPM) to examine the main effects of menstrual cycle phase (follicular minus luteal) on women’s responses to faces and houses. These contrasts were thresholded at a voxel-wise probability of p<0.001 with a cluster-size threshold of 4 voxels (157 mm3). The cluster threshold correction technique used here is the second stage of a two-stage analysis that helps to control false positives, but with a relative sparing of statistical power, which was important for studying the small effect sizes seen between our experimental conditions (Forman et al., 1995). Corrections similar to this have been found to successfully manage the multiple testing problem (Thirion et al., 2007). More specifically, the first stage of our analysis produces a map with all voxels thresholded with a voxel-wise probability of p<0.001. Subsequently, in stage two, a cluster threshold was applied to that map, removing all voxels that did not belong to a group of at least four contiguous voxels. Because voxel clusters of less than three are more likely to represent false positives than clusters that are larger, the cluster correction decreases the type I error rate, without sacrificing power.
Those brain regions determined from the whole-brain SPM group analysis to differ in activation in response to male faces across the two menstrual phases became regions of interest. Activation in these regions of interest was further analyzed by extracting estimated time courses using a deconvolution analysis on each individual. Each woman’s mean BOLD response for men and houses (beta weights) was averaged across the three time points between six to 10 s after stimulus onset (3–5 TR). This average BOLD response was then entered into SPSS (Version 14.02, SAS Institute Inc., Cary, NC) as a dependent measure for linear regression analyses with hormone levels (estradiol, progesterone, free testosterone, total testosterone, and the estradiol to progesterone ratio). Separate regression analyses were also performed within each ROI with participants’ response times and subjective ratings entered into the model. Each subject’s hormone levels, and (separately) subjects’ subjective ratings and response times, were entered in as regressors with participants’ average activation for each region of interest. Data from both test sessions were entered in the same regression models, for a total of 20 data points for each of the regression models. Regression analyses were conducted for both responses to faces and houses, for a total of four linear regression analyses; i.e. for both houses and faces there was one regression model for hormones and one for subjective ratings and response times. A Repeated Measures Multivariate ANOVA was performed on participants’ subjective ratings and response times to look for menstrual cycle phase effects. Subjective ratings and response times within each cycle phase were also correlated with hormone levels using one-tailed Spearmen bivariate correlations. One-tailed analyses were used due to directional hypotheses, specifically we expected testosterone and estradiol to be positively, and progesterone to be negatively, associated with ratings and response times.
Results
Hormones
All follicular level progesterone levels were low, below 2 ng/mL. However, two of the 12 participants had luteal progesterone levels below 2 ng/mL, indicating that they were either tested too early to be considered as in the luteal phase, or that they were tested in their luteal phase and did not have an ovulatory cycle (Israel et al., 1972). These two participants were therefore excluded from further analysis, leaving ten participants in the final data set. Two of the remaining ten began testing in the follicular phase, the other eight started in their luteal phase.
For the remaining ten participants, average day of testing for the follicular phase was day 11 (SD = 2.16) following menses, and average day of testing for the luteal phase was day 23 (SD = 3.14) following menses. Average menstrual cycle length for participants’ test cycles was approximately 28 days (Mean ± SD = 28.1 ± 2.77, N = 10), based on follow-up mail in reports from participants of the date of their next period following their last test session. Women’s estradiol (Follicular, Mean ± SD = 131.71 ± 63.33 pg/mL; Luteal, Mean ± SD = 136.44 ± 52.67 pg/mL) and testosterone (free testosterone, Follicular, Mean ± SD = 1.29 ± 0.36 pg/mL; Luteal, Mean ± SD = 1.21 ± 0.30 pg/mL; total testosterone, Follicular, Mean ± SD = 0.49 ± 0.14 ng/mL; Luteal, Mean ± SD = 0.46 ± 0.14 ng/mL) levels did not differ between the follicular and luteal phase, although progesterone levels were higher during the luteal phase (Follicular, Mean ± SD = 1.06 ± 0.48 ng/mL; Luteal, Mean ± SD = 10.21 ±7.86 ng/mL; paired samples t-test, t(9) = −3.64, p = 0.005), as would be expected based on normal cyclical hormone profiles (Israel et al., 1972; Schreiner-Engel et al., 1982).
Stimuli evaluations, “How likely would you be to…”
One-Way ANOVA demonstrated that stimuli evaluations of male faces or houses did not differ by menstrual cycle phase (Faces, Mean ± SD = 2.38 ± 0.56; Houses, Mean ± SD = 2.44 ± 0.19). Women’s response times also did not change across the two test sessions (Faces, Mean s ± SD = 1.74 ± 0.37; Houses, Mean s ± SD = 1.86 ± 0.38). Results from correlation analyses between behavioral measures and women’s hormone levels demonstrated a negative association between subjective ratings and progesterone during the follicular phase (R9 = −0.55, p = 0.05). Subjective ratings of male faces during the luteal phase were positively associated with women’s estradiol to progesterone ratios (R9 = 0.54, p = 0.05). Estradiol to progesterone ratios were also positively associated with women’s subjective evaluations of houses during the follicular phase (R9 =0.69, p = 0.01), although there was no relationship between hormones and house evaluations during the luteal phase. There were no significant associations observed between women’s response times during the evaluation of faces or houses and hormone levels at either phase. Together these correlation analyses demonstrate increased positive appraisal of faces and houses when progesterone levels were lower relative to estradiol levels.
Neural activation
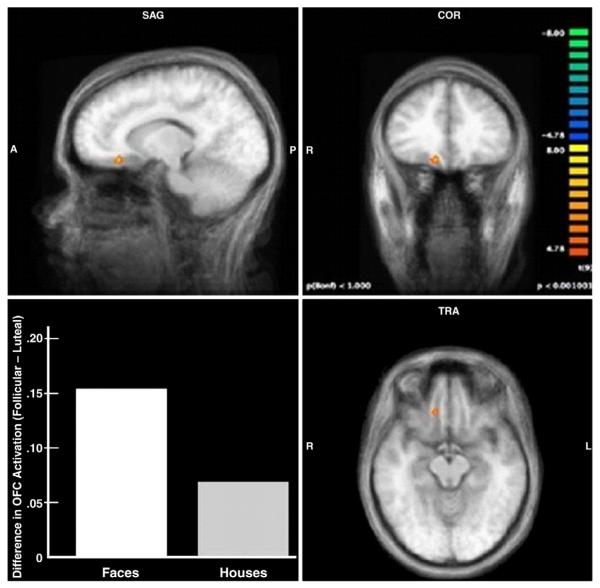
An initial whole-brain group-average SPM (face follicular − faces luteal; p<0.001, cluster threshold correction of 4 voxels) demonstrated increased neural activation in the right medial orbitofrontal cortex during follicular versus luteal testing (OFC, Talairach coordinates 12, 29, × 10; max t-value = 7.88, uncorrected p-value = 0.000025; Fig. 1; Kringelbach and Rolls, 2004; Lacerda et al., 2003).
Fig. 1.
Increased neural activation in response to male faces during the late follicular versus luteal test sessions in the right medial orbitofrontal cortex (12, 29, −10). The bar graph shows the difference in activation in this region in response to faces and houses (follicular minus luteal).
This brain region was treated as a region of interest for subsequent analyses. There were no areas of increased activation in response to male faces observed during the luteal versus follicular phase. Of note, the same pattern of menstrual cycle effect in the OFC was observed when the analysis was conducted within each masculinity condition, and also with high risk partner stimuli, although the pattern was not as strong (p<0.005).
We also looked at differences in neural activation across the two test sessions in response to houses (house follicular − house luteal; p<0.001, cluster threshold correction of 4 voxels). There were no differences in activation across cycle phase using the cluster correction. However, when the cluster correction was removed, we did observe increased activation in the right medial orbitofrontal cortex (Talairach coordinates 12, 32, × 8; max t-value = 6.22, uncorrected p-value = 0.0002) in response to houses during the follicular versus luteal phase.
The outcome of these separate univariate analyses of face and house stimuli suggest that the effect of cycle phase may be stronger with faces than houses, and a direct test of the interaction between stimulus type (houses or faces) and cycle phase ([Face Follicular − Houses Follicular]−[Faces Luteal − Houses Luteal]; p<0.001, uncorrected) supported this claim. The interaction isolated a region at the same coordinates as the contrast on faces alone (Talairach coordinates 12, 29, −8; max t-value = 5.70, uncorrected p-value = 0.0003). (Note: this multivariate analysis accounts for the differences in number of trials for face and house stimuli). The graph in Fig. 1 shows that there were phase effects for both face and house stimuli, but that the effect was larger for faces than houses.
Linear regression analyses demonstrated a significant association of activation in response to faces in the right medial orbitofrontal cortex and women’s estradiol to progesterone ratios (R2 change = 0.23, p = 0.03, partial r = 0.53; Fig. 2). There was no association observed with women’s absolute levels of estradiol, progesterone, free, or total testosterone and activation in the medial OFC in response to houses. There were also no associations between activation in this region and women’s subjective ratings or response times during the evaluation of either male faces or houses.
Fig. 2.
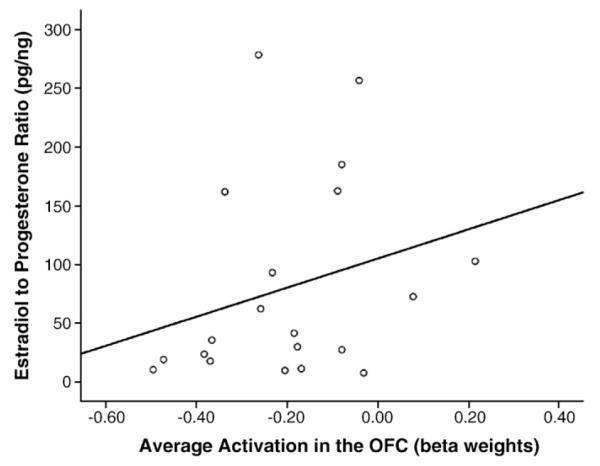
Scatterplot displaying a significant relationship between women’s estradiol to progesterone ratios ([pg/mL]/[ng/mL])and their observed BOLD response (beta weights) to male faces across both sessions.
Discussion
Women’s brains responded differently to male faces at different phases of their menstrual cycle. Women demonstrated cyclic differences in neural activation in response to male faces in the right medial orbitofrontal cortex, a brain region related to reinforcement-guided decision making and reward and risk evaluation (reviewed in Balleine, 2007; Cohen et al., 2005; Doya, 2008; Kringelbach and Rolls, 2004; Rushworth et al., 2007), including financial decision making (Dreher et al., 2007), and attractiveness evaluations of faces (O’Doherty et al., 2003; Winston et al., 2007). We observed this region to be more responsive to male faces during the late follicular compared to the luteal phase, possibly suggesting increased positive appraisal at this time. Neural activation in this region was positively associated with women’s estradiol to progesterone ratios, consistent with the observed hormonal relationships with their subjective ratings. Together, these results suggest that activation in the medial orbitofrontal cortex is related to a woman’s assessment of a man as a possible sexual partner, is influenced by hormonal status, and may contribute to changes in women’s sexual behavior and interest across the menstrual cycle.
The phase-dependent activation observed in the current study suggests that women’s decision making and weighing of potential reinforcers varies across the menstrual cycle to possibly bias evaluations towards a positive appraisal during the late follicular phase. Subjective ratings of both faces and houses were higher when either progesterone, or levels of progesterone in relation to levels of estradiol were lower, which is the characteristic hormone profile of the follicular phase. Because activation in the medial orbitofrontal cortex was also related to the estradiol to progesterone ratio, we may speculate that the hormonal state characteristic of the periovulatory phase biases neural systems towards a more positive appraisal of appetitive stimuli. This interpretation is consistent with data from EEG and EMG studies suggesting a general increase in the positive appraisal of sexually relevant stimuli closer to ovulation (Krug et al., 2000; Mass et al., 2008).
We also observed a difference in activation in response to houses across the menstrual cycle in the right medial orbitofrontal cortex. However, the observed interaction of phase and stimulus type demonstrated that the observed phase effect was stronger in response to faces versus houses. Even so, that we observed differences in response to both faces and houses across the cycle is notable and consistent with previous work showing that women’s brains respond differently to both social and nonsocial stimuli depending on where they are in their menstrual cycle (Dreher et al., 2007; Goldstein et al., 2007; Rupp et al., 2009b). These data may be interpreted in the context of a recent study that found changes in EEG asymmetry across the menstrual cycle suggesting increased behavioral activation around ovulation, possibly increasing appetitive behaviors in general at this (Hwang et al., 2008). Therefore, we speculate that some of the observed differences in response to male faces between cycle phases in the current study are the consequence of more global fluctuations in women’s general appetitive motivation, biasing the system towards positive appraisal during the follicular phase, and that these cyclic changes may generalize across stimulus domains.
Contrary to our hypotheses, we did not observe changes in subcortical limbic regions, including the amygdala and nucleus accumbens, in response to male faces across the cycle. Previous fMRI work using financial rewards (Dreher et al., 2007) and sexually explicit stimuli (Gizewski et al., 2006) did find changes in amygdala activity across the cycle. We did observe widespread limbic activation in response to faces compared to houses overall, suggesting that the male face stimuli were more arousing and possibly rewarding than house stimuli. However activation of the limbic system did not changes across the menstrual cycle. This may, be due in part to the relatively unarousing nature of the male face stimuli used in the current study, compared to more arousing sexually explicit stimuli commonly used in studies in which menstrual cycle changes are observed in the striatum and amygdala (Gizewski et al., 2006). This may also be due, in part, to the fact that the majority of women entered the study in the luteal phase of their cycle. Habituation effects from the first to second session may have interacted with cycle effects to mask the expected increased follicular activation of the limbic system. Future studies examining changes in neural activation should strive for a more equal distribution of the initial test phases to control for habituation effects.
In sum, this study demonstrates differences in neural responses to potential sexual partners in women tested at two time points across their menstrual cycles. The brain region in which changes were observed, the medial orbitofrontal cortex, is a region associated with reward and risk processing and cyclic changes in activation may reflect hormone-mediated changes in appetitive motivation and positive appraisal. Such phase-dependent fluctuations in neural activation during the evaluation of men as sexual partners could contribute to previously reported menstrual cycle effects on women’s arousal and proceptivity and may influence women’s subsequent sexual behavior. Furthermore, because activation patterns found here in response to male faces and houses are consistent with those previously reported for monetary reward, fluctuations in appetitive motivation across the menstrual cycle may generalize beyond sexual stimuli to influence women’s appraisal of stimuli in general.
Acknowledgments
We would like to thank Dr. Ronald McClintock and the GCRC for hormone assays (GCRC Grant: M01 RR00750). We gratefully acknowledge Sunah Kim and Ryan Stevenson for programming, analysis, and technical help. This work was supported by the NIH funded Common Themes in Reproductive Diversity training grant NICHHD-T32-HD-49339-0.
Appendix A
Please make your decision regarding the likelihood of your having sex with the man portrayed in the image based on how attractive you find him and any supplementary information you are provided.
Please imagine yourself in the situation described below and make your decision as if you were in that situation.
You are not in a committed relationship and are open to a sexual encounter. You and some friends are out Friday night. While out, you meet the man presented in the image for the first time. You two have a good time talking together and that continues into the evening. You and he end up back at his place to continue hanging out. It is clear to you that he would have sex with you if you want to.
Imagine that you are in this scenario and open to a sexual encounter. Based on the image and information presented, please indicate using the button box:
How likely would you be to have sex with him?
1 = Very Unlikely
2 = Unlikely
3 = Likely
4 = Very Likely
Appendix B
Please make your decision regarding the likelihood of renting the house portrayed in the image based on how attractive you find it and any supplementary information you are provided.
Please imagine yourself in the situation described below and make your decision as if you were in that situation.
You are not in a long term lease and open to moving. You and some friends are out Saturday afternoon. You walk by the house presented in the image for the first time while you are out that afternoon. You stop and look at the house from the outside. You end up reading the brochure for more information. It is clear to you that the house is currently vacant and available.
Imagine that you are in this scenario are open to moving. Based on the image and information presented, please indicate using the button box:
How likely would you be to rent this house?
1 = Very Unlikely
2 = Unlikely
3 = Likely
4 = Very Likely
References
- Archer JS, Love-Geffen TE, Herbst KL, Swinney DA, Chang JR. Effect of estradiol versus estradiol and testosterone on brain-activation patterns in postmenopausal women. Menopause. 2006;13:528–537. doi: 10.1097/01.gme.0000188737.46746.cd. [DOI] [PubMed] [Google Scholar]
- Balleine BW. The neural basis of choice and decision making. J. Neurosci. 2007;27:8159–8160. [Google Scholar]
- Bullivant SB, Sellergren SA, Stern K, Spencer NA, Jacob S, Menella JA, McClintock MK. Women’s sexual experience during the menstrual cycle: identification of the sexual phase by noninvasive measurement of luteinizing hormone. J. Sex Res. 2004;41:82–93. doi: 10.1080/00224490409552216. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn. Brain Res. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat. Neurosci. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Dreher J, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Res. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R. Human oestrus. Proc. R. Soc. 2008;275:991–1000. doi: 10.1098/rspb.2007.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizewski ER, Krause E, Karama S, Baars A, Senf W, Forsting M. There are differences in cerebral activation between females in distinct menstrual phases during viewing or erotic stimuli: a fMRI study. Exp. Brain Res. 2006;174:101–108. doi: 10.1007/s00221-006-0429-3. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, McClure SM, van den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cogn. Affect. Behav. Neurosci. 2007;7:309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Harvey SM. Female sexual behavior: fluctuations during the menstrual cycle. J. Psychosom. Res. 1987;31:101–110. doi: 10.1016/0022-3999(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc. R. Soc. Biol. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang R, Chen L, Yeh T, Tu P, Tu C, Hsieh J. The resting frontal alpha asymmetry across the menstrual cycle: a magnetoencephalographic study. Horm. Behav. 2008;54:28–33. doi: 10.1016/j.yhbeh.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. Am. J. Obstet. Gynecol. 1972;112:1043–1046. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr. Biol. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Krug R, Plihal W, Fehm HL, Born J. Selective influence of the menstrual cycle on perception of stimuli with reproductive significance: an event related potential study. Psychophysiology. 2000;37:111–122. [PubMed] [Google Scholar]
- Lacerda ALT, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: a validation study of a new method. NeuroImage. 2003;19:665–673. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Lee D. Game theory and neural basis of social decision making. Nat. Neurosci. 2008;11:404–409. doi: 10.1038/nn2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Jackson LW, Lewis G.M. Buck. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr. Perinat. Epidemiol. 2006;20:3–12. doi: 10.1111/j.1365-3016.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- Mass R, Holldorfer M, Moll B, Bauer R, Wolf K. Why we haven’t died out yet: changes in women’s mimic reactions to visual erotic stimuli during their menstrual cycles. Horm. Behav. 2008;55(2):265–266. doi: 10.1016/j.yhbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Chandra A, Jones J. Advance Data from Vital and Health Statistics no. 362. National Center for Health Statistics; Hyattsville, MD: 2005. Sexual Behavior and Selected Health Measures: Men and Women 15–44 Years of Age, United States, 2002. [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Pfaus JG. What’s behind her smile? Horm. Behav. 2008;55(2):267–271. doi: 10.1016/j.yhbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila GA. What can animal models tell us about human sexual response? Annu. Rev. Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Puts DA. Cyclic variation in women’s preferences for masculine traits. Potential hormonal causes. Hum. Nat. 2006;17:114–127. doi: 10.1007/s12110-006-1023-x. [DOI] [PubMed] [Google Scholar]
- Rowland DA, Perrett DI. Manipulating facial appearance through shape and color. IEEE Comput. Graph. App. 1995;15:70–76. [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. The role of the anterior cingulate in women’s sexual decision making. Neurosci. Lett. 2009a;449:42–47. doi: 10.1016/j.neulet.2008.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Women’s neural activation in response to masculinized versus feminized male faces; mediation by hormones and psychosexual factors. Evol. Hum. Behav. 2009b;30:1–10. doi: 10.1016/j.evolhumbehav.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for the cingulate cortex in decisions and social behavior. Trends Cogn. Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schreiner-Engel P, Schiavi RC, Smith H, White D. Sexual arousability and the menstrual cycle. Psychosom. Med. 1982;43:199–214. doi: 10.1097/00006842-198106000-00002. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V. Do women have a hidden heat period? Hum. Reprod. 2002;17:2243–2248. doi: 10.1093/humrep/17.9.2243. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline J. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–215. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Hum. Reprod. 2004;19:1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]