Abstract
Maternal licking of rat pups affects the development of the spinal nucleus of the bulbocavernosus (SNB), a sexually dimorphic motor nucleus that controls penile reflexes involved with copulation. Maternal licking influences SNB motoneurons, with reductions in licking producing decreased SNB number, size, and dendritic length in adulthood. Reduced maternal licking also produces deficits in adult male copulatory behavior. In this experiment, we used an artificial rearing paradigm to assess the potential role of tactile stimulation in mediating the effects of maternal licking on the SNB neuromuscular system. During artificial rearing, pups were stroked with a paintbrush to mimic maternal licking, receiving low, medium, or high levels of daily stimulation. In adulthood, ex copula penile reflex behavior was tested and the morphology of SNB motoneurons assessed. SNB motoneurons were retrogradely labeled with cholera toxin-conjugated HRP and dendritic arbor was reconstructed in three dimensions. Animals that received low levels of stimulation showed deficits in penile reflexes relative to maternally reared controls, including a longer latency to erection, fewer cup erections, and fewer erection clusters. SNB dendritic morphology was also shaped by stimulation condition, with animals that received low or medium levels of stimulation showing an average 27% reduction in dendritic length. In addition, several reflex behaviors were significantly correlated with dendritic length, including latency to first erection, percent of cup erections, and number of erection clusters. These results suggest that tactile stimulation provided by maternal licking mediates some of the effects of maternal care on the development of male copulatory behavior.
Keywords: artificial rearing, maternal care, motoneuron, development, dendrites
INTRODUCTION
Early social contact between mother and offspring shapes the neural and behavioral development of off-spring (Hofer, 1978). In rats, the maternal care repertoire consists of nursing, licking, grooming, incubating, and retrieving of pups (Stern, 1996). Natural variations in these maternal behaviors from dam to dam produce offspring that differ on many neural and behavioral dimensions. Pups that experience higher levels of maternal licking, grooming, and arched-back nursing, for example, show a greater neuron density in the hippocampus (Bredy et al., 2003), greater levels of neurotrophin expression throughout the brain (Liu et al., 2000), altered dopamine expression in the prefrontal cortex (Zhang et al., 2005), altered GABA receptor subunit expression in the amygdala (Caldji et al., 1998, 2000), altered hypothalamic-pituitaryadrenal axis development (Liu et al., 1997), altered oxytocin receptor expression (Francis et al., 2000), and consequent changes in the many behaviors mediated by these structures. These effects even result when pups are cross-fostered from low maternal care dams to high maternal care dams, indicating the epigenetic nature of the phenomenon (Francis et al., 1999).
In addition to these studies using natural variations in maternal behavior, maternal licking can also be experimentally reduced by interfering with the dam’s ability to detect olfactory cues in pup urine that normally drive the licking behavior (Moore, 1981, 1985; Moore et al., 1992). Such experimental reductions in maternal licking produce behavioral deficits in adult male sexual behavior. These deficits include increased latency to ejaculation, increased latency to postejaculatory intromission, and increased interintromission intervals (Moore, 1984). Reductions in licking influence neural development as well. One of many neural structures that controls male copulatory behavior is the spinal nucleus of the bulbocavernosus (SNB). The SNB (also known as the dorsomedial nucleus, or DM; Schroder, 1980) is a sexually dimorphic population of motoneurons in the lumbar spinal cord, which innervates the anal sphincter of both males and females and additionally in males, the bulbocavernosus (BC) and levator ani (LA) muscles of the perineum (Breedlove and Arnold, 1980; Schroder, 1980; McKenna and Nadelhaft, 1986). The BC and LA muscles encircle the base of the penis and their fast, robust contractions produce an intense penile erection with flaring of the glans. This “cupping” allows seminal plug formation and removal, essential components of successful copulation and insemination (Sachs, 1982; Hart and Melese-D’Hospital, 1983). The development of SNB motoneurons extends well into the early postnatal period (Nordeen et al., 1985; Goldstein et al., 1990) and, interestingly, is sensitive to maternal care. Specifically, reductions in maternal anogenital licking produce decreased motoneuron number, motoneuron size, and dendritic length in the SNB (Moore et al., 1992; Lenz and Sengelaub, 2006). Thus, changes in adult copulatory behavior that result from decreased maternal licking may in part reflect underlying changes in the development of SNB motoneurons.
Previous work has shown that the tactile component of maternal licking behavior, when simulated experimentally, can offset the negative behavioral and physiological effects of maternal deprivation (Suchecki et al., 1993; Lévy et al., 2003; Chatterjee et al., 2007). Similarly, supplementing the amount of tactile stimulation that pups receive neonatally alters their HPA axis phenotype to match that of pups that naturally receive higher levels of maternal care (Jutapakdeegul et al., 2003). Moreover, tactile stimulation alters the expression of many proteins throughout the brain which are known to regulate structural plasticity (Chatterjee et al., 2007). These examples suggest that the tactile stimulation component of maternal care plays a crucial role in the neural development of offspring. Given that maternal licking influences the development of the SNB, it is possible that differences in the amount of tactile stimulation provided by maternal licking may differentially influence its development.
In this experiment, we utilized an artificial rearing paradigm to isolate the tactile stimulation component of maternal licking behavior. Artificial rearing has previously been used in this way to study the influence of tactile stimulation on neural and behavioral development (Gonzalez et al., 2001; Gonzalez and Fleming, 2002; Lévy et al., 2003; Lovic et al., 2006; Burton et al., 2007; Chatterjee et al., 2007). In the artificial rearing paradigm, pups are reared in isolation from the mother and littermates, with experimenters providing temperature controlled housing conditions, food, and licking-like stimulation until weaning (e.g., Gonzalez et al., 2001). Therefore, the paradigm allows for the amount of licking-like stimulation provided to individual pups to be reliably varied during the neonatal period. The purpose of this study was to investigate the influence of neonatal tactile stimulation on the morphological development of the SNB neuromuscular system and resultant penile reflex behavior.
METHODS
Artificial Rearing
Subjects were obtained from litters of primiparous Sprague Dawley dams, which were culled to seven males and three females on the day of birth. On the second postnatal day, four male pups were removed for artificial rearing and assigned to one of several stimulation groups. The remaining pups were either sham operated for cheek cannulation (sham group) or removed from the cage and handled similarly to sham-operated pups, but not otherwise manipulated (unmanipulated group). These two groups (sham and unmanipulated) were then returned to the dam following handling on the second postnatal day and otherwise unmanipulated until weaning at the 22nd postnatal day. After weaning, all pups were pair housed with nonexperimental agematched male controls in a standard cage (W 27 cm × L 47 cm × H 15 cm) with shaved woodchip bedding and a metal grid roof allowing ad libitum access to Purina rat chow and water. Light was provided in a 12:12 dark/light cycle, starting at 08:00 h. Room temperature was set at approximately 25°C, with humidity set at approximately 40–50%. All procedures involving animals were approved by the University of Toronto Animal Care Committee.
Artificial rearing was carried out essentially as previously described (Gonzalez et al., 2001), with minor modifications described later to the cannulation procedure and the type of stimulation received by pups. Briefly, rats were weighed, implanted with a cheek cannula, and assigned to one of several stimulation groups described later. Cheek cannulation was used rather than gastric cannulation as this procedure is equally efficacious in supplying adequate nutrition, but has a lower rate of mortality. Cheek cannulae were implanted following topical Lidocaine application, by puncturing the cheek with a stainless steel leader wire (0.25 mm in diameter, VWR), which served as a guide for polyethylene tubing (PE 10), flared at one end to anchor the cannula within the cheek. A polyethylene flat washer and a T-washer (made from flared PE 50 tubing) were attached to the outside of the cheek cannula and bonded in place with superglue. Sham-operated pups had a cheek puncture as described but a cannula was not attached. After surgery, Polysporin triple antibiotic cream (Pfizer) was applied to the cheeks of all pups.
Artificially reared pups received daily stimulation to both the anogenital skin and the dorsal skin to best simulate natural maternal licking behavior. Dorsal stimulation was directed along the spine, from the base of the head to the base of the tail. Stimulation was administered in bouts of 30 s using a moistened paintbrush and spaced regularly between the hours of 8:00 and 22:00. The number of stimulation bouts each animal received was varied, with each animal receiving either low stimulation (0–2 stimulation bouts daily, n = 7), medium stimulation (4–8 stimulation bouts daily, n = 8), and high stimulation (10–16 stimulation bouts daily, n = 10). All pups received a consistent number of daily stimulation bouts throughout the period of artificial rearing. To increase statistical power, sham operated and unmanipulated rats were collapsed to form a single maternally reared control group (n = 17).
Artificial milk (Messer Diet, University of Iowa) was then delivered through the cannulae of artificially reared pups as previously described (Gonzalez et al., 2001). Pups were fed milk until weaning, which occurred between postnatal day 17 and 19 depending on body weight (pups weighing at least 35 g were weaned). At weaning, the cheek cannulae of artificially reared pups were removed. After weaning, pups were housed in small cages (W 15 cm × L 22 cm × H 10 cm) until postnatal day 22 and fed a mixture of powdered rat chow and formula, which increased in solidity each day. At postnatal day 22, artificially reared rats were housed with a nonexperimental, age-matched male social control.
Ex Copula Penile Reflexes
Penile reflexes were evaluated essentially as described by Leipheimer and Sachs (1988). At approximately 90 days of age and before testing, the suspensory ligament of the penis was excised under isoflourane anesthesia to facilitate visualization of the glans. Subjects were allowed to recover for 6 days prior to testing. On the 6th day, habituation to the testing apparatus began, and continued for three consecutive days. The test apparatus consisted of a rectangular aluminum tube (25.4 cm long × 7.6 cm wide × 5.1 cm high), tapered at one end and secured to a platform. Each male was habituated for 10 min to supine restraint in the tube with its body posterior to the hips exposed and its hips, legs, and tail secured to the platform with masking tape. On the day following the last habituation, subjects were restrained as described earlier and the preputial sheath was retracted to expose the glans. Maintained retraction of the preputial sheath produces spontaneous erections (Hart, 1968; Sachs and Garinello, 1978), likely due to tonic pressure of prepuce retraction on the base of the glans (Lumia et al., 1979). Therefore, following preputial sheath retraction, spontaneous erections began and the visible penis was videotaped for 30 min to document reflex behavior.
Videotape was subsequently scored for glans and penile body erections. Glans erections were scored on a three point scale with the following criteria, reflecting a progressive intensity: E1 = engorgement of glans without glans distension; E2 = distension of glans with some widening of the tip; and E3 = a “cup” erection in which the width of the tip of the glans exceeded that of the base, resulting in a cuplike morphology. Additionally, penile body erections or “flips” were scored when the penis extended ventrally, with a designation being made between flips that resulted in an angle between glans and the ventrum which was acute to perpendicular (short flip) and those where the angle of the glans relative the ventrum exceeded perpendicular (long flip). During ex copula reflex testing, penile reflexes tend to occur in clusters of 1–5 erections, cups, and flips, with longer intervals, often several minutes, separating reflex clusters (Hart, 1968; Sachs and Garinello, 1978). We therefore measured the number of reflex clusters, which were defined by grouping all successive erections in which an interval of no more than 45 s separated individual erections. Intervals greater than 45 s separating individual erections were averaged to obtain the mean intercluster interval (ICI). Additionally, both the total number of clusters and the number of erections per cluster were tabulated. The latency to the first erection was also measured.
Histochemistry
Horseradish peroxidase conjugated to the cholera toxin β subunit (BHRP; List Biological Laboratories) was used to retrogradely label SNB motoneurons innervating the BC muscle. BHRP labeling permits population-level quantitative analysis of SNB motoneuron somal and dendritic morphologies (Kurz et al., 1986; Goldstein et al., 1990; Lenz and Sengelaub, 2006). Following reflex testing, at approximately 230 days of age (range 201–256), animals were anesthetized with isoflourane, and the left BC muscle was exposed and injected with 0.5 μL of a 0.2% solution of BHRP. Forty-eight hours after BHRP injection, a period that ensures optimal labeling of SNB motoneurons (Kurz et al., 1986; Goldstein et al., 1990), animals were weighed, overdosed with sodium pentobarbital, and perfused intracardially with saline followed by cold fixative (1% paraformaldehyde/1.25% glutaraldehyde). The entire BC/LA muscle complex was removed from each animal and weighed, as were the seminal vesicles. The lumbar portion of the spinal cord was removed, postfixed in the same fixative for 5 h, and then transferred to sucrose phosphate buffer (10% w/v, pH 7.4) overnight for cryoprotection. Spinal cords were then embedded in gelatin and frozen-sectioned transversely at 40 μm, and sections were collected into four alternate series. For visualization of BHRP, the tissue was immediately reacted using a modified tetramethyl benzidine protocol (Mesulam, 1982), mounted on gelatin-coated slides, counterstained with thionin, and cover-slipped with Permount.
Motoneuron Somata
The number of BHRP-filled motoneurons was assessed in all sections through the entire rostrocaudal extent of the SNB for all animals. Counts of BHRP-labeled motoneurons in the SNB were made under brightfield illumination, where somata and nuclei could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed. Estimates of the total number of labeled SNB motoneurons were obtained using the optical dissector method outlined by Coggeshall (1992) and a procedure similar to that of West and Gundersen (1990). This method yields an unbiased count of SNB motoneurons (Raouf et al., 2000). Counts were made at 500× under brightfield illumination, and motoneuron somata could be easily visualized in multiple focal planes. BHRP-labeled motoneurons were counted as their somata first appeared in focus while focusing through the z axis. Somata in the first focal plane (i.e., tops) were not counted. Within each section, all labeled somata within the SNB were counted. For each animal, counts were derived from sections spaced at 160 μm intervals throughout the rostrocaudal extent of the SNB and corrected for the proportion of sections sampled.
The area of labeled SNB motoneuron somata was assessed in at least one set of alternate sections (160 μm apart) by measuring the cross-sectional area of BHRP-filled motoneurons. Soma areas of an average of 22.46 motoneurons were measured for each animal using a video-based morphometry system (Stereo Investigator; MicroBrightField, Williston, VT) at a final magnification of 1350×. Soma areas within each animal were then averaged for statistical analysis.
Dendritic Length
For each animal, dendritic lengths in a single representative set of alternate sections were measured under dark field illumination. Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the SNB dendritic field was assessed in every other section (320 μm apart) in three dimensions using a computer-based morphometry system (Neurolucida; MicroBrightField) at a final magnification of 250×. Average dendritic length per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by two to correct for sampling, then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons (Kurz et al., 1991), but has been shown to be a sensitive and reliable indicator of changes in dendritic morphology in normal development (Goldstein et al., 1990, 1993; Goldstein and Sengelaub, 1993), in response to hormonal manipulation (Kurz et al., 1986; Forger and Breedlove, 1987; Goldstein et al., 1990; Kurz et al., 1991; Goldstein and Sengelaub, 1992, 1994; Burke et al., 1997, 1999; Hebbeler and Sengelaub, 2003), after changes in dendritic interactions (Goldstein et al., 1993), after alterations in afferent input (Kalb, 1994; Hebbeler et al., 2002; Hebbeler and Sengelaub, 2003), and after maternal care manipulations (Lenz and Sengelaub, 2006).
Past work has shown that maternal licking manipulations alter SNB morphology in a regionally specific manner (Moore et al., 1992; Lenz and Sengelaub, 2006), therefore dendritic length was computed for the rostral and caudal halves of the nucleus. For each animal, the sections that contained BHRP-labeled processes were divided into rostral and caudal portions. Average dendritic lengths per labeled motoneuron for each half of the nucleus were obtained using the same method outlined earlier. If an animal had an odd number of sections in which BHRP label was present, the summed length of dendrites in the middle section was divided in half and added to the dendritic length computed for the rostral and caudal halves.
Dendritic Distribution
To assess potential redistributions of dendrites across treatment groups, the composite dendritic arbor created in the length analysis for each animal was divided using a set of axes oriented radially around the central canal. These axes divided the spinal cord into 12 bins of 30° each. The portion of each animal’s dendritic arbor per labeled motoneuron contained within each location was then determined. This method provides a sensitive measure of dendritic redistribution in response to changes in dendritic interactions (Goldstein et al., 1993) and afferent input (Hebbeler et al., 2002; Hebbeler and Sengelaub, 2003).
Dendritic Extent
The comparability of BHRP labeling across groups was assessed by quantifying both the rostrocaudal and the radial dendritic extent of SNB arbors. The rostrocaudal extent of the dendritic arbor was determined by recording the rostrocaudal distance spanned by SNB dendrites for each animal. In the mediolateral plane, for each animal the maximal radial extent of the dendritic arbor was measured using the same radial axes and resultant 30° bins used for the dendritic distribution analysis. For each bin, the linear distance between the central canal and the most distal BHRP-filled process was measured.
Statistical analysis consisted of analyses of variance (one-way between subjects or one-way with repeated measures) followed by appropriate planned comparisons (Fisher’s LSD). Digital light photomicrographs were obtained by using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop.
RESULTS
Ex Copula Penile Reflexes
Several aspects of penile reflex behavior were influenced by tactile stimulation condition. Latency to first erection showed a significant effect of tactile stimulation condition [F(3, 32) = 3.99, p < 0.05; Fig. 1]. Post-hoc comparisons showed low stimulation animals to have significantly longer latencies to erection than both maternally reared controls and medium stimulation animals, averaging 147% longer [maternally reared = 571.93 ± 161.98 s (M ± SEM); low stimulation = 1208.16 ± 124.88 s; medium stimulation = 427.76 ± 81.87 s; LSD, ps < 0.05].
Figure 1.
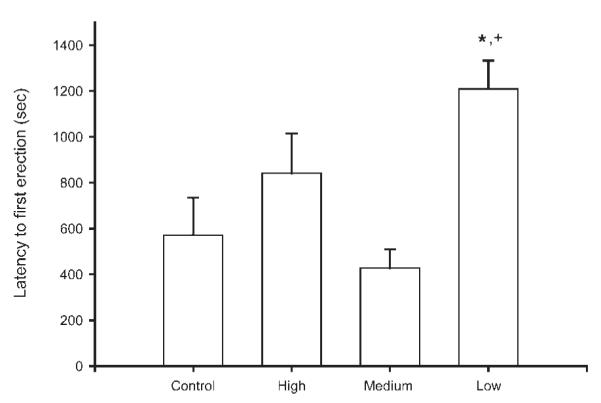
Latency to first erection (in seconds) of maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation. Low tactile stimulation animals had significantly longer latencies to erection than either maternally reared controls or medium stimulation animals. Medium and high stimulation animals did not differ from controls. Bar heights represent means ± SEM. *Indicates a significant difference from control animals; +Indicates a significant difference from medium stimulation animals, p < 0.05.
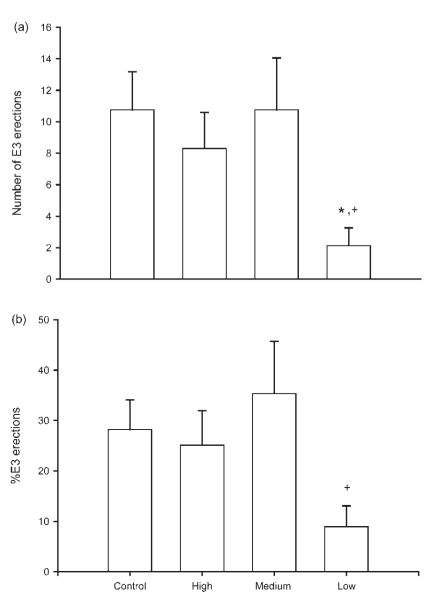
Both the number and percentage of E3 erections were affected by tactile stimulation condition. The number of E3 erections showed a marginal overall effect of tactile stimulation [F(3, 33) = 2.21, p = 0.10; Fig. 2(a)]. Low stimulation animals had 80% fewer E3 erections than either maternally reared controls or medium stimulation animals [maternally reared = 10.75 ± 2.43 erections; low stimulation = 2.14 ± 1.12 erections; medium stimulation = 10.75 ± 3.30 erections; LSD, ps < 0.05]. With regard to percentage of E3s, while there were no overall effects of stimulation condition, planned contrasts showed that low stimulation animals had a smaller percentage of E3s than medium stimulation animals [maternally reared = (28.21 ± 5.87)%; low stimulation = (8.95 ± 4.10)%; = medium stimulation = (35.39 ± 10.31)%; LSD, p < 0.05; Fig. 2(b)]. In contrast to these effects on E3s, neither the number nor percentage of E1 erections or E2 erections were influenced by tactile stimulation condition [all Fs < 1.56, ns].
Figure 2.
(a) The number of erections with flared cups (E3s) displayed by maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation during ex copula reflex testing. Low tactile stimulation animals had significantly fewer E3s than either maternally reared controls or medium stimulation animals. Medium and high stimulation animals did not differ from controls. (b) The percentage of total erections that were flared cups (E3s). Low tactile stimulation animals had a significantly smaller percentage of E3s than medium stimulation animals. Bar heights represent means ± SEM. *Indicates a significant difference from control animals; +Indicates a significant difference from medium stimulation animals, p < 0.05.
With regard to flip erections, the number of short flips showed a marginal effect of tactile stimulation [F(3, 33) = 2.36, p = 0.09], with low stimulation animals showing 95% fewer short flips than medium stimulation animals [low stimulation = 0.14 ± 0.14 flips; medium stimulation = 3.13 ± 1.47 flips; LSD, p < 0.05]. Neither the number of long flips nor the total number of flips was influenced by tactile stimulation condition [Fs < 2.25, ns].
With regard to reflex clusters, while there was no overall effect of stimulation on the number of clusters [F(3, 33) = 1.91, ns], planned comparisons showed that low stimulation animals had significantly fewer reflex clusters than both maternally reared control animals and medium stimulation animals, averaging 52% fewer clusters [maternally reared = 8.83 ± 1.58 clusters; low stimulation = 4.29 ± 1.11 clusters; medium stimulation = 9.13 ± 1.57 clusters; LSD, p < 0.05; Fig. 3]. Other measures of reflex clusters were not affected by tactile stimulation condition, including average number of erections per cluster, intercluster interval, and cluster duration [all Fs < 0.84, ns].
Figure 3.
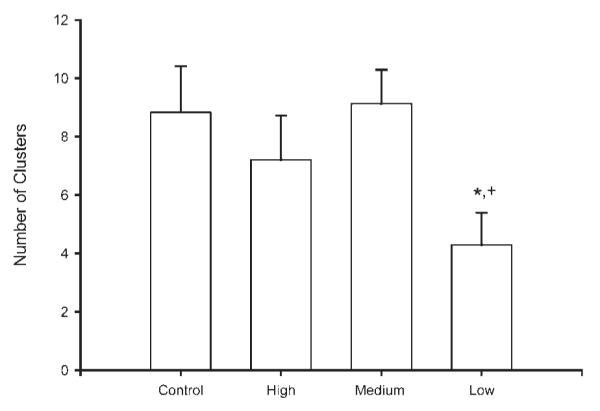
Number of erection clusters displayed by maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation. Low tactile stimulation animals had significantly fewer erection clusters than either maternally reared controls or medium stimulation animals. Medium and high stimulation animals did not differ from controls. Bar heights represent means ± SEM. *Indicates a significant difference from control animals; +Indicates a significant difference from medium stimulation animals, p < 0.05.
Gross Morphology
Body weights and BC/LA muscle weights were collected for a total of 48 animals (maternally reared: n = 17; low stimulation: n = 9; medium stimulation: n = 11; high stimulation: n = 11). The adult body weight of animals did not differ between stimulation groups [maternally reared = 712.77 ± 21.49 g; low stimulation = 776.44 ± 43.06 g; medium stimulation = 752.33 ± 40.78 g; high stimulation = 682.09 ± 24.85 g; F(3, 44) = 1.52, ns]. However, the adult BC/LA muscle weight of animals was affected by stimulation condition [F(3, 44) = 2.68, p < 0.05; Fig. 4]. Overall, BC/LA muscle weights of artificially reared animals were 2.5% lighter than controls, but the muscle weights of low stimulation animals were 14.7% lighter than controls. Post-hoc comparisons showed that low stimulation animals were significantly lighter BC/LA muscles than maternally reared controls [LSD, p < 0.05], whereas medium and high stimulation animals did not significantly differ from controls [maternally reared = 1.96 ± 0.01 g; low stimulation = 1.67 ± 0.11 g; =medium stimulation = 1.78 ± 0.08 g; high stimulation 1.82 ± 0.09 g]. With regard to seminal vesicle weights, there was an overall effect of stimulation group, [F(3, 45) = 3.66, p < 0.05]. Post-hoc tests showed high stimulation animals to have significantly lighter seminal vesicles than control animals [LSD, p < 0.05], whereas low and medium stimulation animals did not differ from controls [maternally reared = 2.14 ± 0.11 g; low stimulation = 2.41 ± 0.21 g; medium stimulation = 2.41 ± 0.21 g; high stimulation = 1.75 ± 0.09 g].
Figure 4.
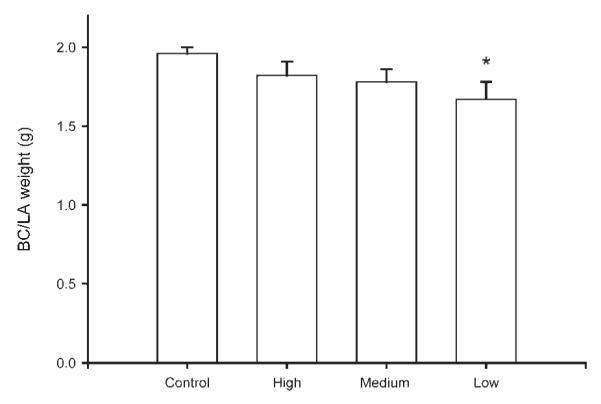
The adult BC/LA muscle weights of maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation. Low tactile stimulation animals had significantly lighter BC/LA muscles relative to maternally reared controls. Medium and high stimulation animals did not differ from controls. Bar heights represent means ± SEM. *Indicates a significant difference from control animals, p < 0.05.
Morphometry
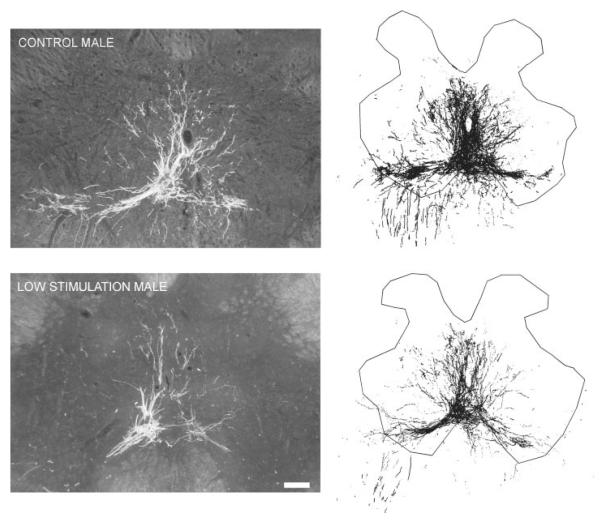
Injection of BHRP into the left BC successfully labeled ipsilateral SNB motoneurons of all animals in a manner consistent with previous studies (Goldstein et al., 1990; Goldstein and Sengelaub, 1994; Burke et al., 1997, 1999). SNB motoneurons displayed their characteristic multipolar morphologies, with dendritic arbors projecting ventrolaterally, dorsomedially, and across the midline into the area of the contralateral SNB [Fig. 5]. The number of BHRP-labeled SNB motoneurons (62.58 ± 2.74 cells per animal) did not differ between groups [F(3, 27) = 0.99, ns].
Figure 5.
Left: Dark field photomicrographs of transverse sections through the lumbar spinal cords of a maternally reared control male (top) and an artificially reared male that received low levels of tactile stimulation (bottom). Right: Computer-generated composites of BHRP-labeled somata and processes drawn at 320 μm intervals through the rostrocaudal extent of the SNB. Composites were selected as they are representative of their respective group average dendritic lengths. Scale bar = 500 μm.
Soma Area
Soma areas were collected for a total of 36 animals (maternally reared: n = 12; low stimulation: n = 8 medium stimulation: n = 8; high stimulation: n = 8). Tactile stimulation during artificial rearing produced a marginally significant difference in SNB soma area [F(3, 32) = 2.67, p = 0.06; Fig. 6]. While mean soma size was 13% smaller over all artificially reared groups relative to maternally reared controls, it was only significantly so in medium stimulation animals [maternally reared = 1017.95 ± 26.64 sq μm; low stimulation = 902.58 ± 58.93 sq μm; medium stimulation = 852.83 ± 47.58 sq μm; high stimulation = 906.70 ± 57.75 sq μm; LSD, p < 0.05].
Figure 6.
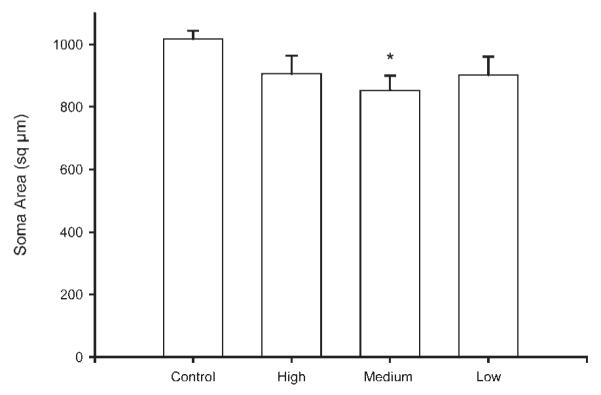
The adult SNB motoneuron soma sizes of maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation. Medium tactile stimulation animals had significantly smaller soma sizes relative to maternally reared controls. Bar heights represent means ± SEM. *Indicates a significant difference from control animals, p < 0.05.
Dendritic Length
Dendritic lengths were computed for a total of 31 animals (maternally reared: n = 11; low stimulation: n = 5; medium stimulation: n = 7; high stimulation: n = 8). Tactile stimulation influenced the overall dendritic arbor per labeled SNB motoneuron [F(3, 27) = 3.02, p < 0.05]. Both low stimulation animals and medium stimulation animals had significantly shorter dendritic lengths relative to maternally reared controls, producing an average 27% decrease in dendritic length [maternally reared = 11069.87 ± 921.40 μm; low stimulation = 8100.618 ± 457.83 μm; medium stimulation = 7907.60 ± 1076.54 μm, LSD ps < 0.05; Fig. 7]. In contrast, the dendritic length of high stimulation animals did not significantly differ from maternally reared controls [high stimulation = 9976.68 ± 826.33 μm]. The observed reductions in dendritic length were not regionally specific, with no significant effects of tactile stimulation condition in either the rostral or caudal portions of the nucleus when analyzed alone [Fs < 1.92, ns].
Figure 7.
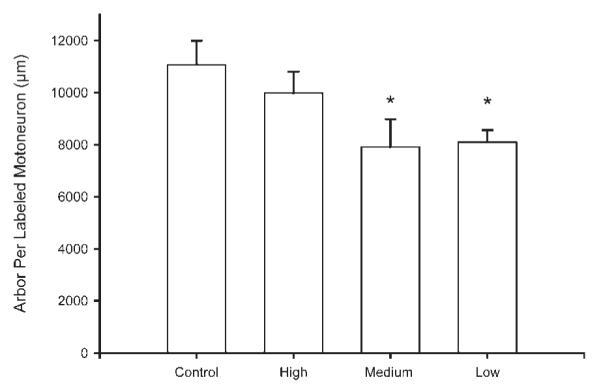
The dendritic length per labeled SNB motoneuron of maternally reared controls and artificially reared animals receiving differing amounts of tactile stimulation. Low and medium tactile stimulation animals had significantly shorter dendritic lengths than maternally reared controls. High stimulation animals did not differ from controls. Bar heights represent means ± SEM. *Indicates a significant difference from control animals, p < 0.05.
Dendritic Distribution
As previously noted (Goldstein et al., 1993; Hebbeler et al., 2002; Hebbeler and Sengelaub, 2003), the SNB dendritic arbor of normal males is radially organized but not uniformly distributed, with more than 50% of the arbor concentrated ventrolaterally between 240° and 300°. A one-way repeated measures analysis of variance showed a significant effect of location [F(11, 297) = 187.84, p < 0.0001; Fig. 8], in accordance with the characteristic nonuniform distribution of SNB motoneurons. There was also a main effect of group on distribution [F(3, 297) = 3.15, p < 0.05]. Post-hoc contrasts showed that this main effect was the result of low and medium stimulation animals having an average 22% less arbor across all locations than maternally reared controls, reflecting the overall decreases in dendritic length per motoneuron produced by low and medium stimulation [percent decreases ranged from 3% to 41%; LSD, p < 0.05]. There was also a significant group by interaction effect [F(33, 297) = 2.86, p < 0.0001], with low and medium stimulation animals showing more dramatic reductions in dendritic length ventromedially. Posthoc comparisons showed that both low and medium stimulation animals had significantly decreased dendritic arbor per cell in several ventrally located bins, averaging 34% lower than maternally reared controls between 210° and 300° (LSD; ps < 0.05; percentages ranged from 22.3% to 41.3%).
Figure 8.
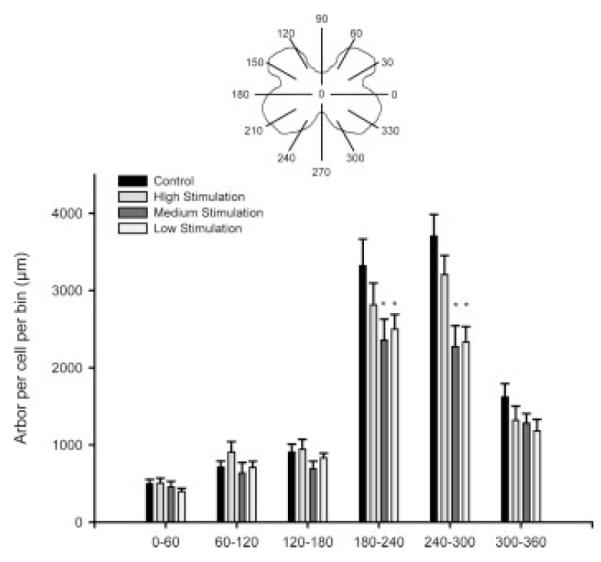
Length per radial bin of SNB dendrites in maternally reared control animals and artificially reared animals receiving differing amounts of tactile stimulation. For graphic purposes, dendritic length measures have been collapsed into six bins of 60° each. SNB motoneuron dendritic arbors display a nonuniform distribution, with a majority of the arbor located between 240° and 300°. This nonuniform distribution is apparent in all groups. Low and medium stimulation animals show shorter dendritic lengths in all bins, reflecting the overall differences in dendritic length, but show a particularly substantial loss of dendrites in the arbor between 180° and 300°. Bar heights represent means ± SEM. *Indicates a significant difference from control animals, p < 0.05.
Dendritic Extent
A one-way repeated measures ANOVA showed that the radial extent of dendrites was distributed nonuniformly, as is typical in the SNB (Goldstein et al., 1993), with a significant effect of location [F(11, 297) = 124.69, p < 0.0001; Fig. 9]. There was also a main effect of group [F(3, 297) = 6.36, p < 0.01], with post-hoc comparisons showing low and medium stimulation animals to have consistently shorter radial extent across locations than both maternally reared controls and high stimulation animals [LSD, ps < 0.05]. There was no group by location interaction [F(33, 297) = 0.73, ns]. With regard to rostrocaudal extent, the distance spanned by SNB dendrites throughout the rostrocaudal axis did not differ across groups [maternally reared = 3389.09 ± 90.75 μm; low stimulation = 3697.14 ± 140.44 μm; medium stimulation = 3704.00 ± 331.93 μm; high stimulation = 3410.00 ± 170.67 μm, F(3, 27) = 1.09, ns].
Figure 9.
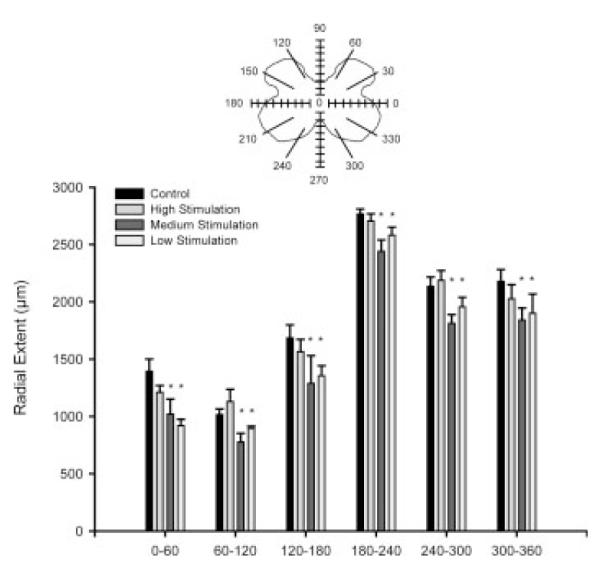
Radial extents of SNB dendrites in maternally reared control animals and artificially reared animals receiving differing amounts of tactile stimulation. Low and medium stimulation caused reductions in radial extent, which likely reflect the overall decreased dendritic arbor in these groups. For graphic purposes, dendritic length measures have been collapsed into six bins of 60° each. Bar heights represent means ± SEM. *Indicates a significant difference from control animals, p < 0.05.
Reflex-Morphology Correlations
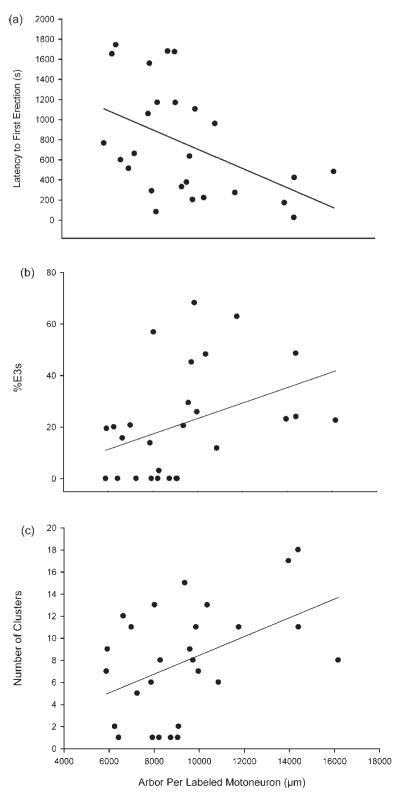
Several aspects of penile reflex behavior were correlated with SNB morphological parameters. Latency to first erection was negatively correlated with overall dendritic arbor per cell [r = −0.47, p < 0.05; Fig. 10(a)], thus the longer the SNB dendritic arbor, the shorter the latency to first erection. In addition, overall arbor per cell was positively correlated with the percentage of E3 erections [r = 0.39, p < 0.05; Fig. 10(b)] as well as the number of reflex clusters [r = 0.46, p < 0.05; Fig. 10(c)]. Therefore the longer the dendritic arbor, the more E3 erections and clusters. Also, overall soma size was negatively correlated with percentage of E2 erections [r = −0.36, p < 0.05; data not shown].
Figure 10.
Scatterplots and regression lines showing the correlation between dendritic arbor per labeled motoneuron and (a) latency to first erection; (b) percent of erections with flared cups (E3s); and (c) number of erection clusters. As the dendritic length increases, the latency to first erection time decreases (r = −0.47), the percent of E3 erections increases (r = 0.39), and the number of clusters increases (r = 0.46).
DISCUSSION
Reduced maternal licking during the early postnatal period has previously been shown to negatively impact adult male copulatory behavior as well as decrease motoneuron number, soma size, and dendritic length in the SNB (Moore, 1984; Moore et al., 1992; Lenz and Sengelaub, 2006). In this experiment, we found that adult penile reflexes and SNB morphology are influenced by the amount of tactile stimulation animals received neonatally. When the amount of tactile stimulation given to artificially reared pups is varied, pups that receive low levels of tactile stimulation have deficits in penile reflex behavior relative to maternally reared controls, specifically showing a longer latency to first erection, fewer cup erections, and fewer erection clusters. With regard to SNB morphology, low and medium tactile stimulation conditions produced decreases in SNB soma size and dendritic length per motoneuron relative to high stimulation and maternally reared control conditions. Furthermore, the observed stimulation-induced decreases in dendritic arbor were regionally concentrated in the ventromedial portion of the arbor. These results show that the development of the SNB and of penile reflex behavior is influenced by the amount of tactile stimulation received neonatally. In addition, we found significant correlations between SNB dendritic morphology and several penile reflex parameters, suggesting that the dendritic and somal morphology of SNB motoneurons is linked to the expression of penile reflex behavior.
Gross Morphological Measures
There were significant differences in adult BC/LA weight, with low stimulation animals having significantly lower BC/LA weights than maternally reared controls. The BC/LA muscle complex is dependent on androgen levels, both developmentally and in adulthood (Wainman and Shipounoff, 1941; Cihak et al., 1970; Tobin and Joubert, 1991), thus it must be considered that low stimulation causes reductions in androgen levels. However, seminal vesicle weights, which are very sensitive to androgen levels (Wainman and Shipounoff, 1941), were no different between low stimulation animals and controls, suggesting that low stimulation does not result in reduced testosterone. It is also possible that the effects of low maternal licking-like stimulation on BC/LA muscle weight are the result of disuse atrophy, brought on indirectly by the negative effects of lower levels of stimulation on both the somal and dendritic morphology of the SNB motoneurons that innervate the muscles.
Penile Reflexes
Previous research has shown that male copulatory behavior is shaped by early maternal licking experience, with animals that receive reduced maternal licking showing longer latencies to ejaculation, longer latencies to post-ejaculatory intromission, and longer interintromission intervals (Moore, 1984). In this experiment, we found that the amount of licking-like tactile stimulation that animals receive neonatally also shapes their ex copula penile reflex behavior in adulthood, with animals that received low levels of tactile stimulation during artificial rearing showing deficits relative to maternally reared controls. These deficits included an increased latency to first erection and a decreased percentage of cup erections. Animals that received medium or high levels of tactile stimulation during artificial rearing, however, were not significantly different from maternally reared controls or from one another on any penile reflex measures. These results suggest that a minimum amount of neonatal tactile stimulation is necessary for the development of normal adult penile reflex behavior.
The observed deficits in penile reflex behavior could result from the effects of low levels of stimulation on SNB morphology, given that SNB motoneurons are active during erections, particularly those with a flared cup (Sachs, 1982; Hart and Melese-D’Hospital, 1983). It is also possible, given the effects of low stimulation on the SNB target muscles, that the effects of low stimulation on reflex behavior result from muscle atrophy, and not a change in SNB motoneuron morphology. However, BC/LA muscle weight was not significantly correlated with any of the measured reflex behaviors, suggesting that target muscle weight is not responsible for the effects of low stimulation on reflex behavior.
Motoneuron Morphology
There were no group differences in the number of BHRP-labeled motoneurons, which match our previous findings in the SNB following reduced maternal licking. However, these results contrast with those of Moore et al. (1992), wherein reductions in the number of SNB motoneurons in adult males were reported as a consequence of reductions in maternal licking. Moore et al. counted all Nissl-stained motoneurons in the SNB, whereas we examined only those motoneurons projecting to the BC musculature. Thus, it is possible that the reductions in SNB motoneuron number reported by Moore et al. could be due to a loss of motoneurons projecting to other SNB target musculature (i.e., the LA and anal sphincter muscles).
With regard to soma sizes, only the medium tactile stimulation animals showed significantly smaller soma sizes than maternally reared controls. However, low and high stimulation resulted in a trend toward smaller soma sizes, suggesting that artificial rearing may produce overall effects on soma size irrespective of tactile stimulation condition. SNB somal growth occurs largely during the first 4 postnatal weeks (Goldstein et al., 1990), and both somal growth and maintenance are dependent on androgens (Breedlove and Arnold, 1983a,b; Goldstein et al., 1990; Goldstein and Sengelaub, 1992). However, seminal vesicle weights suggest no overall effects of artificial rearing on androgen levels, and other androgen-sensitive targets, including BC/LA muscle weight and dendritic length, similarly showed no overall effects of artificial rearing. Therefore, it is unlikely that artificial rearing, irrespective of tactile stimulation condition, produces decreased soma size via effects on androgen levels.
Low tactile stimulation produced an overall decrease in dendritic length per labeled SNB motoneuron, whereas medium or high levels of stimulation were sufficient to produce normal dendritic lengths in adulthood. These results suggest that the tactile component of maternal licking behavior is crucial to producing the normal dendritic development of SNB motoneurons, and that dropping stimulation levels below a certain threshold negatively impacts development. These results further support the idea that SNB dendritic morphology is sensitive to various early maternal care manipulations.
Interestingly, the effects of stimulation condition on the dendritic arbor were not restricted to the rostral portion of the arbor, as was seen previously following reductions in maternal licking (Lenz and Sengelaub, 2006). Given that artificial rearing is a more complex manipulation that differs from maternal rearing in several aspects, it seems reasonable that the artificial rearing manipulation may alter SNB dendritic development more extensively than does reducing maternal licking alone. In addition, in this experiment, artificially reared animals received low, medium, or high levels of both dorsal and anogenital stimulation to more accurately simulate maternal behavior, whereas our previous work used experimental anosmia (Lenz and Sengelaub, 2006), which only reduces licking behavior directed at the anogenital region (Moore and Power, 1992). These changes in both dorsal body stimulation as well as anogenital stimulation may account for the more widespread effects on the development of the SNB. However, overall we have found a very similar pattern of neural and behavioral deficits following low levels of dorsal and anogenital tactile stimulation as has previously been documented following low anogenital stimulation alone.
Low and medium tactile stimulation conditions produced average regional decreases of 34% in dendritic length in the ventromedial portion of the arbor. Past work from our laboratory has documented similar localized reductions in the radial distribution of the dendritic arbor following spinal transection, which deprived SNB motoneurons of connectivity with specific afferent populations, in this example, supraspinal inputs (Hebbeler and Sengelaub, 2003). In the present experiment, similar reductions in the radial distribution of SNB dendrites suggest that a specific afferent population was altered by low levels of tactile stimulation. In the spinal transection experiment, the reductions in dendritic arbor were located ventrolaterally, where many supraspinal afferents terminate. In this experiment, the reductions also occurred ventrally, but more ventromedially, possibly due to the involvement of both sensory and/or other afferents (see later).
Previous studies have demonstrated that neither axonal transport of BHRP (Leslie et al., 1991) nor dendritic transport as demonstrated by the rostrocaudal or mediolateral extent of dendritic labeling (Kurz et al., 1991; Goldstein and Sengelaub, 1994) are affected by hormone levels. Similarly, reduced maternal licking does not alter the dendritic transport of BHRP (Lenz and Sengelaub, 2006). In the present experiment, the possibility that hormonal differences or confounds arising from the artificial rearing manipulation could affect retrograde transport is an important consideration, as such an artifact could potentially result in apparent alterations in dendritic morphology. In this experiment, there was a significant difference in radial extent between maternally reared controls and both low and medium stimulation animals, which could reflect a transport artifact. However, given that no differences in the rostrocaudal extent of dendrites were observed between groups, and furthermore, that the radial extents of high stimulation animals were no different from controls, a rearing related labeling artifact seems unlikely. Furthermore, for the differences in dendritic length between groups to be caused by labeling artifact, such artifact would have had to occur only in the ventromedial portion of the SNB dendritic arbor. Finally, there were no differences in the density of BHRP labeling of SNB motoneurons between groups (F(3, 11) = 0.39, ns). Thus, the dendritic labeling across groups was comparable, allowing direct comparisons of dendritic lengths and distribution across groups.
Possible Mechanisms
Glucocorticoids
Manipulations of maternal care are often used as neonatal stress manipulations, with maternal deprivation or naturally occurring lower levels of maternal care causing altered development of the HPA axis and resultant increases in glucocorticoid levels (Liu et al., 1997; Kalinichev et al., 2002). SNB motoneurons possess glucocorticoid receptors (Blanco et al., 2002), and could thus be directly sensitive to changes in HPA axis activity brought about by the neonatal stimulation manipulation used in this experiment. Furthermore, stress has been shown to indirectly influence synaptic connectivity in the SNB, likely via HPA inhibition of the HPG axis (Matsumoto, 2005). Therefore, the observed effects of artificial rearing with low tactile stimulation on the SNB could result from changes in HPA axis signaling.
It is unlikely that alterations in glucocorticoid levels could occur locally within the nucleus and thus account for the reductions in dendritic length we observed in the ventromedial portion of the SNB. Moreover, there are no regional differences in glucocorticoid receptor distribution in the SNB (Lenz and Sengelaub, 2007), thus glucocorticoids acting locally in the spinal cord do not likely mediate the effects of low tactile stimulation on SNB dendritic morphology. HPA axis activity following low tactile stimulation could also act on other central targets to change the supraspinal or suprasegmental information that the SNB system receives, and thereby indirectly mediate the effects of low stimulation and artificial rearing on the SNB. However, artificial rearing does not evoke increased corticosterone (CORT) levels relative to maternally reared controls during the neonatal period (Ward et al., 2004). Similarly, artificial rearing with low stimulation does not produce changes in either basal or stress-induced CORT levels in adulthood relative to maternally reared controls (Burton et al., 2007). Therefore, the effects of artificial rearing and tactile stimulation on SNB motoneurons are not likely mediated by glucocorticoids.
Sensory Afferents
Tactile stimulation during artificial rearing was applied to both the dorsal and anogenital regions of pups, to best replicate the maternal licking stimulus. This tactile stimulation is relayed to the spinal cord via primary sensory afferents from the perineal and dorsal skin. The development of dendrites is shaped by afferent input (Morest, 1969; Rakic, 1975), and thus it is possible that alterations in afferent activity through low tactile stimulation could alter SNB development. Dendritic arborization is concurrent with the establishment of afferent connectivity in the spinal cord (Vaughn et al., 1988), with dendrite growth guided by afferents into synaptogenic regions of neuropil (Vaughn et al., 1988; Vaughn, 1989; O’Hanlon and Lowrie, 1996). Conversely, developmental deafferentation results in decreased dendrite length and arbor complexity (Smith, 1974; Bradley and Berry, 1976; Parks, 1981; Mizrahi and Libersat, 2002), and as was previously discussed, eliminating supraspinal input to the SNB produces localized redistributions of the dendritic arbor (Hebbeler and Sengelaub, 2003).
In this experiment, the effects of tactile stimulation were combinatorial effects of dorsal and anogenital stimulation. Perineal sensory afferents innervate the L6-S1 spinal cord segments, (McKenna and Nadelhaft, 1986; Ueyama et al., 1987), making no monosynaptic contact with SNB motoneurons, but extensive polysynaptic contact via local interneurons (McKenna and Nadelhaft, 1989; Collins et al., 1991). Most local interneurons that synapse onto SNB motoneurons are located either near the central canal or in the ventral, but particularly ventrolateral, portion of the SNB dendritic arbor (Collins et al., 1991). In contrast, cutaneous sensory afferents from the dorsal surface innervate a wide range of spinal segments, from the late thoracic to the early lumbar segments (Kow and Pfaff, 1975), and therefore make no direct synaptic contact with SNB motoneurons. Interestingly, the pattern generator that drives ejaculatory behavior is located in the early lumbar spinal segments and in turn innervates the lumbosacral cord (Truitt and Coolen, 2002). Dorsal cutaneous afferents may thus possibly make contact with this pattern generator and suprasegmentally influence SNB motoneuron development.
Oxytocin
Oxytocin (OT) inputs from the paraventricular nucleus (PVN) of the hypothalamus innervate the lumbosacral spinal cord (Swanson and McKellar, 1979; Cechetto and Saper, 1988; Wagner and Clemens, 1993; Tang et al., 1998; Veronneau-Longueville et al., 1999; Hallbeck et al., 2001). OT receptors are expressed in the superficial lamina of the dorsal horn and near the central canal (Veronneau-Longueville et al., 1999), and neurophysin containing fibers have been localized dorsally in the lumbosacral cord as well as ventromedially, in close apposition to SNB motoneurons (Wagner and Clemens, 1993). Oxytocin is a proerectile modulator of male copulatory behavior (Argiolas et al., 1986; Giuliano et al., 2001). Interestingly, oxytocin is also centrally released following various types of sensory stimulation (Stock and Uvnas-Moberg, 1988; Uvnas-Moberg et al., 1993), and both stroking the perineum and stimulating the dorsal penile nerve activate OT neurons in the PVN (Yanagimoto et al., 1996). Differences in tactile stimulation experienced by the artificially reared animals in this experiment may thus alter OT signaling in the lumbosacral spinal cord, and explain the effects of the manipulation on the ventromedial portion of the SNB dendritic arbor and on penile reflex behavior.
Morphology-Behavior Correlations
Several aspects of penile reflex behavior were significantly correlated with the length of the SNB dendritic arbor. The dendritic arbor plays a critical role in motoneuron function, accommodating an estimated 20,000 to 50,000 synaptic inputs (Ulfhake and Cullheim, 1988; Burke, 1990). Differences in dendritic branching patterns, distribution, and overall shape determine important functional properties in motoneurons (e.g., Ulfhake and Cullheim, 1981; Schoenen, 1982; Cameron et al., 1985; Cullheim et al., 1987a,b; Furicchia and Goshgarian, 1987; Rall et al., 1992; Ritz et al., 1992; Mainen and Sejnowski, 1996; Koch and Segev, 2000; Lu et al., 2001; Vetter et al., 2001; Grudt and Perl, 2002). In this experiment, the behaviors that were significantly correlated with dendritic length included latency to first erection, percentage of cup erections (E3s), and the number of erection clusters. SNB activation of the BC/LA muscles creates intense erections, and the relationship between these particular reflex behaviors and dendritic length likely reflect the role that SNB motoneurons play in erectile behavior. These correlations between ex copula reflexes and dendritic length underscore that penile reflex behavior is shaped by the morphological properties of SNB motoneurons and the role of the dendritic arbor in receiving and integrating multiple afferent signals.
CONCLUSIONS
Maternal licking during the early postnatal period has previously been shown to influence the development of cell number, somal, and dendritic morphology in the SNB (Moore et al., 1992; Lenz and Sengelaub, 2006) and adult male copulatory behavior (Moore, 1984). In this experiment, we tested whether the tactile stimulation component of early maternal licking is the crucial feature of maternal care shaping neural and behavioral development, by artificially rearing rats and varying the amount of licking-like stimulation they received. Both SNB morphology and ex copula penile reflex behavior were shaped by early life tactile stimulation, with animals that received lower levels of licking-like stimulation showing decreased soma size, dendritic length, and deficits in penile reflexes. The effects of tactile stimulation on the dendritic arbor were particularly robust in the ventromedial arbor, suggesting that an afferent population, possibly sensory, suprasegmental, or supraspinal, was altered by the decrease in tactile stimulation. In addition, the length of the SNB dendritic arbor was significantly correlated with several aspects of penile reflex behavior, confirming the important role of dendritic morphology in the functional output of the SNB neuromuscular system. Overall, this experiment illustrates the important role of early life experience in the development of the neuromuscular system controlling penile reflex behavior in rats.
Acknowledgments
We are grateful to Dr. Benjamin Sachs for instruction on the measurement of penile reflexes. Additional assistance with artificial rearing and behavior testing was provided by Emis Akbari and Radik Budin. We also wish to thank our anonymous reviewers for their helpful comments on the manuscript.
Contract grant sponsor: NIH; contract grant number: 5T32HD049336-03.
Contract grant sponsor: NSERC Discovery; contract grant number: RGPIN 312458-06.
REFERENCES
- Argiolas A, Melis MR, Gessa GL. Oxytocin: An extremely potent inducer of penile erection and yawning in male rats. Eur J Pharmacol. 1986;130:265–272. doi: 10.1016/0014-2999(86)90277-3. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Peltz A, Staley R, Kim F. Effects of pharmacologic androgen treatment duration on glucocorticoid receptor α immunoreactivity of lumbosacral motor neurons in the male rat. Neuroscience. 2002;115:941–949. doi: 10.1016/s0306-4522(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Bradley P, Berry M. The effects of reduced climbing and parallel fibre input on Purkinje cell dendritic growth. Brain Res. 1976;109:133–151. doi: 10.1016/0006-8993(76)90384-x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983a;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983b;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Spinal cord: Ventral horn. In: Sheperd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; New York: 1990. pp. 88–32. [Google Scholar]
- Burke KA, Kuwajima M, Sengelaub DR. Aromatase inhibition reduces dendritic growth in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1999;38:301–312. [PubMed] [Google Scholar]
- Burke KA, Widows MR, Sengelaub DR. Synergistic effects of testosterone metabolites on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1997;33:1–10. doi: 10.1002/(sici)1097-4695(199707)33:1<1::aid-neu1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, Fleming AS. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague-Dawley rats. Brain Res. 2007;1158:28–38. doi: 10.1016/j.brainres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ. Quantitative analysis of the dendrites of cat phrenic motoneurons stained intracellularly with horseradish peroxidase. J Comp Neurol. 1985;231:91–101. doi: 10.1002/cne.902310108. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Cihak R, Gutmann E, Hanzlikova V. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J Anat. 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Collins WF, III, Erichsen JT, Rose RD. Pudendal motor and premotor neurons in the male rat: A WGA transneuronal study. J Comp Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol. 1987a;255:68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Three-dimensional architecture of dendritic trees in typeidentified alpha-motoneurons. J Comp Neurol. 1987b;255:82–96. doi: 10.1002/cne.902550107. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Seasonal variation in mammalian striated muscle mass and motoneuron morphology. J Neurobiol. 1987;18:155–165. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Furicchia JV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol. 1987;96:621–634. doi: 10.1016/0014-4886(87)90224-x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, McKenna K, Longueville F, Rampin O. Spinal proerectile effect of oxytocin in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1870–R1877. doi: 10.1152/ajpregu.2001.280.6.R1870. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Kalkbrenner AE, Sengelaub DR. Changes in dendritic morphology of rat spinal motoneurons during development and after unilateral target deletion. Developmental Brain Research. 1993;73:151–163. doi: 10.1016/0165-3806(93)90133-u. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 1992;326:147–157. doi: 10.1002/cne.903260113. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1994;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Fleming AS. Artificial rearing causes changes in maternal behavior and c-fos expression in juvenile female rats. Behav Neurosci. 2002;116:999–1013. doi: 10.1037//0735-7044.116.6.999. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol. 2001;38:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Hart BL. Sexual reflexes and mating behavior in the male rat. J Comp Physiol Psychol. 1968;65:453–460. doi: 10.1037/h0025842. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D’Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: Morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. Nmethyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Hidden regulatory processes in early social relationships. In: Bateson PPG, Klopfer PH, editors. Perspectives in Ethology. Plenum Press; New York City: 1978. pp. 135–166. [Google Scholar]
- Jutapakdeegul N, Casalotti SO, Govitrapong P, Kotchabhakdi N. Postnatal touch stimulation acutely alters corticosterone levels and glucocorticoid receptor gene expression in the neonatal rat. Dev Neurosci. 2003;25:26–33. doi: 10.1159/000071465. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in longlasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002;27:518–533. doi: 10.1016/S0893-133X(02)00326-3. [DOI] [PubMed] [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nat Neurosci. 2000;3(Suppl):1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Dorsal root recording relevant for mating reflexes in female rats: identification of receptive fields and effects of peripheral denervation. J Neurobiol. 1975;6:23–37. doi: 10.1002/neu.480060107. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: A cholera toxin-HRP study. J Neurobiol. 1991;22:976–988. doi: 10.1002/neu.480220909. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Leipheimer RE, Sachs BD. GABAergic regulation of penile reflexes and copulation in rats. Physiol Behav. 1988;42:351–357. doi: 10.1016/0031-9384(88)90276-4. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Cutaneous sensory afferents, but not spinal glucocorticoid receptors, may mediate the effects of early maternal care on dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Soc Neurosci Abstr. 2007;37:293–16. [Google Scholar]
- Leslie M, Forger NG, Breedlove SM. Does androgen affect axonal transport of cholera toxin HRP in spinal motoneurons? Neurosci Lett. 1991;126:199–202. doi: 10.1016/0304-3940(91)90553-6. [DOI] [PubMed] [Google Scholar]
- Lévy F, Melo AI, Galef BG, Jr, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial learning in adult rats. Dev Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitaryadrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS, Fletcher PJ. Early life tactile stimulation changes adult rat responsiveness to amphetamine. Pharmacol Biochem Behav. 2006;84:497–503. doi: 10.1016/j.pbb.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Lu Y, Inokuchi H, McLachlan EM, Li JS, Higashi H. Correlation between electrophysiology and morphology of three groups of neuron in the dorsal commissural nucleus of lumbosacral spinal cord of mature rats studied in vitro. J Comp Neurol. 2001;437:156–169. doi: 10.1002/cne.1276. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Sachs BD, Meisel RL. Sexual reflexes in male rats: restoration by ejaculation following suppression by penile sheath removal. Physiol Behav. 1979;23:273–277. doi: 10.1016/0031-9384(79)90367-6. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Testosterone prevents synaptic loss in the perineal motoneuron pool in the spinal cord in male rats exposed to chronic stress. Stress. 2005;8:133–140. doi: 10.1080/10253890500140642. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The pudendo-pudendal reflex in male and female rats. J Auton Nerv Syst. 1989;27:67–77. doi: 10.1016/0165-1838(89)90130-6. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tracing Neural Connections With Horseradish Peroxidase. Wiley; Chichester: 1982. [Google Scholar]
- Mizrahi A, Libersat F. Afferent input regulates the formation of distal dendritic branches. J Comp Neurol. 2002;452:1–10. doi: 10.1002/cne.10275. [DOI] [PubMed] [Google Scholar]
- Moore CL. An olfactory basis for maternal discrimination of sex of offspring in rats (Rattus norvegicus) Animal Behav. 1981;29:383–386. [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL. Sex differences in urinary odors produced by young laboratory rats (Rattus norvegicus) J Comp Psychol. 1985;99:336–341. [PubMed] [Google Scholar]
- Moore CL, Power KL. Variation in maternal care and individual differences in play, exploration, and grooming of juvenile norway rat offspring. Dev Psychobiol. 1992;25:165–182. doi: 10.1002/dev.420250303. [DOI] [PubMed] [Google Scholar]
- Moore CL, Dou H, Juraska JM. Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Res. 1992;572:52–56. doi: 10.1016/0006-8993(92)90449-j. [DOI] [PubMed] [Google Scholar]
- Morest DK. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128:290–317. doi: 10.1007/BF00522529. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- O’Hanlon GM, Lowrie MB. The effects of neonatal dorsal root section on the survival and dendritic development of lumbar motoneurons in the rat. Eur J Neurosci. 1996;8:1072–1077. doi: 10.1111/j.1460-9568.1996.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Parks TN. Changes in the length and organization of nucleus laminaris dendrites after unilateral otocyst ablation in chick embryos. J Comp Neurol. 1981;202:47–57. doi: 10.1002/cne.902020105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Role of cell interaction in development of dendritic patterns. Adv Neurol. 1975;12:117–134. [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiol Rev. 1992;72:S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Raouf S, Van Roo B, Sengelaub D. Adult plasticity in hormone-sensitive motoneuron morphology: Methodological/behavioral confounds. Horm Behav. 2000;38:210–221. doi: 10.1006/hbeh.2000.1620. [DOI] [PubMed] [Google Scholar]
- Ritz LA, Bailey SM, Murray CR, Sparkes ML. Organizational and morphological features of cat sacrocaudal motoneurons. J Comp Neurol. 1992;318:209–221. doi: 10.1002/cne.903180206. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Garinello LD. Interaction between penile reflexes and copulation in male rats. J Comp Physiol Psychol. 1978;92:759–767. doi: 10.1037/h0077498. [DOI] [PubMed] [Google Scholar]
- Schoenen J. Dendritic organization of the human spinal cord: The motoneurons. J Comp Neurol. 1982;211:226–247. doi: 10.1002/cne.902110303. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Smith DE. The effect of deafferentation on the postnatal development of Clarke’s nucleus in the kitten—A Golgi study. Brain Res. 1974;74:119–130. doi: 10.1016/0006-8993(74)90115-2. [DOI] [PubMed] [Google Scholar]
- Stern JM. Somatosensation and maternal care in Norway rats. In: Slater PJB, Rosenblatt JS, Snowdon CT, Milinski M, editors. Advances in the Study of Behavior. Academic Press; San Diego: 1996. pp. 243–294. [Google Scholar]
- Stock S, Uvnas-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol Scand. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Brain Res Dev Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Swanson LW, McKellar S. The distribution of oxytocinand neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol. 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S. A quantitative light microscopic study of the dendrites of cat spinal γ-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1981;202:585–596. doi: 10.1002/cne.902020410. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S. Postnatal development of cat hind limb motoneurons. III. Changes in size of motoneurons supplying the triceps surae muscle. J Comp Neurol. 1988;278:103–120. doi: 10.1002/cne.902780107. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993;149:199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Barber RP, Sims TJ. Dendritic development and preferential growth into synaptogenic fields: A quantitative study of golgi-impregnated spinal motor neurons. Synapse. 1988;2:69–78. doi: 10.1002/syn.890020110. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, et al. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Vetter P, Roth A, Hausser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Clemens LG. Neurophysin-containing pathway from the paraventricular nucleus of the hypothalamus to a sexually dimorphic motor nucleus in lumbar spinal cord. J Comp Neurol. 1993;336:106–116. doi: 10.1002/cne.903360109. [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff GC. The effects of castration and testosterone propionate on the striated perineal musculature in the rat. Endocrinology. 1941;29:975–978. [Google Scholar]
- Ward G, Xing HC, Carnide N, Slivchak J, Wainwright P. Adrenocortical response to stress in fasted and unfasted artificially reared 12-day-old rat pups. Dev Psychobiol. 2004;45:245–250. doi: 10.1002/dev.20029. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Yanagimoto M, Honda K, Goto Y, Negoro H. Afferents originating from the dorsal penile nerve excite oxytocin cells in the hypothalamic paraventricular nucleus of the rat. Brain Res. 1996;733:292–296. doi: 10.1016/0006-8993(96)00800-1. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]