Abstract
Context
While the lifetime risk of heart failure at age 40 is about 1 in 5 in the general population, little is known about the association between modifiable lifestyle factors and the remaining lifetime risk of heart failure.
Objective
To examine the association between modifiable lifestyle factors and the lifetime risk of heart failure in a large cohort of men.
Design, Setting, and Participants
Prospective cohort using data from 20,900 men from the Physicians’ Health Study I (1982–2008) who were apparently healthy at baseline.
Main Outcome
Lifetime risk of heart failure.
Results
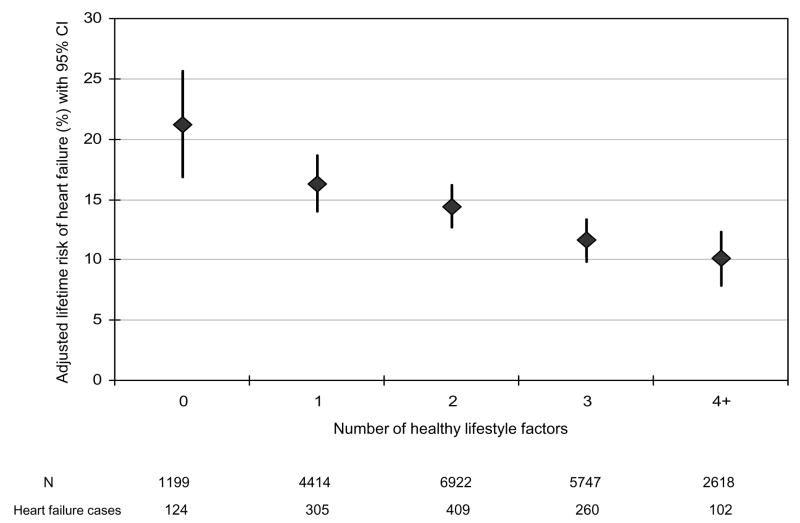
During a mean follow up of 22.4 years, 1,200 men developed heart failure. The average age at baseline was 53.6 y. Overall, the lifetime risk of heart failure was 13.79 (95% CI: 12.85% to 14.73%) at age 40. The lifetime risk remained constant in people who survived free of heart failure through age 70 and reached 10.58% (95% CI: 9.42% to 11.74%) at age 80. The lifetime risk of heart failure was higher in hypertensive than normotensive men. Healthy lifestyle habits (not smoking, regular exercise, normal weight, moderate alcohol intake, and consumption of breakfast cereal and fruits and vegetables) were individually and jointly associated with a lower lifetime risk of heart failure, with the highest risk in men who did not follow any of the six lifestyle factors (risk= 21.20%; 95% CI: 16.80% to 25.60%) and the lowest risk in men with four or more desirable factors (risk = 10.10%, 95% CI: 7.90% to 12.30%).
Conclusions
These data suggest that adherence to healthy lifestyle factors is associated with a substantially lower risk of lifetime risk of heart failure in a male population.
With an annual incidence of 550, 000, heart failure (HF) remains a major public health issue1–5 and is the leading cause of hospitalization among older adults in the US.6,7 Despite improved medical and surgical management of HF, mortality after onset of HF remains high,8–10 ranging from 20% to 50%.11–14 A large proportion of HF cases is accounted for by antecedent coronary heart disease (CHD) and hypertension,15–19 suggesting that predictors of CHD and hypertension might influence the risk of HF.
The concept of lifetime risk is important in public health practice. It is defined as cumulative incidence adjusted for mortality or, simply, the risk of ever developing a disease during one’s remaining lifetime before dying from other causes. At age 40, it is estimated that 1 in every 5 adults will develop HF during their remaining lifetime.20 Thus, it remains important to focus on the primary prevention of HF. Several predictors of HF can be influenced by modifiable lifestyle factors. For example, healthy diet, regular exercise, not smoking, and maintaining healthy weight have been shown to favorably influence HF risk factors including coronary artery disease,21–25 diabetes mellitus,26–28,28,29 and hypertension.30,31 However, it is unclear whether adherence to the healthy lifestyle factors mentioned above could further lead to the reduction in lifetime risk of HF. A demonstration of beneficial influence of healthy lifestyle habits on the lifetime risk of HF has potential clinical and public health implications. With its careful prospective collection of lifestyle factors and health outcomes and over 20 years of follow-up, the Physicians’ Health Study (PHS) I offers a unique opportunity to address these important questions. Therefore, the current project sought to prospectively assess the association between modifiable lifestyle factors and the remaining lifetime risk of HF in a large cohort of men.
Methods
Study population
Participants in these analyses are members of the PHS I which is a completed randomized, double-blind, placebo-controlled trial designed to study low-dose aspirin and beta-carotene for the primary prevention of cardiovascular disease and cancer. Detailed description of the PHS I has been published.32 Of the total 22,071 participants, we excluded 25 men with prevalent HF at baseline, one HF case that occurred after age 100, and 1,145 participants with missing information on lifestyle factors (exercise, body mass index, fruit and vegetable, breakfast cereal consumption, alcohol consumption, and smoking). Thus, a final sample of 20,900 participants was used for current analyses. Each participant signed an informed consent and the Institutional review Board at Brigham and Women’s Hospital approved the study protocol.
Ascertainment of incident HF in the PHS
Ascertainment of endpoints including HF in the PHS has been achieved using follow-up questionnaires. A questionnaire was mailed to each participant every 6 months during the first year and annually thereafter to obtain information on compliance with the intervention and the occurrence of new outcomes including HF. Detailed description of HF validation in the PHS using self-reported information and the Framingham criteria33 has been published elsewhere.34 Furthermore, we conducted an additional validation of self-reported HF using a review of medical records. In the PHS, a systematic request of medical records is only available for the trial primary endpoints (myocardial infarction, stroke, cancer, death, and pulmonary embolus). We selected all participants who reported a diagnosis of HF on a yearly questionnaire and had a subsequent diagnosis of one the trial primary endpoint within 30 days after the reported HF. The rationale for selecting these conditions was that medical records for a cardiovascular event are more likely to contain pertinent information on cardiovascular signs, symptoms, treatments, and diagnostic work up including echocardiogram, cardiac catheterization, and other cardiac imaging techniques. In contrast, cancer are frequently confirmed by histologic reports. Two physicians (one general internist and a cardiologist) independently reviewed 55 charts that met above criteria. A diagnosis of HF was made if there was sufficient evidence in the chart defined as one of the following: a) diagnosis of HF on the discharge summary prior to the current stroke or myocardial infarction, b) major signs and symptoms from the Framingham criteria for HF diagnosis (2 major criteria or 1 major criterion plus 2 minor criteria for HF diagnosis), c) evidence of congestive HF on chest X-ray, echocardiography, or other left ventricular imaging techniques, or d) minor signs and symptoms with concomitant treatment for HF (use of diuretics, digoxin in the absence of atrial fibrillation, angiotensin converting-enzyme inhibitors, angiotensinogen receptor blockers, and beta-blockers). Using these criteria, HF was confirmed in 50 out of 55 cases (~91%). For 5 study participants, we did not find sufficient evidence in the chart to confirm the diagnosis of HF. There was an excellent agreement between the two examiners (kappa=92.3%). For present analyses, we used heart failure and death cases ascertained through February 2008.
Assessment of lifestyle factors and other factors
We focused on modifiable lifestyle factors that have been shown to influence the risk of cardiovascular disease. These included physical activity, smoking, alcohol consumption, adiposity, and dietary habits. At baseline, each subject provided information on exercise [how often do you exercise vigorously enough to work up sweat? Possible answers included rarely/never, 1–3/month, 1/week, 2–4/week, 5–6/week, and daily]; smoking (never, former, and current smoker); and alcohol intake (rarely/never, 1–3 per month, 1 per week, 2–4/week, 5–5/week, daily, and 2+/day). We used an abbreviated food frequency questionnaire to obtain self-reported information on selected foods such as fruits and vegetables and breakfast cereal. Participants were asked to report how often, on average, they have consumed each food item during the past year (possible responses were “rarely/never”, “1–3/month”, “1/week”, “2–4/week”, “5–6/week”, “daily”, and “2+/day”). In this cohort, repeated information was collected on smoking (at 2, 5, and 12 y post-randomization), breakfast cereal (1, 2, 4, 6, 8, and 10 y post-randomization), exercise (at 3 and 9 y post-randomization), fruit and vegetables (at 2, 4, 6, 8, and 10 y post-randomization), and body weight (at 8 through 13 y post-randomization). Pearson’s correlation coefficients between baseline value and subsequent measurements ranged from 0.86 to 0.80 for body mass index and 0.58 to 0.47 for fruit and vegetables. For categorical variables, weighted kappa between baseline and subsequent measures ranged from 0.95 to 0.84 for smoking; 0.37 to 0.28 for exercise; and 0.60 to 0.38 for breakfast cereals. The validity of the semi-quantitative food frequency questionnaire has been published.35 Self-reported baseline weight and height were used to compute body mass index (weight in kilograms divided by height in meter squared). An endpoint committee adjudicated incident cardiovascular disease and deaths through review of medical records, death certificates, search of national death index, and information from family members or next of kin.
Definition of lifestyle groups
To investigate the association between healthy lifestyle factors and the lifetime risk of HF, each lifestyle risk factor was dichotomized as follows: never smoker vs. ever smoker; normal weight (BMI <25 kg/m2) vs. overweight/obese (BMI >25 kg/m2); regular exercise (5 or more times per week) vs. infrequent/no exercise (< 5 times per week); breakfast cereal consumption (1 or more per week vs. none); moderate drinking (4 or more times per week vs. less than 4 times per week); fruit and vegetables (4 or more servings per day vs. less than 4 per day); Of note is that a small proportion (3%) of the total sample reported alcohol consumption of 2+/d. These cut points were chosen based on prior associations between individual lifestyle factors and HF risk in this cohort or public health recommendations. Each subject could have a minimum of 0 and a maximum of 6 healthy lifestyle factors. Since only 397 (1.9%) and 32 (0.2%) men were in the categories with 5 and 6 healthy lifestyle factors respectively, we collapsed the upper 3 categories to obtain stable estimates (subsequently referred to as the 4+ group). Thus, study participants were categorized according to the number of desirable lifestyle factors (0, 1, 2, 3, and 4+).
Statistical analyses
Means and percentages of baseline characteristics of the study participants are presented according to the number of healthy lifestyle factors. To calculate the lifetime risk of HF, a modifiedtechnique of survival analysis was used, as described previously.36,37 Because few men survived past age 98, lifetime riskestimates were calculated only through age 98. Each subject in the study sample was followed up from baselineuntil either the year of a first HF event, the year of death, or attainment of age 98 years. The lifetime risk was calculatedseparately for each index ages of 40, 50, 60, 70, and 80 years. Risk estimates were produced using the Practical Incidence Estimators Macro which has been described in detail.38
The calculation of lifetime risks was stratified by individual lifestyle factors and number of healthy lifestyle factors as described above. In addition, stratification by prevalent hypertension was completed. Because (a) alcohol consumption may raise blood pressure and heavy drinking has been associated with cardiomyopathy39,40 and (b) the lack of complete dietary information to fully assess the role of diet, we repeated our analyses restricted to the 3 remaining lifestyle factors (exercise, adiposity, and smoking). In a sensitivity analysis, we repeated our analysis restricted to HF with and without antecedent coronary disease (angina, myocardial infarction, revascularization, or bypass) or with antecedent myocardial infarction, type 2 diabetes, and hypertension. In addition, we repeated analyses accounting for possible changes in BMI, smoking, exercise, and dietary factors where available. Specifically, for continuous variables, we used a cumulative average from baseline to censoring date or development of HF to classify participants. For smoking, a never smoker was required to remain never smoker throughout all smoking variables assessed prior to HF occurrence or censoring date. A similar approach was used for exercise and dietary factors. All analyses were performed using SAS (SAS version 9.1, NC) and the alpha level was set at 0.05. All p values were 2-sided.
Results
Characteristics
During an average follow-up of 22.4 years, 1200 (5.7%) new cases of HF and 4999 (23.9%) confirmed deaths occurred in the study. Baseline characteristics according to the number of healthy lifestyle factors are presented in Table 1. Compared with participants with zero healthy lifestyle factors, those with 4 or more factors tended to be older and had a lower prevalence of hypertension and diabetes mellitus.
Table 1.
Baseline characteristics of the 20,900 US male physicians according to healthy lifestyle factors*
| Number of healthy lifestyle factors |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4+ | |
| Characteristics | (N=1,199) | (N=4,414) | (N=6,922) | (N=5,747) | (N=2,618) |
| Age (y) | 52.6±8.1 | 53.2±8.9 | 53.7±9.4 | 53.7±9.8 | 54.2±10.4 |
| Body mass index (kg/m2) | 27.6±2.5 | 26.3±2.9 | 24.9±2.7 | 23.7±2.0 | 23.1±1.8 |
| Vegetable intake (servings/d) | 1.82±0.88 | 1.97±0.93 | 2.15±1.02 | 2.36±1.12 | 2.84±1.37 |
| Breakfast cereal 1+/week (%) | 0 | 1055 (23.9) | 3514 (50.8) | 4438 (77.2) | 2386 (91.1) |
| Moderate drinking (%) | 0 | 920 (20.8) | 2471 (35.7) | 2588 (45.0) | 1828 (69.8) |
| Never smokers (%) | 0 | 1126 (25.5) | 3216 (46.5) | 3857 (67.1) | 2161 (82.5) |
| Exercise 5+ times per week (%) | 0 | 126 (2.9) | 647 (9.4) | 1131 (19.7) | 1476 (56.4) |
| Hypertension (%) | 343 (28.6) | 1245 (28.2) | 1685 (24.3) | 1158 (20.2) | 503 (19.2) |
| Atrial fibrillation (%) | 12 (1.0) | 59 (1.3) | 96 (1.4) | 86 (1.5) | 48 (1.8) |
| Left ventricular hypertrophy (%) | 3 (0.3) | 11 (0.3) | 15 (0.2) | 11 (0.2) | 6 (0.2) |
| Valvular heart disease (%) | 1 (0.1) | 4 (0.1) | 15 (0.2) | 5 (0.1) | 6 (0.2) |
| Diabetes mellitus (%) | 33 (2.8) | 119 (2.7) | 140 (2.0) | 99 (1.7) | 44 (1.7) |
Healthy lifestyle factors includes never smoking, moderate alcohol consumption, regular exercise (5 or more times per week), 4 or more servings of fruit and vegetable consumption per day, 1 or more serving of breakfast cereal per week, and normal weight (BMI below 25 kg/m2).
Lifetime risk of HF
Overall, the lifetime risk of HF was 13.79% (95% CI: 12.85%–14.73%) at age 40 years, and remained constant through age 70; at age 80, the lifetime risk for HF was 10.58 % (95% CI: 9.42% to 11.74%) (Table 2). As expected, the remaining lifetime risk of HF was higher (about 2% to 4% higher) in men with hypertension than those without hypertension (Table 2). For men with HF without antecedent myocardial infarction, the lifetime risk (95% CI) was about 1 in 9 or 11.50% (10.64% to 12.35%) at age 40; 11.48% (10.62% to 13.34%) at age 50; 11.51% (10.63% to 12.39%) at age 60; 10.91%(9.98% to 11.83%) at age 70; and 8.67% (7.63% to 9.70%) at age 80.
Table 2.
Lifetime risk (%) of heart failure according to age attained free of heart failure and prevalent hypertension
| Lifetime risk (95% CI)* |
|||
|---|---|---|---|
| All subjects (n=20,900) | Normotensive subjects (n=15,966) | Hypertensive subjects (n=4,934) | |
| (Total deaths: 5673) | (Total deaths: 3506) | (Total deaths: 2167) | |
| Age (y) | (Heart failure: 1200) | (Heart failure: 711) | (Heart failure: 489) |
| 40 | 13.79 (12.85–14.73) | 12.34 (11.15–13.52) | 16.77 (15.23–18.31) |
| 50 | 13.78 (12.83–14.78) | 12.31 (11.12–13.50) | 16.82 (15.28–18.37) |
| 60 | 13.81 (12.84–14.78) | 12.34 (11.13–13.56) | 16.74 (15.18–18.31) |
| 70 | 13.09 (12.07–14.11) | 11.80 (10.52–13.08) | 15.38 (13.72–17.05) |
| 80 | 10.58 (9.42–11.74) | 9.64 (8.21–11.07) | 11.99 (10.06–13.92) |
Lifetime risk is the mortality–adjusted cumulative risk conditional on disease-free survival to the age specified CI denotes confidence interval
Lifestyle factors and lifetime risk of HF
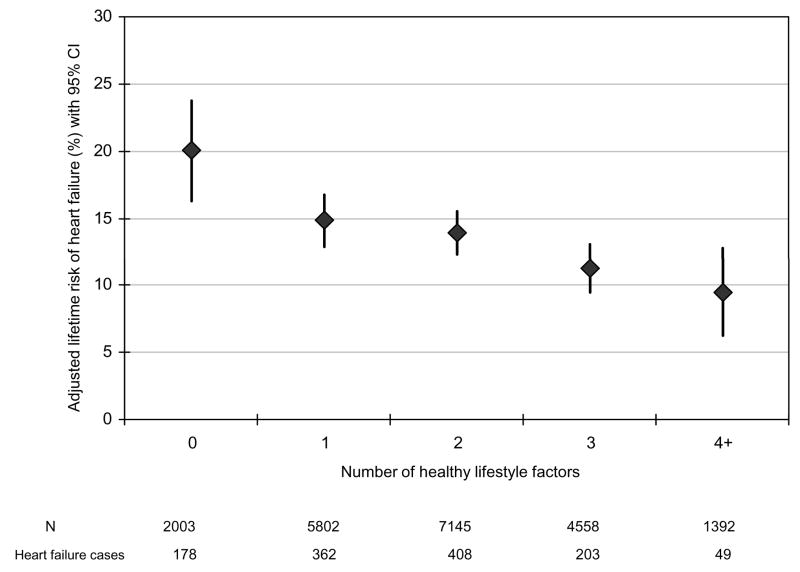
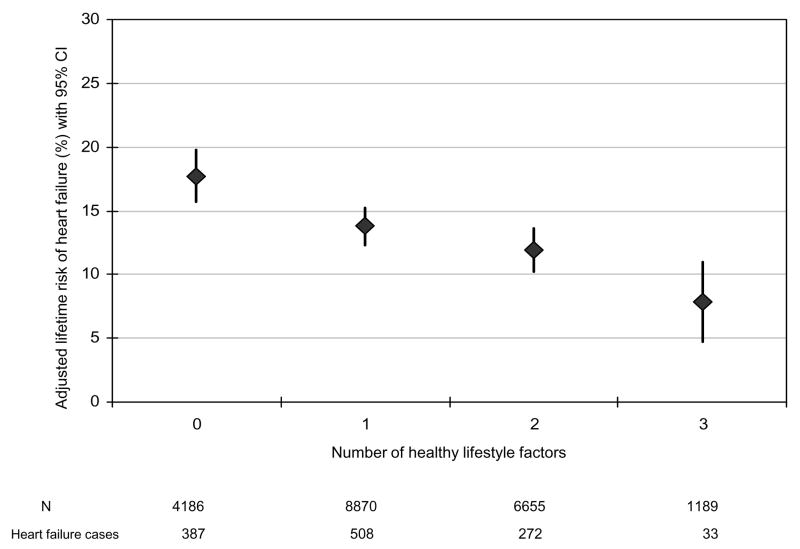
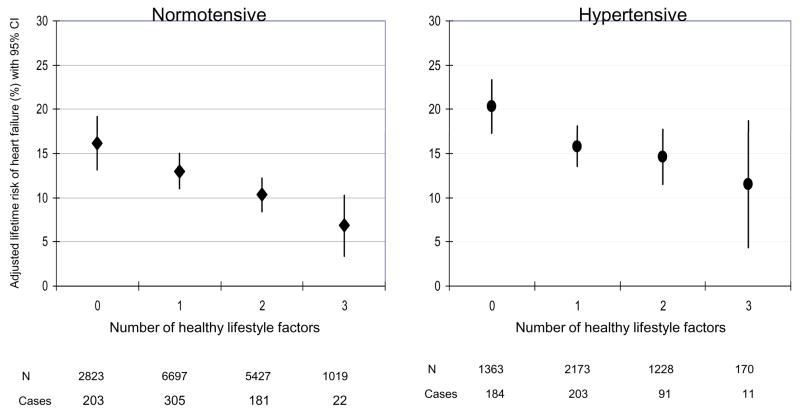
Normal body weight, regular exercise, being a never smoker, moderate alcohol intake, and consumption of breakfast cereal and fruit and vegetables were individually associated with a lower lifetime risk of HF compared to the corresponding undesirable behavior (Table 3). There was an inverse and graded association between the number of healthy lifestyle factors and lifetime risk of HF (Figure 1). For example, the lifetime risk for HF was about 1 in 5 or 21.2% (95% CI: 16.8% to 25.6%) in men with none of the desirable lifestyle factors compared to 1 in 10 or 10.10% (95% CI: 7.9% to 12.3%) in those with 4 or more healthy lifestyle factors. We evaluated the possible influence of change in lifestyle factors over time on the lifetime risk of HF using the cumulative average of repeated measures (BMI and fruit/vegetables) or by requiring healthy behavior on repeated measurements prior to HF or censoring date and found similar and stronger results (Figure 2). When restricted to smoking, exercise, and adiposity, the association between lifestyle factors and lifetime risk of HF persisted in the overall population (Figure 3) as well as in people with and without hypertension (Figure 4). We also observed a similar association between healthy lifestyle factors and the lifetime risk of HF with antecedent myocardial infarction, type 2 diabetes, or hypertension (please see online material, Figure 5).
Table 3.
Lifetime risk (%) of heart failure at age 40 according to lifestyle factors
| Lifestyle factors | N | Total deaths | Heart failure cases | Lifetime risk (95% CI)* |
|---|---|---|---|---|
| Smoking | ||||
| Never smoking | 10,360 | 227 | 473 | 13.20 (11.73–14.66) |
| Ever smoking | 10,540 | 3399 | 727 | 14.35 (13.12–15.59) |
| Fruit and vegetable intake | ||||
| ≥ 4 servings/d | 1,485 | 546 | 97 | 11.94 (9.47–14.42) |
| < 4 servings/d | 19,415 | 5127 | 1103 | 14.03 (13.00–15.05) |
| Breakfast cereal | ||||
| ≥ 1 serving/week | 11,393 | 3094 | 620 | 12.92 (11.71–14.13) |
| < 1 serving/week | 9,507 | 2579 | 580 | 15.02 (13.49–16.54) |
| Exercise | ||||
| ≥ 5 times/week | 3,380 | 930 | 161 | 11.44 (9.42–13.45) |
| < 5 times/week | 17,520 | 4743 | 1039 | 14.28 (13.21–15.35) |
| Alcohol intake | ||||
| 5–14 times/week | 7,807 | 2431 | 460 | 13.13 (11.72–14.54) |
| < 5 times/week | 13,093 | 3242 | 740 | 14.22 (12.95–15.48) |
| Overweight | ||||
| BMI < 25 kg/m2 | 12,007 | 3021 | 517 | 11.34 (10.15–12.53) |
| BMI ≥ 25 kg/m2 | 8,893 | 2652 | 683 | 16.88 (15.36–18.41) |
Lifetime risk is the mortality–adjusted cumulative risk conditional on disease-free survival to the age of 40. CI denotes confidence interval.
Figure 1.
Lifetime risk of heart failure (%) according to the number of healthy lifestyle factors
Figure 2.
Lifetime risk of heart failure (%) according to the number of healthy lifestyle factors accounting for change over time in exercise, body mass index, smoking, breakfast cereal intake, and fruit and vegetable intake.
Figure 3.
Lifetime risk of heart failure (%) according to the number of healthy lifestyle factors restricted to exercise, smoking, and adiposity
Figure 4.
Lifetime risk of heart failure (%) according to the number of healthy lifestyle factors restricted to exercise, smoking, and adiposity stratified by hypertension status
Discussion
Lifetime risk of HF
In this cohort of apparently healthy male physicians, we observed that the remaining lifetime risk of HF was about 1 in 7 at age 40, 50, 60, and 70 years. Despite the homogeneity in educational attainment and socioeconomic status, we noted that adherence to healthy lifestyle factors was associated with the remaining lifetime risk of HF in this cohort. As expected, the lifetime risk of HF was higher in hypertensive than normotensive men. We observed a similar relation between HF with antecedent myocardial infarction, type 2 diabetes, or hypertension.
Few studies have examined the remaining lifetime risk of this condition. In the Framingham Heart Study, Lloyd-Jones and colleagues20 found that the lifetime risk of HF in 3757 men age 40 was 21.0%. In the Rotterdam Study,41 the lifetime risk of HF was found to be 33.0%. Contrary to the Framingham Heart Study, where the lifetime risk remained constant from age 40 through age 80, there was a decrease in lifetime risk with advancing age in the Rotterdam Study from 33% at age 55 to 23% at age 85. In the PHS I, we observed a constant lifetime risk of HF from age 40 through age 70 and a drop was only observed in people aged 80. Such lower risk in the oldest age group could be attributable to the shorter remaining time at risk as well as the depletion of susceptible individuals and/or decreased disease ascertainment or reporting with very old ages. Although the lifetime risk of HF in our study (about 1 in 7) was high, it was noticeably lower than the 1 in 5 observed in the Framingham heart Study20 or 1 in 3 reported in the Rotterdam study41 in men of similar ages. What factors could possibly account for the discrepancy?
First, we acknowledge the difficulty of direct comparison of lifetime risk across populations in the absence of comparable mortality rates. A high mortality rate from other causes can lead to lower lifetime risks of HF due to a shorter period a risk. In contrast, longevity can lead to a higher lifetime risk than expected, particularly if the disease is prevalent in old ages. At age 40, PHS I participants have a life expectancy of 49.3 additional years42; 12 years longer than that of 40-year-old men in the general US population.43 It is noticeable that even though our cohort is longer lived (thus had a longer period at risk for HF) than either the Framingham or the Rotterdam studies, we observed substantially lower lifetime risks of HF. This may be due to the healthy lifestyle factors in our population leading to a decreased incidence of HF. Second, the study period may be a possible contributing source for the variability in estimates of lifetime risk of HF across studies. While there was limited overlap between the Framingham study period (1971 to 1996) and the PHS I (1982 to 2008), the Rotterdam study period (1989 to 2000) was completely included in the PHS study period. A reduction in annual incidence over time (due to better treatment) would partially explain the lower lifetime risk of HF in the most recent study. However, published data on secular trends in HF incidence suggest no substantial change in rate over time.44–47 Third, it is possible that the variability in diagnosis criteria for HF could have led to heterogeneity in cases across studies. Fourth, the PHS I population consisted of adult male physicians recruited for a primary prevention trial. Consequently, it is possible that the fact that physicians may have lower risk of HF given their medical knowledge, their access to state of the art treatment, and early recognition of signs and symptoms leading to the detection of milder cases of HF which may have been missed in the Framingham or the Rotterdam Study. However, early detection of HF in the PHS I would have led to increased rate of HF and not explain the observed lower lifetime risk of HF compared to the Framingham and the Rotterdam studies. Lastly, the healthy volunteer effect could have contributed to the lower lifetime risk of HF in this cohort.
Our finding for HF without antecedent coronary disease was similar to the Framingham data20 in men. As expected, hypertensive men had a higher lifetime risk of HF than normotensive.
Influence of lifestyle factors and lifetime risk of HF
In this cohort, we noted that regular exercise, not smoking, maintaining a normal weight, consumption of fruit and vegetables, breakfast cereal, and moderate alcohol consumption were individually and jointly associated with a lower lifetime risk of HF. The lowest risk was observed in people with 4 or more healthy lifestyle factors. Our data were robust in that restriction to 3 common lifestyle factors (exercise, smoking, and adiposity) yielded similar results and accounting for change in lifestyle factor overtime further strengthened the results.
To the best of our knowledge, this is the first study to examine the influence of modifiable lifestyle factors on the remaining lifetime risk of HF in a large cohort. Of note is that the lifetime risk was 22% in people without any of the desirable healthy lifestyle factors considered. This is about the same risk observed among men in the Framingham Heart Study who were the same age (40 y) and suggests that education alone without adherence to healthy lifestyle factors may not be adequate to lower the lifetime risk of HF. To the contrary, our data suggest that maintenance of healthy habits known to lower the risk of cardiovascular disease remains critical to lowering the risk of HF.
Study strengths and limitations
The large number of participants, 22+ years of follow up, and the standardized methods of endpoints ascertainment are major strengths of this study. On the other hand, the fact that all participants were male physicians, most of whom were Caucasians limits the generalizabilty of the current findings. In addition, we were unable to examine the lifetime risk of systolic versus diastolic HF and we did not have data on the etiology of HF. Although the ascertainment of HF in this study was self-reported, the high confirmation rates of HF diagnosis using Framingham criteria and review of medical records on 2 subsamples is reassuring that we had a reasonable case ascertainment in this study. Nevertheless, we can not exclude misclassification of some cases of HF in this study. It is possible that change in lifestyle factors before incident HF may have led to an underestimation of the effect measure. However, findings accounting for change in lifestyle factors over time yielded similar conclusions, suggesting that such bias may not completely explain our findings. Furthermore, we had reasonable Pearson’s correlation coefficients or levels of agreement across repeated measures as noted above. We were unable to account for early lifestyle factors for men who entered the study at an older age (i.e. 75+ y) despite the 22 years of follow up. Lastly, in the absence of randomization of studied lifestyle factors, we can not exclude unmeasured or residual confounding as partial or complete explanation of these findings.
Public health and clinical implications
Our data provide further evidence supporting a high burden of HF, even among those with a higher educational attainment. Our estimate of lifetime risk of HF could help public health official allocate resources for the prevention and management of this condition. Our findings of a low lifetime risk in people who adhere to modifiable lifestyle factors emphasize the need for incorporation of these behaviors in prevention strategies against HF both at individual and population level.
Conclusions
In this cohort of apparently healthy men, our findings suggest that adherence to healthy lifestyle factors is associated with a lower lifetime risk of HF as compared to the general population. Confirmation of the influence of modifiable lifestyle factors on the lifetime risk of HF in the other populations is warranted.
Supplementary Material
Lifetime risk of heart failure (%) with antecedent myocardial infarction, type 2 diabetes, or hypertension, according to the number of healthy lifestyle factors accounting for change over time in exercise, body mass index, smoking, breakfast cereal intake, and fruit and vegetable intake.
Acknowledgments
We are indebted to the participants in the PHS for their outstanding commitment and cooperation and to the entire PHS staff for their expert and unfailing assistance.
Funding/Support: The Physicians’ Health Study is supported by grants CA-34944 and CA-40360, and CA-097193 from the National Cancer Institute and grants HL-26490 and HL-34595 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Role of the Sponsor: The sponsors of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial disclosures: Dr. Djoussé is currently recipient of investigator-initiated grants from the National Institutes of Health. Over the past five years, he has received investigator-initiated grants from the Huntington’s Disease Society of America, the National Institutes of Health, and the Biomedical Research Institute, Brigham and Women’s Hospital.
Dr. Driver has received research grants from the Parkinson’s Disease Foundation, the Hartford Foundation’s Center of Excellence in Geriatric Medicine at Harvard Medical School and the Eleanor and Miles Shore/Harvard Medical School Scholars in Medicine Program. She is currently the recipient of a Career Development Award from the Department of Veterans Affairs. During the past 5 years, Dr. Gaziano has received investigator-initiated grants from the National Institutes of Health, the Veterans Administration, Veroscience, and Amgen; has served as a consultant or received honoraria from Bayer AG; and has served as an expert witness for Merck.
Footnotes
Author Contributions: Drs. Djoussé and Gaziano have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Djoussé
Acquisition of data: Gaziano
Analysis and interpretation of data: Djoussé, Driver, and Gaziano
Drafting of the manuscript: Djoussé
Critical revision of the paper for important intellectual content: Djoussé, Driver, and Gaziano
Statistical analysis: Djoussé and Driver
Obtaining funding: Gaziano
Administrative, technical, or material support: Gaziano
Study supervision: Gaziano
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Garg R, Packer M, Pitt B, Yusuf S. Heart failure in the 1990s: evolution of a major public health problem in cardiovascular medicine. J Am Coll Cardiol. 1993;22(4 Suppl A):3A–5A. doi: 10.1016/0735-1097(93)90454-9. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133(6):703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994;13(4):S107–S112. [PubMed] [Google Scholar]
- 5.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 6.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137(2):352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 7.Koelling TM, Chen RS, Lubwama RN, L’Italien GJ, Eagle KA. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004;147(1):74–78. doi: 10.1016/j.ahj.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 9.Gillum RF. Epidemiology of heart failure in the United States. Am Heart J. 1993;126(4):1042–1047. doi: 10.1016/0002-8703(93)90738-u. [DOI] [PubMed] [Google Scholar]
- 10.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21):2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118(7):728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer FA, Meyer T. Trends in mortality attributed to heart failure in Worcester, Massachusetts, 1992 to 2001. Am J Cardiol. 2005;95(11):1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 13.Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10(5):374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 15.Ho KKL, Pinsky JL, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6a–13a. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 16.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97(3):282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 18.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 19.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38(7):2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 21.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 22.Djoussé L, Pankow JS, Eckfeldt JH, Folsom AR, Hopkins PN, Province MA, et al. Relation between dietary linolenic acid and coronary artery disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2001;74(5):612–619. doi: 10.1093/ajcn/74.5.612. [DOI] [PubMed] [Google Scholar]
- 23.Djoussé L, Arnett DK, Carr JJ, Eckfeldt JH, Hopkins PN, Province MA, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Circulation. 2005;111:2921–2926. doi: 10.1161/CIRCULATIONAHA.104.489534. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 25.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160–167. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Manson JE, Stampfer MJ, Hu FB, Giovannucci E, Colditz GA, et al. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. 2000;90(9):1409–1415. doi: 10.2105/ajph.90.9.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochar J, Djoussé L, Gaziano JM. Breakfast cereals and risk of type-2 diabetes in the Physicians’ Health Study I. Obesity. 2007;15:3039–3044. doi: 10.1038/oby.2007.362. [DOI] [PubMed] [Google Scholar]
- 28.Djousse L, Kochar J, Gaziano JM. Dietary factors and risk of heart failure: a systematic review. Curr Cardiovasc Risk Rep. 2007;1:330–334. [Google Scholar]
- 29.Djousse L, Biggs ML, Mukamal KJ, Siscovick D. Alcohol Consumption and Type 2 Diabetes Among Older Adults: The Cardiovascular Health Study. Obesity. 2007;15:1758–1765. doi: 10.1038/oby.2007.209. [DOI] [PubMed] [Google Scholar]
- 30.Moore LL, Singer MR, Bradlee ML, Djousse L, Proctor MH, Cupples LA, et al. Intake of fruits, vegetables, and dairy products in early childhood and subsequent blood pressure change. Epidemiology. 2005;16(1):4–11. doi: 10.1097/01.ede.0000147106.32027.3e. [DOI] [PubMed] [Google Scholar]
- 31.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 32.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 33.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Djousse L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation. 2007;115(1):34–39. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 35.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease [see comments] Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 37.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 38.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19(11–12):1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Lazarevic AM, Nakatani S, Neskovic AN, Marinkovic J, Yasumura Y, Stojicic D, et al. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol. 2000;35(6):1599–1606. doi: 10.1016/s0735-1097(00)00565-9. [DOI] [PubMed] [Google Scholar]
- 40.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121(5):1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- 41.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 42.Driver J, Logroscino G, Gaziano JM, Kurth T. The incidence and remaining lifetime risk of Parkinson’s disease in advanced age. Neurology. 2009;72:432–438. doi: 10.1212/01.wnl.0000341769.50075.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54(14):1–40. [PubMed] [Google Scholar]
- 44.Djousse L, Kochar J, Gaziano JM. Secular Trends of Heart Failure among US Male Physicians. Am Heart J. 2007;154:855–860. doi: 10.1016/j.ahj.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159(1):29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 46.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 47.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lifetime risk of heart failure (%) with antecedent myocardial infarction, type 2 diabetes, or hypertension, according to the number of healthy lifestyle factors accounting for change over time in exercise, body mass index, smoking, breakfast cereal intake, and fruit and vegetable intake.