Abstract
Background
Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS), induces endothelial dysfunction. Although elevated ADMA has been associated with an increased risk of cardiovascular disease (CVD) events and mortality in referral samples, the prognostic significance of ADMA in the community has not been adequately evaluated.
Methods and Results
We related plasma ADMA, L-arginine, and the Arg/ADMA ratio to the incidence of CVD (fatal or non-fatal myocardial infarction, coronary insufficiency, angina pectoris, stroke or transient ischemic attack, intermittent claudication, or heart failure) and death in 3319 Framingham Offspring participants (1769 women; mean age 59 years). Over a follow-up period of 10.9 years, 281 individuals of 2956 free of CVD at baseline developed incident CVD, and 285 participants died. In multivariable models adjusting for established risk factors and other biomarkers (B-type natriuretic peptide, renin, homocysteine, urine albumin excretion and C-reactive protein), ADMA and the Arg/ADMA ratio were significantly associated with all-cause mortality (adjusted-hazard ratio [HR] per SD increment 1.21, 95% CI 1.07-1.37, and 0.80, 95% CI 0.69-0.93, respectively), whereas L-arginine was not (HR per SD 0.89, 95% CI 0.77-1.02). We noted effect modification by diabetes status: ADMA was associated with mortality in individuals without diabetes (adjusted-HR per SD increment 1.30, 95% CI 1.13-1.50), but not in individuals with diabetes (adjusted-HR per SD increment 0.85, 95% CI 0.62-1.16). ADMA, L-arginine and the Arg/ADMA ratio were not associated with CVD incidence (p >0.10).
Conclusions
In our large community-based sample, ADMA was significantly associated with all-cause mortality, particularly in non-diabetic individuals.
Keywords: nitric oxide, l-arginine, ADMA, cardiovascular disease, epidemiology
Introduction
The endothelium plays a major role in regulating vascular tone, mainly by secreting the potent vasodilator, nitric oxide (NO), which is anti-atherogenic.1 NO is synthesized from its precursor, L-arginine (Arg), by endothelial nitric oxide synthase (NOS).2 NOS is competitively inhibited by asymmetric dimethylarginine (ADMA), an endogenous compound that is elevated in renal failure, cardiovascular disease (CVD), and diabetes mellitus.3,4 A low L-arginine to ADMA ratio (Arg/ADMA) is also a marker of endothelial dysfunction. Prospective investigations of ADMA have highlighted its role as a predictor of CVD events or death in patients with established coronary artery disease (CAD),5-7 advanced renal failure,8 or other high-risk conditions.9
Only limited information is available from comparatively small studies regarding whether higher ADMA or a diminished Arg/ADMA ratio are associated with risk of death and CVD events in the general population. Two recent studies10,11 suggested that elevated ADMA is associated with a two-fold risk of death in non-smoking healthy men. Given the lack of data on the prognostic significance of ADMA in the community, we related ADMA, L-arginine and the Arg/ADMA ratio to incidence of CVD and death in a large ambulatory community-based cohort, adjusting for traditional risk factors, including newer biomarkers.12
Methods
Study Sample
The design of the Framingham Offspring Study has been described previously.13 The 3,532 attendees at the sixth examination (1995-1998) were eligible for the present investigation. We excluded 212 individuals for the following reasons: serum creatinine >2.0 mg/dl (n=15), non-available ADMA or L-arginine (n=79), and missing covariates (n=118). After exclusions, 3,320 participants remained eligible. At each Heart Study examination, participants undergo standardized measurements of blood pressure (BP), anthropometry, medical history, physical examination, and laboratory assessment of risk factors.14 The study protocol was approved by the Institutional Review Board of the Boston University Medical Center and the Ethics Committee of the Hamburg Board of Physicians. All participants provided written informed consent.
Assays for ADMA and L-Arginine and other biomarkers
Laboratory assessment of several biomarkers was conducted on fasting samples drawn at the sixth examination cycle (details in Appendix). Plasma samples that were stored for about 8 years at −80°C without freeze-thaw cycles were used for mass spectrometric determination of ADMA and L-arginine using a validated high throughput LC-MS/MS assay.15,16 The following biomarkers were used as covariates because of prior association with CVD/mortality in our cohort12: high-sensitivity C-reactive protein (CRP); plasma B-type natriuretic peptide (BNP); plasma renin concentration; plasma homocysteine; and the urinary albumin-to-creatinine ratio (UACR) assessed on a spot urine specimen.
Outcomes
The outcomes of interest were incidence of a first CVD event and death due to all causes during follow-up from the baseline examination through December 2006. Major CVD events included fatal or non-fatal coronary heart disease (myocardial infarction, coronary insufficiency, and angina pectoris), stroke or transient ischemic attack, intermittent claudication or heart failure. Criteria for these events have been described elsewhere (see Appendix).14
Statistical analysis
We used multivariable linear regression to relate ADMA, and L-arginine to the following variables: age, sex, systolic and diastolic BP, hypertension treatment, ratio of total/HDL cholesterol, serum triglycerides, diabetes, body mass index (BMI), glomerular filtration rate, smoking status, and alcohol consumption. The Modification of Diet in Renal Disease (MDRD) equation17 was used to calculate an estimated glomerular filtration rate (eGFR).
We used Cox regression18 to relate ADMA, L-arginine and the Arg/ADMA ratio to the incidence of a first CVD event, and death (separate analyses for each biomarker and for each outcome) after confirming the assumption of proportionality of hazards. Individuals with prior CVD were excluded for analyses of incident CVD (leaving 2956 eligible participants), but were eligible for mortality analyses. We performed pooled sex analyses to maximize statistical power because tests of interaction for sex with these biomarkers were not statistically significant. The biomarkers were normally distributed and analyzed as continuous variables and as quartiles.
Multivariable analyses adjusted for: age, sex, diabetes, systolic and diastolic BP, treatment for hypertension, smoking, total/high-density lipoprotein (HDL) cholesterol ratio, and serum creatinine. For CVD analyses, we additionally adjusted for BNP and UACR because these two biomarkers have been associated with CVD in our sample.12 For mortality analyses, we adjusted also for prevalent CVD, BNP, UACR, homocysteine, CRP, and renin (given associations of the latter 4 with mortality in our sample).12 We evaluated the contributions of ADMA, L-arginine and Arg/ADMA to prediction of CVD and mortality by evaluating the increment in C-statistic (to models incorporating established risk factors and other biomarkers).12
We examined whether the relations of ADMA, L-arginine and the Arg/ADMA ratio to CVD/mortality varied according to age, obesity (BMI≥30 kg/m2), hypertension, diabetes, smoking, and low eGFR (<60 m/min). A two-sided P<0.05 was considered statistically significant. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The baseline characteristics of our sample are shown in Table 1. ADMA was positively correlated with L-arginine (age-, sex-adjusted r 0.30, p<0.0001).
Table 1. Baseline characteristics of study participants*.
| Characteristic | Men | Women |
|---|---|---|
| (N= 1551) | (N= 1769) | |
| Age, years | 59±10 | 59±10 |
| Smoking (%) | 14 | 16 |
| Serum cholesterol (mg/dl) | ||
| Total | 197±35 | 211±38 |
| High-density lipoprotein | 44±12 | 58±16 |
| Total/HDL Ratio | 4.79±1.37 | 3.91±1.32 |
| Body Mass Index (kg/m2) | 28.5±4.4 | 27.4±5.7 |
| Blood Pressure (mm Hg) | ||
| Systolic | 130±17 | 127±20 |
| Diastolic | 77±10 | 74±9 |
| Hypertension (%) | 35 | 28 |
| Use of antihypertensive agents (%) | 31 | 25 |
| Blood glucose (mg/dl) | 107 (27) | 100 (25) |
| Diabetes mellitus (%) | 14 | 9 |
| Serum creatinine (mg/dl) | 1.10±0.17 | 0.95±0.15 |
| eGFR (ml/min) | 87±24 | 86±26 |
| Prevalent CVD (%) | 15 | 7 |
| Median Biomarker Levels (25th percentile – 75th percentile) | ||
| ADMA (μmol/l) | 0.54 (0.47-0.62) | 0.51 (0.46-0.61) |
| L-Arginine (μmol/l) | 78.0 (66.8-91.0) | 75.7 (64.1-88.6) |
| Arg/ADMA | 146.0 (120.0-174.6) | 144.0 (120.0-172.1) |
| CRP (mg/dl) | 1.8 (0.9-3.8) | 2.3 (1.0-5.7) |
| BNP (pg/ml) | 6.7 (4.0-17.2) | 10.0 (4.0-20.3) |
| Renin (mU/l) | 14.0 (8.0-25.0) | 11.0 (6.0-19.0) |
| Homocysteine (μmol/l) | 9.8 (8.3-11.8) | 8.4 (7.0-10.3) |
| UACR (mg/g) | 4.9 (2.2-10.9) | 8.6 (3.6-17.5) |
Values are means±SD. ADMA, asymmetric dimethylarginine; Arg/ADMA, ratio of plasma L-arginine to ADMA; BNP, b-type natriuretic peptide; CRP, C-reactive protein; eGFR, glomerular filtration rate; UACR, urinary albumin-to-creatinine excretion ratio.
Cross-sectional correlates of ADMA
ADMA was associated positively with age, BMI and smoking but inversely related to diastolic BP (Table 2). Importantly, ADMA was not related to renal function. L-arginine was positively related to age, male sex, smoking and eGFR, but inversely associated with BMI, diabetes, and alcohol consumption. The Arg/ADMA ratio was positively related to male sex and inversely associated with age, BMI, diabetes and alcohol consumption. Clinical variables explained only about 3.5% of interindividual variation in ADMA. All three biomarkers studied were only weakly associated with other novel biomarkers included in multivariable models (all pair-wise r<0.10, except ADMA and homocysteine, r=0.13).
Table 2. Cross-sectional correlates (multivariable linear regression) of ADMA, L-arginine, and the Arg/ADMA ratio.
| Variable | Unit of Increase/Categories | Regression Coefficient | P Value |
|---|---|---|---|
| ADMA | |||
| Age | 10 year | 0.02 | <0.0001 |
| BMI | 1 unit | 0.002 | <0.0001 |
| Diastolic blood pressure | 10 mm Hg | -0.007 | 0.0044 |
| Smoking | Smoker vs. non-smoker | 0.015 | 0.0137 |
| L-Arginine | |||
| Age | 10 year | 1.113 | 0.0054 |
| Sex | Male vs. female | 2.946 | <0.0001 |
| BMI | 1 unit | -0.170 | 0.0166 |
| Diabetes | Present vs. absent | -4.933 | <0.0001 |
| Smoking | Smoker vs. non-smoker | 3.848 | 0.0002 |
| Alcohol consumption | Drinks/week | -0.260 | 0.0117 |
| eGFR | 1 unit | 0.037 | 0.0180 |
| Arg/ADMA Ratio | |||
| Age | year | -4.921 | <0.0001 |
| Sex | Male vs. female | 3.606 | 0.0242 |
| BMI | 1 unit | -0.845 | <0.0001 |
| Diabetes | Present vs. absent | -5.205 | 0.0387 |
| Alcohol consumption | Drinks/week | -0.689 | 0.0019 |
Independent variables reported are those that remained in the model after stepwise backward elimination analysis and were statistically significant in the final model (p<0.05). Independent variables were chosen on the basis of significant univariate associations and pathophysiological mechanisms. R2 of the final model was 0.0347 for ADMA, 0.0179 for L-arginine, 0.0247 for the Arg/ADMA ratio.
BMI denotes body mass index. eGFR denotes estimated glomerular filtration rate.
Relations of ADMA to CVD incidence
Over a follow-up period of 10.9 years, there were 281 incident CVD events (119 in women) among 2956 individuals at risk. Neither of the biomarkers nor their ratio was associated with CVD incidence (Table 3). These results were similar to multivariable analyses that did not adjust for novel biomarkers (data not shown). There was no effect modification by age, sex, hypertension, obesity, diabetes, smoking, or eGFR. With our sample size and at an alpha of 0.05, we had good power (82% and 96%) to detect hazard ratios (HR) per SD increment of 1.5 and 1.75, respectively. We had limited power to detect HR smaller than 1.5.
Table 3. Biomarkers and CVD Risk.
| Number of Events/number at risk (%) | Unadjusted Hazard Ratio (95% CI) | p | Multivariable-adjusted Hazard Ratio (95%CI)* | p | |
|---|---|---|---|---|---|
| ADMA | |||||
| ADMA (per SD increase) | 1.00 (0.90-1.12) | 0.94 | 0.92 (0.82, 1.05) | 0.21 | |
| Quartile 1 | 54/754 (7.2) | Referent | - | Referent | - |
| Quartile 2 | 63/746 (8.5) | 1.19 (0.83-1.71) | 0. 35 | 1.05 (0.70-1.55) | 0.83 |
| Quartile 3 | 87/744 (11.7) | 1.34 (0.95-1.88) | 0.10 | 1.17 (0.80-1.70) | 0.42 |
| Quartile 4 | 77/712 (10.8) | 1.00 (0.71-1.42) | 0.998 | 0.85 (0.58-1.25) | 0.41 |
| Trend | 1.00 (0.90-1.11) | 0.97 | 0.96 (0.85-1.08) | 0.47 | |
| L-arginine | |||||
| L-Arg (per SD increase) | 1.01 (0.91-1.12) | 0.85 | 0.99 (0.87-1.11) | 0.83 | |
| Quartile 1 | 57/727 (7.8) | Referent | - | Referent | - |
| Quartile 2 | 65/757 (8.6) | 1.26 (0.88-1.79) | 0.21 | 1.25 (0.85-1.83) | 0.26 |
| Quartile 3 | 82/753 (10.9) | 1.17 (0.84-1.65) | 0.35 | 1.04 (0.72-1.51) | 0.84 |
| Quartile 4 | 77/719 (10.7) | 1.12 (0.80-1.58) | 0.52 | 0.95 (0.65-1.38) | 0.77 |
| Trend | 1.02 (0.92-1.14) | 0.66 | 0.97 (0.86-1.09) | 0.56 | |
| Arg/ADMA | |||||
| Arg/ADMA (per SD increase) | 0.98 (0.88-1.09) | 0.70 | 1.01 (0.91-1.14) | 0.80 | |
| Quartile 1 | 74/725 (10.2) | Referent | referent | ||
| Quartile 2 | 63/730 (8.6) | 0.94 (0.67-1.32) | 0.72 | 0.94 (0.64-1.37) | 0.74 |
| Quartile 3 | 77/752 (10.2) | 1.07 (0.78-1.48) | 0.66 | 1.10 (0.77-1.56) | 0.60 |
| Quartile 4 | 67/749 (9.0) | 0.99 (0.71-1.38) | 0.96 | 1.17 (0.81-1.69) | 0.40 |
| Trend | 1.01 (0.91-1.12) | 0.84 | 1.06 (0.95-1.20) | 0.30 | |
adjusted for age, sex, systolic and diastolic BP, hypertension treatment, smoking, diabetes, total/HDL cholesterol, creatinine, log-BNP and log-UACR. N=2,544 for the multivariable adjusted model. Mean (SD) was 0.54 (0.13) μmol/l for ADMA, 78.8 (20.8) μmol/l for L-arginine, and 149.8 (44.6) for the Arg/ADMA ratio.
Relations of ADMA to all-cause mortality
On follow-up, there were 285 deaths among the 3320 at-risk participants. Mortality rose across ADMA quartiles but declined with increasing Arg/ADMA ratio (Table 4). While ADMA was positively associated with mortality, L-arginine was not (Table 4). The Arg/ADMA ratio was inversely associated with mortality, which was especially evident for the fourth quartile. In analyses adjusting for all covariates other than the novel biomarkers, results were similar to those shown in Table 4 (data not shown). Figure 1 shows the relations of ADMA, L-Arginine and Arg/ADMA ratio with mortality using regression splines.
Table 4. Biomarkers and Mortality Risk.
| Number of Events/number at risk (%) | Unadjusted Hazards Ratio (95% CI) | p | Multivariable-adjusted Hazards Ratio (95%CI)* | p | |
|---|---|---|---|---|---|
| ADMA | |||||
| ADMA (per SD increase) | 1.10 (0.99-1.22) | 0.09 | 1.21 (1.07-1.37) | 0.003 | |
| Quartile 1 | 50/832 (6.0) | Referent | - | Referent | - |
| Quartile 2 | 61/827 (7.4) | 1.23 (0.85-1.79) | 0.27 | 1. 08 (0.71-1.64) | 0.71 |
| Quartile 3 | 79/834 (9.5) | 1.38 (0.97-1.97) | 0.07 | 1.15 (0.77-1.71) | 0.51 |
| Quartile 4 | 95/827 (11.5) | 1.33 (0.95-1.88) | 0.10 | 1.53 (1.05-2.23) | 0.03 |
| Trend | 1.09 (0.98-1.21) | 0.08 | 1.15 (1.02-1.30) | 0.02 | |
| L-arginine | |||||
| L-Arg (per SD increase) | 0.87 (0.77-0.98) | 0.02 | 0.89 (0.77-1.02) | 0.08 | |
| Quartile 1 | 71/821 (8.7) | Referent | - | Referent | - |
| Quartile 2 | 64/833 (7.7) | 1.03 (0.73-1.44) | 0.88 | 0.96 (0.66-1.41) | 0.85 |
| Quartile 3 | 88/850 (10.4) | 0.98 (0.71-1.34) | 0.88 | 0.86 (0.60-1.24) | 0.43 |
| Quartile 4 | 62/816 (7.6) | 0.70 (0.50-0.98) | 0.04 | 0.73 (0.50-1.07) | 0.10 |
| Trend | 0.90 (0.81-0.997) | 0.04 | 0.90 (0.80-1.02) | 0.09 | |
| Arg/ADMA | |||||
| Arg/ADMA (per SD increase) | 0.82 (0.72-0.94) | 0.003 | 0.80 (0.69-0.93) | 0.004 | |
| Quartile 1 | 92/824 (11.2) | Referent | - | Referent | - |
| Quartile 2 | 77/827 (9.3) | 0.85 (0.63-1.15) | 0.30 | 0.76 (0.53-1.07) | 0.12 |
| Quartile 3 | 70/835 (8.4) | 0.85 (0.62-1.16) | 0.30 | 0.69 (0.48-1.01) | 0.05 |
| Quartile 4 | 46/834 (5.5) | 0.52 (0.36-0.74) | 0.0003 | 0.49 (0.33-0.74) | 0.0007 |
| Trend | 0.83 (0.75-0.93) | 0.0006 | 0.80 (0.71-0.91) | 0.0007 | |
adjusted for age, sex, systolic and diastolic BP, hypertension treatment, smoking, diabetes, total/HDL cholesterol, creatinine, prevalent CVD, and logBNP, log renin, log Hcy, log UACR, and log CRP. N=2,750 for the multivariable adjusted model. Mean (SD) was 0.55 (0.13) μmol/l for ADMA, 78.9 (20.7) μmol/l for L-arginine, and 149.4 (44.4) for the Arg/ADMA ratio.
Figure 1.

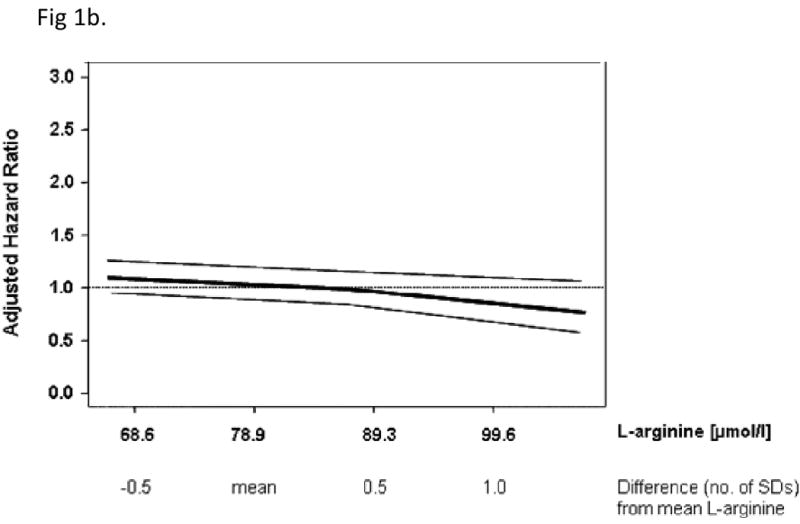
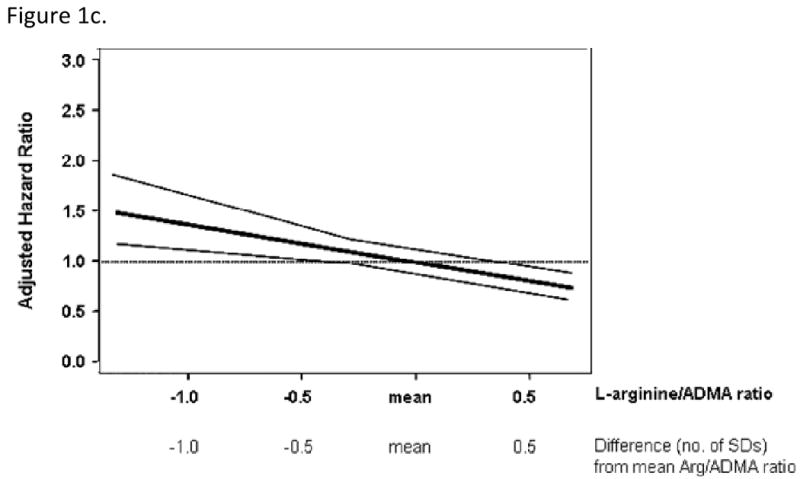
Spline graphs displaying the relationship between mortality and ADMA (panel A), L-arginine (panel B) and the Arg/ADMA ratio (panel C) at baseline. Mean (SD) were: ADMA, 0.55 (0.13) μmol/l; L-arginine, 78.9 (20.7) μmol/l; L-arginine/ADMA, 149.4 (44.4). Data are expressed as SD difference from the mean for each biomarker. The bold line shows the association of each biomarker with mortality risk, 95% CI are plotted as fine grey lines. The horizontal, grey line is the line of no association between the biomarker and risk.
Neither ADMA nor the Arg/ADMA ratio improved the model C-statistic incrementally over traditional risk factors (C for models without and with ADMA 0.772 and 0.768, respectively; C for models without and with Arg/ADMA 0.772 and 0.775, respectively), or when other novel biomarkers were added (C for models without and with ADMA 0.805 and 0.801, respectively; C for models without and with Arg/ADMA 0.805 and 0.804, respectively).
As the pathogenetic mechanism relates to a molecular interaction between ADMA and L-arginine, we modelled these biomarkers together as quartiles (instead of as a ratio) for display purposes (Figure 2). The increase in mortality risk with increasing ADMA was consistent across L-arginine quartiles. However, this trend was less clear for L-arginine; the highest mortality risk was associated with high ADMA and low L-arginine, whereas the lowest mortality risk was associated with low ADMA and high L-arginine, respectively.
Figure 2.
Hazards ratio for death according to cross-classification of quartiles of plasma ADMA and L-arginine at baseline. The first quartile of ADMA and fourth quartile of L-arginine were defined as referent.
In secondary analyses, we evaluated the associations of the 3 biomarkers to CVD mortality versus non-CVD mortality (Appendix Tables A and B). We observed strong and statistically significant associations of ADMA (positive) and the Arg/ADMA ratio (inverse) to non-CVD mortality, but did not find a significant association with CVD mortality. Of note, the lower proportion of deaths due to CVD in these analyses is because of an emphasis on specificity in the adjudication process in the Framingham study, which may have limited our statistical power.
Interaction between ADMA and diabetes for mortality risk
Despite the fact that people with and without diabetes had similar mean ADMA levels (0.553 [SD 0.13] μmol/l versus 0.546 [0.126] μmol/l; p=NS), we observed significant interactions between ADMA (and Arg/ADMA ratio) and diabetes for mortality risk (p = 0.03 for both); no such interaction was evident for L-arginine. Therefore, the analyses of mortality for ADMA and the Arg/ADMA ratio were stratified by diabetes status (Table 5). Of 285 deaths, 216 occurred in subjects without diabetes (N=2,948), and 69 deaths occurred among individuals with diabetes (N=372). In multivariable analyses, ADMA and the Arg/ADMA ratio were associated with mortality in participants without diabetes but not in participants with diabetes.
Table 5a. ADMA and Mortality Risk in Participants with Diabetes versus those without Diabetes.
| No. events/no. at risk (%) | Unadjusted Hazards Ratio (95% CI) | p | Multivariable-adjusted Hazards Ratio (95%CI)* | p | |
|---|---|---|---|---|---|
| Subjects with no Diabetes (n=2,948) | |||||
| ADMA (per SD increase) | 1.16 (1.03-1.31) | 0.02 | 1.30 (1.13-1.50) | 0.0002 | |
| Quartile 1 | 36/746 (4.8) | Referent | Referent | - | |
| Quartile 2 | 41/737 (5.6) | 1.16 (0.74-1.82) | 0.51 | 1.04 (0.63-1.73) | 0.88 |
| Quartile 3 | 62/736 (8.4) | 1.62 (1.07-2.44) | 0.02 | 1.44 (0.90-2.31) | 0.12 |
| Quartile 4 | 77/729 (10.6) | 1.45 (0.97-2.15) | 0.07 | 1.75 (1.13-2.72) | 0.01 |
| Trend | 1.14 (1.01-1.28) | 0.04 | 1.23 (1.07-1.41) | 0.004 | |
| Subjects with Diabetes (n=372) | |||||
| ADMA (per SD increase) | 0.92 (0.74-1.15) | 0.47 | 0.85 (0.62-1.16) | 0.30 | |
| Quartile 1 | 14/86 (16.3) | Referent | Referent | - | |
| Quartile 2 | 20/90 (22.2) | 1.25 (0.63-2.49) | 0.52 | 0.84 (0.37-1.91) | 0.68 |
| Quartile 3 | 17/98 (17.4) | 0.83 (0.41-1.69) | 0.61 | 0.44 (0.18-1.07) | 0.07 |
| Quartile 4 | 18/98 (18.4) | 0.98 (0.48-1.97) | 0.94 | 0.71 (0.30-1.71) | 0.45 |
| Trend | 0.95 (0.76-1.18) | 0.62 | 0.84 (0.63-1.12) | 0.24 | |
adjusted for age, sex, systolic and diastolic BP, hypertension treatment, smoking, total/HDL cholesterol, creatinine, prevalent CVD, logBNP, log renin, log Hcy, log UACR, and log CRP.
SD in Subjects with no Diabetes: ADMA=0.13
SD in Subjects with Diabetes: ADMA=0.13
Discussion
Principal Findings
First, higher ADMA and a lower Arg/ADMA ratio were associated with mortality in our community-based sample. Regression splines confirmed these linear relations. The strength of the association (increase in risk of 21% per SD increase [0.13 μmol/l] in ADMA) is comparable to that for a 4.2-year (0.4 SD) increase in age in our sample. However, we observed no incremental contribution of ADMA to prediction of mortality risk. Prior observations indicate modest increments to the C-statistic with the addition of biomarkers.12 Accordingly, we do not propose ADMA as a predictive marker for mortality, but as an important pathophysiological mechanism. Second, the association of ADMA with mortality was strongest in participants without diabetes, whereas there was no association in those with diabetes. Third, neither ADMA nor L-arginine (or their ratio) was associated with CVD incidence in our sample.
Potential Mechanisms Underlying the Association with Mortality
ADMA is an endogenous inhibitor of all three NOS. ADMA impairs NO production, possibly by competing with L-arginine for the substrate-binding site of NOS.19
Clinical studies indicate that reduced NO release in the presence of elevated ADMA results in endothelial dysfunction that is reversible by L-arginine.20,21 A recent study demonstrated that ADMA is a strong determinant of flow-mediated, endothelium-dependent vasodilation in healthy people,22 similar to findings reported in hypercholesterolemic individuals.20 Patients with endothelial dysfunction are at increased risk of CVD/death.23 A systemic infusion of ADMA results in increased peripheral resistance, elevated BP, and impaired cerebral blood flow in healthy people.24,25 Recent studies also indicate that higher ADMA increases mortality risk in patients who are critically ill due to cardiac (e.g., cardiogenic shock),26 or non-cardiac illnesses (e.g., septic shock),27 suggesting that depletion of arginine accompanied by increased ADMA may be a marker of mortality risk due to cardiovascular or non-cardiovascular causes. In secondary analyses, we observed an association of higher ADMA with non-CVD mortality but not with CVD mortality. The latter observations are hypothesis-generating and warrant evaluation in further studies with a greater number of outcome events.
Interestingly enough, mice that lack all three NOS isoforms develop spontaneous myocardial infarction, renal disease, ileus, and glucose intolerance more frequently than wild-type mice or mice lacking a single NOS isoform.28 As ADMA is an unselective inhibitor of all three NOS isoforms, slight changes in its concentration may affect multi-organ function much more than that anticipated from reduced eNOS activity alone – a phenomenon which might as well explain the stronger association of ADMA with mortality than with incident CVD in our cohort. Indeed, recent data show that even small changes of ADMA in the extracellular space, which correspond to the difference between the lowest and highest quartiles of ADMA, or to those between wild-type versus DDAH-transgenic mice, are sufficient to induce significant changes in the Arg/ADMA ratio and in NOS activity in cultured endothelial cells.21
In a recent study, ADMA was predictive of mortality in patients with coronary disease (CAD), but not in those without CAD.29 In the Population Study of Women in Gothenburg, ADMA was associated with a HR of 1.12 for mortality in healthy women,30 which is consistent with our study.
Experimental studies have substantiated the adverse consequences of higher ADMA. Genetically-modified mice that overexpress dimethylarginine dimethylaminohydrolase-1 (DDAH-1, the enzyme that clears ADMA), show enhanced NO production,31 whereas heterozygous DDAH-1 knockout mice show impaired NO signalling and pulmonary hypertension, and homozygotes are not viable.32
Taken together these data may explain the relation between high ADMA and mortality in our cohort. Our data suggest that ADMA may be a marker of mortality risk, but its role as a causal factor or as a promising therapeutic target merits further study.
Lack of Association with CVD Incidence: Comparison with the Literature
We did not observe an association of ADMA with CVD incidence. This may be related to a true lack of association, or to a limited statistical power to discern modest hazards in our sample. In two recent studies10,11 ADMA was positively related with coronary events in non-smoking men. A recent population-based study of women revealed that those in the highest ADMA quintile (>0.71 μmol/l) had a 29% increased risk of myocardial infarction and stroke over 24 years of follow-up;30 however, the incidence curves for women with ADMA above versus below this threshold did not separate until 13 years of follow-up. It is possible that the follow-up period in our study was too short to demonstrate a significant increase in CVD incidence in our sample, which overall had an intermediate risk of vascular events. Previous studies in high-risk samples have shown that ADMA is associated with CVD events in CAD patients,5,7 patients with peripheral arterial occlusive disease,33 chronic renal disease,8 and in unselected patients undergoing major elective surgery.34
ADMA and Mortality: Effect Modification by Diabetes
In pre-specified analyses, we observed significant association of ADMA and the Arg/ADMA ratio with mortality in individuals without diabetes but no association in those with diabetes. There are several possible explanations for these observations. First, studies suggest that in patients with diabetes, ADMA may change with progression of the disease; lower ADMA concentrations are observed during the initial period of mild renal damage and increased GFR,35 whereas levels are higher in diabetes-associated chronic renal failure.36 Second, in some studies the elevation of ADMA in diabetes may actually have been overestimated due to the technical difficulty of separating ADMA and its biologically inactive isomer, symmetric dimethylarginine (SDMA) by chromatography.37 Our method was designed to specifically separate dimethylarginines by differences in their mass spectra rather than by differences in retention times, and thus allows highly reliable and accurate quantitation of ADMA.16 Third, physiological effects of ADMA in diabetes may be complex. In diabetes, the burden of reactive oxygen species (ROS) is generated by hyperglycemia in the vessel wall.38 ROS such as superoxide anion or hydrogen peroxide almost instantly react with NO, forming hazardous species like peroxinitrite and nitroxyl anion. Also, there are conditions in which NOS turns into a generator of oxygen-derived free radicals rather than NO, e.g. during substrate depletion or co-factor deficiency.38,39 There is evidence for enhanced NOS-derived free radical production in diabetes.40,41 ADMA competes with L-arginine for binding to NOS and to the amino acid transporter and thereby reduces intracellular availability of substrate for NOS. Thus, inhibition of NO production by ADMA may result in variable effects in terms of oxidative damage to the vessel wall in diabetes. Additional studies evaluating a larger sample of individuals with diabetes are warranted.
Strengths and Limitations
The strengths of our investigation are its prospective design, longitudinal surveillance for outcomes, and adjustment for novel biomarkers. The biological plausibility that higher ADMA, lower L-arginine or a low Arg/ADMA mediate mortality risk is reflected by the strength of the associations, temporal relations, and the overall consistency of the associations across analyses. Several limitations merit comment. Establishing that ADMA is a ‘risk factor’ for death would require additional mechanistic studies that assess systemic oxidative/nitrosative stress, and cause-specific mortality. The modest sample size of participants with diabetes and limited number of deaths in this group may have limited our statistical power to detect associations in this subgroup. Also, future studies that compare the relative predictive utilities of endothelial function measures versus ADMA may help clarify if factors other than NO availability may influence CVD risk. Lastly, our sample was white of European descent, and comprised of middle-aged individuals with a vascular risk factor profile consistent with this age group, limiting the generalizability to other ethnicities and to younger and more healthy samples.
Conclusions
In our large community-based sample, higher ADMA was significantly associated with all-cause mortality, but not with CVD incidence. This relation was observed in individuals without baseline diabetes, whereas no association was noted in persons with diabetes. Additional investigations are warranted to confirm these observations and elucidate the mechanisms underlying the associations in select strata.
Table 5b. L-Arginine/ADMA Ratio and Mortality Risk in Participants with Diabetes versus those without Diabetes.
| No. events/no. at risk (%) | Unadjusted Hazards Ratio (95% CI) | p | Multivariable-adjusted Hazards Ratio (95%CI)* | p | |
|---|---|---|---|---|---|
| Subjects with no Diabetes (n=2,948) | |||||
| ARG/ADMA (per SD increase) | 0.77 (0.66-0.90) | 0.0009 | 0.72 (0.60-0.87) | 0.0005 | |
| Quartile 1 | 66/697 (9.5) | Referent | Referent | - | |
| Quartile 2 | 62/735 (8.4) | 0.86 (0.61-1.22) | 0.40 | 0.68 (0.45-1.02) | 0.06 |
| Quartile 3 | 57/765 (7.5) | 0.86 (0.60-1.22) | 0.39 | 0.66 (0.43-1.00) | 0.05 |
| Quartile 4 | 31/751 (4.1) | 0.43 (0.28-0.67) | 0.0001 | 0.37 (0.23-0.62) | 0.0001 |
| Trend | 0.80 (0.71-0.90) | 0.0003 | 0.75 (0.65-0.87) | 0.0002 | |
| Subjects with Diabetes (n=372) | |||||
| ARG/ADMA (per SD increase) | 0.99 (0.80-1.22) | 0.91 | 1.07 (0.82-1.40) | 0.62 | |
| Quartile 1 | 26/127(20.5) | Referent | Referent | - | |
| Quartile 2 | 15/92 (16.3) | 0.77 (0.41-1.46) | 0.42 | 0.79 (0.35-1.75) | 0.56 |
| Quartile 3 | 13/70 (18.6) | 0.87 (0.44-1.70) | 0.67 | 0.59 (0.24-1.45) | 0.25 |
| Quartile 4 | 15/83 (18.1) | 0.90 (0.48-1.71) | 0.75 | 0.97 (0.43-2.16) | 0.93 |
| Trend | 0.97 (0.79-1.19) | 0.76 | 0.95 (0.73-1.24) | 0.71 | |
adjusted for age, sex, systolic and diastolic BP, hypertension treatment, smoking, total/HDL cholesterol, creatinine, prevalent CVD, logBNP, log renin, log Hcy, log UACR, and log CRP.
SD in Subjects with no Diabetes: Arg/ADMA=43.77
SD in Subjects with Diabetes: Arg/ADMA=48.20
Acknowledgments
The authors thank Mariola Kastner and Anna Steenpaβ for their technical assistance.
Funding/Support: This work was supported through NIH/NHLBI Contract N01-HC-25195, K23-HL074077-01 (Dr. Wang), N01HV28178, and 2K24HL04334 (Dr. Vasan), and by the Deutsche Forschungsgemeinschaft grant Bo 1431/4-1 (Dr. Böger).
Footnotes
Conflict of interest/Disclosures: Drs. Böger, Schwedhelm, and Maas are named as inventors on patents relating to analytical assays for methylarginines and receive royalties from these. No other authors reported financial disclosure.
The endothelium plays a major role in regulating vascular tone by secreting the potent vasodilator, nitric oxide (NO), which is synthesized from L-arginine (Arg) by endothelial nitric oxide synthase (NOS). NOS is competitively inhibited by asymmetric dimethylarginine (ADMA), an endogenous compound that is elevated in renal failure, cardiovascular disease (CVD), and diabetes mellitus. Higher ADMA and low L-arginine to ADMA ratio (Arg/ADMA) are markers of endothelial dysfunction. Prospective investigations have highlighted the role of ADMA as a predictor of CVD events and death in patients with coronary artery disease (CAD), renal failure, and other high-risk conditions. Data are limited regarding the relations of ADMA and Arg/ADMA to CVD incidence and death in the general population.
We related plasma ADMA, L-arginine, and the Arg/ADMA ratio to the incidence of CVD and death in 3319 participants from the community-based Framingham Study cohort who were followed for 10.9 years. In multivariable models adjusting for established risk factors, ADMA was associated positively with mortality while the Arg/ADMA ratio was inversely related. We noted effect modification by diabetes status: ADMA was associated with mortality in individuals without diabetes but not in individuals with diabetes. ADMA and the Arg/ADMA ratio were not associated with CVD incidence. Additional studies evaluating a larger sample of individuals (including those with diabetes) and with longer follow-up are warranted to confirm these observations.
References
- 1.Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–6. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 3.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–33. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 4.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 5.Krempl TK, Maas R, Sydow K, Meinertz T, Boger RH, Kahler J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur Heart J. 2005;26:1846–51. doi: 10.1093/eurheartj/ehi287. [DOI] [PubMed] [Google Scholar]
- 6.Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912–9. doi: 10.1016/j.ehj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e53–e59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frolich J, Boger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–7. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 9.Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–36. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 10.Maas R, Schulze F, Baumert J, Lowel H, Hamraz K, Schwedhelm E, Koenig W, Boger RH. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clin Chem. 2007;53:693–701. doi: 10.1373/clinchem.2006.081893. [DOI] [PubMed] [Google Scholar]
- 11.Valkonen VP, Paiva H, Salonen JT, Lakka TA, Lehtimaki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–8. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D′Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Wolf PA, Garrison RJ, editors. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. Framingham Heart Study, 30 year follow-up. Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 15.Schwedhelm E, Tan-Andresen J, Maas R, Riederer U, Schulze F, Boger RH. Liquid chromatography-tandem mass spectrometry method for the analysis of asymmetric dimethylarginine in human plasma. Clin Chem. 2005;51:1268–71. doi: 10.1373/clinchem.2004.046037. [DOI] [PubMed] [Google Scholar]
- 16.Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Boger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:211–9. doi: 10.1016/j.jchromb.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables. (B).J Royal Stat Soc. 1972;34:187–220. with discussion. [Google Scholar]
- 19.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- 20.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 21.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–87. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 22.Ardigo D, Stuehlinger M, Franzini L, Valtuena S, Piatti PM, Pachinger O, Reaven GM, Zavaroni I. ADMA is independently related to flow-mediated vasodilation in subjects at low cardiovascular risk. Eur J Clin Invest. 2007;37:263–9. doi: 10.1111/j.1365-2362.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 23.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 24.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–9. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 25.Kielstein JT, Impraim B, Simmel S, Bode-Boger SM, Tsikas D, Frolich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–7. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls SJ, Wang Z, Koeth R, Levison B, DelFraino B, Dzavik V, Griffith OW, Hathaway D, Panza JA, Nissen SE, Hochman JS, Hazen SL. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–24. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- 27.O′Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care. 2006;10:R139. doi: 10.1186/cc5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakata S, Tsutsui M, Shimokawa H, Suda O, Morishita T, Shibata K, Yatera Y, Sabanai K, Tanimoto A, Nagasaki M, Tasaki H, Sasaguri Y, Nakashima Y, Otsuji Y, Yanagihara N. Spontaneous Myocardial Infarction in Mice Lacking All Nitric Oxide Synthase Isoforms. Circulation. 2008;117:2211–23. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- 29.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–83. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 30.Leong T, Zylberstein D, Graham I, Lissner L, Ward D, Fogarty J, Bengtsson C, Bjorkelund C, Thelle D. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol. 2008;28:961–7. doi: 10.1161/ATVBAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 31.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–7. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 32.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O′Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 33.Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, Minar E, Muller M, Wolzt M, Schillinger M. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–40. doi: 10.1161/01.ATV.0000242801.38419.48. [DOI] [PubMed] [Google Scholar]
- 34.Maas R, Dentz L, Schwedhelm E, Thoms W, Kuss O, Hiltmeyer N, Haddad M, Kloss T, Standl T, Boger RH. Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine predict adverse events in patients undergoing noncardiac surgery. Crit Care Med. 2007;35:1876–81. doi: 10.1097/01.CCM.0000277038.11630.71. [DOI] [PubMed] [Google Scholar]
- 35.Paiva H, Lehtimaki T, Laakso J, Ruokonen I, Rantalaiho V, Wirta O, Pasternack A, Laaksonen R. Plasma concentrations of asymmetric-dimethyl-arginine in type 2 diabetes associate with glycemic control and glomerular filtration rate but not with risk factors of vasculopathy. Metabolism. 2003;52:303–7. doi: 10.1053/meta.2003.50048. [DOI] [PubMed] [Google Scholar]
- 36.Krzyzanowska K, Mittermayer F, Shnawa N, Hofer M, Schnabler J, Etmuller Y, Kapiotis S, Wolzt M, Schernthaner G. Asymmetrical dimethylarginine is related to renal function, chronic inflammation and macroangiopathy in patients with Type 2 diabetes and albuminuria. Diabet Med. 2007;24:81–6. doi: 10.1111/j.1464-5491.2007.02018.x. [DOI] [PubMed] [Google Scholar]
- 37.Schwedhelm E. Quantification of ADMA: analytical approaches. Vasc Med. 2005;10 1:S89–S95. doi: 10.1177/1358836X0501000113. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard KA, Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–8. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 39.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387:1521–33. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- 40.Dixon LJ, Hughes SM, Rooney K, Madden A, Devine A, Leahey W, Henry W, Johnston GD, McVeigh GE. Increased superoxide production in hypertensive patients with diabetes mellitus: role of nitric oxide synthase. Am J Hypertens. 2005;18:839–43. doi: 10.1016/j.amjhyper.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]



