Abstract
Two kinds of border guards control the incessant traffic of ions across cell membranes: ion channels and ion pumps. When open, channels let selected ions diffuse rapidly down electrical and concentration gradients, whereas ion pumps labour tirelessly to maintain the gradients, by consuming energy to slowly move ions against them. Because of their diametrically opposed tasks and their divergent speeds, channels and pumps have traditionally been viewed as completely different entities, as alike as chalk and cheese. But new structural and mechanistic information about both classes of these molecular machines challenges this comfortable separation, forcing its reevaluation.
Precisely controlled movements of ions into and out of cells and organelles are essential for all life. For example, in cells ion flows mediate processes as disparate as signalling, pH balance, volume regulation, and the cell cycle, and in higher organisms they underlie fertilization, immune responses, secretion, muscle contraction, and all electrical signals in nerves, muscles, and synapses. The proteins that transport ions across membranes fall into two general classes: passive conduits called ion channels, through which ions rush down gradients of concentration and electric potential, and pumps that release energy from ATP or other source to actively push ions against those gradients and so build them up.
Ion flow through channels generates transmembrane electric currents. Currents of Na or K ions cause changes in membrane potential that act as physical signals, whereas for Ca currents the Ca ions themselves usually represent the signal, in that case chemical. And currents of Cl ions tend to stabilize membrane potentials, e.g. at inhibitory synapses and in skeletal muscle fibers, or to facilitate transmembrane salt and water movement. Because ion flow through channels dissipates the very gradients that drive it, channels contain gates regulated to turn the ion flow on only when needed.
“Pumps” encompasses all transporters capable of thermodynamically uphill transport (so-called active transport). Ion pumps that hydrolyze ATP, ATPases, are sometimes called primary pumps to distinguish them from secondary pumps. The latter exploit the energy stored in ion (often Na) electrochemical gradients by coupling thermodynamically downhill movements of those ions to drive the uphill transport of another substrate; secondary pumps are sometimes called co-transporters, or counter-transporters or exchangers, according to the relative directions of the coupled downhill and uphill ion flows.
The very different behaviours of ion channels and ion pumps – passive, thermodynamically downhill, and high speed ion movement through channels, versus active, thermodynamically uphill transport, frequent incorporation of enzyme-like reaction mechanisms, and low speed of ion movement through pumps – led to a separation of the efforts to understand them. And only recently have atomic-resolution X-ray crystal structures and high-resolution functional measurements of examples from both classes begun to suggest that ion channels and ion pumps are not as different as once thought and, in fact, have much in common. So now seems an appropriate time to reconsider their similarities and differences. This review begins with a theoretical appraisal of what distinguishes channels from pumps, then examines clear-cut examples from each class, addresses how one kind might be transformed into the other, evaluates a protein family that straddles the increasingly blurred boundary between the two, and ends with a look at apparently hybrid molecules. Only a few instructive examples from the many different kinds of ion channels1 and pumps (e.g., http://www.tcdb.org/) are chosen for discussion, and their myriad roles in biology are not covered at all.
One gate versus two gates
The principal difference, in principle, between channels and pumps is that a channel needs no more than a single gate whereas a pump needs at least two gates that should never be open at once. So what is a gate? A gate can be considered to be the part of the protein that precludes ion movement along the translocation pathway in the prohibitive conformation but not in the permissive conformation. Progress in determining the locations and structures of gates and the mechanisms of gating remains slow, and is a major unsolved problem in the field of ion transport. Nevertheless, it is unlikely that any door-shaped gate like those in the diagrams of Fig. 1 will be found in nature. As we will see below, examples of gates identified so far include a single amino-acid side chain in chloride-ion transport proteins of the ClC family, and an iris-like bundle crossing of four transmembrane helices in potassium-ion channels.
Figure 1. Ion channels versus ion pumps, in principle.

a/, Schematic representation of an ion channel as membrane-spanning pore through which movement of ions (red circles) is controlled by a single gate, cartooned here as a hinged door. b/, Ion pump as membrane-spanning pore with two gates that open and close alternately. Coupling of an energy source to switch the relative binding affinity for red vs. blue ions between the left-and right-hand states enables active exchange of red for blue ions across the membrane. c/, Occluded states, with both gates closed around bound ions, preclude inadvertent opening of the second gate before closure of the first gate, which would otherwise allow ions to flow down their electrochemical potential gradient several orders of magnitude faster than the pump can move them against that gradient; pumped ion movement is rate limited by the gating reactions, rather than by electrodiffusion. [Modified with permission from refs. 65, 84 ]
How does the one-gate-versus-two-gates principle work? As depicted in Fig. 1a, an ion channel can be viewed as a selective, hydrophilic and hence energetically favourable, pathway for conducting the chosen ions through the hostile hydrophobic interior of the membrane's phospholipid bilayer1. Passage of ions through the channel is controlled by a gate, whose opening and closing is modulated by appropriate signals. The direction ions move in depends on the sign of their charge, the magnitude and direction of their transmembrane concentration gradient, and the size and sign of the transmembrane electric potential difference. Net flow of an ion through a channel is always down its electrochemical potential gradient. Tens of millions of ions per second can cross the membrane through just one open ion channel, a rate large enough for sensitive amplifiers to record the resulting electric current in a single channel2 (for examples, see below). Sudden starting and stopping of that current reveals the timing of individual channel opening and closing events, which occur comparatively infrequently, typically after intervals on the timescale of several milliseconds. This means that the protein conformational changes that open and close the channel gate take place on the order of a hundred times per second.
Though a single gate suffices to control ion flow through a channel, many have more than one1. For example, voltage-gated sodium-, or calcium-, or potassium-ion channels are opened when a change of membrane potential displaces voltage sensors connected to a cytoplasmic-side “activation gate”, and they can be closed by reversal of those displacements (“de-activation”) in response to an opposite change of membrane voltage. But even with their activation gates in the permissive position, the ion pathway through those channels can by closed by a separate gating process called “inactivation”. Both gates must be in their permissive positions for the channel to conduct ions, but closure of either gate precludes ion flow.
An ion pump can similarly be viewed as an ion-selective pathway, but one to which access is controlled by two gates whose opening and closing is timed so that they are never both open simultaneously3 (Fig. 1b). Instead, the gates open and close alternately, allowing the chosen ions to enter the pathway from one side of the membrane while one of the gates is open, and then to leave at the other side of the membrane while the other gate is open, after the first gate has shut4-6. The speed of ion movement through pumps is thus limited by the gating reactions, which, like those of ion channels, occur with frequencies around a hundred times per second. This accounts for the several orders of magnitude slower ion transport through pumps than through open channels. A practical consequence of this lower speed is that, even if a pump moves net charge across the membrane and so generates an electric current, that current will be far too small to be detectable for a single pump molecule. The vast difference in the rates of ion movements mediated by channels and pumps, together with the different directions of those ion movements -- downhill, dissipating the gradients, for channels, and uphill, generating the gradients, for pumps -- are the major reasons that channels and pumps have been considered unrelated.
But the one-gate-versus-two-gates formalism of Fig. 1a,b lays bare their relatedness. It emphasizes that the crux of what distinguishes a pump from a channel is the timing of the closure of the first gate with respect to the opening of the second gate. The far faster ion flow downhill through an open channel than uphill through alternating gating of an ion pump means that any communication failure between a pump's two gates that left both momentarily open would allow channel-like dissipative ion flow that would quickly undo the pump's hard work. The briefest period of simultaneously open gates, even for less than 0.001% of the time given the above-mentioned typical transport rates, would render the pump useless. In fact, the resulting molecule would function like ion channels that include separate gates for activation and inactivation. Because the consequences of such a communication breakdown between their gates would be so catastrophic, pumps have evolved a fail-safe mechanism (Fig. 1c) in which both gates first close, occluding the bound ions inside the protein (see below), before the second gate opens to release them.
How well does this formalism hold?
The one-gate-versus-two-gates formalism is heuristically useful, but how well does it hold up in real life? X-ray crystal structures and detailed functional measurements provide incontrovertible evidence for the existence of honest-to-goodness examples of both ion channels and ion pumps.
Bona fide ion channel exemplars
X-ray crystal structures have been obtained for several kinds of K ion channels, which serve as examples of ion channels that have been exquisitely refined by evolution. These K-ion channels are all tetramers, and they all contain an axial pore with conserved architecture. The pore comprises a narrow selectivity filter that spans the outer third of the distance across the membrane and contains closely-spaced binding sites for a queue of K ions (4 green spheres in Fig. 2a); the selectivity filter leads to a water-filled cavity, containing another K ion, near the middle of the membrane and then the pore narrows again until it becomes closed to ion flow by converging helices near the intracellular surface7.
Figure 2. Crystal structures of a real channel and a real pump reveal clear differences.

a/ The transmembrane regions of three subunits of a KcsA K-channel tetramer (front subunit removed for clarity) show the narrow axial selectivity filter (yellow sections of α-carbon chain), containing four closely-spaced sites for K ions (green spheres), towards the extracellular side of the membrane, and a fifth K ion in a large cavity half-way across the membrane; the single gate (activation gate) is near the cytoplasmic side. Horizontal lines mark approximate membrane boundaries. [Reproduced with permission from ref. 9]. b/ Na,K-ATPase pump complex in E2P-related conformation (with MgF42- as stable phosphate mimic; pink spheres in cytoplasmic phosphorylation, P, domain), containing two Rb ions (purple spheres) occluded within the transmembrane domain. Labels indicate the catalytic α-subunit (with grey membrane-spanning, blue phosphorylation, red nucleotide-binding, and yellow actuator, domains), auxiliary β-subunit (green), and regulatory γ-subunit (red helix). [Modified with permission from ref. 20].
The K ions in the selectivity filter are dehydrated but each one sits at the centre of a cage of eight backbone carbonyl oxygens that are arranged just like the oxygens of the waters that contact, and solvate, the K ion in the large central cavity8. Evidently, the selectivity filter structure has evolved to compensate for the large energetic cost of dehydrating K ions so that they can be selectively transported across the lipid bilayer. Because the selectivity filter so closely mimics the inner hydration shell of K ions in solution, an open K-ion channel can conduct hundreds of millions of K ions per second, essentially as fast as they can diffuse up to the mouth of the channel9. And it can do that while excluding Na ions, with a discrimination ratio of around 1000 to 1 (e.g., refs. 1,7).
No overt gate-like structure is seen at the extracellular end of the pore near the selectivity filter (but see ref. 8), and the main gate is formed near the intracellular end where the four inner transmembrane helices approach each other (Fig. 2a). In different kinds of K channels, the disposition of that so-called activation gate can be controlled by a variety of devices connected to the cytoplasmic end of the inner or outer transmembrane helix. Those devices include ligand-binding domains and voltage sensors7, and their movement splays the inner helices apart, thereby opening the pore.
Bona fide ion pump exemplars
P-type ATPases are quintessential ion pumps. They are exemplified by the Na,K-ATPase (Fig. 2b) that maintains Na and K gradients across the surface membranes of animal cells, the sarcoplasmic- and endoplasmic-reticulum Ca-ATPase (SERCA) that fills cellular Ca ion stores, the H,K-ATPase that acidifies our stomachs, and the H-ATPase that extrudes cellular protons in plants and fungi. In these pumps, a conserved aspartate residue in a central cytoplasmic domain (blue P-domain, Fig. 2b) becomes phosphorylated (whence “P-type”) by ATP once transported ions have entered the binding pocket within the transmembrane domain through the open cytoplasmic-side entryway, while the exit pathway remains closed. This autophosphorylation event shuts the cytoplasmic-side gate, temporarily occluding the bound ions before a large conformational change opens the gate to the extra-cytoplasmic side. The lowered affinity for the transported ions in that conformation prompts their release to the extra-cytoplasmic compartment, an event followed by binding of the counter-transported ions: K in the case of the Na,K-, and H,K-ATPases, or H in SERCA. Closure of the extra-cytoplasmic-side gate, triggered by counter-ion binding, leads to dephosphorylation of the aspartyl-phosphate and stabilization of another occluded state10, this time enclosing counter ions (2 magenta spheres in Fig. 2b). A second large rearrangement restores the conformation with open cytoplasmic gate that has lowered affinity for the counter-transported ions, but relatively high affinity for the transported ions.
For SERCA, X-ray crystal structures have been obtained in all four of these principal conformations11-19. The structures reveal that, in P-type pumps, “gates” correspond to closely apposed segments of transmembrane helices that form 10-20 Å-thick barriers on either side of the binding pocket (e.g., refs. 11,12,18). The two major conformations (called E1 and E2) correspond to the left- and right-hand cartoons in Fig. 1b or c, above, and the two occluded states to the upper and lower cartoons depicting trapped ions in Fig. 1c. In the crystal structure of Na,K-ATPase (Fig. 2b) containing two occluded Rb ions (as K ion surrogates)20, as in the structures of SERCA with two occluded Ca ions11,15,19, the occluded ions sit almost side by side, more than a third of the way across the membrane from the cytoplasm.
Lessons from the exemplars
These examples illustrate three distinctions between highly-evolved ion channels and perfected pumps. First, in contrast to the K-channel structure, but as expected for an occluded-ion conformation of a pump, a space-filling representation of the Na,K-ATPase structure of Fig. 2b confirms the absence of any pathway through which the trapped ions could escape from the binding pocket20. Second, the side-by-side arrangement of bound K, Rb, or Ca ions in the pumps (Fig. 2b) stands in stark contrast to the single-file arrangement of ions in K channels, along the fourfold axis of symmetry, of ions in K channels (Fig. 2a); the high throughput capability of the latter arrangement9 provides no advantage when pumped ion flow is rate-limited by large conformational changes. Third, the structures involved in ion selectivity and activation gating are separate in the channels, permitting a variety of gating modules to control the conserved pore. But these features are intertwined in the pumps, in which the binding pocket becomes remodelled during the gating conformational changes that switch access from one side of the membrane to the other and alter relative ion-binding affinity14.
Straddling the pump-channel divide
These structural distinctions served to reinforce the division between the pump and channel camps. They also relegated to intellectual curiosity status the understanding that, in principle, subtle timing differences in the opening and closing of two gates could separate a pump from a channel3-6. But the recent discovery21 that a single family of Cl transport proteins (the ClC family) includes examples of Cl pumps as well as of Cl channels was an in-your-face reminder that small structural differences can indeed underlie very different functions.
The first member of the family, ClC-0, was identified in electric fish over a quarter of a century ago as a double-barrelled channel with independent fast gates that open and close each barrel on a timescale of tens of milliseconds (Fig. 3a) and a common slow gate that operates on a timescale of seconds22 (not shown). The double-barrelled behaviour was later explained by the homodimeric structure of ClC proteins, in which each subunit contains its own ion transport pathway23-25. Comparable mammalian Cl channels were found that belong to the same ClC family which, in humans, has nine members26. The crystal structure (Fig. 3c) of a homodimeric prokaryotic ClC protein, ClC-ec1, was a breakthrough25. The structure revealed a narrow pathway with multiple transported anions (Cl or Br) in a row (Fig. 3c,d) 25; not a straight row like that in K channels, but a row nonetheless. Whether there were two or three anions depended on the position of the side chain of a glutamate residue (E148) that occupied the pathway near its extracellular end, replacing the third anion found there after neutralization of the side chain (by mutation to glutamine27; Fig. 3d). As neutralization of the equivalent glutamate in ClC-0 channels essentially abolished their fast gating (Fig. 3b), the glutamate was identified as the fast gate and was proposed to swing out of the ion pathway upon protonation, so opening the pore to Cl-ion flow27. The structures of ClC-ec1 and their analysis were equally successful in rationalizing other results from examinations of structure and mechanism in ClC-0 and mammalian ClC-1 and ClC-2 channels (e.g., refs. 28-30).
Figure 3. A Cl channel evolved from a Cl/H exchanger.
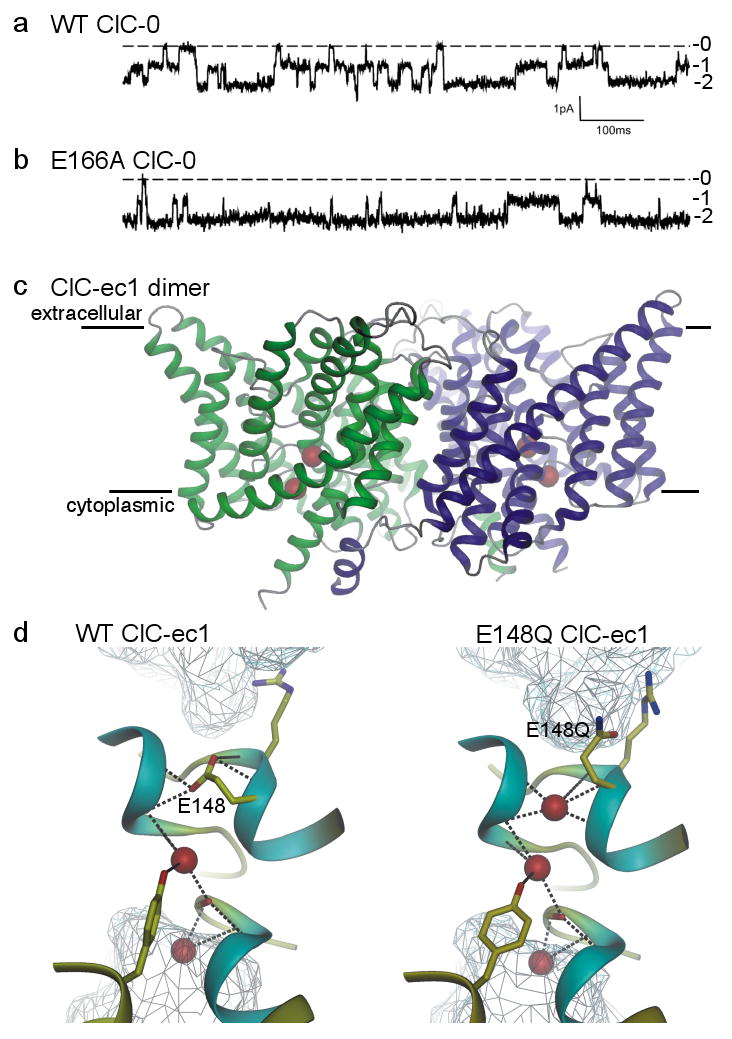
a/, b/ Current recordings showing independent opening and closing of the 2 pores in a wild-type (WT) ClC-0 channel (a), and the mostly open behaviour of an E166A ClC-0 channel (b); labels 0, 1, and 2 indicate number of open pores in each dimeric channel [Modified with permission from ref. 27]. c/ Transmembrane region of WT ClC-ec1 dimer viewed from within the membrane (membrane boundaries indicated). The ion pathway in each monomer (green, blue) contains 2 anions (red spheres). [Modified with permission from ref. 85]. d/ Pore region connecting extra- and intracellular aqueous vestibules (cyan mesh) in WT (left) and E148Q (right) ClC-ec1, containing 2 or 3 Cl ions (red spheres) respectively; the third Cl ion occupies the space vacated by the swung-out side chain of the external gate residue, 148 (E148 is equivalent to E166 of ClC-0). [Modified with permission from ref. 86].
So it came as a shock when a year later ClC-ec1 was discovered not to be a Cl channel, but a Cl/H exchange pump that couples downhill movement of one proton to uphill transport of 2 Cl ions in the opposite direction, or vice versa21. The unavoidable conclusion is that the structure of a ClC pump must so closely resemble that of a ClC channel that they cannot yet be told apart, even at near atomic resolution. Subsequent work has confirmed that some of the human ClCs are indeed channels, but at least as many are Cl/H exchange pumps31.
This outcome violated the comfortable notion that these two classes of ion transport protein ought to be visibly dissimilar. But does it violate the one-gate-versus-two-gates formalism? Not really, because the largely conserved glutamate side chain appears to fulfill the role of a fast gate in ClC channels27, and one of the two required gates in the ClC pumps21,25,32,33.
So what constitutes the second gate in ClC pumps? A plausible candidate is a conserved tyrosine (Y445 in Fig. 3d) that coordinates the central Cl ion, as ever smaller side chains at this position in ClC-ec1 allow Cl flow that is increasingly uncoupled from proton transport34. And, when both the glutamate at the external site and the tyrosine at the central site are replaced with the smallest amino acids, the passive Cl flow is accelerated to ∼4×104 ions/sec, consistent with shrunk gates33. But, even in these double-gate mutants, something else must restrain Cl-ion movement, because this flow rate is still more than a hundred times slower than Cl ion transit through human ClC-1 channels35 and a thousand times slower than through fish ClC-0 channels22; this is despite the fact that both ClC-1 and ClC-0 contain the conserved tyrosine, which is retained in the channels as well as the pumps.
Ion channels evolved from perturbed pumps?
So there are pumps that look like pumps, channels that reek of channels, and a family of proteins that includes some pumps and some channels. And we know that, in theory, disruption of the coordinated timing between a pump's two gates, or of the structure of one of them, could convert the pump into a channel. Are any natural examples of such conversion known? Indeed, there are examples of ion channels, and of ion channel-like proteins, that belong to families of transporters, their close genetic relationship implying that the channels arose from pumps following evolutionary degradation of a gate32,36-41.
Even ClC channels still transport protons!
The channel/pump dichotomy within the ClC family, together with the non-equilibrium nature of ClC channel gating42 and its characteristic dependence28 on pH, raise the question of whether the ClC channels evolved from an ancestral ClC Cl/H pump in which one of the gates became incompetent to hold back Cl ions32,36. Recent direct measurement of pH changes close to the extracellular surface of ClC-1 channels during gating43, and the demonstration that non-equilibrium gating of ClC-0 channels depends on the proton electrochemical potential gradient36, imply that even today gating of the Cl-ion pore in ClC channels involves proton transport. This supports a view of ClC channels as broken ClC Cl/H exchange pumps32,36.
A chloride channel evolved from an ABC transporter
Another example of a family of transport proteins begetting an ion-channel child is the CFTR (cystic fibrosis transmembrane conductance regulator) Cl channel44 (Fig. 4a). CFTR is the only one of thousands of ABC (ATP binding cassette) proteins known to function as an ion channel. Most of the others are believed to be ATP-driven transporters, each with its own designated cargo. A few, including the SURs (suphonylurea receptors)45, appear to use the ATP-induced conformational changes to signal to other proteins45-47. ABC proteins bind and hydrolyze ATP in composite catalytic sites formed within the interface between paired cytoplasmic nucleotide-binding domains (the ABCs; Fig. 4a), which are drawn together by bound ATP and later separated by its hydrolysis45,48,49. These movements are transmitted to the transmembrane domains via their extended links into the cytoplasm to alternately open and close gates to the substrate-binding site and so effect substrate transport. In CFTR, ATP binding opens the ion channel pore, and hydrolysis of that ATP triggers channel closure (reviewed in ref. 37); thus, CFTR channels remain closed until exposed to ATP (Fig. 4b,c), and mutation of a key catalytic residue to prevent ATP hydrolysis makes channel closure upon ATP withdrawal nearly 1000 fold slower than normal (Fig. 4c).
Figure 4. A Cl channel evolved from an ABC transporter.
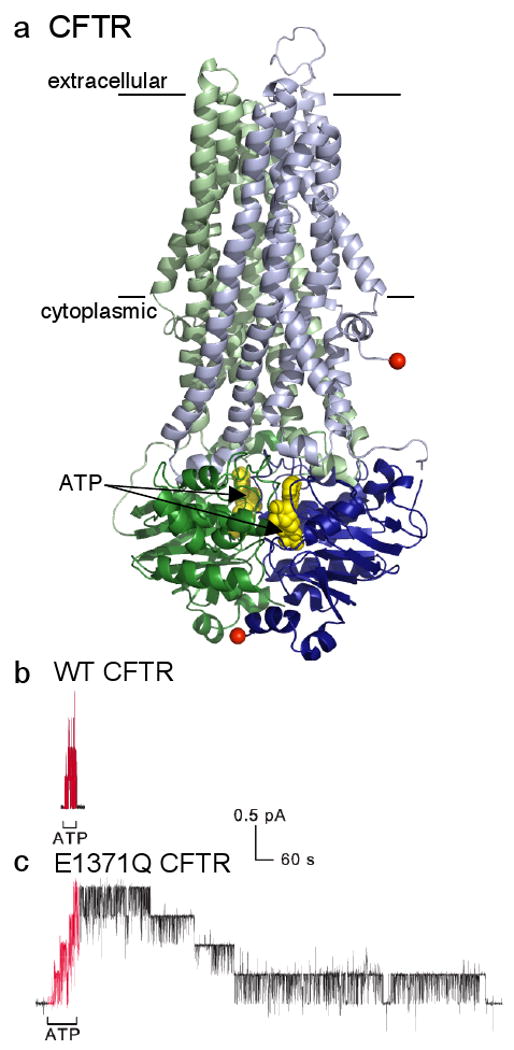
a/ Model of CFTR (N-terminal half green, C-terminal half blue) based largely on homology with prokaryotic ABC transporter50,51 Sav1866; the transmembrane domains (lines mark membrane boundaries) and cytoplasmic linkers are lighter in colour, and the dimerized cytoplasmic nucleotide-binding domains containing 2 bound ATPs (yellow spacefill) are darker; absent is the ∼200-residue regulatory domain of unknown structure that somehow links the N- and C-terminal halves by connecting the two red spheres. [Modified with permission from ref. 87]. b/ , c/ Recordings of CFTR channel currents in excised inside-out membrane patches. Exposure to cytoplasmic ATP (red segments) allows opening and closing of WT CFTR channels (b), and of mutant E1371Q CFTR channels (c) bearing a point mutation that prevents ATP hydrolysis; each 0.4-pA current step reflects opening or closing of a single CFTR channel. The greatly delayed closing of all four E1371Q channels (c), compared to the four WT CFTR channels (b), after ATP removal shows that ATP hydrolysis times normal channel closing, and that the channel open-burst state corresponds to a conformation with ATP bound within the dimerized nucleotide-binding domains (cf. part a). [Reproduced with permission from ref. 37].
No X-ray crystal structure of a eukaryotic ABC transporter is presently available, but crystal structures of prokaryotic relatives have revealed that in the nucleotide-bound conformation the extracellular-side gate in the transmembrane domains is open, while the cytoplasmic side is closed50-53. Since ∼107 Cl ions/sec flow through an ATP-bound open CFTR channel (from the size of single-channel currents; Figs. 4b,c) in which, by analogy, the extracellular-side gate ought to be open, it seems reasonable to surmise that its cytoplasmic-side gate has become atrophied, or uncoupled from the outer gate. In other words, CFTR can be considered to be a broken ABC transporter (e.g., refs. 38,39).
Although CFTR is unquestionably an anion channel, it is not yet fully ruled out that CFTR might also be able to transport something; glutathione has been suggested54,55. Regardless, mutant cycle analysis based on multiple sequence alignment40 suggests that the ATP-dependent cycle of dynamic rearrangement of the nucleotide-binding domains that drives opening and closing of the channel gates in CFTR is the same as that which powers substrate transport in most ABC proteins.
Light-activated pump-channels
Channelrhodopsins41 may be further examples of ion channels that evolved, by loss or uncoupling of a gate, from microbial pumps such as bacteriorhodopsin (a proton pump) and halorhodospin (a Cl pump). The two channelrhodopsins are light-activated channels selective for protons (channelrhodopsin-1), or permeable to a range of cations (channelrhodopsin-2)41. However, preliminary estimates41 suggest that only ∼3×104 cations/sec flow through channelrhodopsin-2, a rate which, like that of double-gate mutant ClC-ec1 pumps33, puts channelrhodopsins squarely in the twilight zone between pumps and channels (see below).
Pharmacological transformation of a pump into a channel
Nature has also found a means to acutely subvert the fail-safe mechanism of the Na,K-ATPase pump, in which strict coupling between its two gates ensures that each gate opening is followed by an occluded state. The sophisticated marine toxin, palytoxin, binds specifically to extracellularly-exposed parts of the Na,K pump and thereby disrupts the tight communication between the gates, allowing both to sometimes be open at the same time, so transforming the pump into a cation channel56-59 (see Box, part a). The channels are not highly selective, however; they distinguish poorly among small monovalent cations, and even slowly conduct organic cations up to 3 times larger than a Na ion60.
Box. Acute transformation of pumps into channels.
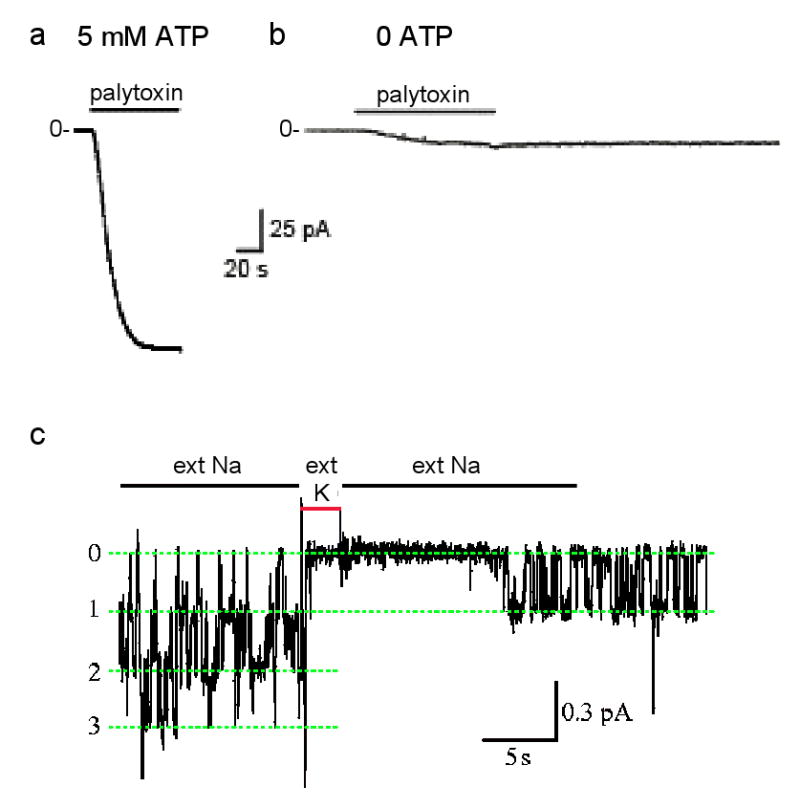
Each Na,K-ATPase pump in a cell membrane exports 3 Na ions and imports 2 K ions for each ATP hydrolyzed77 and can repeat this up to a hundred times a second78. The net charge movement generates a current that can reach ∼20 attoamperes for a single Na,K pump, too tiny for present recording equipment. But the >20 picoamperes generated by the millions of Na,K pumps in an entire cell can be measured (e.g., ref. 79). If all K ions are removed, leaving only Na, Na,K pumping is precluded, and steady current through the pump becomes zero regardless of the membrane potential (e.g., ref. 80). This means that there is no channel-like electrodiffusive flow of Na ions through the stalled Na,K pumps, confirming that their two gates remain tightly coupled. This is the condition (zero current at -40-mV membrane potential) at the beginning of the recordings of current flow through excised outside-out patches of cell membrane each containing thousands of Na,K pumps (see figure, parts a,b). However, in presence of millimolar ATP a saturating concentration (100 nM) of palytoxin quickly elicits a relatively large current that reflects rapid Na-ion flow through Na,K pumps after palytoxin has transformed them all into cation channels60,61,81 (part a; modified with permission from ref. 81).
The gates are uncoupled, but still functional Without ATP, a much weaker current is activated by the same palytoxin concentration (part b; modified with permission from ref. 81) in a comparable patch, with presumably similar numbers of palytoxin-bound Na,K pump-channels. The current is weaker because individual palytoxin-bound pump-channels spend a much greater fraction of the time closed in the absence of ATP than in its presence61. This channel-opening effect of ATP is mimicked by AMPPNP or ADP, and reflects the known action of nucleotides on unmodified Na,K pumps to open the cytoplasmic-side gate60,61,82,83.
External cations also modulate the probability of palytoxin-bound pump-channels being open. Just as external K ions enter unmodified Na,K pumps and become occluded in their binding sites after closure of the extracellular-side gate (Fig. 2b), brief replacement of external Na ions with K ions temporarily shuts palytoxin-bound pump-channels61 (part c; labels 0, 1, 2, 3 indicate number of simultaneously open pump-channels; modified with permission from ref. 65). Evidently, palytoxin transforms Na,K pumps into channels in which the two gates still respond to their physiological ligands but are no longer in synch: palytoxin breaks the fundamental rule of the one-gate-versus-two-gates formalism, which is that a pump's two gates should never be open at the same time.
The fact that the gates of palytoxin-bound Na,K pump-channels continue to respond to extracellular K (Box, part c) ions and to cytoplasmic ATP (parts a,b) bolsters the interpretation that the two gates still work, just no longer in synch61. The size of the current through single palytoxin-bound pump-channels (Box, part c) indicates that, when both gates are open, more than a million Na ions per second flow through each pump. So these transformed pumps provide a sobering illustration of the orders of magnitude increase in ion throughput, albeit dissipative, posited above to result from a breakdown in communication between a pump's two gates.
An ion pump from a tricked-out channel?
Though conceptually simpler for a transporter to lose function of a gate during evolution and so become a channel, an ion pump could conceivably evolve from a channel by gaining a second gate. In fact Kdp, a P-type ATPase that pumps K ions into bacteria62, appears to have evolved by association of an ATPase component with a K-ion channel polypeptide with similarities in sequence to the tetrameric KcsA pore of Fig. 2a. However, neither the translocation route of the K ions transported by the Kdp complex nor the mechanism is known yet62. And, since the ATPase component itself retains seven transmembrane helices homologous to those that in other P-type ATPases contain the ion-binding pocket and ion-translocation pathway11,13,17-19,63-65, it seems premature to classify Kdp as a channel that has simply adopted a second gate.
Pump-channels with hybrid behaviour
Thus far, we have discussed examples of channels, of pumps, and of pumps converted to channels by evolutionary degradation of a gate (or inter-gate communication), or by acute pharmacological subversion of the tight coupling between a pump's two gates. But we have not explicitly considered molecules that can simultaneously display both pump and channel function. Given the orders of magnitude faster ion flow downhill through a typical channel than uphill via a pump, a single molecule combining those functions for the same ion travelling the same pathway makes no sense. But the proton transport apparently conserved throughout evolution to gate electrodiffusive Cl current in ClC-channels36,43, in part by protonating the external gate glutamate27,32, is a striking example of hybrid behaviour, involving different ions, in a single molecule. And, at least in the ClC Cl/H exchange pumps, the protons and Cl ions travel some of the way along separate paths66. The full route taken by the protons transported by ClC channels, however, and whether their transport serves any metabolic, or other, function is unknown.
Chloride channels in neurotransmitter pumps
But there are other examples of hybrid pump-channels. Released excitatory and inhibitory transmitters and biogenic amines are cleared from synaptic spaces by two families of Na-coupled neurotransmitter pumps67,68, prokaryotic homologues of which have begun to be structurally characterized (reviewed in ref. 69). Despite their distinct architectures, in each homologue binding of extracellular Na ions with substrate triggers occlusion of both, before a large conformational rearrangement releases them to the cytoplasm69. The inward-directed electrochemical gradient for Na ions thus powers, and ensures, net uptake of neurotransmitter.
Both in vertebrate glutamate pumps and in their purified, reconstituted, prokaryotic homologues, Na-dependent transport is accompanied by a thermodynamically uncoupled electrodiffusive flow of Cl ions67,70-74. The Cl current is not required for substrate transport, because that persists when the current is diminished by Cl replacement with impermeant anions70,73 or by mutation of the pump73,75. The latter finding, the reconstitution of both transport and Cl current by purified protein73, and impairment of both by inhibitors73,76, all demonstrate that glutamate transporters are hybrid molecules that unite a stoichiometric pump with a separate channel-like pathway. Just how channel-like remains unclear, as estimates of the Cl current size71,72 indicate flows of only 104-105 Cl ions/sec, again, like that of double-gate mutant ClC-ec1 pumps33, too small for single-molecule recordings. Nevertheless, correlations between changes in stoichiometric transport and in Cl current suggest that only a subset of the pump conformations visited during the transport cycle are able to conduct Cl ions71,72,74,76.
The one-versus-two-gates perspective
It is now certain that integral-membrane ion-transport proteins span a broad and continuous spectrum, from clear-cut, highly-evolved, exquisitely-selective, ion channels at one extreme to prototypical, primary, stoichiometric, ion pumps at the other. And the evidence emerging from the ClC protein family mandates a blurry region near the middle of this continuum. There we will find exceedingly fast pumps in which the gates are small and so can be moved rapidly, as well as extraordinarily slow ion channels in which the ion pathway embraces, and is deformed by, the conducted ions, thereby slowing their diffusion. In fact, we should not be surprised if the fastest pumps turn out to be capable of higher ion transport rates than the slowest open ion channels.
But there is still no reason to abandon the one-versus-two-gates formalism to distinguish pumps from channels. It is worth reemphasizing that, more specifically, the distinction boils down to the requirement of strict coordination between a pump's two gates (whatever their physicochemical form). The ultimate functional distinction between a pump and a channel will always rest on whether the membrane protein is capable of thermodynamically uphill transport, against the electrochemical potential gradient. Those that meet this test are pumps, and may be further subdivided into primary or secondary active transporters according to the coupled energy source. Let's call all the others channels, regardless of how many gates they have, or of how tight their grip on the ions they conduct. And as we ponder the sloth of the slowest ion channels, the growing structural understanding of membrane-spanning ion transport proteins should help us avoid the obfuscation and semantic diversion of applying to them outmoded terms like carrier, uniporter, and facilitated diffusion. Across the full spectrum of ion transport proteins there is much to learn about the features that define them – that is to say, about the structures of their gates, and the mechanisms of gating and its coordination -- even if we now have a clearer appreciation of the significance of those features.
Acknowledgments
I thank present and past lab members for their contributions to the research and ideas encapsulated here, A. Takeuchi, A. Gulyás-Kovács, P. Vergani, P. Artigas, and P. Hoff for help with figures, R. Dutzler and R. MacKinnon for images, and the NIH for funding via grants HL36783, HL49907, and DK51767.
Glossary
- membrane potential
the difference in electrical potential between one side of a membrane and the other usually the electrical potential inside a cell measured with respect to that of the extracellular space.
- activation gate
the gate in an ion channel that when opened initiates ion flow through the channel pore.
- gating reaction
a change in conformation of a transmembrane transport protein that alters access to the substrate translocation pathway.
- selectivity filter
the narrow region of an ion translocation pathway in which the transport protein interacts with the ions to select among them on the basis of their physicochemical characteristics.
References
- 1.Hille B. Ion channels of excitable membranes. Vol. 814. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 2.Sakmann B, Neher E. Single-Channel Recording. Plenum; New York: 1995. [Google Scholar]
- 3.Läuger P. A channel mechanism for electrogenic ion pumps. Biochim Biophys Acta. 1979;552:143–161. doi: 10.1016/0005-2736(79)90253-0. [DOI] [PubMed] [Google Scholar]
- 4.Patlak CS. Contributions to the theory of active transport. II. The gate-type non-carrier mechanism and generalization concerning tracer glow efficiency, and measurement of energy expenditure. Bull Math Biophys. 1957;19:209–235. [Google Scholar]
- 5.Vidaver GA. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J Theor Biol. 1966;10:301–306. doi: 10.1016/0022-5193(66)90128-7. [DOI] [PubMed] [Google Scholar]
- 6.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;27:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 7.MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]; This high-resolution structure revealed the mechanism of the K channel selectivity filter. Each of its four K-ion sites lies at the centre of a cage of eight carbonyl oxygens that mimic those of the eight water molecules found surrounding the hydrated K ion in the large central cavity. So the selectivity filter minimizes the energetic cost of transferring K ions from the aqueous environment to the K channel interior.
- 9.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 10.Post RL, Hegyvary C, Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972;247:6530–6540. [PubMed] [Google Scholar]
- 11.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]; This was the first X-ray crystal structure of any P-type ATPase ion pump. The two transported Ca ions were found buried side by side in their binding sites, deep within the transmembrane domain and inaccessible from either side.
- 12.Toyoshima C, Nomura H, Tsuda T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature. 2004;432:361–368. doi: 10.1038/nature02981. [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima C, Norimatsu Y, Iwasawa S, Tsuda T, Ogawa H. How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump. Proc Natl Acad Sci USA. 2007;104:19831–19836. doi: 10.1073/pnas.0709978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyoshima C, Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature. 2002;418:605–611. doi: 10.1038/nature00944. [DOI] [PubMed] [Google Scholar]
- 15.Toyoshima C, Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- 16.Møller JV, Nissen P, Sorensen TLM, le Marie M. Transport mechanism of the sarcoplasmic reticulum Ca2+-ATPase pump. Curr Opin Struct Biol. 2005;15:387–393. doi: 10.1016/j.sbi.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Olesen C, Sorensen TL, Nielsen RC, Møller JV, Nissen P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science. 2004;306:2251–2255. doi: 10.1126/science.1106289. [DOI] [PubMed] [Google Scholar]
- 18.Olesen C, et al. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen TLM, Møller JV, Nissen P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science. 2004;304:1672–1675. doi: 10.1126/science.1099366. [DOI] [PubMed] [Google Scholar]
- 20.Morth JP, et al. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 21.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]; Using electrophysiological and biochemical functional assays on the same preparation of prokaryotic ClC proteins (ClC-ec1) that had been used for X-ray crystallography, this study came to the shocking conclusion that ClC-ec1 is a Cl/H exchange pump, rather than the Cl channel initially assumed.
- 22.Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982;299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- 23.Middleton RE, Pheasant DJ, Miller C. Homodimeric architecture of a CIC-type chloride ion channel. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- 24.Ludewig U, Pusch M, Jentsch TJ. Two physcially distinct pores in the demeric CIC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- 25.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 26.Jentsch TJ, Poët M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl-channels gleaned from human genetic disease and mouse models. Annu Rev Physiol. 2005;67:779–807. doi: 10.1146/annurev.physiol.67.032003.153245. [DOI] [PubMed] [Google Scholar]
- 27.Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]; In this structural study, a glutamate side chain that occupies an anion site in the ion pathway of ClC-ec1 was found to swing away on protonation, unblocking the pathway and allowing a Cl ion to take its place. The residue was identified as a fast gate, because mutation of the analogous glutamate in ClC-0 channels to glutamine almost abolished fast gating, leaving the channels mostly open.
- 28.Chen MF, Chen TY. Different fast-gate regulation by external Cl(−) and H(+) of the muscle-type ClC chloride channels. J Gen Physiol. 2001;118:23–32. doi: 10.1085/jgp.118.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estévez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 2003;38:47–59. doi: 10.1016/s0896-6273(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 30.Engh AM, Maduke M. Cysteine accessibility in ClC-0 supports conservation of the ClC intracellular vestibule. J Gen Physiol. 2005;125:601–617. doi: 10.1085/jgp.200509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zifarelli G, Pusch M. CLC chloride channels and transporters: a biophysical and physiological perspective. Rev Physiol Biochem Pharmacol. 2007;158:23–76. doi: 10.1007/112_2006_0605. [DOI] [PubMed] [Google Scholar]
- 32.Miller C. CLC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 33.Jayaram H, Accardi A, Wu F, Williams C, Miller C. Ion permeation through a Cl--selective channel designed from a CLC Cl-/H+ exchanger. PNAS. 2008;105:11194–11199. doi: 10.1073/pnas.0804503105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walden M, Accardi A, Wu F, Xu C, Williams C, Miller C. Uncoupling and turnover in a Cl-/H+ exchange transporter. J Gen Physiol. 2007;129:317–329. doi: 10.1085/jgp.200709756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saviane C, Conti F, Pusch M. The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia. J Gen Physiol. 1999;113:457–468. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lísal J, Maduke M. The ClC-0 chloride channel is a ‘broken’ Cl-/H+ antiporter. Nat Struct Mol Biol. 2008;15:805–810. doi: 10.1038/nsmb.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using single-channel recordings to show that the gating pattern of ClC-0 channels depends on transmembrane movement of protons, this study firmly established the evolutionary link between ClC pumps and ClC channels, and suggested that the channels evolved from a dysfunctional pump.
- 37.Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muallem D, Vergani P. ATP hydrolysis-driven gating in cystic fibrosis transmembrane conductance regulator. Philos Trans R Soc Lond B Biol Sci. 2009;364:247–255. doi: 10.1098/rstb.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan K, Kota KC, Cui G, Thompson CH, McCarty NA. Evolutionary and functional divergence between the cystic fibrosis transmembrane conductance regulator and related ATP-binding cassette transporter. Proc Natl Acad Sci USA. 2008;105:18865–18870. doi: 10.1073/pnas.0806306105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;24:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used CFTR-channel gating kinetics to demonstrate ATP-dependent energetic interaction between residues at positions shown to structurally interact across the interface of prokaryotic nucleotide-binding domain dimers. Evolutionary conservation at these interacting positions in most ABC proteins suggests that nearly all undergo the same ATP-driven cycle of conformational changes established for CFTR.
- 41.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richard EA, Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990;247:1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- 43.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 44.Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 45.Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 46.Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]; This was the first demonstration that the nucleotide-binding domains of ABC proteins form head-to-tail dimers in the presence of ATP, with one ATP molecule enclosed in each of the two composite catalytic sites formed within the dimer interface. As ADP did not support dimerization, it was proposed that cycles of ATP-induced dimerization and hydrolysis-triggered dissociation provide the basis for function of all ABC proteins.
- 47.Sixma TK. DNA mismatch repair: MutS structures bound to mismatches. Curr Opin Struct Biol. 2001;11:47–52. doi: 10.1016/s0959-440x(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 48.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu G, Westbrooks JM, Davidson AL, Chen J. ATP hydrolysis is required to reset the ATP-binding cassette dimer into the resting-state conformation. Proc Natl Acad Sci U S A. 2005;102:17969–17974. doi: 10.1073/pnas.0506039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]; This paper reported the first high-resolution structure of an entire ABC transporter homologous to clinically relevant human ABC proteins. The structure revealed that the transmembrane helices extend long linker helices into the cytoplasm where they connect to the nucleotide-binding domains via short conserved coupling helices. It also revealed a domain-swapped architecture in which each nucleotide-binding domain receives connections from both N- and C-terminal transmembrane domains.
- 51.Dawson RJ, Locher KP. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 52.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 53.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linsdell P, Hanrahan JW. Adenosine triphosphate-dependent asymmetry of anion permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol. 1998;111:601–614. doi: 10.1085/jgp.111.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Habermann E. Palytoxin acts through Na+ K+ ATPase. Toxicon. 1989;27:1171–1187. doi: 10.1016/0041-0101(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 57.Tosteson MT. Mechanism of action, pharmacology and toxicology. In: Botana LM, editor. Seafood and Freshwater: Toxins Pharmacology, Physiology, and Detection. Marcel Dekker; New York: 2000. pp. 549–566. [Google Scholar]
- 58.Scheiner-Bobis G, Meyer zu Heringdorf D, Christ M, Habermann E. Palytoxin induces K+ efflux from yeast cells expressing the mammalian sodium pump. Mol Pharmacol. 1994;45:1132–1136. [PubMed] [Google Scholar]
- 59.Hirsh JK, Wu CH. Palytoxin-induced single-channel currents from the sodium pump synthesized by in vitro expression. Toxicon. 1997;35:169–176. doi: 10.1016/s0041-0101(96)00136-5. [DOI] [PubMed] [Google Scholar]
- 60.Artigas P, Gadsby DC. Large diameter of palytoxin-induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands. J Gen Physiol. 2004;123:357–376. doi: 10.1085/jgp.200308964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Artigas P, Gadsby DC. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc Natl Acad Sci USA. 2003;100:501–505. doi: 10.1073/pnas.0135849100. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided support for the view of the Na/K pump as a channel controlled by two gates that open strictly alternately. Current recordings showed that palytoxin interferes with this strict coupling, allowing the two gates to sometimes both be open, but that each gate in a palytoxin-bound pump-channel still responds to its physiological ligand, external K or cytoplasmic ATP.
- 62.Greie JC, Altendorf K. The K+-translocating KdpFABC complex from Escherichia coli: a P-type ATPase with unique features. J Bioenerg Biomembr. 2007;39:397–402. doi: 10.1007/s10863-007-9111-0. [DOI] [PubMed] [Google Scholar]
- 63.Reyes N, Gadsby DC. Ion Permeation through the Na+,K+-ATPase. Nature. 2006;443:470–474. doi: 10.1038/nature05129. [DOI] [PubMed] [Google Scholar]
- 64.Takeuchi A, Reyes N, Artigas P, Gadsby DC. The ion pathway through the opened Na(+),K(+)-ATPase pump. Nature. 2008;456:413–416. doi: 10.1038/nature07350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gadsby DC, Takeuchi A, Artigas P, Reyes N. Peering into an ATPase ion pump with single-channel recordings. Philos Trans R Soc Lond B Biol Sci. 2009;364:229–238. doi: 10.1098/rstb.2008.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Accardi A, Walden M, Nguitragool W, Jayaram H, Williams C, Miller C. Separate ion pathways in a Cl-/H+ exchanger. J Gen Physiol. 2005;126:563–70. doi: 10.1085/jgp.200509417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 68.Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Gouaux E. The molecular logic of sodium-coupled neurotransmitter transporters. Philos Trans R Soc Lond B Biol Sci. 2009;364:149–154. doi: 10.1098/rstb.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 71.Larsson HP, Picaud SA, Werblin FS, Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996;70:733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryan RM, Mindell JA. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2007;14:365–371. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]; This study used reconstituted purified glutamate transporter protein to establish that the uncoupled channel-like Cl current flows through the same protein that carries out stoichiometric Na-dependent transport of glutamate.
- 74.Vandenberg RJ, Huang S, Ryan RM. Slips, leaks and channels in glutamate transporters. Channels (Austin) 2008;2:51–58. doi: 10.4161/chan.2.1.6047. [DOI] [PubMed] [Google Scholar]
- 75.Ryan RM, Mitrovic AD, Vandenberg RJ. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem. 2004;279:20742–20751. doi: 10.1074/jbc.M304433200. [DOI] [PubMed] [Google Scholar]
- 76.Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20:2749–2757. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Post RL, Jolly PC. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957;25:118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- 78.Jørgensen PL. Isolation of (Na+ plus K+)-ATPase. Methods Enzymol. 1974;32:277–290. [PubMed] [Google Scholar]
- 79.Gadsby DC, Kimura J, Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985;315:63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- 80.Nakao M, Gadsby DC. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986;323:628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- 81.Artigas P, Gadsby DC. Ion channel-like properties of the Na+/K+ Pump. Ann N Y Acad Sci. 2002;976:31–40. doi: 10.1111/j.1749-6632.2002.tb04711.x. [DOI] [PubMed] [Google Scholar]
- 82.Simons TJB. The interaction of ATP-analogues possessing a blocked gamma-phosphate group with the sodium pump in human red cells. J Physiol. 1975;244:731–739. doi: 10.1113/jphysiol.1975.sp010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forbush B., 3rd Rapid release of 42K 86Rb from an occluded state of the Na, K-pump in the presence of ATP or ADP. J Biol Chem. 1987;244:731–739. [PubMed] [Google Scholar]
- 84.Gadsby DC. Ion transport: spot the difference. Nature. 2004;427:795–797. doi: 10.1038/427795a. [DOI] [PubMed] [Google Scholar]
- 85.Lobet S, Dutzler R. Ion-binding properties of the CIC chloride selectivity filter. EMBOJ. 2006;25:24–33. doi: 10.1038/sj.emboj.7600909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dutzler R. A structural perspective on CIC channel and transporter function. FEBS Letters. 2007;581:2839–2844. doi: 10.1016/j.febslet.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Mornon JP, Lehn P, Callebaut I. Atomic model of human cystic fibrosis transmembrane conductance regulator: membrane-spanning domains and coupling interfaces. Cell Mol Life Sci. 2008;65:2594–2612. doi: 10.1007/s00018-008-8249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


