Abstract
Background
Signal transduction cascade of anesthetic-induced preconditioning has been extensively studied, yet many aspects of it remain unsolved. Here we investigated the roles of reactive oxygen species (ROS) and mitochondrial uncoupling in cardiomyocyte preconditioning by 2 modern volatile anesthetics: desflurane and sevoflurane.
Methods
Adult rat ventricular cardiomyocytes were isolated enzymatically. The preconditioning potency of desflurane and sevoflurane was assessed in cell survival experiments by evaluating myocyte protection from the oxidative stress-induced cell death. ROS production and flavoprotein fluorescence, an indicator of flavoprotein oxidation and mitochondrial uncoupling, were monitored in real-time by confocal microscopy. The functional aspect of enhanced ROS generation by the anesthetics was assessed in cell survival and confocal experiments using the ROS scavenger Trolox.
Results
Preconditioning of cardiomyocytes with desflurane or sevoflurane significantly decreased oxidative stress-induced cell death. That effect coincided with increased ROS production and increased flavoprotein oxidation detected during acute myocyte exposure to the anesthetics. Desflurane induced significantly greater ROS production and flavoprotein oxidation than sevoflurane. ROS scavenging with Trolox abrogated preconditioning potency of anesthetics and attenuated flavoprotein oxidation.
Conclusion
Preconditioning with desflurane or sevoflurane protects isolated rat cardiomyocytes from oxidative stress-induced cell death. Scavenging of ROS abolishes the preconditioning effect of both anesthetics and attenuates anesthetic-induced mitochondrial uncoupling, suggesting a crucial role for ROS in anesthetic-induced preconditioning and implying that ROS act upstream of mitochondrial uncoupling. Desflurane exhibits greater effect on stimulation of ROS production and mitochondrial uncoupling than sevoflurane.
INTRODUCTION
Multiple mechanisms are involved in adaptation of the heart to ischemia/reperfusion injury, including changes in myocardial metabolism that enable cardiac cells to withstand prolonged ischemia and reperfusion.1 This endogenous defense mechanism can be elicited by pre-exposing myocardium to volatile anesthetics (VAs), a phenomenon termed anesthetic preconditioning (APC).2 Although APC has been known for more than a decade, little is known about similarities and differences in preconditioning potency of various VAs and the essential steps of APC signal transduction cascade. Explaining differences in preconditioning may have important implications on selection of VAs in clinical practice. The available data concerning the cardioprotective potency of desflurane and sevoflurane are controversial. One study showed that desflurane is a potent cardioprotective anesthetic, whereas sevoflurane did not exhibit such a property in the rabbit heart in vivo.3 By contrast, 2 other studies demonstrated that desflurane and sevoflurane both effectively precondition the myocardium in vivo4 and in vitro5 with similar potency. Further, very few studies compared the components of preconditioning signaling cascades of desflurane and sevoflurane. The outcome of such studies would be important for explaining the mechanisms of VA-induced cardioprotection and discriminating the effects of desflurane and sevoflurane on myocardial function.
An increase in generation of reactive oxygen species (ROS)5–7 and mitochondrial uncoupling via opening of mitochondrial potassium channels8 appear to be critical events at the onset of the APC signaling cascade. However, the temporal relationship between these 2 events remains controversial. Enhanced ROS production in APC has been attributed to opening of the mitochondrial ATP-sensitive K+ (mitoKATP) channel.9 However, other studies suggested that the opening of the mitoKATP channel is induced by ROS.10,11 Whole animal,6 isolated heart,7 and human right atrial trabeculae5 studies have shown that APC can be abolished by ROS scavengers, suggesting that a transient increase in ROS production participates in the APC signaling cascade. Although it has been shown in an isolated heart model that VAs increase ROS production,12 there is no evidence that ROS are generated in myocardial cells. The goal of the present study, therefore, was to document and investigate the time course of VA-induced ROS generation in isolated, intact, ventricular cardiomyocytes. We hypothesized that the preconditioning potency of desflurane and sevoflurane correlates with the magnitude of ROS production and mitochondrial uncoupling elicited by acute exposure to VAs. We investigated the functional importance of enhanced ROS production in the triggering phase of APC by desflurane and sevoflurane and hypothesized that an increase in ROS production occurs upstream of mitochondrial uncoupling.
METHODS
The animal use and experimental protocols of this study were approved by the Institutional Animal Use and Care Committee of the Medical College of Wisconsin, Milwaukee, Wisconsin.
Isolation of ventricular myocytes
Ventricular cardiomyocytes were isolated from adult male Wistar rats by enzymatic dissociation with collagenase and protease as reported previously.8 Myocytes were stored in Tyrode solution (in mM: 132 NaCl, 10 HEPES, 5 glucose, 5 KCl, 1 CaCl2, 1.2 MgCl2, adjusted to pH 7.4) at room temperature and were used for experiments within 5 hours after isolation.
Myocyte survival protocols
Survival experiments were conducted using glucose-free Tyrode solution as previously described.8 Cell viability was evaluated by cellular morphology and trypan blue staining (Sigma-Aldrich, St. Louis, MO) at the beginning and at the end of experiment. The cells were exposed to oxidative stress using 200 μM H2O2 (Calbiochem, La Jolla, CA) and 100 μM FeSO4. VAs, alone or with ROS scavenger Trolox (Calbiochem, La Jolla, CA), were applied before initiation of oxidative stress. Due to incomplete washout of Trolox that altered oxidative stress-induced cell death, a modification of the cell survival protocol was necessary for the experiments with Trolox to achieve comparable cell death in all stress groups (Fig. 1). Trolox washout was extended from 5 to 15 minutes, the H2O2 concentration was increased from 200 μM to 325 μM, and Trolox was present in the stress group as well.
Figure 1.
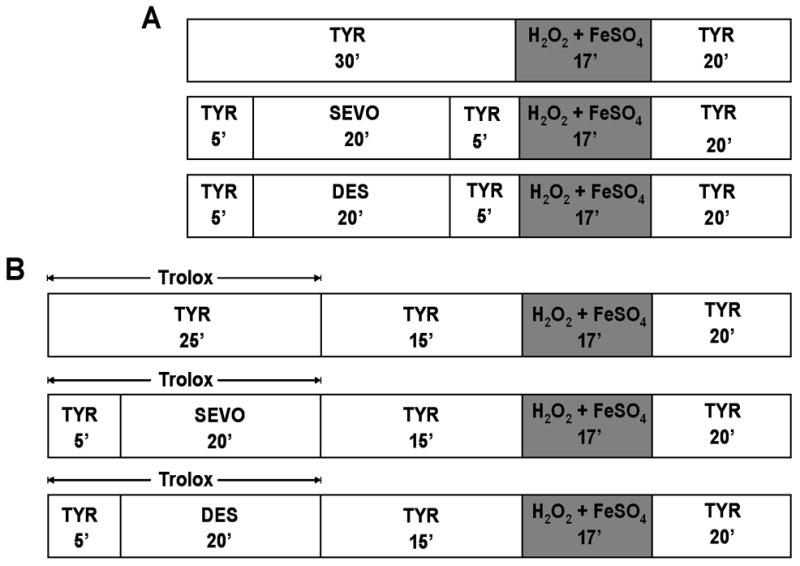
Protocols used in cell survival experiments (Fig. 2 and Fig. 4). TYR = glucose-free Tyrode solution; SEVO = sevoflurane; DES = desflurane.
Analysis of ROS production
The ROS-sensitive indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Molecular Probes, Eugene, OR) was used for assessment of ROS production. CM-H2DCFDA is a membrane permeable form of indicator that diffuses into cardiomyocytes where cellular esterases cleave the acetate group yielding membrane impermeable, non-fluorescent, CM-H2DCF. ROS, mainly H2O2, oxidize CM-H2DCF, generating fluorescent CM-DCF. Isolated cardiomyocytes were loaded with 2 μM CM-H2DCFDA for 20 minutes, followed by 20-minute washout. Cells were superfused with Tyrode solution at room temperature and fluorescence was recorded with a laser scanning confocal microscope (Leica TCS SP5, Mannheim, Germany) using the 40×/0.85 dry objective and 8000 Hz resonant scanner. Data were analyzed using LAS AF software (Leica, Mannheim, Germany). Spectrofluorometrical analysis showed that concentrations of VAs 10 times larger than used in the current study did not change CM-H2DCF fluorescence in Tyrode solution, excluding a possibility that VAs directly enhance fluorescence signal. Rates of increase in CM-DCF fluorescence before and during anesthetic application were analyzed statistically. The cells showing a baseline increase in the CM-DCF signal more than 15% over 10 minutes were excluded from the study.
Analysis of mitochondrial redox state
Autofluorescence of flavoproteins (FP) was measured for the assessment of mitochondrial redox state.13 Fluorescence signal that increases with oxidation of FPs is compensatory to mitochondrial uncoupling.14 Used as positive control, protonophore 2,4-dinitrophenol (DNP, 100 μM) induced maximal oxidation of FPs. Isolated cardiomyocytes were superfused with Tyrode solution in the recording chamber, and fluorescence was recorded at room temperature using the laser-scanning confocal microscope (Eclipse TE2000-U; Nikon, Tokyo, Japan) with a 40×/1.3 oil-immersion objective (Nikon). Data were analyzedusing MetaMorph 6.1 software (Universal Imaging, West Chester, PA). For the purpose of statistical analyses, the averaged fluorescence of time points recorded before anesthetic exposure (Baseline) was compared to the averaged fluorescence of time points during anesthetic exposure and anesthetic washout. Results are presented as percent change in fluorescence intensities relative to baseline (F0) before the exposure to anesthetics (F/F0 × 100).
Anesthetics
Sevoflurane (Abbot Laboratories, North Chicago, IL) and desflurane (Baxter, Deerfield, IL) were dispersed in experimental solution by sonication. Anesthetic-containing solution was delivered from airtight glass syringes, and the recording chamber was covered with a glass coverslip to maintain a constant concentration of anesthetics. At the end of each experiment, samples were taken from the outflow of the recording chamber, and anesthetic concentrations were analyzed by gas chromatography. The mean desflurane and sevoflurane concentrations were 1.26±0.28 mM and 0.62±0.11 mM, respectively, equivalent to 0.6 minimum alveolar concentration (MAC) and 0.7 MAC, respectively, in rats. A 5-minute washout was sufficient to eliminate anesthetic from the recording chamber.
Statistical analysis
Data are presented as means ± SD, and n indicates number of experiments. Results were analyzed using analysisof variance with the Bonferroni’s post hoc test. In the cell survival experiments, each anesthetic group, with or without Trolox, was compared to a matching stress group using Student t test, while different experimental groups were analyzed using analysisof variance with the Bonferroni’s post hoc test. Differences at P<0.05 were considered significant.
RESULTS
ROS are involved in desflurane and sevoflurane-induced cardiomyocyte protection from oxidative stress
To test for cytoprotective effects by desflurane and sevoflurane, cardiomyocytes were exposed to oxidative stress (H2O2 + FeSO4) with or without pretreatment with anesthetics. Percent cell death was determined relative to matching stress groups. Cell death in stress groups was as follows: desflurane control 68±12%, sevoflurane control 59±16%, desflurane-Trolox control 62±10%, and sevoflurane-Trolox control 50±15%. Pretreatment with desflurane or sevoflurane significantly reduced cell death to 69±27% (n=12) and 81±30% (n=21), respectively, suggesting that in vitro preconditioning with either anesthetic effectively protects isolated cardiomyocytes from oxidative stress. Although differences between anesthetics were not significant, desflurane tended to have greater preconditioning potency than sevoflurane. To test whether ROS are involved in initiation of APC, the ROS scavenger Trolox (250 μM) was applied together with anesthetics. In the presence of Trolox the protection by anesthetics was abolished and cell death was 90±32% (n=12) in the desflurane and 99±28% in the sevoflurane group (Fig. 2). These findings suggested that APC with desflurane and sevoflurane protects cardiomyocytes from oxidative stress via an increase in ROS production.
Figure 2.
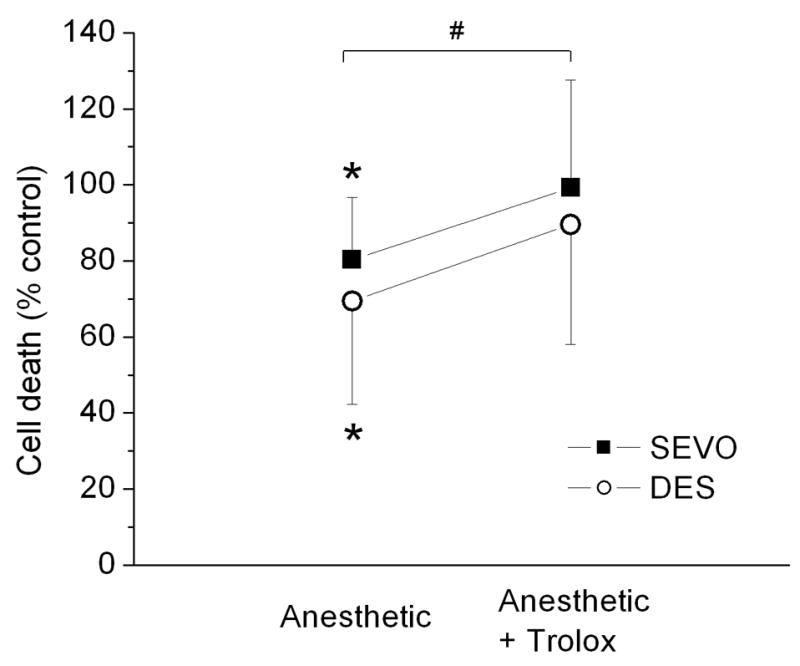
Protection of ventricular myocytes from oxidative stress by desflurane or sevoflurane preconditioning is abrogated by reactive oxygen species (ROS) scavenger Trolox. Percent cell death was expressed relative to that of matching stress group. Preexposure of cardiomyocytes to sevoflurane (SEVO, 0.7 MAC) or desflurane (DES, 0.6 MAC) significantly attenuated cell death. Co-application of Trolox with anesthetics abrogated preconditioning effect. Shown are summary data for 12–25 experiments in each group. Values are means ± SD. *P<0.05 vs. matching stress control; #P<0.05 anesthetic vs. anesthetic + Trolox.
Acute treatment with desflurane and sevoflurane increases ROS production in isolated cardiomyocytes
To confirm whether acute treatment with anesthetics enhances ROS generation, the ROS-sensitive fluorescence indicator CM-H2DCFDA was used. The rate of relative increase in fluorescence prior to anesthetic application (Baseline) was 0.65±0.50 in the desflurane and 0.66±0.41 in the sevoflurane group (Fig. 3A). During application of desflurane or sevoflurane, the rate of relative increase in fluorescence was 1.48±0.64 and 1.08±0.27, respectively, which was significantly greater than baseline values, and the effect of desflurane was significantly greater than sevoflurane. Fig. 3B shows fluorescence data corrected for increase in the baseline fluorescence. During the anesthetic washout, CM-DCF fluorescence did not increase further (data not shown). Since CM-DCF is irreversibly oxidized by ROS,15 no change in fluorescence suggested a decrease in the rate of ROS production during anesthetic washout.
Figure 3.
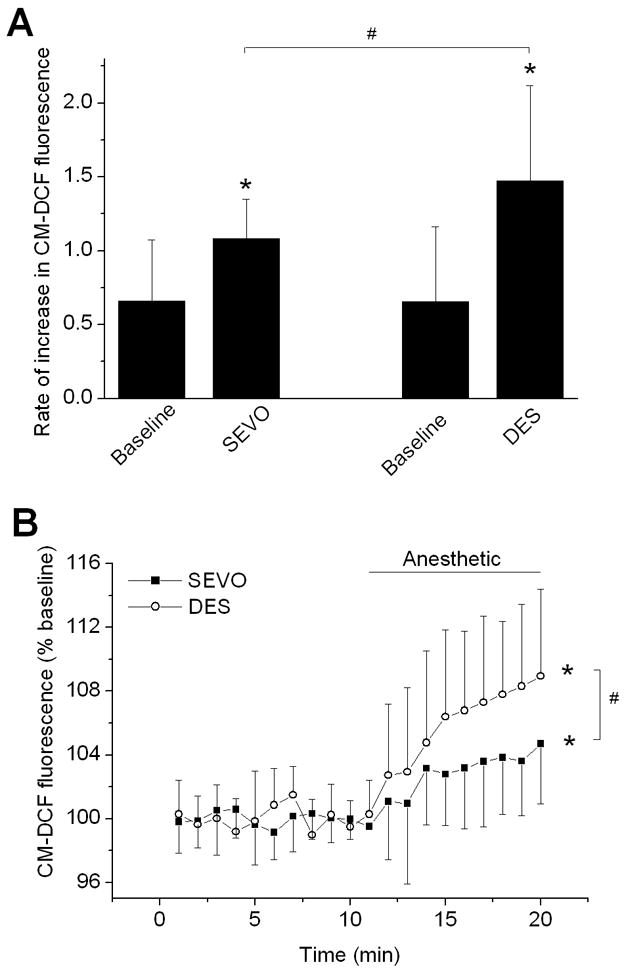
Sevoflurane and desflurane induce reactive oxygen species (ROS) production in isolated ventricular myocytes. Cells were loaded with the ROS indicator CM-H2DCFDA (2 μM) and changes in fluorescence were recorded over time by confocal microscopy. (A) The rates of relative change in CM-DCF fluorescence before anesthetic exposure (Baseline) and during anesthetic application (SEVO and DES). Sevoflurane (0.7 MAC) and desflurane (0.6 MAC) increased production of ROS. The effect of desflurane was significantly greater than the effect of sevoflurane. (B) Summary of data corrected for changes in baseline fluorescence. Values are means ± SD (n=15–20). *P<0.05 vs. baseline; #P<0.05 DES vs. SEVO.
Desflurane and sevoflurane have differential effects on oxidation of FPs
Enhancement of FP fluorescence by isoflurane is attributed to activation of the mitoKATP channel that uncouples mitochondria and causes oxidation of FPs.14,16 We used FP autofluorescence for assessment of mitochondrial uncoupling that likely reflects opening of the mitoKATP channel.17 Desflurane and sevoflurane significantly increased oxidation of mitochondrial FPs to 168±36% (n=19) and 141±37% (n=25) of baseline, respectively, with desflurane exerting a significantly greater effect than sevoflurane (Fig. 4). Greater mitochondrial uncoupling by desflurane appears to correlate with greater ROS production evoked by desflurane.
Figure 4.
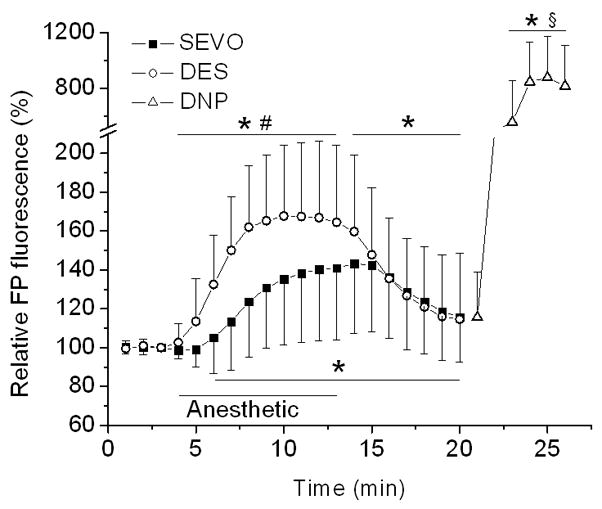
Desflurane induces greater oxidation of mitochondrial flavoproteins (FPs) than sevoflurane in isolated cardiomyocytes. Autofluorescence of FPs is used as an indicator of mitochondrial redox state, in which an increase in fluorescence signal indicates shift toward more oxidized state and mitochondrial uncoupling. An increase in FP fluorescence induced by desflurane (DES, 0.6 MAC) and sevoflurane (SEVO, 0.7 MAC) indirectly reflects depolarization of mitochondria, with desflurane showing a significantly greater effect than sevoflurane. DNP (100 μM), a mitochondrial uncoupler, was added at the end of experiment to produce complete oxidation of FPs. Data are expressed as changes from the baseline. Summary data are means ± SD, n=19–25. *P<0.05 vs. baseline; #P<0.05 vs. SEVO; §P<0.05 vs. DES and SEVO.
ROS mediate mitochondrial uncoupling induced by desflurane and sevoflurane
To test whether enhanced ROS production precedes mitochondrial uncoupling during anesthetic treatment, Trolox was applied 5 min before and during application of anesthetics. Application of desflurane and sevoflurane in the presence of Trolox increased peak FP fluorescence to 136±17% (n=10) and 111±8% (n=8) of baseline, respectively, which is significantly less compared to the effect of anesthetics alone (Fig 5). Although sevoflurane-induced oxidation of FPs was completely abolished by Trolox, the desflurane effect was only attenuated. Trolox alone did not have any significant effect on FP fluorescence. These findings suggested that ROS mediate the mitochondrial uncoupling induced by desflurane and sevoflurane.
Figure 5.
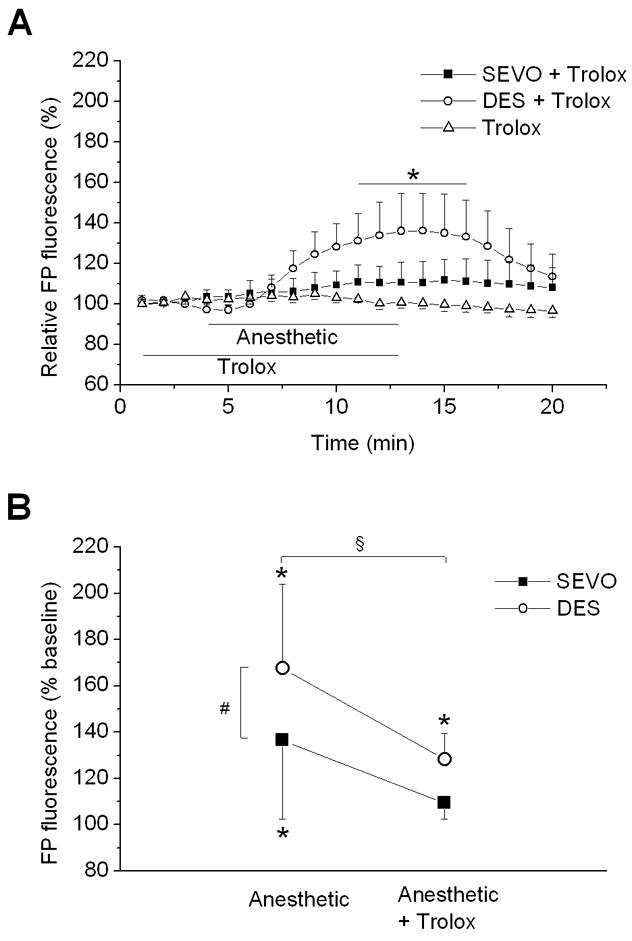
Effect of Trolox on anesthetic-induced changes in flavoprotein (FP) autofluorescence. (A) Scavenging reactive oxygen species (ROS) with Trolox abrogated sevoflurane (SEVO, 0.7 MAC) and attenuated desflurane (DES, 0.6 MAC)-induced increase in FP fluorescence, suggesting that anesthetic-induced mitochondria uncoupling is mediated by ROS. Summary data are from 8–10 experiments. (B) Fluorescence measured at 8 min of exposure to either anesthetic or anesthetic + Trolox is expressed as percent of baseline for comparison between groups. Data are means ± SD. *P<0.05 vs. baseline; #P<0.05 vs. SEVO; §P<0.05 anesthetic vs. anesthetic + Trolox.
DISCUSSION
The results of the present study show that preconditioning of freshly isolated cardiomyocytes with either desflurane or sevoflurane decreases cell death caused by oxidative stress. Cytoprotective effects of desflurane and sevoflurane were completely abolished by the ROS scavenger Trolox, suggesting involvement of ROS in the triggering phase of preconditioning. This was confirmed by demonstration of increased ROS production during acute application of both VAs. Furthermore, both anesthetics increased the oxidation of mitochondrial FPs, implying mitochondrial uncoupling. Application of Trolox together with anesthetics significantly attenuated the desflurane-induced FP oxidation and abrogated FP oxidation induced by sevoflurane, suggesting that ROS generation precedes mitochondrial uncoupling. Furthermore, desflurane exhibited a significantly greater effect on ROS production and FP oxidation than sevoflurane. Our study is the first to demonstrate differences in ROS production and mitochondrial uncoupling, 2 important components of the APC signaling cascade, between desflurane and sevoflurane. This may reflect different preconditioning potency of these VAs. We also showed that VAs enhance ROS production by direct action on cardiomyocytes. Contrary to many other studies,9,18,19 our study suggests that enhanced ROS production precedes mitochondrial uncoupling in the APC signaling cascade.
The present study demonstrates that, similar to isoflurane,8 preconditioning by desflurane and sevoflurane increases survival of isolated cardiomyocytes during oxidative stress. To test the functional importance of ROS for preconditioning by desflurane and sevoflurane, the ROS scavenger Trolox was applied together with VAs resulting in abrogated cytoprotection. Interestingly, Trolox abolished sevoflurane-induced oxidation of FPs, but only attenuated FP oxidation induced by desflurane, suggesting that the signaling cascade in preconditioning by desflurane is only partly mediated by ROS, or more likely that Trolox incompletely scavenged ROS induced by desflurane since desflurane stimulated greater ROS production than sevoflurane. The latter was also indicated by another study that demonstrated complete abrogation of desflurane-induced preconditioning by ROS scavenging.5
Although previous studies suggested that ROS mediate APC signaling cascade,5–7 to our knowledge, the present study is the first documentation that VAs enhance ROS production by direct action on cardiomyocytes under non-ischemic conditions.20 It has been reported that treatment of the whole heart with sevoflurane increases ROS production,12 but those results did not exclude the possibility that other types of cells, besides cardiomyocytes, were involved in this process. Since a mild increase in ROS production contributes to the signal transduction cascade of ischemic preconditioning,21 it is possible that APC, by inducing ROS production, initiates an endogenous defense program against ischemia. Among identified cellular ROS targets that may be related to preconditioning, the HIF–1α,22 PKC,23 mitoKATP channel,11 and MAP kinases24 are implicated in APC.8,25–27
Oxidation of FPs is a compensatory response to mitochondrial depolarization and uncoupling,13 which can be induced by pharmacological agents or by opening mitochondrial ion channels, such as mitoKATP and calcium-activated potassium (mitoBKCa) channels. Although both channels are implied in APC,14,16,28 most studies implicate mitoKATP channel opening as a part of the APC signaling cascade8,9 and isoflurane-induced FP oxidation.14 Thus, the effect of desflurane and sevoflurane on oxidation of FPs is likely dominated by the extent of mitoKATP channel opening, suggesting a significantly greater effect of desflurane than sevoflurane on mitoKATP channel opening. Compared to complete FP oxidation by DNP, the effects of desflurane and sevoflurane were moderate, further suggesting that VAs only partly uncouple mitochondria.
Previous demonstration that ROS10,11 and isoflurane10 directly open mitoKATP channels reconstituted into lipid bilayer brought additional complexity to the relationship between mitoKATP channel and ROS in APC, since some studies reported that an increase in ROS production is a consequence of mitoKATP channel opening and mitochondrial potassium influx.9,18,19 Our results suggest that an increase in ROS production precedes oxidation of FP (i.e. mitoKATP channel opening) since ROS scavenging attenuated desflurane-induced FP oxidation and completely abolished sevoflurane-induced FP oxidation. This is in agreement with other studies showing that sevoflurane-induced generation of ROS is independent of mitoKATP channel opening7 and that H2O2 acts upstream of mitoKATP channel opening.29 It is possible that both concepts are correct and that there is positive feedback between ROS and mitoKATP channel opening. Potassium influx may enhance ROS production by mitochondria while ROS, in turn, may further stimulate opening of the mitoKATP channel. Initiation of this positive feedback loop could be mediated by PKC30 or directly by VAs which open the mitoKATP channel.10 Another mechanism by which VAs may induce ROS production is inhibition of electron flux across the electron transport chain,31 resulting in increased “electron leak” and generation of ROS.32
Few studies compared the preconditioning signaling cascades of desflurane and sevoflurane. In contrast to our study that indicates differences between signaling cascades of these VAs, a comprehensive proteome analysis of left ventricular tissue after 3h exposure to desflurane or sevoflurane did not show differences in patterns of protein expression between anesthetics.33 However, it is possible that the modest changes in posttranslational protein modifications in signaling cascades of desflurane and sevoflurane were not detected in that study.
Our study has several limitations. The cellular model of APC using isolated cardiomyocytes provides promising evidence for a direct effect of anesthetics on cardiac cells, but these results cannot be extrapolated to humans since they were obtained from different species. Also, our study did not address the possible effects of VAs on vasculature, neuronal elements and the immune system, all of which could be relevant to APC.4,34 Another limitation is that our experiments were performed at room temperature in non-beating, quiescent cardiomyocytes. We are aware that cellular processes, such as ROS generation, may have different kinetics at room temperature in quiescent cardiomyocytes.35
Important findings from our study are that desflurane induces greater ROS production, greater mitochondrial uncoupling, and shows a tendency for greater cytoprotection than sevoflurane. Based on these findings, we anticipate that desflurane would be a more potent cardioprotective drug than sevoflurane in clinical use. However, the drawbacks mentioned in the paragraph above need to be considered before extrapolating these data to patients. Also, observed effects may be beneficial in isolated healthy cardiomyocytes, but this may not be the case in patients with myocardial dysfunction, since both ROS and mitochondrial uncoupling may also have detrimental effects.
Our study showed that desflurane and sevoflurane alter ROS production and induce mitochondrial uncoupling in ventricular cardiomyocytes, with desflurane having a significantly greater effect than sevoflurane. This coincides with a tendency for greater cardioprotection by desflurane preconditioning than sevoflurane. Our data imply that desflurane has greater preconditioning potency due to greater activation of preconditioning signaling cascade which involves mitochondrial uncoupling, most likely via mitoKATP channel opening, and is preceded by enhanced ROS production.
Acknowledgments
Financial Support: Supported in part by grants P01GM066730 and R01HL034708 (ZJ Bosnjak) from the National Institutes of Health, Bethesda, Maryland
We thank Samantha J. Mueller, B.S. for technical assistance, Terri Misorski, A.A.S. for editorial assistance, Mary Ziebell for anesthetic measurements and Aniko Szabo, Ph.D. for assistance in statistical analysis.
Footnotes
Conflicts of Interest: None
References
- 1.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 2.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007;21:212–9. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 3.Piriou V, Chiari P, Lhuillier F, Bastien O, Loufoua J, Raisky O, David JS, Ovize M, Lehot JJ. Pharmacological preconditioning: comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br J Anaesth. 2002;89:486–91. [PubMed] [Google Scholar]
- 4.Lange M, Smul TM, Blomeyer CA, Redel A, Klotz KN, Roewer N, Kehl F. Role of the beta1-adrenergic pathway in anesthetic and ischemic preconditioning against myocardial infarction in the rabbit heart in vivo. Anesthesiology. 2006;105:503–10. doi: 10.1097/00000542-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Hanouz JL, Zhu L, Lemoine S, Durand C, Lepage O, Massetti M, Khayat A, Plaud B, Gerard JL. Reactive oxygen species mediate sevoflurane- and desflurane-induced preconditioning in isolated human right atria in vitro. Anesth Analg. 2007;105:1534–9. doi: 10.1213/01.ane.0000286170.22307.1a. table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Jr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97:1485–90. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Riess ML, Kevin LG, McCormick J, Jiang MT, Rhodes SS, Stowe DF. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in Guinea pig isolated hearts. Anesth Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- 8.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–43. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Jiang MT, Nakae Y, Ljubkovic M, Kwok WM, Stowe DF, Bosnjak ZJ. Isoflurane activates human cardiac mitochondrial adenosine triphosphate-sensitive K+ channels reconstituted in lipid bilayers. Anesth Analg. 2007;105:926–32. doi: 10.1213/01.ane.0000278640.81206.92. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89:1177–83. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- 12.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96:949–55. doi: 10.1213/01.ANE.0000052515.25465.35. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 14.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–90. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 15.Acker T, Fandrey J, Acker H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc Res. 2006;71:195–207. doi: 10.1016/j.cardiores.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–340. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, O’Rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–4. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–74. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 19.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–6. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 20.Dworschak M, Breukelmann D, Hannon JD. The impact of isoflurane during simulated ischemia/reoxygenation on intracellular calcium, contractile function, and arrhythmia in ventricular myocytes. Anesth Analg. 2004;99:1302–7. doi: 10.1213/01.ANE.0000134803.28029.7E. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–69. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- 22.Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, Ha J. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–21. doi: 10.1093/carcin/bgn032. [DOI] [PubMed] [Google Scholar]
- 23.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–61. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee HT, Xu H, Ota-Setlik A, Emala CW. Oxidant preconditioning protects human proximal tubular cells against lethal oxidant injury via p38 MAPK and heme oxygenase-1. Am J Nephrol. 2003;23:324–33. doi: 10.1159/000072914. [DOI] [PubMed] [Google Scholar]
- 25.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–25. doi: 10.1097/ALN.0b013e318164cab1. [DOI] [PubMed] [Google Scholar]
- 26.Bouwman RA, Musters RJ, van Beek-Harmsen BJ, de Lange JJ, Lamberts RR, Loer SA, Boer C. Sevoflurane-induced cardioprotection depends on PKC-alpha activation via production of reactive oxygen species. Br J Anaesth. 2007;99:639–45. doi: 10.1093/bja/aem202. [DOI] [PubMed] [Google Scholar]
- 27.Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003;99:138–47. doi: 10.1097/00000542-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Redel A, Lange M, Jazbutyte V, Lotz C, Smul TM, Roewer N, Kehl F. Activation of mitochondrial large-conductance calcium-activated K+ channels via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg. 2008;106:384–91. doi: 10.1213/ane.0b013e318160650f. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HY, McPherson BC, Liu H, Baman TS, Rock P, Yao Z. H(2)O(2) opens mitochondrial K(ATP) channels and inhibits GABA receptors via protein kinase C-epsilon in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1395–403. doi: 10.1152/ajpheart.00683.2001. [DOI] [PubMed] [Google Scholar]
- 30.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–83. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 31.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–93. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 33.Kalenka A, Maurer MH, Feldmann RE, Kuschinsky W, Waschke KF. Volatile anesthetics evoke prolonged changes in the proteome of the left ventricule myocardium: defining a molecular basis of cardioprotection? Acta Anaesthesiol Scand. 2006;50:414–27. doi: 10.1111/j.1399-6576.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 34.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–99. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]


