Abstract
The objective of this study was to determine if dietary selenium could inhibit pulmonary cell proliferation in control and cigarette smoke-exposed female A/J mice. Selenium in the form of sodium selenite was supplemented to purified diets similar to the AIN-93M diet to yield 0.15, 0.5, or 2.0 mg selenium/kg diet. After 3 weeks, mice in each dietary group were divided into two subgroups; one used as control, whereas the other was exposed to cigarette smoke for five consecutive days. Mice from both groups were euthanized 3 days later. Mice were administered bromodeoxyuridine in the drinking water starting 5 days before the initiation of the smoke exposure and continuing until they were euthanized. After euthanasia, the left lung lobe was processed for histology and cell proliferation analysis. Cigarette smoke increased cell proliferation in the terminal bronchioles and large airways, but not in alveoli. High-selenium diets inhibited cell proliferation in the alveoli, terminal bronchioles and large airways areas in both control and smoke-exposed mice. Increasing the dietary selenium level led to increased selenium levels in the blood and lung, and increased glutathione peroxidase (GPx) activity in the lung. Cytochrome P-450 1A1 protein levels in the lung were increased by cigarette smoke but were not affected by dietary selenium. It is concluded that dietary selenium inhibits pulmonary cell proliferation in both control and cigarette smoke-exposed mice, indicating that selenium is inhibiting cell proliferation independently of smoke exposure, and that this inhibition may be related to selenium concentration and GPx activity in the lung.
Keywords: selenium, cigarette, smoke, cell proliferation, lung
Lung cancer is the leading cause of death from cancer in the United States. The major risk factor for lung cancer development is cigarette smoking. Tobacco smoke is a complex chemical mixture which has been found to contain approximately 4800 different compounds (Hoffmann et al., 2001); approximately 100 of them are known carcinogens, co-carcinogens and/or mutagens. Polycyclic aromatic hydrocarbons (PAHs) and N-nitrosamines have received most attention in relation to tobacco carcinogenesis. Many other smoke constituents like free radicals, aromatic amines, catechols, aldehydes, and inorganics like nickel, chromium, and cadmium may be equally important, however, because some of these are present in smoke at substantially high levels. The presence of high levels of pro-oxidants like free radicals in smoke is well documented, and a puff of cigarette smoke is estimated to contain over 1015 free radicals, which are detectable in both mainstream and sidestream cigarette smoke (Pryor et al., 1983). Steady state reactions in smoke can prolong the half-lives of many radicals that continue to generate superoxide anions and other free radicals (Pryor, 1997), thus increasing the oxidative potential and possibly tumorigenic activity of cigarette smoke.
Although the tumorigenicity of cigarette smoke condensates on mouse skin was demonstrated long ago, it has been generally difficult to induce respiratory cancers in animals by inhalation exposure to cigarette smoke (Coggins, 1998, 2007). However, Witschi and coworkers (Witschi et al., 1997a, b, 2000, 2002) have conclusively demonstrated the carcinogenicity of inhaled cigarette smoke in the A/J strain of mice. The A/J mouse strain used in the smoking model is relatively more sensitive to carcinogens and has been extensively employed to examine the carcinogenicity of many structurally diverse chemicals and to develop chemopreventive interventions for lung tumorigenesis (Stoner, 1991; Stoner et al., 1993). Treatment of these mice with single carcinogens, some known to be present in tobacco smoke, for example, PAH and N-nitrosamines, has been found to significantly increase the number of lung tumors in this strain. These observations and others have led to the widely held belief that PAH metabolites like diolepoxides and tobacco specific N-nitrosamine metabolites like 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) are the primary tobacco carcinogens (Hecht, 1999). However, studies of Witschi and coworkers (Witschi et al., 1997a, 1998, 2000) suggest that other smoke constituents may also be involved in tobacco carcinogenesis. Two observations in particular support this contention. First, chemopreventive agents which inhibited PAH- and NNK-induced lung tumors in this mouse model were found to be ineffective against smoke-induced lung tumors (Witschi et al., 1998, 2000). Second, exposure to gas phase of smoke alone, which is largely devoid of the particulate carcinogens, increased lung tumors in A/J mice to the same extent as whole smoke (Witschi et al., 1997a). More recent studies have implicated a role of other tobacco smoke constituents such as 1,3-butadiene in smoke-induced lung tumorigenesis (Witschi, 2005). Free radicals and other pro-oxidants of tobacco smoke, which induce oxidative stress, can also be expected to play a role in lung tumorigenesis. Overall, these studies demonstrate the utility of Witschi model for testing complex mixture like cigarette smoke and for examining the effects of putative chemopreventive agents.
Dietary selenium (Se) has been found to inhibit the development of several types of cancer in epidemiological and experimental studies. The beneficial effect of Se in cancer chemoprevention has been recognized for nearly nine decades (Schrauzer, 2000). The Se supplementation trial by Clark et al. (1996), primarily designed to prevent skin cancer recurrence, demonstrated that treatment with Se decreased the risk of cancer of the prostate, lung, and colon and rectum. In addition, the concentration of Se in tobaccos from low lung cancer-incidence countries is significantly higher (0.49 ± 0.22 μg/g) than that in tobaccos from high lung cancer-incidence countries (0.16 ± 0.05 μg/g) (Bogden et al., 1981). However, the recently-published SELECT trial failed to detect inhibitory effects of Se on lung, colon, or prostate cancer (Lippman et al., 2009). Other clinical trials are currently ongoing (Facompre and El-Bayoumy, 2009). Although the Clark et al. study and similar clinical trials as well as epidemiologic studies and animal studies pointed to the potential use of Se for cancer prevention and therapy, the mechanisms by which Se could protect from cancer have not been well defined. Proposed mechanisms that may explain the anti-cancer effect of Se include antioxidant protection from glutathione peroxidases (GPx) and thioredoxin reductase (TrxR), cell proliferation inhibition, increased apoptosis, effects on the cell cycle, transcription factor activation, increased expression of the tumor suppressor gene p53, impaired glutathione (GSH) metabolism, and formation of Se metabolites that are anti-tumorigenic (Combs and Gray, 1998; Ganther, 1999; Ip, 1998). Selenium is an essential nutrient for cell growth, but in higher amounts can inhibit cell proliferation (Zeng and Combs, 2008).
In this study, we tested the hypothesis that dietary Se inhibits cell proliferation in the lungs of A/J mice exposed to cigarette smoke. Witschi et al. (1995, 1997b) previously observed that smoke exposure increased cell proliferation in the lungs of mice. Mice were fed diets containing the recommended level of Se as well as two higher levels to determine if supplemental Se would influence cell proliferation in the lung.
MATERIALS AND METHODS
Materials.
The University of Kentucky reference research cigarettes 3R4F (Lexington, KY) were used. Materials for purified diets were obtained from Harlan Teklad (Madison, WI). Se was purchased from Sigma Chemical Company (St Louis, MO). The anti-CYP 1A1 antibody was purchased from XenoTech (Lenexa, KS) and the anti-rabbit horseradish peroxidase (HRP) antibodies were purchased from Sigma. All other chemicals, unless noted, were purchased from Sigma.
Smoke exposure system.
Inhalation exposures to smoke was carried out in a whole-body Hinners type stainless steel/glass chamber as described earlier (Gairola, 2006). Cigarette smoke was generated from 3R4F University of Kentucky research cigarettes. The concentration of smoke particulates in the exposure chamber atmosphere averaged 46 ± 3 mg TPM/m3. The mice received smoke exposure for a total of 6 h each day for 5 days, Monday through Friday, and then were euthanized the next Monday morning after the weekend.
Experimental design.
Female A/J mice, 7–8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME), and allowed to acclimate for one week before the beginning of the study. A total of 48 mice were split into six different groups containing eight mice per group and fed a purified diet similar to the AIN-93M diet formulation (Table 1), which contained Se at the concentration of 0.15 mg/kg (Reeves et al., 1993). Se (as sodium selenite) was added to the diet to obtain Se concentrations of 0.5 and 2.0 mg Se/kg diet, respectively (Table 1). Mice were fed the diets for 3 weeks before beginning smoke exposure, to allow them to adjust to the diets. At this time, one-half of the mice in each dietary group were exposed to cigarette smoke for 5 days (6 h/day). Mice were kept an additional 3 days (64 h) without being further exposed to smoke and then were euthanized, by anesthesia with isoflurane followed by exsanguination. Mice were administered bromodeoxyuridine (BrdU) in the drinking water (0.5 mg/ml) starting 5 days before the initiation of the smoke exposure and continuing until they were euthanized. The lungs were removed during autopsy with part of the lung frozen in liquid nitrogen and then stored at −80°C, whereas the remainder was fixed in buffered neutral formalin and then processed for histology. Blood was also collected during autopsy and serum was prepared and then stored at −80°C.
TABLE 1.
Purified Diet Composition
| Ingredient | % of diet |
| Torula yeast | 30 |
| Corn starch | 36 |
| Dextrose | 19.95 |
| Cellulose fiber | 5 |
| AIN-93M Mineral Mix | 3.5 |
| AIN-93 Vitamin Mix | 1 |
| Choline bitartrate | 0.25 |
| D,L-Methionine | 0.3 |
| Soybean oil | 4 |
| Total | 100 |
Analysis of cell proliferation.
After fixation and processing, 5-μm sections were prepared from paraffin blocks. The sections of paraffin-embedded tissue samples were deparaffinized, thoroughly washed in water, and then placed in 3% H2O2 in methanol for 10 min. The sections were then stained immunohistochemically by the avidin-biotin-peroxidase complex method using Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) with monoclonal antibodies specific for BrdU (Bectin Dickinson Labware, Franklin Lakes, NJ), at 100 μl/slide using a 1:40 dilution of BrdU. The reaction product was then visualized using diaminobenzidine (Vector Laboratories Peroxidase Substrate Kit) and the slides were counterstained with hematoxylin. Having brown nuclei identified cells that had incorporated BrdU. Labeling indexes in lung epithelial cells were determined for three regions: the alveolar zone, the terminal bronchioles, and the intrapulmonary large airways. In the alveolar zone 1000 cells per slide were counted in random fields, and in the terminal bronchioles and large airways 500 cells per slide were counted. Terminal bronchioles were identified by their opening into the alveolar ducts and large airways by their diameter (0.5–1.5 mm).
Exposure markers.
Microsomes were prepared from the frozen lung tissue by differential centrifugation (Fadhel et al., 2002), and were then used for determining CYP 1A1 protein levels by Western analysis (Subramaniam et al., 1999).
Determination of selenium status.
GPx activity was measured in lung homogenates and serum using the method of Lawrence and Burk (1976), using hydrogen peroxide as the substrate, which is specific for the Se-dependent forms of GPx. The absorbance was measured at 340 nm in spectrophotometer. Immediately cumene hydroperoxide was added and the absorbance was measured again at 340 nm in spectrophotometer. Lung and serum Se was determined using the method of Spallholz et al. (1978). For the GPx and serum Se methods, tissues from every mouse in the group were used, with two tissues combined before analysis, for a total of four samples per group. For the lung Se analysis, four lungs per group were used with two lungs being combined before analysis, for a total of two samples per group.
Statistical analyses.
All statistical analyses were conducted using SYSTAT V.8 (SPSS, Inc., Chicago, IL) software. Results were first analyzed by 2 × 3 ANOVA. Two-way ANOVA was used because the study was a two factorial study (Se level being one factor and smoke exposure being the other factor). Differences between means were determined using Bonferroni's post hoc test. The results are reported as means ± SEM. A probability level ≤ 0.05 was required for significance.
RESULTS
In this study, we examined if dietary Se could inhibit lung cell proliferation induced by cigarette smoke. Mice were initially fed diets varying in Se for 3 weeks. Mice fed the 0.5 mg/kg Se diet weighed significantly more than mice fed the 2.0 mg/kg diet at the end of this period (Table 2). One-half of the mice were then exposed to cigarette smoke for 5 days; mice were kept an additional 64 h without being further exposed to smoke and then were euthanized. All the mice survived cigarette smoke exposure except one (in the 0.15 mg/kg group). The control mice gained body weight, whereas the smoke-exposed mice lost body weight during the smoke exposure; mice exposed to cigarette smoke weighed less than control mice at the end of the study (Table 2). Final body weights were not affected by dietary Se.
TABLE 2.
Body Weights
| Body weight (g) |
||||
| Selenium (mg/kg diet) | Treatment | Mice per group | Beginning of exposure | End of study |
| 0.15 | Control | 8 | 18.85 ± 1.53a,b | 19.29 ± 1.67 |
| Smoke | 7 | 18.79 ± 1.01a,b | 16.54 ± 1.48* | |
| 0.5 | Control | 8 | 19.54 ± 1.07b | 20.00 ± 1.14 |
| Smoke | 8 | 19.95 ± 1.03b | 17.14 ± 1.04* | |
| 2.0 | Control | 8 | 18.90 ± 1.37a | 19.50 ± 1.33 |
| Smoke | 8 | 17.98 ± 1.14a | 16.24 ± 0.82* | |
| Results of two-way ANOVA (p values) | ||||
| Main effect for selenium | 0.01 | 0.24 | ||
| Main effect for smoking | 0.59 | < 0.001 | ||
| Selenium × Smoking interaction | 0.29 | 0.84 | ||
Note. Results are means ± SEM. Significant differences in smoke-exposed mice for the same selenium level are indicated with an asterisk (*); for differences due to dietary selenium, values with different superscript letters are significantly different (p < 0.05).
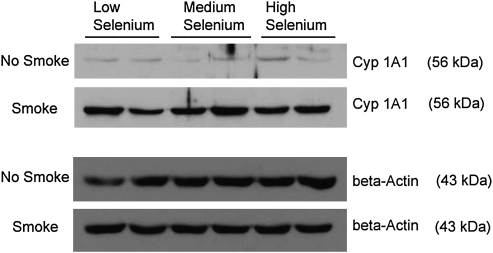
Lung CYP1A1 protein levels were quantified to verify smoke exposure. After 5 days exposure to cigarette smoke, the CYP1A1 protein levels of lung microsomes were higher than in the control mice in the three groups (Fig. 1). Dietary Se did not affect the CYP1A1 levels.
FIG. 1.
Western blot of CYP 1A1 in the lung of mice exposed to smoke and fed different levels of selenium.
Se concentrations in the lung and serum as well as GPx activity in the lung were quantified to determine if tissue Se levels responded to dietary Se. The Se levels in lung and serum increased as the intake of dietary Se increased (Table 3). In the lung, each dietary group was significantly different from the other groups; in the serum the medium and high dose groups were significantly different from the low dose group but not from each other (p < 0.05). There were no significant differences in Se levels, however, between the control and smoke-treated mice in either the lung or serum. Se-dependent GPx activity in the lung of the medium dose group was higher than that for the low dose group, but there were no significant differences between the high dose group and either of the other two groups. Selenium-dependent GPx activity was significantly higher in mice exposed to cigarette smoke. Total GPx levels were not affected by either dietary Se or smoke exposure (Table 4).
TABLE 3.
Selenium Concentrations in Lung and Serum
| Se concentration (ppm) |
|||
| Selenium (mg/kg diet) | Treatment | Lung | Serum |
| 0.15 | Control | 0.104 ± 0.008a | 0.109 ± 0.026a |
| Smoke | 0.098 ± 0.011a | 0.097 ± 0.023a | |
| 0.5 | Control | 0.117 ± 0.005b | 0.119 ± 0.021b |
| Smoke | 0.134 ± 0.008b | 0.148 ± 0.031b | |
| 2.0 | Control | 0.143 ± 0.021c | 0.139 ± 0.036b |
| Smoke | 0.169 ± 0.013c | 0.161 ± 0.034b | |
| Results of two way ANOVA (p values) | |||
| Main effect for Selenium | < 0.01 | 0.01 | |
| Main effect for Smoking | 0.13 | 0.29 | |
| Selenium × Smoking interaction | 0.22 | 0.35 | |
Note. Results are means ± SEM. Significant differences in smoke-exposed mice for the same selenium level are indicated with an asterisk (*); for differences due to dietary selenium, values with different superscript letters are significantly different (p < 0.05).
TABLE 4.
GPX Activities in the Lung
| Dietary Selenium (mg/kg diet) | Treatment | n | Se-dependent GPx activity (nmol NADPH/min/mg protein) | Total GPx activity (nmol NADPH/min/mg protein) |
| 0.15 | Control | 4 | 160.8 ± 51.8a | 321 ± 62 |
| Smoke | 4 | 227.8 ± 62.7*a | 305 ± 51 | |
| 0.5 | Control | 4 | 225.1 ± 26.3b | 330 ± 37 |
| Smoke | 4 | 262.6 ± 39.6*b | 346 ± 19 | |
| 2.0 | Control | 4 | 203.6 ± 31.6ab | 299 ± 46 |
| Smoke | 4 | 211.7 ± 49.0*a,b | 302 ± 27 | |
| Results of two way ANOVA (p values) | ||||
| Main effect for selenium | 0.10 | 0.24 | ||
| Main effect for Smoking | 0.05 | 0.96 | ||
| Selenium × Smoking interaction | 0.44 | 0.75 | ||
Note. Results are means ± SEM. Significant differences in smoke-exposed mice for the same selenium level are indicated with an asterisk (*); for differences due to dietary selenium, values with different superscript letters are significantly different (p < 0.05).
Cell proliferation was measured in the alveolar zone, terminal bronchioles, and large airways after a 12-day exposure to BrdU in the drinking water. In mice exposed to cigarette smoke, cell proliferation was increased in the terminal bronchioles and large airways, but not in the alveolar zone. In all three zones, cell proliferation in the medium and high Se dose groups was significantly lower than the low dose group but was not significantly different from each other, in both control and smoke-exposed mice (p < 0.05) (Table 5).
TABLE 5.
Labeling Indices in Alveolar Zone and Airways
| Labeling Index (%) |
|||||
| Selenium (mg/kg diet) | Treatment | n | Alveolar zone | Terminal bronchioles | Large airways |
| 0.15 | Control | 8 | 8.9 ± 4.0a | 9.8 ± 3.9a | 7.7 ± 3.0a |
| Smoke | 7 | 7.7 ± 4.3a | 11.3 ± 3.8*a | 11.6 ± 3.8*a | |
| 0.5 | Control | 8 | 5.3 ± 1.8b | 5.6 ± 2.1b | 2.9 ± 1.5b |
| Smoke | 8 | 4.3 ± 2.3b | 8.3 ± 2.7*b | 10.3 ± 4.5*b | |
| 2.0 | Control | 8 | 3.4 ± 1.7b | 5.6 ± 1.9b | 2.4 ± 1.6b |
| Smoke | 8 | 2.5 ± 1.3b | 7.4 ± 3.5*b | 7.7 ± 2.7*b | |
| Results of two-way ANOVA (p values) | |||||
| Main effect for selenium | < 0.001 | < 0.01 | < 0.001 | ||
| Main effect for Smoking | 0.22 | 0.04 | < 0.001 | ||
| Selenium × Smoking interaction | 0.99 | 0.86 | 0.28 | ||
Note. Results are means ± SDs. Significant differences in smoke-exposed mice for the same selenium level are indicated with an asterisk (*); for differences due to dietary selenium, values with different superscript letters are significantly different (p < 0.05).
DISCUSSION
In this study, we tested the hypothesis that cell proliferation induced by cigarette smoking may be inhibited by dietary Se. We observed that increased dietary Se inhibited cell proliferation in the lung, and that these effects were consistent with higher levels of Se in the lung. Dietary Se appeared to inhibit cell proliferation independently of smoke exposure, because it significantly inhibited cell proliferation in both control and smoke-exposed mice, with no significant interactions in the ANOVA.
We observed that cigarette smoke induced increases in cell proliferation in the terminal bronchioles and large airways but had no effect in alveoli. The results in the large airways and alveoli are in agreement with the earlier study of Witschi et al. (1995), who used a similar protocol (6 h/day, 5 days/week exposure with euthanasia one wk after beginning exposure; 7-day Alzet pumps were used for BrdU administration). However, we observed a significant increase in the labeling index in the terminal bronchioles, whereas Witschi et al. did not. Witschi et al. did observe an 89% increase, but this was not listed as being statistically significant, and the P value obtained was not listed. However, Witschi et al. (1997b) observed increases in cell proliferation in large airways and terminal bronchioles with 1–6 weeks exposure and in alveoli with 1–2 weeks exposure, using the same protocol. Prolonged exposure to smoke in mice did not induce increases in cell proliferation in any of the cell types, however (Witschi et al., 1995, 1997b). In rats, after exposure to cigarette smoke for 6 h/day, 5 days/week, bronchiolar cell proliferation was increased after 5 days but not after 28 or 90 days of smoke exposure, using a 3-day Alzet pump; alveolar cell proliferation was not affected at any time (Ayres et al., 1995). March et al. (1999), however, observed that exposing rats to cigarette smoke for 6 h/day, 5 days/week for 2 weeks, with a 7-day BrdU exposure using Alzet pumps resulted in increased cell proliferation in the terminal bronchioles and axial airways. In another study using rats, Zhong et al. (2005) found that a 14-week exposure (6 h/day, 3 days/week) increased cell proliferation in the central and distal airways but not the parenchyma. In hamsters, exposure to cigarette smoke did not result in increased cell proliferation in lung epithelium (Takahashi et al., 1992; Witschi and Rajini, 1994). Therefore species differences clearly exist in the proliferative response to cigarette smoke exposure, and variation among studies also exists.
The role of cell proliferation in lung carcinogenesis remains unclear. The induction of lung tumors clearly involves increased cell proliferation and/or decreased apoptosis. The results of the present study as well as those of Witschi et al. (1995; 1997b) show that the rate of cell proliferation in terminal bronchioles and large airways correlates well with the induction of tumors by smoke exposure, although not as well as in alveoli, one of the sites of lung tumorigenesis. With other lung carcinogens, cell proliferation is often correlated with carcinogenicity, but not always (Witschi et al., 1987; Witschi, 1986). Several metabolic pathways that lead to enhanced cell proliferation have been found to be activated during lung carcinogenesis, including cyclooxygenase (COX)-2 and its downstream signal transduction pathways, the Wnt-signaling pathway, ras-mediated signaling, and the epidermal growth factor signaling pathway (Adjei, 2001; Capdevila et al., 2009; He et al., 2005; Huber and Stratakis, 2004; Lee et al., 2008).
Se decreased cell proliferation in the alveolar zone, in the terminal bronchioles, and in the large airways, both in smoke-exposed and control mice. The medium dose level produced a significant decrease, with no further decrease in the high dose group. The concentration of Se in the lung was further increased in the high dose group, but this did not result in further reductions in cell proliferation. However, the highest activity of selenium-dependent GPx was observed in the medium dose group; the activity in the high dose group was not significantly different from that in the medium dose group. Therefore, the effects of Se on cell proliferation correlate better with selenium-dependent GPx activity than with Se concentrations. No previous studies have examined the effect of dietary Se on cell proliferation in the lung in vivo. Se has been found to inhibit the proliferation of lung cells in vitro (Ao et al., 1987; Chen et al., 2003; Swede et al., 2003).
These studies support a chemopreventive role for Se in lung carcinogenesis. 1,4-Phenylenebis(methylene)selenocyanate (p-XSC) but not sodium selenite or Se-enriched yeast was found to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)–induced lung tumors in A/J mice (Das et al., 2003; el-Bayoumy et al., 1993). p-XSC but not selenomethionine was subsequently found to inhibit both the initiation and promotion phases of NNK-induced carcinogenesis in A/J mice (Prokopczyk et al., 1997). Li et al. (2005) found that 2-oxo-selenazolidine-4(R)-carboxylic acid (2-oxo-SCA) and selenocystine inhibited NNK-induced lung tumors in mice, whereas sodium selenite, L-selenomethionine, Se-methyl-L-selenocysteine, SCA, and 2-methyl-SCA did not. In addition, dietary Se as sodium selenite did not inhibit NNK-induced lung tumors in A/J mice (Castonguay et al., 1991). Therefore, some forms of dietary Se appear to be effective in inhibiting lung carcinogenesis in mice, whereas others do not.
In summary, cell proliferation in the lungs of A/J mice was inhibited by dietary Se. Se inhibited cell proliferation in both control and smoke-exposed mice, indicating that it acted independently of smoke exposure. The effect of dietary Se on the smoke-induced A/J mouse model of lung cancer (Witschi et al., 1997b) should be examined in future studies. In addition, further research needs to be carried out to determine the molecular mechanisms by which Se is inhibitory, and thus provide a mechanistic basis for possible dietary recommendations for the prevention of lung cancer.
FUNDING
National Cancer Institute (grant number CA125788); China Scholarship Council; and Kentucky Agricultural Experiment Station.
Acknowledgments
Technical assistance of Ruth Holland and H. David Gillespie is gratefully acknowledged.
References
- Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- Ao P, Yu SY, Zhao M, Sun J. [Different effects of selenium on proliferation of human lung adenocarcinoma cells and human embryonic lung diploid cells in vitro] Zhonghua Zhong Liu Za Zhi. 1987;9:408–411. 22. [PubMed] [Google Scholar]
- Ayres PH, McKarns SC, Coggins CRE, Doolittle DJ, Sagartz JE, Payne VM, Mosberg AT. Replicative DNA synthesis in tissues of the rat exposed to aged and diluted sidestream smoke. Inhal. Toxicol. 1995;7:1225–1246. [Google Scholar]
- Bogden JD, Kemp FW, Buse M, Thind IS, Louria DB, Forgacs J, Llanos G, Moncoya Terrones I. Composition of tobaccos from countries with high and low incidences of lung cancer. I. Selenium, polonium-210, Alternaria, tar, and nicotine. J. Natl. Cancer Inst. 1981;66:27–31. [PubMed] [Google Scholar]
- Capdevila J, Elez E, Macarulla T, Ramos FJ, Ruiz-Echarri M, Tabernero J. Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment. Cancer Treat. Rev. 2009;35:354–363. doi: 10.1016/j.ctrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Castonguay A, Pepin P, Stoner GD. Lung tumorigenicity of NNK given orally to A/J mice: Its application to chemopreventive efficacy studies. Exp. Lung Res. 1991;17:485–499. doi: 10.3109/01902149109064434. [DOI] [PubMed] [Google Scholar]
- Chen WX, Cao XZ, Zhu RZ. [Effect of selenium dioxide on proliferation, apoptosis, and elomerase activity of human lung cancer cell line in vitro] Ai Zheng. 2003;22:927–931. [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- Coggins CR. A review of chronic inhalation studies with mainstream cigarette smoke in rats and mice. Toxicol. Pathol. 1998;26:307–314. doi: 10.1177/019262339802600301. discussion 315. [DOI] [PubMed] [Google Scholar]
- Coggins CR. An updated review of inhalation studies with cigarette smoke in laboratory animals. Int. J. Toxicol. 2007;26:331–338. doi: 10.1080/10915810701490190. [DOI] [PubMed] [Google Scholar]
- Combs GF, Gray WP. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- Das A, Desai D, Pittman B, Amin S, El-Bayoumy K. Comparison of the chemopreventive efficacies of 1,4-phenylenebis(methylene)selenocyanate and selenium-enriched yeast on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced lung tumorigenesis in A/J mouse. Nutr. Cancer. 2003;46:179–185. doi: 10.1207/S15327914NC4602_11. [DOI] [PubMed] [Google Scholar]
- el-Bayoumy K, Upadhyaya P, Desai DH, Amin S, Hecht SS. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenicity in mouse lung by the synthetic organoselenium compound, 1,4-phenylenebis(methylene)selenocyanate. Carcinogenesis. 1993;14:1111–1113. doi: 10.1093/carcin/14.6.1111. [DOI] [PubMed] [Google Scholar]
- Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: Implications for cancer survivors. Cancer Res. 2009;69:2699–2703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl on the induction of hepatic lipid peroxidation and cytochrome P-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Gairola CG. Animal models of 2nd hand smoking. In: Wang XL, Scott D, editors. Molecular Mechanisms for Tobacco-Induced Diseases. New York: Nova Science Publishers; 2006. pp. 121–132. [Google Scholar]
- Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- He B, Barg RN, You L, Xu Z, Reguart N, Mikami I, Batra S, Rosell R, Jablons DM. Wnt signaling in stem cells and non-small-cell lung cancer. Clin. Lung Cancer. 2005;7:54–60. doi: 10.3816/CLC.2005.n.022. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- Huber RM, Stratakis DF. Molecular oncology—Perspectives in lung cancer. Lung Cancer. 2004;45(Suppl. 2):S209–S213. doi: 10.1016/j.lungcan.2004.07.973. [DOI] [PubMed] [Google Scholar]
- Ip C. Lessons from basic research in selenium and cancer prevention. J. Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. Inflammation in lung carcinogenesis: New targets for lung cancer chemoprevention and treatment. Crit. Rev. Oncol. Hematol. 2008;66:208–217. doi: 10.1016/j.critrevonc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, Roberts JC. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J. Biochem. Mol. Toxicol. 2005;19:396–405. doi: 10.1002/jbt.20105. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March TH, Kolar LM, Barr EB, Finch GL, Menache MG, Nikula KJ. Enhanced pulmonary epithelial replication and axial airway mucosubstance changes in F344 rats exposed short-term to mainstream cigarette smoke. Toxicol. Appl. Pharmacol. 1999;161:171–179. doi: 10.1006/taap.1999.8798. [DOI] [PubMed] [Google Scholar]
- Prokopczyk B, Amin S, Desai DH, Kurtzke C, Upadhyaya P, El-Bayoumy K. Effects of 1,4-phenylenebis(methylene)selenocyanate and selenomethionine on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mouse lung. Carcinogenesis. 1997;18:1855–1857. doi: 10.1093/carcin/18.9.1855. [DOI] [PubMed] [Google Scholar]
- Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 1997;105(Suppl. 4):875–882. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: Nature of the free radicals in gas-phase smoke and in cigarette tar. Environ. Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN. Anticarcinogenic effects of selenium. Cell. Mol. Life Sci. 2000;57:1864–1873. doi: 10.1007/PL00000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallholz JE, Collins GF, Schwarz K. A single test tube method for the fluorometric microdetermination of selenium. Bioinorg. Chem. 1978;9:453–459. [Google Scholar]
- Stoner GD. Lung tumors in strain A mice as a bioassay for carcinogenicity of environmental chemicals. Exp. Lung Res. 1991;17:405–423. doi: 10.3109/01902149109064428. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Adam-Rodwell G, Morse MA. Lung tumors in strain A mice: Application for studies in cancer chemoprevention. J. Cell. Biochem. Suppl. 1993;17F:95–103. doi: 10.1002/jcb.240531014. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Srinivasan S, Bummer PM, Gairola CG. Perinatal sidestream cigarette smoke exposure and the developing pulmonary surfactant system in rats. Hum. Exp. Toxicol. 1999;18:206–211. doi: 10.1191/096032799678839923. [DOI] [PubMed] [Google Scholar]
- Swede H, Dong Y, Reid M, Marshall J, Ip C. Cell cycle arrest biomarkers in human lung cancer cells after treatment with selenium in culture. Cancer Epidemiol. Biomarkers Prev. 2003;12:1248–1252. [PubMed] [Google Scholar]
- Takahashi M, Imaida K, Mitsumori K, Okamiya H, Shinoda K, Yoshimura H, Furukawa F, Hayashi Y. Promoting effects of cigarette smoke on the respiratory tract carcinogenesis of Syrian golden hamsters treated with diethylnitrosamine. Carcinogenesis. 1992;13:569–572. doi: 10.1093/carcin/13.4.569. [DOI] [PubMed] [Google Scholar]
- Witschi HP. Separation of early diffuse alveolar cell proliferation from enhanced tumor development in mouse lung. Cancer Res. 1986;46:2675–2679. [PubMed] [Google Scholar]
- Witschi H. Carcinogenic activity of cigarette smoke gas phase and its modulation by beta-carotene and N-acetylcysteine. Toxicol. Sci. 2005;84:81–87. doi: 10.1093/toxsci/kfi043. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Dance ST, Miller MS. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol. Sci. 2002;68:322–330. doi: 10.1093/toxsci/68.2.322. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997a;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997b;18:575–586. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Yu M, Willits NH. The effects of phenethyl isothiocyanate, N-acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19:1789–1794. doi: 10.1093/carcin/19.10.1789. [DOI] [PubMed] [Google Scholar]
- Witschi H, Godfrey G, Frome E, Lindenschmidt RC. Pulmonary toxicity of cytostatic drugs: Cell kinetics. Fundam. Appl. Toxicol. 1987;8:253–262. doi: 10.1016/0272-0590(87)90124-2. [DOI] [PubMed] [Google Scholar]
- Witschi H, Oreffo VI, Pinkerton KE. Six-month exposure of strain A/J mice to cigarette sidestream smoke: Cell kinetics and lung tumor data. Fundam. Appl. Toxicol. 1995;26:32–40. doi: 10.1006/faat.1995.1072. [DOI] [PubMed] [Google Scholar]
- Witschi HP, Rajini P. Cell kinetics in the respiratory tract of hamsters exposed to cigarette sidestream smoke. Inhal. Toxicol. 1994;6:321–333. [Google Scholar]
- Witschi H, Uyeminami D, Moran D, Espiritu I. Chemoprevention of tobacco-smoke lung carcinogenesis in mice after cessation of smoke exposure. Carcinogenesis. 2000;21:977–982. doi: 10.1093/carcin/21.5.977. [DOI] [PubMed] [Google Scholar]
- Zeng H, Combs GF., Jr Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008;19:1–7. doi: 10.1016/j.jnutbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis. 2005;26:2187–2195. doi: 10.1093/carcin/bgi189. [DOI] [PubMed] [Google Scholar]