Abstract
Data from experiments conducted almost exclusively in the rat have established that some phthalates have deleterious effects on the fetal testis probably due to their antiandrogenic and/or estrogenic effects, but their mechanisms of action remain unknown. A recent study reported that phthalates also have deleterious effects on human fetal testis with germ cell number, but not steroidogenesis altered. Therefore, we used organ culture of fetal testes at different stages of development to analyze the direct effects of phthalates on both steroidogenesis and gonocyte development and to determine if the effects of MEHP on these functions reported in the rat can be extended to other mammalian species. We defined specific periods of sensitivity of the fetal mouse testis to MEHP for these two functions and showed that the effects of phthalates on steroidogenesis vary with the developmental stage. Conversely, the strong deleterious effects of phthalates on germ cells were constantly present during the active phases of gonocyte development and thus share no relationship with the steroidogenic status. Moreover, all the effects of phthalates were unchanged in testes from mice deficient for estrogen (ERαKO or ERβKO) or androgen (Tfm) receptors. In conclusion, our results demonstrate that phthalates impair mouse fetal germ cell number similarly to other mammalian species, but are neither estrogenic nor antiandrogenic molecules because their effects do not involve, directly or indirectly, ER or AR.
Keywords: MEHP, phthalates, fetal testis, gonocytes, steroidogenesis, Leydig cell, development, estrogen receptor, androgen receptor
Phthalate esters are a class of environmental endocrine disrupting chemicals which are mainly used as plasticizers in PVC plastics. They are found in numerous consumer goods and, because they are not covalently bound to the plastic product, they can leach out over time from these products and can be ingested (Silva et al., 2004). After absorption, phthalates are rapidly hydrolyzed by esterases in the gut and other tissues into a monoester, which is the active molecule (Latini, 2005). For example, di-(2-ethylhexyl) phthalate (DEHP), one of the most widely used phthalates, is metabolized to its monoester metabolite, mono-(2-ethylhexyl) phthalate (MEHP), which is a recognized active testicular toxicant (Fisher, 2004).
Numerous studies in the rat have shown that in utero exposure to phthalates results in male reproductive disorders including altered seminiferous cord formation, multinucleated gonocyte (MNG) formation, epididymal agenesis, nipple retention, reduced ano-genital distance, hypospadias and cryptorchidism (Foster, 2006; Gray et al., 2006), and also reduction of fetal testosterone production and Leydig cell Insl3 gene expression (Lehmann et al., 2004; McKinnell et al., 2005; Sharpe and Skakkebaek, 2008), all these effects attesting to a profound alteration of testis development.
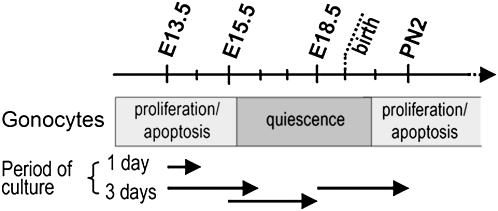
During fetal and neonatal development of the testis (Jost and Magre, 1993; Olaso and Habert, 2000), after migration of the primordial germ cells (PGCs) from extra-embryonic mesoderm to the genital ridge at embryonic day 11.5 (E11.5) in the mouse (Yoshimizu et al., 2001), the differentiating Sertoli cells (Wilhelm et al., 2007) surround the germ cells, therefore called gonocytes, to form the seminiferous cords from E12.5. The gonocytes divide actively, many of them undergoing apoptosis by E12.5-14.5 (Wang et al., 1998) and then enter a quiescent period (Nagano et al., 2000; Vergouwen et al., 1991). After birth, they resume mitosis while a second wave of apoptosis occurs (Boulogne et al., 2003; Wang et al., 1998) (Fig. 1) and start to differentiate into spermatogonia. The interstitial region contains mesenchymal cells and steroid-secreting Leydig cells from E12.5 (Livera et al., 2006; O'Shaughnessy et al., 2005). Interactions between the different cell lineages occur early in morphogenesis of the testis and are crucial for its normal development. Androgens mediate a wide range of developmental and physiological responses that are critical for the male reproductive and nonreproductive system (Sharpe, 2006; Welsh et al., 2008). During fetal life, testis development is physiologically modulated by, among other factors (Olaso and Habert, 2000), estrogen and androgen because estrogen receptor beta (ERβ) and androgen receptor (AR) inactivation lead to an increase in germ cell number (Delbes et al., 2004; Merlet et al., 2007), whereas testosterone production is enhanced by ERα inactivation (Delbes et al., 2005).
FIG. 1.
Schematic representation of the development of gonocytes during mouse fetal and neonatal life in relation to the timing and duration of the organ cultures as described in “Material and Methods.”
Until now, the mechanisms of action of phthalates have not been elucidated. MEHP activates peroxisome proliferators–activated receptor (PPAR-α) in cell transactivation assays (Corton and Lapinskas, 2005) but phthalate-treated PPAR-α−/− mice develop toxic lesions in the testis (Ward et al., 1998), suggesting that phthalates can act through PPAR-α–independent pathways in mediating testicular toxicity (Bhattacharya et al., 2005). In addition, phthalates show little estrogenic activity (Harris et al., 1997; Takeuchi et al., 2005), but can also reduce estradiol production (Lovekamp-Swan and Davis, 2003), and there is a growing consensus that they are antiandrogenic (Hu et al., 2009). However, phthalates and their mono-phthalate metabolites do not bind to AR (Parks et al., 2000), indicating that they are not direct AR antagonists. Most studies investigating the in utero effects of phthalates have been performed in the rat in vivo and have focused on the phthalate-induced suppression of testosterone production and Leydig cell aggregation (Fisher et al., 2003; Mylchreest et al., 1999). This has led to a hypothesized causal relationship between reduced testosterone production, altered fetal testicular development and reduced sperm counts in adulthood (Scott et al., 2007; Skakkebaek et al., 2001). However, an in vitro study from our laboratory on human fetal testis evidenced a reduction of the number of gonocytes by MEHP in the absence of any alteration of testosterone production (Lambrot et al., 2009). A recent study in the mouse demonstrated also that, as in the rat, in utero DBP exposure increases the number of MNGs, but unlike the rat this response occurs in the absence of measurable disruption of testicular testosterone concentrations (Gaido et al., 2007).
Therefore, we used organ culture of fetal mouse testis to establish that there is no relationship between phthalate-induced alterations of fetal steroidogenesis and gonocyte development, unlike what has been concluded from observations in the rat. Furthermore, using mice deficient for AR or ER, we showed that the phthalate-induced alterations are independent of these receptors.
MATERIAL AND METHODS
Chemicals and solutions.
The culture medium was phenol red-free Dulbecco's Modified Eagle Medium/Ham F12 (1:1) (Invitrogen, Carlsbad, CA) supplemented with 80 μg/ml gentamicin (Invitrogen). Ovine LH (oLH; NIH.LH S19; 1.01 IU/mg) was a gift from Dr A.F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). MEHP was from TCI Europe (Antwerp, Belgium). A stock solution (4mM) was made up in dimethyl sulfoxide and then diluted in culture medium. db-cAMP (N6, 2′-O-dibutyryl-cAMP) was from Sigma Aldrich (St Louis, MO).
Animals.
C57Bl/6 and transgenic mice were housed under controlled photoperiod (lights on 08:00-20:00) with ad libitum access to tap water and a soy and alfalfa-free breeding diet (Global diet 2019, Harlan Teklad, Indianapolis, IN). Mice lacking ERα (ERα−/−) or ERβ (ERβ−/−) were produced by Dupont et al. (2000) and generously provided by Pierre Chambon (Institut de Genetique et de Biologie Moleculaire et Cellulaire, Illkirch, France). Androgen-insensitive Tfm mice (C57BL/6J-Aw-J.Cg-EdaTa-6J_/_ArTfm, Tfm/Y) were from the Jackson Laboratory (Bar Harbor, ME). Transgenic animals were genotyped as described previously (Delbes et al., 2004, 2005; Merlet et al., 2007). Males were caged with females overnight and the day following overnight mating was counted as E0.5. Pregnant mice were killed by cervical dislocation on E13.5, E15.5, or E18.5 and the fetuses were quickly removed from the uterus. Fetuses were dissected under a binocular microscope, their sex was determined on the basis of the gonad morphology and the testes were collected from male fetuses. All animal studies were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Organ culture and treatment.
Organ cultures were performed as previously described (Livera et al., 2006). Briefly, intact E13.5 testes associated with their mesonephros were placed on 10-mm-diameter Millicell CM filters (Millipore, Billerica, MA) (pore size 0.45 μm). Testes from E15.5 and E18.5 were isolated and cut into six and eight pieces, respectively, and all the pieces from the same testis were placed on a single Millicell filter. The filter bearing the pieces of testes was floated on 0.4 ml of culture medium in tissue culture dishes and incubated at 37°C, in humidified atmosphere containing 95% air/5% CO2. The medium was changed every 24 h and the culture was pursued for 1 or 3 days at E13.5 and for 3 days at E15.5 and E18.5 (Fig. 1). The testes were cultured in the presence or absence of oLH (100 ng/ml) and the effect of 20 or 200μM MEHP was estimated by comparing one testis cultured in the presence of MEHP with the other testis from the same fetus cultured in control medium. At the end of culture, testes were fixed for 1 h at room temperature in Bouin's fluid, embedded in paraffin, and cut into 5-μm sections. For RNA analysis, testes were immediately dry frozen with liquid nitrogen and stored at −80°C. The entire media were kept at −20°C until testosterone radioimmunoassay. The data were obtained from at least three independently repeated cultures with fetuses from different litters.
Immunohistochemistry.
Serial sections were mounted on slides, deparaffinized and rehydrated. They were immunostained by a standard procedure as previously described (Delbes et al., 2007). To unmask the MVH (mouse vasa homolog) protein, sections were microwaved at 750 W for 5 min and at 450W for 3 min in 10mM citrate buffer solution (pH 6.0). For all immunohistological procedures, slides were incubated in 0.3% H2O2 for 15 min and in 5% bovine serum albumin in phosphate-buffered saline for 30 min to block nonspecific antigens and were then incubated with the primary antibody (rabbit polyclonal anti-AMH (anti-Müllerian hormone) antibody (1/500; Santa Cruz Biotechnology, Santa Cruz, CA), the rabbit polyclonal anti-3βHSD antibody (1/5000; provided by Prof A. Payne, Stanford University Medical Center) or the rabbit anti-MVH antibody (1/500; Abcam, Cambridge, UK) overnight at 4°C. The primary antibody was detected by incubation with an appropriate biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for a further 30 min at room temperature followed by incubation with avidin-biotin-peroxidase complex (Vector Laboratories) for 30 min. DAB (Vector Laboratories) was used as the chromogen and hematoxylin as the nuclear counterstained. Negative controls were done by omitting the primary antibody.
Testicular cell counting.
Germ cells and Leydig cells were identified by immunohistochemical detection of MVH and 3βHSD, respectively. The counting was done as previously described (Delbes et al., 2004). All counts were performed blind using Histolab analysis software (Microvision Instruments, Evry, France).
Measurement of germ cell apoptosis.
Because cleaved caspase-3 is involved in most of the apoptotic pathway, we chose its detection as a marker of apoptosis (Delbes et al., 2004). Gonocytes were distinguished by combining staining for AMH (a Sertoli-specific marker) and cleaved caspase-3 (rabbit polyclonal anti-cleaved caspase-3 1:100; Cell Signaling Technology, Beverly, MA). Cleaved caspase-3 was localized first using DAB (Vector Laboratories) and AMH was then detected using VIP substrate (Vector Laboratories). The percentage of apoptotic gem cells was determined by counting the stained and unstained gonocytes in all sections mounted.
Measurement of BrdU incorporation index.
The percentage of germ cells in S-phase was evaluated by measuring the BrdU (5-bromo-2′-deoxyuridine) incorporation by immunohistochemical methods using the Cell Proliferation Kit (GE Healthcare, Buckinghamshire, UK) according to the manufacturer's recommendations. BrdU (1%) was added to the culture medium 3 h before the end of culture. BrdU incorporation into proliferating cells was detected by immunochemistry using a mouse anti-BrdU antibody and a peroxidase-linked anti-mouse IgG. Sertoli cells and gonocytes were distinguished by combining staining for AMH (a Sertoli-specific marker) and BrdU. BrdU localization was performed first using DAB (Vector Laboratories) and AMH was then detected using VIP substrate (Vector Laboratories). The BrdU incorporation index was obtained by a blind counting of at least 1000 Sertoli cell or 400 gonocyte stained and unstained nuclei in five nonconsecutive sections from every treatment group experiment. Histolab analysis software was used for counting (Microvision Instruments, Evry, France).
Measurement of testosterone production.
Testosterone secretion into the medium was determined in duplicate by direct radioimmunoassay, without extraction as previously described (Habert and Picon, 1984).
RNA extraction and reverse transcription.
Total RNA was extracted using the RNeasy Plus mini-Kit (Qiagen, Courtaboeuf, France) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Courtaboeuf, France) according to the manufacturer's instructions.
Real-time quantitative PCR.
Q-PCRs were performed on an ABI PRISM 7000 Sequence Detector System using a TaqMan PCR Master Mix (Applied Biosystems). The primers and probes used were assays on demand designed by Applied Biosystems (Table 1). The primers and probes used for 3βHSD and external standard luciferase were described (Baker and O'Shaughnessy 2001; Merlet et al., 2007). Q-PCR was performed and analyzed as previously described (Merlet et al., 2007). Results were analyzed by using the delta-delta Ct method with the use of luciferase as a normalized control (Merlet et al., 2007). For each treatment, the mRNA levels were determined in samples from three different litters and at least from six to nine testes.
TABLE 1.
Primers Used in Real-Time RT-PCR
| Gene full name | Gene symbol | Applied Biosystem accession number |
| 3-Hydroxy-3-methylglutaryl coenzyme A reductase | HMG-CoA reductase | Mm01282499_m1 |
| Steroidogenic acute regulatory protein | StAR | Mm00441558_m1 |
| P450 side-chain cleavage enzyme | Cyp11a1 | Mm 00490735_m1 |
| P450 17 α hydroxylase | Cyp17a1 | Mm00484040_m1 |
| Steroid 5-alpha reductase | Srd5a1 | Mm00614213_m1 |
| P450 aromatase | Cyp19a1 | Mm00484049_m1 |
| Luteinizing hormone receptor (LH-R) | Lhcgr | Mm00442931_m1 |
| Insulin-like growth factor 3 | Insl-3 | Mm01340353_m1 |
Statistical analysis.
All values are expressed as means ± SEM. The statistical significance of the difference between the mean values for the treated and untreated testes from the same fetus was evaluated using the paired Student's t-test. The unpaired Student's t-test was used to evaluate the difference between the mean values for two different genotypes.
RESULTS
Effects of MEHP on Leydig Cells
Basal and LH-stimulated testosterone production.
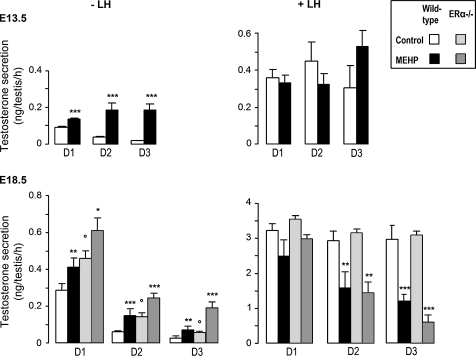
In wild-type control testes, as shown in Figure 2, the presence of 200μM MEHP during 3 days of culture in basal medium induced a high and significant time-dependent stimulation of testosterone production in E13.5 and E18.5 testes. When the testes were cultured in the presence of 100 ng/ml oLH, testosterone production was increased fourfold in E13.5 testes and more than 10-fold in E18.5 testes compared with basal conditions. In these conditions, the daily testosterone production in E13.5 testes was unaffected by 200μM MEHP, but was significantly decreased in a time-dependent manner in E18.5 testes with a strong inhibition on D3.
FIG. 2.
Effect of MEHP on testosterone secretion by fetal testes from wild-type and ERα−/− mice in organ culture. Testes at E13.5 (upper panels) and E18.5 (lower panels) were cultured for 3 days (D1–D3) in control medium or in the presence of 200μM MEHP. Cultures were performed in the absence (left panels) or presence (right panels) of 100 ng/ml oLH as indicated. Values are mean ± SEM from 4 to 18 cultures. *p < 0.05, **p < 0.01, ***p < 0.001 versus corresponding control value in the paired Student's t-test. °p < 0.05 in the unpaired Student's t-test for different genotypes.
As expected (Delbes et al., 2005), testosterone production was significantly higher in ERα−/− animals than in respective wild-type controls in basal conditions. The invalidation of ERα did not modify the stimulatory and inhibitory effects of MEHP on testosterone secretion in E18.5 testes in basal and LH-stimulated conditions, respectively (Fig. 2).
Number of Leydig cells.
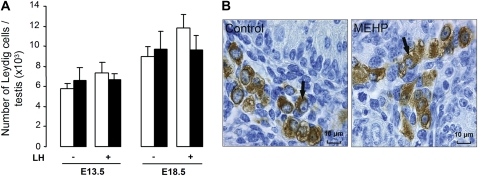
Compared with controls, the number of Leydig cells was not significantly modified after exposure to 200μM MEHP in the presence or absence of LH at all the studied stages (Fig. 3A). In addition, no abnormal distribution or clustering of the Leydig cells was observed in any condition (Fig. 3B).
FIG. 3.
Effect of MEHP on the number and histological appearance of Leydig cells in mouse testes in organ culture. (A) Number of Leydig cells in E13.5 and E18.5 testes after 3 days of culture in the absence (white bars) or presence of 200μM MEHP (black bars) and in the absence or presence of 100 ng/ml oLH. Values are mean ± SEM from four to five cultures. There was no significant difference between control and MEHP. (B) Leydig cells (arrows), as revealed by immunostaining of 3β-HSD, in mouse testes at E18.5 after 3 days of culture in control medium or in the presence of 200μM MEHP. No morphological alteration or distribution was detectable after MEHP exposure.
Leydig cell gene expression.
Because MEHP had no effect on Leydig cell number, we hypothesized that its effect on steroidogenesis was due to the modulation of the expression of genes coding for proteins involved in cholesterol and testosterone biosynthesis or metabolism. Therefore, mRNA expression of key genes was analyzed by real-time PCR (Fig. 4).
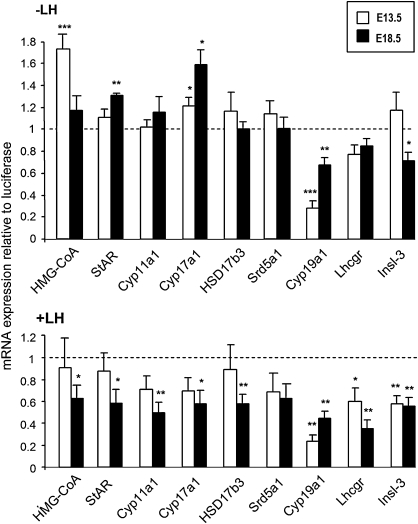
FIG. 4.
Quantitative real-time PCR analysis of the effect of MEHP (200μM) on mRNA levels of a selected set of genes involved in Leydig cell cholesterol metabolism and steroidogenesis, of LH-R (Lhcgr) and Insl3 genes in E13.5 (white bars) and E18.5 (black bars) testes after 3 days of culture in the absence (A) or presence (B) of 100 ng/ml oLH. The results were calculated by the delta-delta Ct method using an external standard (luciferase) added during RNA extraction as an endogenous reference. The levels of mRNA are expressed as mean of relative unit ± SEM of six to nine cultures with the control animals having a value of 1, with each sample processed in duplicate. *p < 0.05, **p < 0.01, ***p < 0.001 versus corresponding control value in the paired Student's t-test.
In basal conditions, significant increases in gene expression likely to explain the stimulation of testosterone production by MEHP were observed at the level of HMG-CoA reductase and Cyp17a1 in E13.5 testes and StAR and Cyp17a1 in E18.5 testes (Fig. 4). In LH-stimulated conditions, the decrease in testosterone production in E18.5 testes was related to a 40–50% reduction of the expression levels of all the genes tested, except 5-alpha-reductase, whereas they were not significantly altered in E13.5 testes (Fig. 4) in agreement with the lack of change in testosterone production. Aromatase (Cyp19a1) and 5-alpha-reductase are required in the conversion of testosterone to estradiol and dihydrotestosterone, respectively, and could modulate the amount of testosterone secreted. The expression of aromatase was decreased in both the absence and presence of LH at all ages studied (Fig. 4), but the expression of 5-alpha-reductase was never modified. The expression of LH-R (LH receptor, Lhcgr) in E13.5 and E18.5 testes was not modified by MEHP in the absence of LH in the culture medium, but was significantly reduced in its presence (Fig. 4). Interestingly, the expression of Insl-3, the protein which plays a major role in testis descent, was decreased by MEHP in all studied conditions except in E13.5 testes cultured in the absence of LH (Fig. 4).
db-cAMP–stimulated testosterone production.
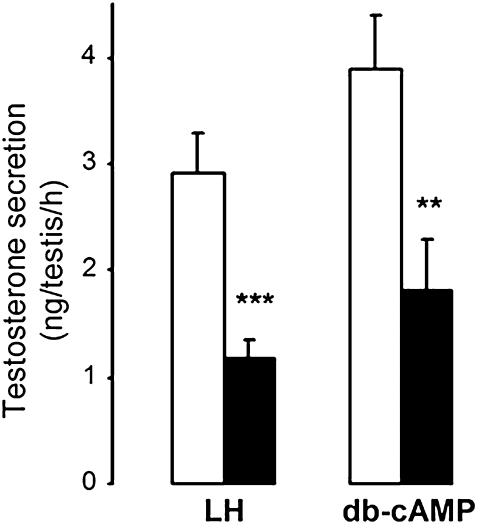
In an attempt to elucidate whether the decrease in LH receptor expression was involved in the inhibition of LH-stimulated testosterone production by MEHP at E18.5, we evaluated the effect of MEHP on db-cAMP–stimulated testosterone production. The inhibition of testosterone production by MEHP (Fig. 5) was similar to that obtained with LH, which means that it exerts its rate-limiting inhibitory effect downstream from LH-R.
FIG. 5.
Testosterone secretion by E18.5 testes cultured in the presence of oLH or db-cAMP and in the absence (white bars) or presence (black bars) of 200μM MEHP. Values are mean ± SEM of testosterone secretion on day 3 of culture (n = 11–18 cultures). **p < 0.01, ***p < 0.001, versus control value in the paired Student's t-test.
Effects of MEHP on Sertoli Cell Proliferation and Function
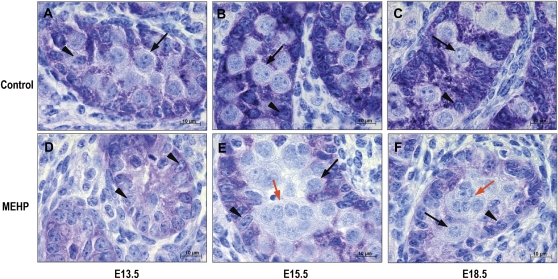
MEHP did not alter the proliferation of Sertoli cells because the BrdU-labeling index was not modified after 3-day treatment with 200μM MEHP at any age studied (results not shown). However, immunostaining of AMH clearly showed that its Sertoli cell content was markedly decreased compared with control in E13.5, E15.5, and E18.5 testes after 3 days of treatment with 200μM MEHP (Fig. 6).
FIG. 6.
AMH immunostaining in mouse testes at E13.5 (A, D), E15.5 (B, E), E18.5 (C, F) cultured for 3 days in control medium (A, B, C) or in the presence of 200μM MEHP (D, E, F). Black arrow: mononucleated gonocyte; orange arrow: MNGs; arrowhead: Sertoli cell.
Effects of MEHP on Germ Cells
Gonocyte distribution and morphology.
After 3 days of culture, the integrity of the seminiferous cord structure was maintained in both control and treated testes at all fetal and neonatal stages studied and at all concentrations of MEHP tested (Fig. 7). However, with 200μM MEHP all the gonocyte have disappeared in E13.5dpc testes (see further) and, in E15.5 and E18.5 testes, the gonocytes stayed aggregated in the center of the cord (Figs. 6E and 6F).
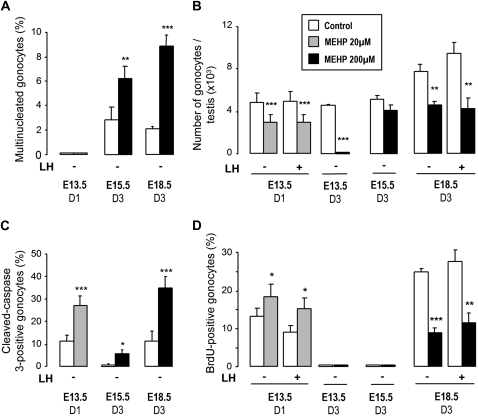
FIG. 7.
Effect of MEHP on the development of the gonocytes in fetal testes in organ culture. Testes were cultured for 1 day (D1) or 3 days (D3) at E13.5 and 3 days at E15.5 and E18.5 in control medium (white bars) or in the presence of 20μM (gray bars) or 200μM (black bars) MEHP and in the presence or absence of 100 ng/ml oLH as indicated. At the end of the culture, MNGs (A) and total gonocytes (B) were counted as described in Material and Methods. The percentage of cleaved caspase-3–positive gonocytes (C) was used to measure apoptotic activity and the percentage of BrdU-positive gonocytes (D) to evaluate proliferating index. Values are mean ± SEM from four to nine cultures. *p < 0.05, **p < 0.01, ***p < 0.001 versus corresponding control value in the paired Student's t-test.
MNGs occurred spontaneously in E15.5 and E18.5 control testes after 3 days of culture, that is, during and after the quiescent period, and their number was increased significantly by 200μM MEHP with the greatest increase observed in E18.5 testes (Fig. 7A). E18.5 testes from ERβ (ERβ−/−) or AR (Tfm) deficient mice, showed similar increase in MNCs (for ERβ−/−: 1.84 ± 0.33% in control vs. 8.79 ± 0.94 in MEHP treated, n = 9; for Tfm: 2.15 ± 0.52% in control vs. 12.5 ± 2.91 in MEHP treated, n = 4) than the wild-type (Fig. 7A: 2.02 ± 0.32% in control vs. 8.79 ± 0.89 in MEHP treated, n = 9). In E13.5 testes, no MNG was detected after 1 or 3 days of culture in control conditions or after 24-h treatment with 20μM MEHP (Fig. 7A).
Number of gonocytes.
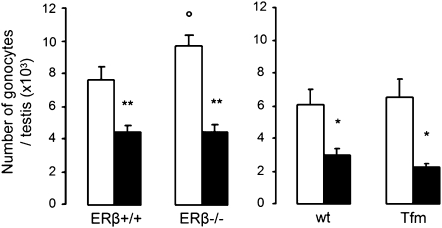
Because MEHP affected testosterone production differently in the presence and absence of LH, we evaluated its effect on gonocytes in these two conditions (Fig. 7B). The presence of LH and, consequently, a high level of testosterone production, did not modify the number of gonocytes in E13.5 and E18.5 control cultures. In contrast, MEHP severely reduced the number of gonocytes in any condition at all the stages studied, except with E15.5 testes cultured for 3 days i.e. during the quiescent period. At E13.5, the gonocytes had completely disappeared after 3 days of culture (D3) in the presence of 200μM MEHP and the number of gonocytes was reduced by 40% by 20μM MEHP after one day of culture (D1). With E18.5 testes, 200μM MEHP reduced the number of gonocytes by ∼40–50% after 3 days of culture (D3), that is, 24 h after resuming mitosis and apoptosis. As expected (Delbes et al., 2004), in testes from E18.5 the number of gonocytes was higher in ERβ−/− mice than in wild-type mice (ERβ+/+) after 3 days of culture. After MEHP treatment, the number of gonocytes was reduced in the same manner in E18.5 testes from wild-type mice and from ERβ−/− or Tfm mice (Fig. 8). Taken together, these results demonstrate that MEHP reduces the number of germ cells throughout fetal/neonatal life except during the quiescent period, independently of the level of testosterone production and of ER and AR pathways.
FIG. 8.
Involvement of ER and AR in the effects of MEHP on the number of gonocytes in fetal testes. Testes at E18.5 from wild-type, ERβ−/− and Tfm mice were cultured for 3 days in control medium (white bars) or in the presence of 200μM MEHP (black bars). At the end of the culture, total gonocytes were counted as described in Material and Methods. Values are mean ± SEM from four to nine cultures. *p < 0.05, **p < 0.01 versus corresponding control value in the paired Student's t- test for wild-type mice; °p < 0.05 in the unpaired Student's t-test for two different genotypes.
Germ cell proliferation and apoptosis.
The decrease in the number of gonocytes in MEHP-exposed testes could be induced by an increased rate of apoptosis or by a reduced rate of proliferation. The effect of MEHP on proliferation and apoptosis was therefore studied by assessing the percentage of BrdU (Fig. 7D) and cleaved caspase 3–positive (Fig. 7C) gonocytes, respectively.
MEHP greatly increased the rate of apoptotic gonocytes at all the ages studied (Fig. 7C). At the same time, the proliferation of the gonocytes was increased by 40% in E13.5 after 24-h exposure to 20μM MEHP (Fig. 7D), but was reduced by 60% in E18.5 testes after 3 days of exposure to 200μM MEHP. Taken together, these results show that MEHP reduces the number of gonocytes essentially by increasing apoptosis.
DISCUSSION
We have previously shown that ER and AR are involved in fetal testis development (Delbes et al., 2004, 2005; Merlet et al., 2007). Using an organotypic culture system of fetal testis from wild-type mice or from mice deficient for ER or AR, we present here the first demonstration that phthalate-induced alterations of both steroidogenesis and germ cell number do not involve ER and AR.
Steroidogenesis
Our findings show that testicular alteration of steroidogenesis by phthalates during fetal life is very complex. We report here for the first time a stimulatory effect of MEHP on testosterone production in fetal mouse testis and that MEHP can stimulate, inhibit or have no effect on testosterone production depending on the age of the testis at explantation and on the culture conditions. These results differ from those of numerous experiments conducted in the rat that have led to the conclusion that the main deleterious effect of phthalates concerns their antiandrogenic capacity, because testosterone production by the fetal testis is reduced after in utero (Fisher et al., 2003; Foster, 2006) or in vitro exposure (Chauvigné et al., 2009; Hallmark et al., 2007) to phthalates. Furthermore, recent experiments on in utero exposure to DBP in the mouse (Gaido et al., 2007) and on in vitro exposure of fetal human testis to MBP (Hallmark et al., 2007) or MEHP (Lambrot et al., 2009) failed to observe any inhibition of testosterone production or changes in the expression of the steroidogenic enzymes. Moreover, phthalates can stimulate steroidogenesis in prepubertal testis in vivo (Lin et al., 2008), in mouse gonadal cell lines or primary Leydig cell cultures (Ge et al., 2007). In all these cases, the mechanisms involved have not been fully elucidated.
In the present study, the MEHP-induced stimulation of steroidogenesis can be ascribed mainly to an increased transcription of p450C17 and also of HMGCoA-reductase, as reported in gonadal cell lines (Gunnarsson et al., 2008) or of StAR, depending on the developmental stage. In contrast, the inhibitory effect of MEHP on steroidogenesis was obtained by a reduction of the level of the mRNAs of the main genes involved in testosterone biosynthesis while, in the rat it was mainly ascribed to p450C17 and P450scc (Chauvigné et al., 2009; Culty et al., 2008; Hallmark et al., 2007). Additionally, in E18.5 testes, MEHP reduced the expression of LH-R, but this does not seem to be involved in the reduction of steroidogenesis. The reduction of aromatase activity could participate in the stimulation of testosterone production in the absence of LH, that is, in conditions of low testosterone production, mostly by reducing the inhibition of steroidogenesis by estradiol (Delbes et al., 2006, 2007) via ERα (Delbes et al., 2005), the reduction of testosterone transformation being probably negligible because intratesticular estradiol concentration is very low (Delbes et al., 2004). However, we show here that the ERα pathway (Delbes et al., 2006) is involved neither in the stimulatory nor in the inhibitory effects of MEHP on testosterone secretion.
The present results provide new insights into the mechanisms underlying the effects of phthalates on fetal testicular steroidogenesis, because they prove that they are independent of the ER pathway but depend on other factors: 1) the developmental stage, which modulates the maturation and the limiting steps of the steroidogenic pathway, the LH level and the cholesterol availability (present results; Gunnarsson et al., 2008; Hallmark et al., 2007); 2) the species because, in vitro, unlike the rat (Chauvigné et al., 2009) and the results we present here in the mouse, human fetal testis is not sensitive to phthalate in terms of steroidogenesis (Hallmark et al., 2007; Lambrot et al., 2009). Aggregation of Leydig cells and even their presence in the seminiferous tubules have also been reported to be a prominent consequence of in utero (Lin et al., 2008; Mahood et al., 2005) or in vitro (Chauvigné et al., 2009; Hallmark et al., 2007) exposure of rat fetal testis to phthalates, but was not observed in the present experiments on mouse testis in vitro or in vivo (Gaido et al., 2007) or in human fetal testis in vitro (Hallmark et al., 2007; Lambrot et al., 2009).
The importance of an alteration of Leydig cell status during fetal life is related to its possible role in inducing cryptorchidism and hypospadia (Foster et al., 2001; Henley and Korach, 2006) and concerns not only testosterone but also Insl3 production. Underdeveloped gubernaculum (Barlow and Foster, 2003) and reduced Insl3 expression have been observed following fetal exposure to phthalates in male rats (Lague and Tremblay, 2008; Lehmann et al., 2004; McKinnell et al., 2005). Indeed, Insl3 mRNA was also reduced in the mouse testes after MEHP exposure, except in E13.5 testis in basal conditions when maturation/stimulation of Leydig cells is low.
Gonocyte Development
Our findings clearly show that phthalates strongly impair germ cell development in the fetal mouse testis in vitro during critical time-specific windows. In our model, MEHP reduces the number of gonocytes by inducing massive apoptosis during early fetal and late fetal/neonatal life, in relation to the high mitotic and apoptotic activity during these periods. During the quiescent phase between E15.5 and E19.5 (day of birth), due to the naturally low apoptotic activity of the gonocytes, phthalates did not significantly reduce their number. This can be compared with the low sensitivity to retinoic acid (Livera et al., 2000) and transforming growth factor (TGFβ1) (Olaso et al., 1998) of rat germ cells during their quiescent period. These results are in accordance with the few studies that have previously focused on the modification of the number of gonocytes by phthalates in other species, with the mouse being particularly sensitive: in the rat in vivo, the number of gonocytes is reduced at E21.5 when the animals are treated from E13.5, but not when they are treated later (Ferrara et al., 2006); in vitro MEHP reduces the number of gonocytes in the fetal testis of E14.5 (Chauvigné et al., 2009) and of PN3 neonate (Li and Kim, 2003); lastly, phthalates have been shown recently to reduce the number of gonocytes in human fetal testis in vitro at an early stage (Lambrot et al., 2009).
Apoptosis could result from a direct effect of phthalates on the gonocytes or from an indirect effect via other cell types. The fact that the deleterious effects of MEHP on the gonocytes are similar whether it stimulates or inhibits steroidogenesis suggests that they do not involve antiandrogenic effects as postulated in the rat (Chauvigné et al., 2009; Scott et al., 2007; Skakkebaek et al., 2001). This hypothesis was strengthened by the fact that MEHP reduces the number of gonocytes in a similar fashion in Tfm mice. The gonocytes of ERβKO mice were also similarly affected, demonstrating that MEHP does not also act via ER, directly, or indirectly via the modulation of aromatase. Therefore, even though we demonstrated previously that ERβ and AR pathways physiologically control fetal germ cell number (Delbes et al., 2004; Merlet et al., 2007), we can definitively eliminate a direct or indirect effect of MEHP on fetal germ cells via antiandrogenic/estrogenic effects.
In the rat, germ cell apoptosis could also be induced by Sertoli cells (Fisher et al., 2003; Kleymenova et al., 2005) which are targets of MEHP because, although their proliferation is not affected, they exhibit a reduction in AMH content. Various nonexclusive pathways have been postulated to be involved in MEHP-induced germ cell apoptosis, such as the TRAIL (McKee et al., 2006) and NF-kB (Rasoulpour and Boekelheide, 2005) pathways and the Fas/FasL system (Giammona et al., 2002; Yao et al., 2008). Lastly, our in vitro observations in the mouse that phthalates disturb gonocyte migration and increase the occurrence of MNGs in late fetal life are in agreement with in vivo observations in the mouse (Gaido et al., 2007) and rat (Ferrara et al., 2006; Kleymenova et al., 2005).
The phthalate-induced alteration in the number of gonocytes at the fetal and neonatal stage could compromise subsequent reactivation of germ cell proliferation and development during puberty as in the rat (Ferrara et al., 2006) and lead to altered fertility in adulthood. Furthermore, the impairment of differentiation of gonocytes is to some extent reminiscent of the failure of fetal gonocyte differentiation that is thought to lead to formation of CIS (carcinoma in situ) cells and subsequent testicular germ cell tumors in humans (Rajpert-De Meyts, 2006). Further studies are needed to establish if in utero exposure to phthalates leads, in the mouse, to altered fertility or occurrence of tumors in adulthood.
CONCLUSION
The present data define specific critical periods of sensitivity of fetal mouse testis to MEHP and clearly establish that the effects of phthalates on steroidogenesis are unrelated to those on gonocyte development. We demonstrated that the strong deleterious effect of phthalates on the germ cell lineage that is observed in various mammalian species is independent of any antiandrogenic effects and that the phthalates are neither estrogenic nor antiandrogenic because their effects do not involve, directly or indirectly, ER or AR.
FUNDING
Université Paris Diderot, by the Institut National de la Santé et de la Recherche Médicale; Commissariat à l'Energie Atomique; Agence Nationale de la Recherche Project Santé-Environnement et Santé-Travail (ANR2007SEST01901); and Ministère de l'Education Nationale de la Recherche et de la Technologie (237132006) fellowship to A.L.
Acknowledgments
We thank Prof. P. Chambon for providing transgenic mice (Institut de Génétique et Biologie Moléculaire et Cellulaire, Illkirch, France) and Prof A. Payne (Stanford University Medical Center, CA) for providing the anti-3βHSD antibody. We also thank C. Chamaillard for expert technical assistance, V. Neuville, S. Leblay, and S. Rodrigues for animal care and A. Gouret for secretarial assistance.
References
- Baker PJ, O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Pakarinen P, Huhtaniemi IT, Abel MH, Charlton HM, Kumar TR, O'Shaughnessy PJ. Failure of normal Leydig cell development in follicle-stimulating hormone (FSH) receptor-deficient mice, but not FSHbeta-deficient mice: Role for constitutive FSH receptor activity. Endocrinology. 2003;144:138–145. doi: 10.1210/en.2002-220637. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 2003;31:397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- Bhattacharya N, Dufour JM, Vo MN, Okita J, Okita R, Kim KH. Differential effects of phthalates on the testis and the liver. Biol. Reprod. 2005;72:745–754. doi: 10.1095/biolreprod.104.031583. [DOI] [PubMed] [Google Scholar]
- Boulogne B, Habert R, Levacher C. Regulation of the proliferation of cocultured gonocytes and Sertoli cells by retinoids, triiodothyronine, and intracellular signaling factors: Differences between fetal and neonatal cells. Mol. Reprod. Dev. 2003;65:194–203. doi: 10.1002/mrd.10311. [DOI] [PubMed] [Google Scholar]
- Chauvigné F, Menuet A, Lesné L, Chagnon M-C, Chevrier C, Regnier J-F, Angerer J, Jégou B. Time- and Dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro. Environ. Health Perspect. 2009;117:515–521. doi: 10.1289/ehp.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ. Peroxisome proliferator-activated receptors: Mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol. Sci. 2005;83:4–17. doi: 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- Culty M, Thuillier R, Li W, Wang Y, Martinez-Arguelles DB, Benjamin CG, Triantafilou KM, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol. Reprod. 2008;78:1018–1028. doi: 10.1095/biolreprod.107.065649. [DOI] [PubMed] [Google Scholar]
- Delbes G, Duquenne C, Szenker J, Taccoen J, Habert R, Levacher C. Developmental changes in testicular sensitivity to estrogens throughout fetal and neonatal life. Toxicol. Sci. 2007;99:234–243. doi: 10.1093/toxsci/kfm160. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132:527–538. doi: 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R. Estrogen receptor {beta}-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology. 2004;145:3395–3403. doi: 10.1210/en.2003-1479. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum. Reprod. Update. 2001;7:231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, Sealfon SC, Bernard DJ, Hardy MP. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J. Androl. 2007;28:513–520. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- Giammona CJ, Sawhney P, Chandrasekaran Y, Richburg JH. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol. Appl. Pharmacol. 2002;185:119–127. doi: 10.1006/taap.2002.9536. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D, Leffler P, Ekwurtzel E, Martinsson G, Liu K, Selstam G. Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction. 2008;135:693–703. doi: 10.1530/REP-07-0460. [DOI] [PubMed] [Google Scholar]
- Habert R, Picon R. Testosterone, dihydrotestosterone and estradiol 17ß levels in maternal and fetal plasma and in fetal testes in the rat. J. Steroid. Biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: Comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ. Health Perspect. 2007;115:390–396. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147:S25–S32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- Hu GX, Lian QQ, Ge RS, Hardy DO, Li XK. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol. Metab. 2009;20:139–145. doi: 10.1016/j.tem.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A, Magre S. Sexual differentiation. In: Thibault C, Levasseur M, Hunter RHF, editors. Reproduction in Mammals and Man. Paris: Ellipses; 1993. pp. 197–226. [Google Scholar]
- Kleymenova E, Swanson C, Boekelheide K, Gaido KW. Exposure in utero to di(n-butyl) phthalate alters the vimentin cytoskeleton of fetal rat Sertoli cells and disrupts Sertoli cell-gonocyte contact. Biol. Reprod. 2005;73:482–490. doi: 10.1095/biolreprod.104.037184. [DOI] [PubMed] [Google Scholar]
- Lague E, Tremblay JJ. Antagonistic effects of testosterone and the endocrine disruptor mono-(2-ethylhexyl) phthalate on INSL3 transcription in Leydig cells. Endocrinology. 2008;149:4688–4694. doi: 10.1210/en.2008-0310. [DOI] [PubMed] [Google Scholar]
- Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates Impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ. Health Perspect. 2009;117:32–37. doi: 10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G. Monitoring phthalate exposure in humans. Clin. Chim. Acta. 2005;361:20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol. Sci. 2004;81:60–68. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Li H, Kim KH. Effects of mono-(2-ethylhexyl) phthalate on fetal and neonatal rat testis organ cultures. Biol. Reprod. 2003;69:1964–1972. doi: 10.1095/biolreprod.103.018895. [DOI] [PubMed] [Google Scholar]
- Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian QQ, Hardy DO, Sottas CM, Li XK, Hardy MP. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7218–7222. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G, Delbes G, Pairault C, Rouiller-Fabre V, Habert R. Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res. 2006;324:507–521. doi: 10.1007/s00441-006-0167-7. [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Durand P, Habert R. Multiple effects of retinoids on the development of Sertoli, germ and Leydig cells of fetal and neonatal rat testis in culture. Biol. Reprod. 2000;62:1303–1314. doi: 10.1095/biolreprod62.5.1303. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- McKee CM, Ye Y, Richburg JH. Testicular germ cell sensitivity to TRAIL-induced apoptosis is dependent upon p53 expression and is synergistically enhanced by DR5 agonistic antibody treatment. Apoptosis. 2006;11:2237–2250. doi: 10.1007/s10495-006-0288-1. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Sharpe RM, Mahood K, Hallmark N, Scott H, Ivell R, Staub C, Jegou B, Haag F, Koch-Nolte F, Hartung S. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di (n-Butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- Merlet J, Racine C, Moreau E, Moreno SG, Habert R. Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3615–3620. doi: 10.1073/pnas.0611421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 1999;156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Nagano R, Tabata S, Nakanishi Y, Ohsako S, Kurohmaru M, Hayashi Y. Reproliferation and relocation of mouse male germ cells (gonocytes) during prespermatogenesis. Anat. Rec. 2000;258:210–220. doi: 10.1002/(SICI)1097-0185(20000201)258:2<210::AID-AR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Johnston H. Neuroendocrine regulation of Leydig cell development. Ann. N. Y. Acad. Sci. 2005;1061:109–119. doi: 10.1196/annals.1336.013. [DOI] [PubMed] [Google Scholar]
- Olaso R, Habert R. Genetic and cellular analysis of male germ cell development. J. Androl. 2000;21:497–511. [PubMed] [Google Scholar]
- Olaso R, Pairault C, Boulogne B, Durand P, Habert R. Transforming growth factor ß1 and ß2 reduce the number of gonocytes by increasing apoptosis. Endocrinology. 1998;139:733–740. doi: 10.1210/endo.139.2.5765. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: Genetic and environmental aspects. Hum. Reprod. Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- Rasoulpour RJ, Boekelheide K. NF-kappaB is activated in the rat testis following exposure to mono-(2-ethylhexyl) phthalate. Biol. Reprod. 2005;72:479–486. doi: 10.1095/biolreprod.104.034363. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O'Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil. Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL, Jr, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology. 2005;210:223–233. doi: 10.1016/j.tox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J. Reprod. Fertil. 1991;93:233–243. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- Wang RA, Nakane PK, Koji T. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol. Reprod. 1998;58:1250–1256. doi: 10.1095/biolreprod58.5.1250. [DOI] [PubMed] [Google Scholar]
- Ward JM, Peters JM, Perella CM, Gonzalez FJ. Receptor and nonreceptor-mediated organ-specific toxicity of di(2-ethylhexyl)phthalate (DEHP) in peroxisome proliferator-activated receptor alpha-null mice. Toxicol. Pathol. 1998;26:240–246. doi: 10.1177/019262339802600208. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. TNF Alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol. Reprod. 2008;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Obinata M, Matsui Y. Stage-specific tissue and cell interactions play key roles in mouse germ cell specification. Development. 2001;128:481–490. doi: 10.1242/dev.128.4.481. [DOI] [PubMed] [Google Scholar]