Abstract
Therapy employing the fluoroquinolone antibiotic, trovafloxacin (TVX) was curtailed due to idiosyncratic hepatotoxicity. Previous studies in mice showed that a nonhepatotoxic inflammatory stress induced by tumor necrosis factor α (TNF) synergized with a nonhepatotoxic dose of TVX to cause liver injury. The purpose of this study was to explore mechanisms by which TVX interacts with TNF to cause liver injury. TVX pretreatment prolonged the peak of plasma TNF after its administration. This prolongation of TNF by TVX was critical to the development of hepatotoxicity. The prolongation of TNF concentration in plasma was primarily due to reduced clearance when compared with secondary biosynthesis. TNF is cleared from plasma by binding to soluble TNF receptors (TNFRs) which are eliminated by the kidney; however, the plasma concentrations of soluble TNFRs were not reduced, and biomarkers of renal dysfunction were not elevated in TVX/TNF-treated mice. Two injections of TNF mimicked the prolongation of the TNF peak by TVX and caused liver injury, but injury was less severe than after TVX/TNF coexposure. TVX enhanced the induction of proinflammatory cytokines by TNF. Additionally, TVX sensitized Hepa1c1c7 cells to TNF-induced killing in a concentration-dependent manner and increased both potency and efficacy of TNF to activate effector caspases that were critically involved in cell death from TVX/TNF coexposure. In summary, TVX reduced the clearance of TNF independent of either receptor shedding or kidney dysfunction. Additionally, TVX interacted with TNF to enhance inflammation and sensitize hepatocytes to TNF-induced cell death.
Keywords: hepatotoxicity, tumor necrosis factor, trovafloxacin, idiosyncratic toxicity, inflammation, liver injury
The use of trovafloxacin (TVX), a fluoroquinolone antibiotic, was severely restricted after 2 years of widespread use due to an association with hepatotoxicity (Lazarczyk et al., 2001; Nicholson et al., 2002). TVX-induced hepatotoxicity was only seen in a small fraction of patients and occurred at variable times in relation to the time of exposure. These characteristic led to the classification of TVX hepatotoxicity as “idiosyncratic.”
The mechanism(s) underlying liver injury from TVX and other drugs with idiosyncratic liability are unknown. Several mechanisms have been proposed; however, none has been proven. One hypothesis is that some drugs synergize with a normally noninjurious inflammatory stress to precipitate an idiosyncratic response. This hypothesis has led to the development of some of the first animal models of hepatotoxicity induced by drugs with idiosyncratic liability, such as sulindac, ranitidine, chlorpromazine, and TVX (Buchweitz et al., 2002; Luyendyk et al., 2003; Waring et al., 2006; Shaw et al., 2007; Zou et al., 2009). TVX synergized with a nonhepatotoxic dose of bacterial lipopolysaccharide (LPS) to cause tumor necrosis factor α (TNF)–dependent liver injury in mice (Shaw et al., 2007). Subsequently, we showed that TVX also interacted with a nonhepatotoxic dose of recombinant TNF to cause liver injury (Shaw et al., 2009b).
TNF is a versatile cytokine that can induce a number of cellular responses, among which is activation of an inflammatory cascade (Bemelmans et al., 1996; Fiers, 1991). It is produced as a type II transmembrane protein which is cleaved by TNF converting enzyme to form the soluble, biologically active cytokine (Tang et al., 1996; Black et al., 1997). The production and cleavage of TNF can be induced by a number of stimuli including bacterial products and other cytokines (Bemelmans et al., 1993b; Joyce and Steer, 1995; Joyce and Steer, 1996). The biological responses induced by TNF are exerted through its interaction with two membrane receptors, TNF receptor (TNFR) 1 (p55) and TNFR2 (p75) (Locksley et al., 2001). The activation of either receptor initiates signaling pathways that lead to activation of nuclear factor κB (NF-κB) and other transcription factors, resulting in proinflammatory cytokine production (Fiers, 1991; Bemelmans et al., 1996; Dopp et al., 2002). In addition to its proinflammatory properties, TNF is a mediator of apoptosis. When TNFR1 is activated, its intracellular domain interacts with a death domain that cleaves and activates caspase 8 (Bradham, 1998). Activated caspase 8 cleaves effector caspases 3 and 7, thereby initiating the apoptotic cascade (Budihardjo et al., 1999).
Both TNFRs can be cleaved by inducible, but incompletely understood, mechanisms to soluble TNFRs (sTNFRs) that circulate in the plasma (Bemelmans et al., 1993b; Seckinger et al., 1989; Spinas et al., 1992). The clearance of TNF from the plasma consists of two phases, both of which involve sTNFRs. First, TNF is rapidly inactivated in the plasma primarily through its binding to sTNFRs (Ferraiolo et al., 1988). The inactivation depends on both of the sTNFRs, but it is believed that sTNFR2 is primarily responsible for the inactivation in mice (Bemelmans et al., 1993a). Second, the TNF:sTNFR complexes, which are immunologically detectable but inactive, are cleared primarily by the kidney and to a lesser extent by the liver (Bemelmans et al., 1993a; Beutler et al., 1985; Pessina et al., 1987). The first step of inactivation occurs with a t1/2 of 12 min., whereas the clearance of TNF:sTNFR complexes takes place with a t1/2 of approximately 100 min (Bemelmans et al., 1993a).
Previously, TVX pretreatment prolonged the LPS-induced increase in plasma concentration of TNF, and this prolongation was critical to TVX/LPS-induced liver injury (Shaw et al., 2007). However, it is unknown if a reduction in clearance of TNF contributes to this prolongation. The ability of a xenobiotic agent to alter the clearance of TNF has not been reported previously. Additionally, we reported that TNF inhibition significantly attenuated TVX/LPS-induced increases in plasma concentrations of several inflammatory cytokines (Shaw et al., 2009b). However, it is unknown if TVX also acts by enhancing TNF-induced inflammation and/or sensitizing cells to TNF-mediated cell death. This study was designed to examine these various potential mechanisms of interaction between TVX and TNF.
MATERIALS AND METHODS
Materials.
Unless otherwise noted, all reagents were purchased from Sigma Chemical Co. (St. Louis, MO). TVX was synthesized by Cayman Chemical (Ann Arbor, MI). Recombinant truncated form of murine TNF (mTNF), recombinant rat TNF (rTNF), and human TNF (hTNF) were purchased from R&D Systems (Minneapolis, MN).
Animals.
All animals received humane care, and all studies complied with Michigan State University guidelines. Nine- to 11-week-old, male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME) were used for all studies. Mice were allowed to acclimate for 1 week in a 12-h light/dark cycle prior to use. They were given continuous access to bottled spring water and fed a standard chow (Rodent Chow/Tek 2018, Harlan Teklad, Madison, WI).
Experimental protocols.
Mice were fasted 12 h prior to treatment. TVX (150 mg/kg) or its saline vehicle was administered orally 3 h before recombinant murine, human or rTNF (50 μg/kg, i.p.), the injection of which is designated as time zero throughout. The dose of TVX was chosen because it was nonhepatotoxic and interacted with TNF to cause liver injury (Shaw et al., 2009b). Food was returned immediately after TNF administration. In some studies, etanercept (Michigan State University Pharmacy) or its vehicle (phosphate-buffered saline [PBS]) was administered at a dose of 12 mg/kg by intraperitoneal injection. It was administered at −1, +1.5, or +4 h in relation to the TNF dose. In the studies involving two administrations of TNF, mice received a second injection of TNF (20 μg/kg, i.p.) 2 h after the initial administration of 50 μg/kg TNF. This second administration was chosen to simulate the prolonged elevation of TNF that occurs in TVX/TNF-treated mice. Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) at designated times and euthanized by exsanguination. Blood was drawn into a syringe containing sodium citrate (final concentration, 0.32%). The left lateral liver lobe was fixed in 10% neutral buffered formalin and blocked in paraffin.
Alanine aminotransferase activity and histopathology.
Alanine aminotransferase (ALT) activity was measured in the plasma spectrophotometrically using Infinity ALT reagent purchased from Thermo Electron Corp. (Louisville, KY). Paraffin-embedded liver sections were cut 5 μm thick and stained with hematoxylin and eosin.
DNA fragmentation immunohistochemistry.
Terminal deoxynucleotidyl transferase nick end labeling (TUNEL) was performed on 5-μm-thick frozen liver sections using the ApoTag Fluorescein In Situ Apoptosis Detection Kit purchased from Millipore Corp. (Billerica, MA) per the manufacturer's instructions. Briefly, the sections were fixed in 1% paraformaldehyde and permeabilized with 2:1 ethanol:acetic acid. Sections were incubated with terminal deoxynucleotidyl transferase (TdT), and the result was visualized with anti-digoxigenin-fluorescein isothiocyanate (FITC) antibody. Sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. An Olympus IX-71 inverted fluorescent microscope and Olympus MicroSuite software (Olympus America, Center Valley, PA) were used to capture images. Image J Software (National Institutes of Health) was used to pseudocolor the DAPI staining as red and FITC staining as green. Overlay images were created, and colocalized staining appeared yellow.
Active caspase 3 immunohistochemistry.
Active caspase 3 staining was performed on 5-μm-thick, paraffin-embedded liver sections using the SignalStain Cleaved Caspase-3 Immunohistochemistry Detection Kit purchased from Cell Signaling Technology (Danvers, MA). Paraffin was removed from sections with xylene, and sections were rehydrated. The sections were immersed in 0.01M sodium citrate, pH 6.0, at a near boiling temperature for 10 min to unmask the antigen. Endogenous peroxidase was blocked per the manufacturer's instructions. Sections were incubated with the primary antibody overnight at 4°C. The biotinylated secondary antibody was applied the next day for 30 min at room temperature. The substrate-chromagen mixture was applied to the slide for 15 min prior to hematoxylin counterstaining. The sections were then dehydrated in ethanol and visualized.
Plasma concentrations of TNF.
Murine and hTNF were measured using bead-plex kits from Bio-Rad Laboratories and a Bio-Plex 100 System (Hercules, CA). Rat TNF was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from R&D Systems (Minneapolis, MN). Cross-reactivity of rat, human and mTNF was measured by running controls of 10 and 100 ng/ml of the appropriate recombinant TNF in assays designed to detect TNF from other species. Neither rTNF nor hTNF (100 ng/ml) was above the detection limit in the assay for mTNF, suggesting no cross-reactivity. Similarly, recombinant mTNF (100 ng/ml) was below the detection limit in both the hTNF bead-plex assay and the rTNF ELISA.
TNF biological activity measurement.
Biological TNF activity was measured using the mouse hepatoma cell line Hepa1c1c7 purchased from American Type Culture Collection (Manassas, VA). Cell death was measured using the CytoTox-Glo Assay purchased from Promega Corp. (Madison, WI). Cells were plated in a 96-well plate at a concentration of 25,000 cells per well 12 h before treatment. Medium was removed and replaced with fresh medium containing various agents as indicated, and cell death was measured 12 h later. A standard curve was generated by making serial dilutions of recombinant mTNF. Treatment of animals with TVX alone did not increase the plasma concentration of TNF (Shaw et al., 2007). To account for any effect TVX in the serum may have on TNF-induced cell death, mice treated only with TVX were killed at 3 h and serum was added to the wells of the TNF standard curve and the samples from Veh/TNF-treated mice. Therefore, the same concentration of TVX was present in all wells.
Plasma concentrations of sTNFRs.
The plasma concentrations of soluble TNFR1 and TNFR2 were measured using ELISA kits purchased from R&D Systems (Minneapolis, MN).
Plasma concentrations of creatinine and urea.
The plasma concentrations of creatinine and urea were measured using Quantichrome assay kits purchased from BioAssay Systems (Hayward, CA).
Plasma cytokine measurements.
The plasma concentrations of TNF-α, interferon γ (IFN-γ), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-1 β (IL-1β), monocyte chemoattractant protein 1 (MCP-1), vascular endothelial growth factor (VEGF), macrophage inflammatory protein-2 (MIP-2), keratinocyte chemoattractant (KC), and MIP-1α were measured using bead-plex kits purchased from Bio-Rad Laboratories and a Bio-Plex 200 System (Hercules, CA).
Hepatic neutrophil accumulation.
Paraffin-embedded liver sections were stained immunohistochemically for neutrophils (PMNs) as described previously (Yee et al., 2003). The primary antibody was a rabbit anti-PMN Ig isolated from the serum of rabbits immunized with rat PMNs (Hewett et al., 1992). PMN accumulation was quantified without knowledge of treatment by counting the number of PMNs in eight randomly selected, high-power fields (×400) for each coded liver.
Hepa1c1c7 cell experiments.
Hepa1c1c7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) purchased from Invitrogen (Carlsbad, CA) and supplemented with 1% antibiotic–antimycotic (Invitrogen) and 10% heat-inactivated fetal bovine serum (SAFC Biosciences, Lenexa, KS). Cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Medium with 0.25% trypsin purchased from Invitrogen was used to detach cells once they were approximately 70% confluent. Cells were plated in 96-well plates at 25,000 cells per well. They were allowed to attach for 18 h before medium was removed and replaced with fresh medium. TVX was reconstituted to a stock solution of 200mM in dimethyl sulfoxide (DMSO) which resulted in a maximum final concentration of 0.1% DMSO in the treated wells. Various concentrations of TVX and/or TNF were added to designated wells, and cells were incubated for 6 or 24 h. EC50 concentrations were calculated using SigmaPlot 8.0 Software. At 6 h, caspase activation was measured using Caspase-Glo 3/7 assay purchased from Promega (Madison, WI). At 24 h, cytotoxicity was measured using the Cytotox-Glo cytotoxicity assay purchased from Promega (Madison, WI). Z-VAD-FMK (40μM) purchased from R&D Systems (Minneapolis, MN) was reconstituted in DMSO and added to designated wells simultaneously with TVX and/or TNF addition.
Statistical analyses.
All results are presented as mean ± SEM. A one- or two-way ANOVA was used as appropriate after data normalization. All multiple pairwise comparisons were done using Tukey's test. The criterion for significance was p < 0.05.
RESULTS
Timecourse of TVX/TNF-Induced Liver Injury
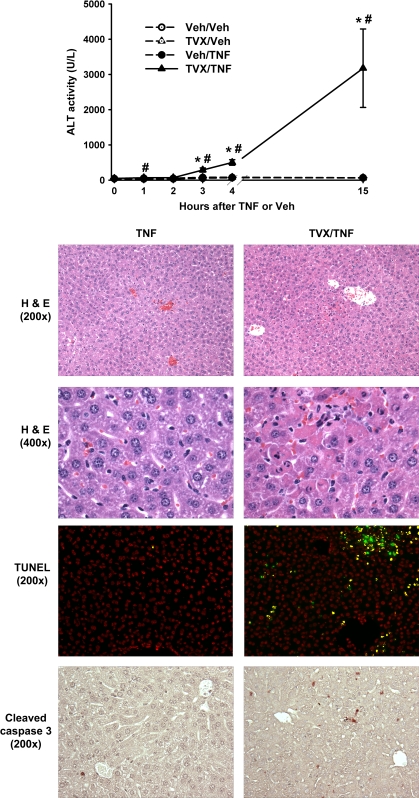
TVX (150 mg/kg) pretreatment synergized with a dose of recombinant mTNF (50 μg/kg) to cause a significant increase in plasma ALT activity as early as 3 h after TNF administration (Fig. 1). This progressed to 15 h. In contrast, treatment with either TVX or TNF alone caused no increase in plasma ALT activity. Liver histopathology and markers of apoptosis were examined at 4 h. Mice treated with Veh or TVX did not develop any lesions of hepatocellular damage (data not shown). Similarly, treatment with Veh/TNF resulted in no hepatocellular oncotic necrosis or apoptosis (Fig. 1). However, TVX/TNF coexposure caused lesions of hepatocellular necrosis primarily localized to midzonal regions of liver lobules (Fig. 1, ×200). Higher magnification revealed that TVX/TNF-treated mice had hepatocytes exhibiting apoptotic morphology, which was not seen in Veh/TNF-treated mice (Fig. 1, ×400). Hepatocellular apoptosis was evident in TVX/TNF-treated mice as shrunken hepatocytes with chromatin margination and presence of apoptotic bodies; whereas oncotic necrosis appeared as swollen hepatocytes with darkly staining, pyknotic nuclei.
FIG. 1.
TVX/TNF-induced hepatocellular oncosis and apoptosis. Mice were treated with TVX (150 mg/kg) or its saline vehicle 3 h before recombinant mTNF (50 μg/kg) or its saline vehicle as described in “Materials and Methods.” Plasma ALT activity was measured at various times. n = 4–7 animals per group. Mice were killed at 4 h for histopathology and immunohistochemistry. Photomicrographs were taken of representative liver sections stained with hematoxylin and eosin at ×200 (top row) and ×400 magnification (second row). Midzonal lesions of hepatocellular oncotic necrosis and apoptosis were observed in TVX/TNF-treated mice. Frozen liver sections were stained for DNA fragmentation (third row) using TUNEL staining. A representative photomicrograph at ×200 magnification is shown. Liver sections were stained for TUNEL (green) and nuclei (red). Staining colocalization (yellow), representing nuclei with DNA fragmentation, was observed only in livers from the TVX/TNF-treated mice. Paraffin-embedded liver sections were stained for cleaved caspase 3 (bottom row), which appears dark brown. A representative photomicrograph at ×200 magnification was taken of each treatment group. TVX/TNF-treated mice had greater cleaved caspase 3 staining compared with Veh/TNF-treated mice. *Significantly different from the respective treatment group at 0 h. #Significant difference between TVX/TNF and Veh/TNF groups at the same time.
To confirm the apoptosis observed morphologically, livers were stained for DNA fragmentation (TUNEL) and active caspase 3. TUNEL staining (green) was counterstained with DAPI (red) to identify apoptotic nuclei. Overlapping staining (yellow) was minimal to nonexistent in livers of Veh-, TVX- (data not shown), and Veh/TNF-treated mice (Fig. 1, TUNEL). In contrast, TVX/TNF coexposure caused an increase in the number of nuclei that stained for DNA fragmentation. Active caspase 3 staining was minimal in Veh-, TVX- (data not shown), and Veh/TNF-treated mice (Fig. 1, cleaved caspase 3). TVX/TNF treatment resulted in an increase in hepatocytes that stained for active caspase 3. Representative photomicrographs of livers of mice near the mean plasma ALT activity within each treatment group are shown.
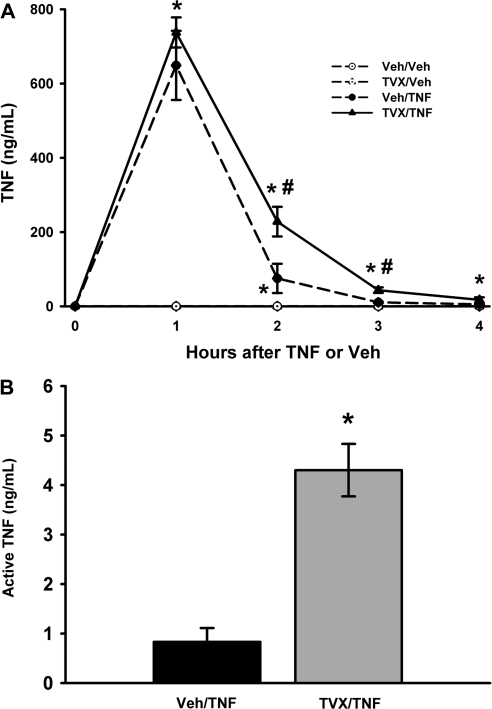
TVX Pretreatment Prolongs the Peak of Plasma TNF Concentration
The effect of TVX on the time course (0–4 h) of plasma TNF concentration was evaluated. TVX alone did not increase the plasma concentration of TNF (Fig. 2A). After intraperitoneal injection of TNF, its concentration in the plasma was markedly elevated at 1 h, and decreased rapidly between 1 and 2 h. TVX pretreatment did not affect the plasma concentration of TNF at 1 h, but it did enhance TNF concentration at 2 and 3 h (Fig. 2A). That is, TVX prolonged the appearance of TNF in the plasma. Similar to the amount of immunologically detectable TNF (Fig. 2A), TVX/TNF-treated mice had ∼4 times as much biologically active TNF compared with Veh/TNF-treated mice at 3 h (Fig. 2B). TNF-induced Hepa1c1c7 cytotoxicity was used to assay biologically active TNF in the serum. Addition of etanercept, a sTNFR mimic that neutralizes TNF activity, protected Hepa1c1c7 cells from cytotoxicity induced by serum from TVX/TNF- or Veh/TNF-treated mice (data not shown). This confirms that the cytotoxicity measured was TNF- mediated.
FIG. 2.
Effect of TVX on plasma concentration of TNF after mTNF administration. Mice were treated with TVX (150 mg/kg) or its saline vehicle 3 h before recombinant mTNF (50 μg/kg) or its saline vehicle as described in “Materials and Methods.” (A) The plasma concentration of immunologically detectable TNF was measured at various times. n = 4–7 animals per group. *Significantly different from the respective treatment group at 0 h. #Significant difference between TVX/TNF and Veh/TNF groups at the same time. (B) Serum was collected at 3 h, and biological TNF activity was measured using the Hepa1c1c7 cell line as described in “Materials and Methods.” n = 5 animals per group. *Significantly different from Veh/TNF treatment group.
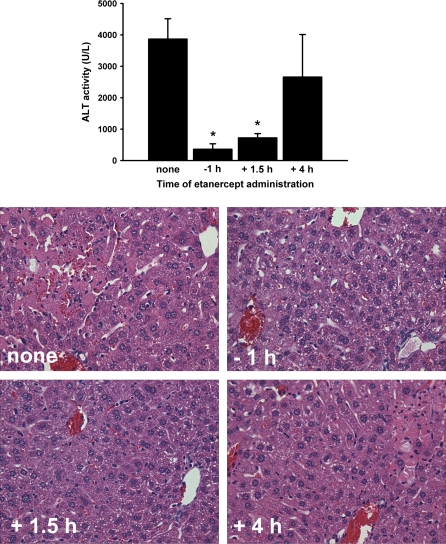
TVX/TNF-Induced Liver Injury Depends on the Prolongation of TNF
Mice dosed with TVX/TNF were cotreated with etanercept (12 mg/kg, i.p.) at various times in relation to the TNF administration. Times were chosen to attenuate TNF activity for the entire duration of exposure to TNF (−1 h), during the period when the concentration was greater in TVX-cotreated mice (+1.5 h), or after the prolonged elevation of TNF in TVX-cotreated mice (+4 h) (Fig. 2A). Etanercept treatment at either 1 h before or 1.5 h after TNF administration protected mice from liver injury induced by TVX/TNF coexposure (Fig. 3). TVX/TNF-induced liver injury was unaffected by etanercept given at +4 h. The increases in plasma ALT activity were reflected in histopathological changes; that is, livers of TVX/TNF-cotreated mice had less necrosis if given etanercept at −1 or +1.5 h but not at +4 h.
FIG. 3.
Effect of the timing of etanercept treatment on TVX/TNF-induced liver injury. Mice were treated with TVX and recombinant mTNF as described in “Materials and Methods.” Etanercept (12 mg/kg, i.p.) was administered at the indicated time relative to TNF dosing or not at all. Plasma ALT activity was measured at 15 h. n = 5–7 animals per group. Photomicrographs were taken of representative livers. *Significantly different from TVX/TNF treatment without etanercept.
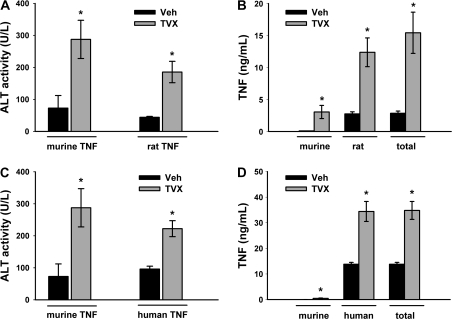
TVX Reduces the Clearance of Rat TNF and Human TNF and Increases Endogenous TNF Production
Mice dosed with TVX or Veh were treated 3 h later with either recombinant rTNF or recombinant hTNF to determine the contribution of injected TNF and endogenously produced TNF (mTNF) to the prolonged TNF peak observed after TVX/TNF coexposure. Unlike mTNF or rTNF, hTNF does not bind to murine TNFR2 (Lewis et al. 1991; Tartaglia et al. 1991), rendering hTNF a useful tool to dissect the roles of each sTNFR. Similar to TVX/mTNF coexposure, plasma ALT activity was increased at 3 h after TVX/rTNF or TVX/hTNF coexposure (Figs. 4A, 4C, respectively). The plasma concentrations of rTNF, hTNF (both are injected TNF), and mTNF (endogenously produced TNF) were measured at 3 h. As described in “Materials and Methods,” the rTNF ELISA and hTNF bio-plex kits were determined not to cross-react with mTNF. Conversely, the mTNF bio-plex kit did not cross-react with rTNF or hTNF. TVX pretreatment significantly increased the plasma concentration of mTNF, and to a much larger extent, rTNF (Fig. 4B). Accordingly, the increase in total TNF was due primarily to an increase in rTNF. Similarly, TVX pretreatment significantly increased the plasma concentration of total TNF at 3 h after administration of hTNF, and this was due predominantly to an increase in hTNF, with only a small contribution from mTNF (Fig. 4D). These data suggest that endogenous production of TNF is slightly enhanced, and TNF elimination is markedly reduced in TVX-cotreated mice.
FIG. 4.
TVX-induced liver injury and plasma TNF concentration after injection of rTNF or hTNF. Mice were treated with TVX (150 mg/kg) or its saline vehicle 3 h before recombinant rTNF (50 μg/kg), recombinant hTNF (50 μg/kg) or its saline vehicle as described in “Materials and Methods.” (A, C) Plasma ALT activity was measured 3 h later and compared with mice treated with TNF alone. n = 4–6 animals per group. *Significantly different from Veh group with same recombinant TNF treatment. (B, D) rTNF, hTNF, and mTNF concentrations were measured at 3 h. Cross-reactivity of antibodies used for measurement was below the detection limit. Total TNF was calculated by adding rTNF or hTNF and mTNF concentrations for each mouse. n = 4–6 animals per group. *Significantly different from Veh within TNF type.
Effect of TVX/TNF Treatment on sTNFRs and Kidney Function
TNF is cleared from plasma by binding to sTNFRs, which are subsequently eliminated in the urine (Bemelmans et al., 1994). The plasma concentrations of both sTNFRs were measured to determine if TVX pretreatment affected TNF-induced increases in these. TVX alone did not alter the concentration of circulating sTNFR1 or sTNFR2 at any time measured (Figs. 5A, 5B). In contrast, TNF injection caused an increase in the plasma concentrations of both soluble receptors at all times measured, and this increase was not attenuated by TVX pretreatment.
FIG. 5.
Effect of TVX/TNF coexposure on plasma sTNFR concentrations and renal function. Mice were treated with TVX or Veh and with recombinant mTNF or Veh as described in “Materials and Methods.” (A, B) The plasma concentrations of sTNFR1 and sTNFR2 were measured at various times. n = 4–6 animals per group. *Significantly different from the respective treatment group at 0 h. (C, D) Plasma concentrations of creatinine and urea were measured at 3 h. n = 4–7 animals per group.
Because biologically inactive TNF:sTNFR complexes are primarily removed by the kidneys via filtration (Bemelmans et al., 1994), kidney function was measured. Renal dysfunction causes a significant increase in plasma concentrations of creatinine and urea (Stonard, 1990). These were unchanged at 3 h in all treatment groups compared with untreated mice (Figs. 5C and 5D).
TVX Pretreatment Enhances TNF-Induced Increases in Plasma Cytokine Concentrations and Hepatic Neutrophil Accumulation
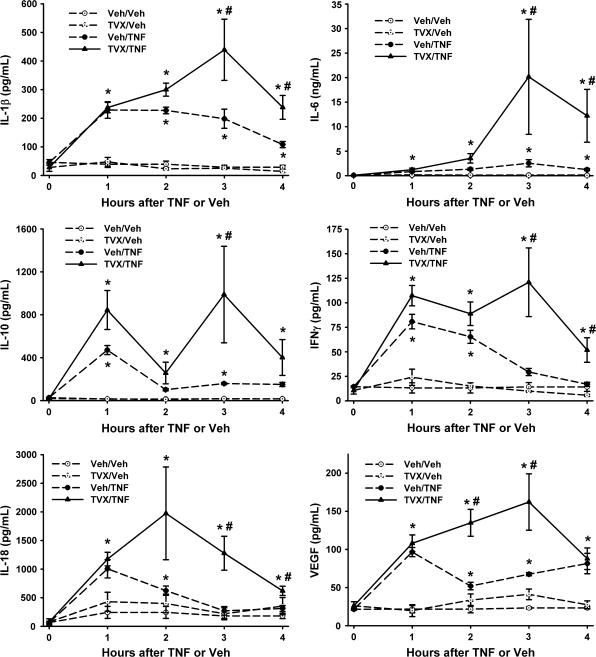
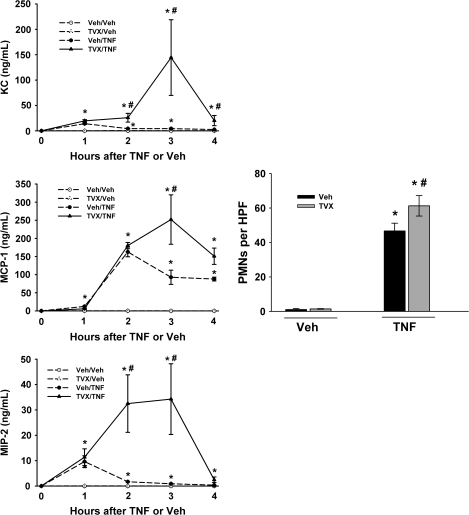
The effect of TVX on TNF-induced increases in inflammatory cytokines was determined during the early pathogenesis (0–4 h). Administration of TVX alone did not significantly increase any of the cytokines or chemokines measured when compared with Veh/Veh-treated mice (Figs. 6 and 7). Administration of recombinant mTNF alone caused a significant increase in the plasma concentrations of IL-1β, IL-6, IL-10, and VEGF at all times measured (Fig. 6). Administration of TNF also increased plasma concentrations of IFN-γ and IL-18 at 1 and 2 h. In addition, TNF administration increased the plasma concentrations of chemokines KC, MIP-2, and MCP-1 at all times measured (Fig. 7). TVX pretreatment enhanced the TNF-induced increase of all of these mediators at or before the onset of liver injury (i.e., 3 h).
FIG. 6.
Effect of TVX pretreatment on TNF-induced increases in plasma cytokines. Mice were treated with TVX and/or TNF as described in “Materials and Methods.” The plasma concentrations of cytokines were measured at various times. n = 4–6 animals per group. *Significantly different from the respective treatment group at 0 h. #Significant difference between Veh/TNF- and TVX/TNF-treated groups at the same time.
FIG. 7.
Effect of TVX pretreatment on TNF-induced increases in plasma chemokines and hepatic neutrophil accumulation. Mice were treated with TVX and/or TNF as described in “Materials and Methods.” The plasma concentrations of chemokines were measured at various times. n = 4–6 animals per group. *Significantly different from the respective treatment group at 0 h. #Significant difference between Veh/TNF- and TVX/TNF-treated groups at the same time. Hepatic PMN accumulation was measured immunohistochemically at 4 h after TNF or Veh treatment. n = 4–6 animals per group. *Significantly different from the respective Veh group not treated with TNF. #Significantly different from Veh/TNF-treated group.
Hepatic neutrophil (PMN) accumulation was measured at 4 h. TNF caused a significant increase in hepatic PMN accumulation which was increased further when mice were pretreated with TVX (Fig. 7).
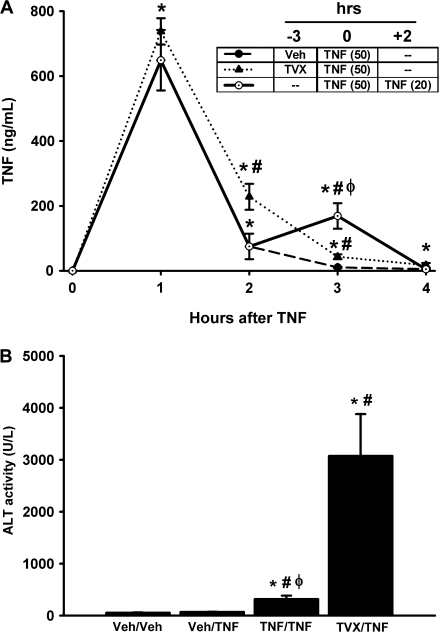
Dual Administration of TNF Causes Liver Injury
When mice were given TVX and then recombinant mTNF (50 μg/kg) 3 h later, the peak of plasma TNF concentration was prolonged (Fig. 2A). To mimic this prolongation, mice were treated with TNF (50 μg/kg) and then given a second injection of TNF (20 μg/kg) 2 h later. This TNF/TNF coexposure resulted in a significantly greater plasma TNF concentration at 3 h compared with Veh/TNF- and TVX/TNF-treated mice (Fig. 8A).
FIG. 8.
Effect of dual administration of TNF to mimic TVX prolongation of plasma TNF. Mice were treated with Veh/TNF, TVX/TNF, or TNF/TNF as described in “Materials and Methods.” (A) Plasma concentration of TNF was measured at various times. The timecourse of the plasma TNF concentration in Veh/TNF- and TVX/TNF-treated mice was also presented in Figure 2 and is represented here for comparison with TNF/TNF treatment. n = 4–6 animals per group. *Significantly different from the respective treatment group at 0 h. #Significantly different from Veh/TNF-treated mice at the same time. ϕSignificantly different from TVX/TNF-treated mice at the same time. (B) Plasma ALT activity was measured at 15 h. n = 6–8 animals per group. *Significantly different from Veh/Veh group. #Significantly different from TNF-treated mice. ϕSignificantly different from TVX/TNF-treated mice at the same time.
To compare the effects of TVX/TNF treatment and TNF/TNF coexposure on liver injury, plasma ALT activity was measured at 15 h after TNF treatment (Fig. 8B). TNF/TNF treatment caused a significant increase in plasma ALT activity compared with Veh/Veh- and TNF/Veh-treated mice. However, TVX/TNF-treated mice had a much greater increase in plasma ALT activity than TNF/TNF-treated mice.
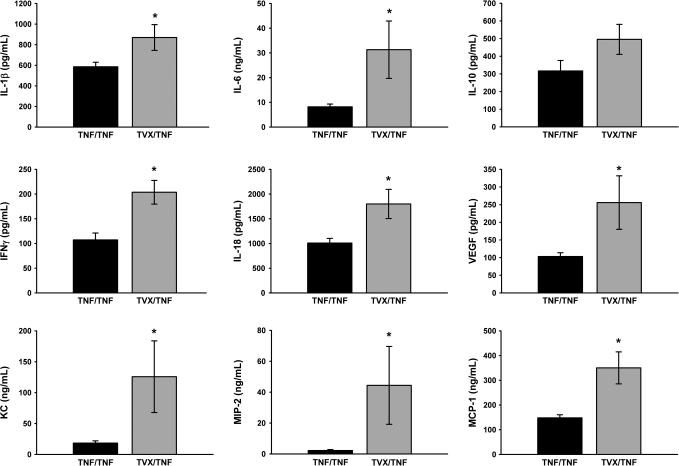
Plasma cytokine Concentrations after TNF/TNF Coexposure
Plasma cytokines were measured at 3 h in TNF/TNF- and TVX/TNF-treated mice to evaluate whether TVX had an effect in addition to prolonging the elevated plasma concentration of TNF that contributed to an increase in plasma cytokine concentrations. TVX/TNF coexposure increased plasma concentrations of IL-1β, IL-6, IFNγ, IL-18, VEGF, KC, MIP-2, and MCP-1 to a greater extent than TNF/TNF treatment (Fig. 9).
FIG. 9.
Comparison of TVX/TNF and TNF/TNF induction of cytokines and chemokines. Mice were treated with TVX/TNF or TNF/TNF as described in “Materials and Methods.” Plasma concentrations of cytokines and chemokines were measured at 3 h. n = 8–10 animals per group. *Significantly different from TNF/TNF group.
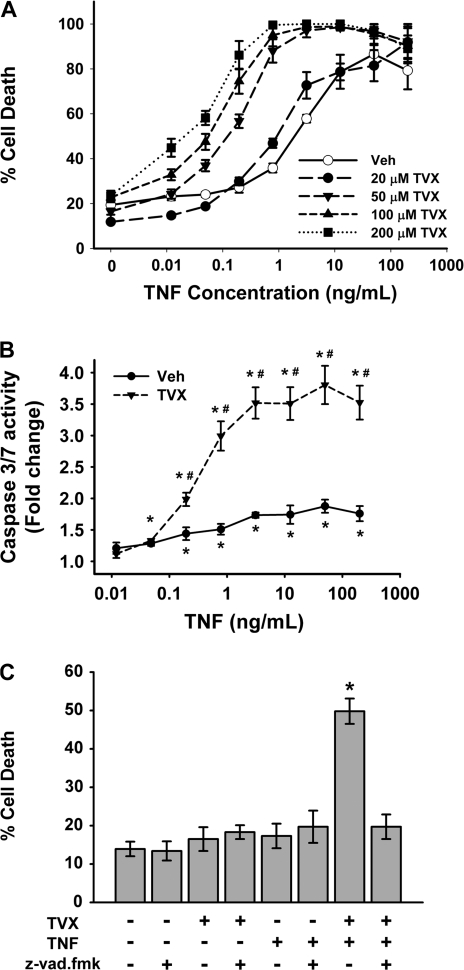
TVX Sensitizes Hepa1c1c7 Cells to TNF-Induced Caspase Activation and Cell Death
Recombinant mTNF caused concentration-dependent cell death of Hepa1c1c7 cells by 24 h (Fig. 10A, open circles). TVX alone, up to 200μM, did not cause cell death. However, TVX coexposure caused a leftward shift in the concentration-response curve for TNF-induced cell death (Fig. 10A). The potency of TNF was markedly increased by TVX in a concentration-dependent manner as reflected by the EC50 concentrations (Supplemental Table 1).
FIG. 10.
Effect of TVX on TNF-induced caspase activation and cell death. (A) Hepa1c1c7 cells were treated with various concentrations of TVX and with recombinant mTNF as described in “Materials and Methods.” Cell death was measured at 24 h. n = 4 separate experiments/group. Open symbols represent TNF-treated cells with 0.1% DMSO vehicle. Two-way ANOVA revealed a significant TVX interaction with TNF. (B, C) Hepa1c1c7 cells were treated with 200 μm TVX and with various concentrations of recombinant mTNF as described in “Materials and Methods.” (B) Caspase 3/7 activity was measured at 6 h. n = 4 separate experiments per group. Cells treated only with the vehicles were the control and set as a fold change of 1. *Significantly different from control group without TNF. #Significantly different from Veh-treated cells at the same TNF concentration. (C) Cells were treated with the caspase inhibitor, z-vad.fmk (40μM), TVX (200μM), and/or TNF (0.2 ng/ml) as TVX (200μM) as described in “Materials and Methods.” Cell death was measured at 24 h. n = 4 separate experiments per group. *Significantly different from all other treatment groups.
Because DNA fragmentation and caspase 3 activation were found in hepatocytes in vivo after TVX/TNF cotreatment of mice, caspase 3/7 activation was measured in Hepa1c1c7 cells after incubation with TVX and TNF. TNF caused a significant increase in caspase 3/7 activity compared with Veh controls at all concentrations greater than 0.1 ng/ml (Fig. 10B). TVX coexposure markedly enhanced the TNF-induced increase in caspase 3/7 activity. To determine if the synergistic interaction between TVX and TNF to cause cytotoxicity was caspase dependent, cells were pretreated with a pan-caspase inhibitor (Z-vad.fmk), and cell death was measured at 24 h (Fig. 10C). Z-vad/fmk was not cytotoxic by itself or in combination with either TVX or TNF alone. TVX synergized with a nontoxic concentration of TNF to cause cytotoxicity. The TVX/TNF-induced cell death was eliminated by z-vad.fmk.
DISCUSSION
We reported previously that TVX interacted with nontoxic LPS exposure to cause hepatocellular injury in mice. TNF was a critical mediator of this response, because etanercept afforded protection against the liver injury and because injection of mTNF could substitute for LPS exposure in interacting with TVX to cause liver injury (Shaw et al., 2007, 2009b). This study was designed to characterize further the hepatotoxic interaction between TVX and TNF and to explore the underlying mechanisms. The timecourse of liver injury induced by TVX/TNF coexposure (Fig. 1) was very similar to that caused by TVX/LPS, inasmuch as both treatments increased plasma ALT activity by 4.5 h (Shaw et al., 2009a). As early as 4 h, TVX/TNF coexposure caused hepatocellular oncotic necrosis and apoptosis primarily in midzonal regions of liver lobules. Apoptotic hepatocytes were seen in mice treated with TVX/TNF, but not with TNF alone (Fig. 1). The results are in accordance with other studies that showed TNF administration alone at a similar dose to be nonhepatotoxic (Schwabe and Brenner, 2006).
In the TVX/LPS model, TVX prolonged the appearance of TNF in the plasma caused by LPS administration (Shaw et al., 2007). Surprisingly, the appearance of TNF in plasma was also prolonged by TVX after the injection of TNF itself (Fig. 2). This TVX-mediated prolongation of elevation of TNF was essential to the pathogenesis as evidenced by the observation that etanercept reduced liver damage but was only effective if given before or during the prolongation (Fig. 3). In the TVX/LPS model, the prolongation of TNF appearance probably did not arise from enhanced gene expression, because TVX cotreatment did not affect the LPS-induced increase in TNF mRNA (Shaw et al., 2009a). This result suggested that TVX cotreatment enhanced post-transcriptional events leading to TNF production and/or slowed the elimination of this cytokine. To distinguish injected TNF from endogenously produced cytokine, recombinant rTNF was administered instead of recombinant mTNF. rTNF is very similar to mTNF and binds to both of the murine TNFRs (Bemelmans et al., 1996). In TVX-treated mice, rTNF caused a similar degree of liver injury as injected mTNF at both 3 (Fig. 4A) and 15 h (data not shown). TVX pretreatment significantly increased the plasma concentrations of both mTNF and rTNF (Fig. 4B). This result indicates that both enhanced production and decreased clearance of TNF contributed to its prolonged appearance in the plasma of TVX/TNF-cotreated mice. Importantly, approximately 70% of the increase in total TNF was rTNF, suggesting that reduced clearance of TNF was primarily responsible for the prolongation of the TNF peak. To our knowledge, this is the first evidence of a xenobiotic agent acting to decrease cytokine clearance.
The first step in inactivation and clearance of TNF is binding to sTNFRs in plasma (Bemelmans et al., 1996). In mice, sTNFR2 is the dominant sTNFR responsible for TNF inactivation and clearance (Bemelmans et al., 1993a). Whereas rTNF binds to both murine TNFRs, hTNF does not bind murine TNFR2 (Lewis et al., 1991; Tartaglia et al., 1991; Bemelmans et al., 1993a). Thus, the role of sTNFR1 can be isolated by evaluating hTNF in this model. Like rTNF, injected hTNF caused a similar degree of liver injury as mTNF in TVX-treated mice at both 3 h (Fig. 4C) and 15 h (data not shown). This finding suggests that TNFR1 is largely responsible for the hepatotoxic TVX/TNF interaction. This was surprising, inasmuch as TNFR2−/− mice were completely protected from TVX/LPS-induced liver injury (Shaw et al., 2009b). However, the peak plasma concentration of TNF after injection of recombinant TNF was approximately 200× greater than after LPS injection (Shaw et al., 2007). It has been proposed that TNFR2 acts to bind TNF and pass it to cell-associated TNFR1, resulting in TNFR1 activation at smaller concentrations of TNF (Tartaglia et al., 1991). If so, at larger concentrations of TNF, as occurred after TNF injection in these studies, TNFR2 might not be required for hepatotoxicity. As with mTNF, TVX pretreatment increased the total plasma TNF concentration after administration of hTNF. hTNF accounted for almost all of the total TNF present, suggesting that TVX reduced its clearance (Fig. 4D). Furthermore, the observation that the plasma concentration of hTNF remained elevated in TVX-treated mice suggests that TVX impairs TNF clearance by a mechanism that involves sTNFR1. The baseline concentration of sTNFR1 in humans is approximately 5 ng/ml, whereas in mice it is approximately 0.1–0.3 ng/ml (Bemelmans et al., 1994). Thus, in humans where sTNFR1 is likely more important in TNF clearance, idiosyncratic TVX hepatotoxicity might involve a mechanism by which TVX reduces sTNFR1-dependent TNF clearance.
Cell-associated TNFR1 and TNFR2 are cleaved to 30- and 40-kDa soluble receptors, respectively (Gatanaga et al., 1990a, b; Katsura et al., 1996). The biologically inactive TNF:sTNFR complexes are primarily removed by glomerular filtration in the kidneys, but the liver and lungs are also believed to be involved in their clearance (Bemelmans et al., 1994). It is unlikely that TVX reduced TNF clearance by affecting the cleavage of its membrane receptors to soluble forms because the plasma concentrations of sTNFRs were not reduced by TVX pretreatment in TNF-treated mice (Figs. 5A, 5B). It is possible that TVX decreased the affinity of TNF binding to sTNFRs; however, this seems unlikely inasmuch as the fraction of immunologically detectable TNF that was biologically active was similar with or without TVX treatment (i.e., the ratio of active:total TNF at 3 h was ∼10% irrespective of TVX treatment [Figs. 2A, 2B]). Additionally, the biologically active fraction of TNF was similar to a previously reported study (Bemelmans et al., 1993a). Finally, it seems unlikely that impaired renal function was responsible for the reduced TNF clearance. Plasma creatinine and urea concentrations reflect decreases in glomerular filtration, and these were unchanged by TVX/TNF coexposure at 3 h (Fig. 5C, D). Thus, it seems unlikely that reduced TNFR shedding or renal dysfunction was responsible for the early reduction in TNF clearance.
In addition to reducing the clearance of TNF, it appears that TVX enhanced the downstream effects of TNF to precipitate liver injury. For example, TVX enhanced the TNF-induced increases in plasma concentration of cytokines and chemokines at a time near the onset of liver injury (Figs. 6 and 7). The increase in chemokines was accompanied by an increase in PMN recruitment into the liver (Fig. 7). TVX enhanced the inflammatory cascade (cytokines, chemokines, and PMN recruitment) induced by TNF, only at times after the prolongation of the TNF plasma concentration. Thus, it might be the prolonged appearance of TNF that drove the enhanced appearance of these proinflammatory factors. However, it is also possible that TVX can reduce the clearance of other inflammatory cytokines as it does for TNF.
The question arises whether the extended exposure to TNF is sufficient to cause changes in inflammation and liver injury, or whether TVX exerts actions in addition to this prolongation that contribute to these effects. To address this question, a second, smaller dose of TNF was administered 2 h after the first dose. This second administration resulted in a greater plasma TNF concentration at 3 h than in TVX/TNF-treated mice (Fig. 8A) but a smaller increase in plasma ALT activity (Fig. 8B). This finding suggests that although the prolongation of TNF is involved in the progression of hepatotoxicity, TVX also contributes to the pathogenesis through additional mechanisms.
Although TNF/TNF-treated mice had a greater plasma concentration of TNF compared with TVX/TNF treatment, TVX/TNF coexposure had greater plasma concentrations of IL-1β, IL-6, IFN-γ, IL-18, VEGF, KC, MIP-2, and MCP-1 (Fig. 9). The only cytokine which was not different between the two groups was the anti-inflammatory cytokine, IL-10. The increased production of IFN-γ and IL-18 are of particular interest, inasmuch as these cytokines play a critical role in TVX/LPS-induced liver injury (Shaw et al., 2009a). Thus, the differences observed in these cytokines may explain, at least in part, the large difference in hepatocellular injury between TVX/TNF- and TNF/TNF-treated mice. Because TVX enhanced the production of all cytokines after TNF treatment, it is likely that TVX either acts proximally in the signaling pathway for cytokine expression or at a common post-transcriptional step.
In addition to augmenting TNF-induced inflammation, TVX acted directly to enhance TNF-induced hepatocellular death (Fig. 10A). The mechanism by which TVX sensitizes cells to TNF-induced cell death is unknown. It is unlikely that TVX upregulated TNFRs on Hepa1c1c7 cells, inasmuch as TVX treatment alone did not affect TNFR expression in mice (Shaw et al., 2009a). It seemed more likely that TVX affected TNF-induced cell death signaling, such as caspase activation. The efficacy of TNF to induce effector caspase activation in Hepa1c1c7 cells was enhanced markedly by TVX (Fig. 10B). This is consistent with the finding that TVX interacted with TNF to cause caspase 3 cleavage in vivo (Fig. 1). Additionally, caspase activation was critical to TVX/TNF-induced cytotoxicity of Hepa1c1c7 cells (Fig. 10C). Therefore, it is likely that increased caspase activation in hepatocytes is involved in the pathogenesis of liver injury from TVX/TNF coexposure.
TNF can be rendered hepatotoxic when coadministered with an inhibitor of RNA synthesis such as galactosamine (GalN) (Gezginci and Bolkent, 2007). Similar to TVX/TNF coexposure, GalN/TNF caused an increase in TUNEL and caspase 3 staining of parenchymal cells (Gezginci and Bolkent, 2007). The antibiotic properties of TVX include inhibition of bacterial topoisomerase (Ernst et al., 1997). It is possible that TVX also affects eukaryotic topoisomerases and thereby inhibits protein synthesis in hepatocytes, and in this manner acts similarly to GalN to increase sensitivity of hepatocytes to TNF. However, it is unknown if TVX acted to enhance TNF-induced caspase activation and sensitize Hepa1c1c7 cells via this mechanism.
In summary, TVX interacted with a nonhepatotoxic dose of TNF to cause liver injury in mice. The pathogenesis of liver injury depended on the prolongation of the peak of TNF, which was caused in part by enhancement of TNF production by TVX, but mostly by a reduction in TNF elimination. The mechanism underlying the early reduction of TNF clearance by TVX did not involve TNFR cleavage, TNF binding to sTNFRs or kidney dysfunction. This finding that a xenobiotic has the potential to reduce the clearance of TNF is novel. Additionally, the induction of inflammation in vivo and cell death in vitro by TNF was enhanced by TVX coexposure. Thus, TVX interacts with TNF in several ways, including reducing its clearance, increasing its ability to induce other cytokines and sensitizing hepatocytes to cell death. Such interactions between TVX and TNF should enter into our thinking about how TVX causes idiosyncratic hepatotoxicity in humans.
FUNDING
National Institutes of Health (Grant DK061315); and National Institute of Environmental Health Sciences (Training Grant GM075685).
Supplementary Material
References
- Bemelmans MH, Gouma DJ, Buurman WA. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J. Immunol. 1993a;150:2007–2017. [PubMed] [Google Scholar]
- Bemelmans MH, Gouma DJ, Buurman WA. LPS-induced sTNF-receptor release in vivo in a murine model. Investigation of the role of tumor necrosis factor, IL-1, leukemia inhibiting factor, and IFN-gamma. J. Immunol. 1993b;151:5554–5562. [PubMed] [Google Scholar]
- Bemelmans MH, Gouma DJ, Buurman WA. Tissue distribution and clearance of soluble murine TNF receptors in mice. Cytokine. 1994;6:608–615. doi: 10.1016/1043-4666(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Bemelmans MH, van Tits LJ, Buurman WA. Tumor necrosis factor: Function, release and clearance. Crit. Rev. Immunol. 1996;16:1–11. doi: 10.1615/critrevimmunol.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- Beutler BA, Milsark IW, Cerami A. Cachectin/tumor necrosis factor: Production, distribution, and metabolic fate in vivo. J. Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am. J. Physiol. 1998;275:6387–9213. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- Buchweitz JP, Ganey PE, Bursian SJ, Roth RA. Underlying endotoxemia augments toxic responses to chlorpromazine: Is there a relationship to drug idiosyncrasy? J. Pharmacol. Exp. Ther. 2002;300:460–467. doi: 10.1124/jpet.300.2.460. [DOI] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Sarafian TA, Spinella FM, Kahn MA, Shau H, de VJ. Expression of the p75 TNF receptor is linked to TNF-induced NFkappaB translocation and oxyradical neutralization in glial cells. Neurochem. Res. 2002;27:1535–1542. doi: 10.1023/a:1021608724117. [DOI] [PubMed] [Google Scholar]
- Ernst ME, Ernst EJ, Klepser ME. Levofloxacin and trovafloxacin: The next generation of fluoroquinolones? Am. J. Health Syst. Pharm. 1997;54:2569–2584. doi: 10.1093/ajhp/54.22.2569. [DOI] [PubMed] [Google Scholar]
- Ferraiolo BL, Moore JA, Crase D, Gribling P, Wilking H, Baughman RA. Pharmacokinetics and tissue distribution of recombinant human tumor necrosis factor-alpha in mice. Drug Metab. Dispos. 1988;16:270–275. [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Gatanaga T, Hwang CD, Kohr W, Cappuccini F, Lucci JA, III, Jeffes EW, Lentz R, Tomich J, Yamamoto RS, Granger GA. Purification and characterization of an inhibitor (soluble tumor necrosis factor receptor) for tumor necrosis factor and lymphotoxin obtained from the serum ultrafiltrates of human cancer patients. Proc. Natl. Acad. Sci. U. S. A. 1990a;87:8781–8784. doi: 10.1073/pnas.87.22.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatanaga T, Lentz R, Masunaka I, Tomich J, Jeffes EW, III, Baird M, Granger GA. Identification of TNF-LT blocking factor(s) in the serum and ultrafiltrates of human cancer patients. Lymphokine Res. 1990b;9:225–229. [PubMed] [Google Scholar]
- Gezginci S, Bolkent S. The effect of Z-FA.FMK on D-galactosamine/TNF-alpha-induced liver injury in mice. Cell Biochem. Funct. 2007;25:277–286. doi: 10.1002/cbf.1352. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Schultze AE, VanCise S, Roth RA. Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab. Invest. 1992;66:347–361. [PubMed] [Google Scholar]
- Joyce DA, Steer JH. Tumor necrosis factor alpha and interleukin-1 alpha stimulate late shedding of p75 TNF receptors but not p55 TNF receptors from human monocytes. J. Interferon Cytokine Res. 1995;15:947–954. doi: 10.1089/jir.1995.15.947. [DOI] [PubMed] [Google Scholar]
- Joyce DA, Steer JH. IL-4, IL-10 and IFN-gamma have distinct, but interacting, effects on differentiation-induced changes in TNF-alpha and TNF receptor release by cultured human monocytes. Cytokine. 1996;8:49–57. doi: 10.1006/cyto.1996.0007. [DOI] [PubMed] [Google Scholar]
- Katsura K, Park M, Gatanaga M, Yu EC, Takishima K, Granger GA, Gatanaga T. Identification of the proteolytic enzyme which cleaves human p75 TNF receptor in vitro. Biochem. Biophys. Res. Commun. 1996;222:298–302. doi: 10.1006/bbrc.1996.0738. [DOI] [PubMed] [Google Scholar]
- Lazarczyk DA, Goldstein NS, Gordon SC. Trovafloxacin hepatotoxicity. Dig. Dis. Sci. 2001;46:925–926. doi: 10.1023/a:1010741510046. [DOI] [PubMed] [Google Scholar]
- Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J. Pharmacol. Exp. Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- Nicholson SC, Webb CD, Moellering RC., Jr Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794–796. doi: 10.1592/phco.22.9.794.34066. [DOI] [PubMed] [Google Scholar]
- Pessina GP, Pacini A, Bocci V, Maioli E, Naldini A. Studies on tumor necrosis factor (TNF): II. Metabolic fate and distribution of human recombinant TNF. Lymphokine Res. 1987;6:35–44. [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Mechanisms of liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- Seckinger P, Isaaz S, Dayer JM. Purification and biologic characterization of a specific tumor necrosis factor alpha inhibitor. J. Biol. Chem. 1989;264:11966–11973. [PubMed] [Google Scholar]
- Shaw PJ, Ditewig AC, Waring JF, Liguori MJ, Blomme EA, Ganey PE, Roth RA. Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol. Sci. 2009a;107:270–280. doi: 10.1093/toxsci/kfn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ganey PE, Roth RA. Tumor necrosis factor alpha is a proximal mediator of synergistic hepatotoxicity from trovafloxacin/lipopolysaccharide coexposure. J. Pharmacol. Exp. Ther. 2009b;328:62–68. doi: 10.1124/jpet.108.143792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol. Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- Spinas GA, Keller U, Brockhaus M. Release of soluble receptors for tumor necrosis factor (TNF) in relation to circulating TNF during experimental endotoxinemia. J. Clin. Invest. 1992;90:533–536. doi: 10.1172/JCI115891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonard MD. Assessment of renal function and damage in animal species. A review of the current approach of the academic, governmental and industrial institutions represented by the Animal Clinical Chemistry Association. J. Appl. Toxicol. 1990;10:267–274. doi: 10.1002/jat.2550100407. [DOI] [PubMed] [Google Scholar]
- Tang P, Hung M-C, Klostergaard J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry. 1996;35:8216–8225. doi: 10.1021/bi952182t. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr., Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring JF, Liguori MJ, Luyendyk JP, Maddox JF, Ganey PE, Stachlewitz RF, North C, Blomme EA, Roth RA. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils. J. Pharmacol. Exp. Ther. 2006;316:1080–1087. doi: 10.1124/jpet.105.096347. [DOI] [PubMed] [Google Scholar]
- Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, Roth RA. Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol. Sci. 2003;72:43–56. doi: 10.1093/toxsci/kfg019. [DOI] [PubMed] [Google Scholar]
- Zou W, Devi SS, Sparkenbaugh E, Younis HS, Roth RA, Ganey PE. Hepatotoxic interaction of sulindac with lipopolysaccharide: Role of the hemostatic system. Toxicol. Sci. 2009;108:184–193. doi: 10.1093/toxsci/kfn259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.