Abstract
Previous statistical voxelwise lesion-behavior mapping (VLBM) studies have demonstrated that spatial neglect is associated with cortical and subcortical gray matter damage. However, it has also been suggested that the disorder may result from white matter injury. Our aim was to investigate the white matter connectivity in a large sample of 140 stroke patients. We combined a VLBM approach with the histological maps of the human white matter fiber tracts provided by the Jülich probabilistic cytoarchitectonic atlas. We found that damage of right perisylvian white matter connections—the superior longitudinal fasciculus, the inferior occipitofrontal fasciculus, and the superior occipitofrontal fasciculus—is a typical finding in patients with spatial neglect. However, the analysis also revealed that the largest portion of the lesion area, namely between 89.1% and 96.6%, affected brain structures other than the perisylvian white matter fiber tracts. Predominantly, these included gray matter structures such as the superior temporal, inferior parietal, inferior frontal, and insular cortices, as well as subcortically the putamen and the caudate nucleus. Damage of gray matter structures thus appears to be a strong predictor of spatial neglect.
Keywords: attention, human, spatial neglect, stroke, superior longitudinal fasciculus, superior occipitofrontal fasciculus, visual search, white matter
Introduction
Spatial neglect is a common consequence of stroke and is typically observed in individuals who suffer from right hemisphere strokes (Pedersen et al. 1997; Hillis et al. 2005; Becker and Karnath 2007). Patients with spatial neglect spontaneously deviate their eyes and head toward the ipsilesional, right side (Fruhmann Berger et al. 2006) and tend to ignore information presented on their contralesional side. Clinically, spatial neglect is a strong predictor for poor recovery on a wide range of everyday tasks (Jehkonen et al. 2000). The syndrome also provides theoretical insight into the normal function of the spatial attentional system in humans.
Structural imaging can be combined with new statistical methods to correlate the location of brain injury with behavioral deficits (for review, Rorden and Karnath 2004). These techniques can offer an objective measure for lesion-behavior relationships. Indeed, a number of rigorous statistical methods have been developed for conducting voxelwise lesion-behavior mapping (VLBM), where one does not make any explicit, a priori assumptions regarding brain anatomy (Frank et al. 1997; Bates et al. 2003; Kimberg et al. 2007; Rorden et al. 2007).
Recently, a series of studies have examined the anatomical correlates of spatial neglect by using these statistical VLBM methods (Mort et al. 2003; Karnath et al. 2004; Committeri et al. 2007). The results from these studies were interpreted as implicating cortical and subcortical gray matter structures. However, none of these studies explicitly attempted to assess involvement of white matter connections. This raises the possibility that these studies might have a bias toward identifying gray matter structures rather than nearby white matter fiber bundles. It was even speculated that “…a particular form of disconnection might have greater predictive value than the localization of gray matter lesions concerning the [neglect] patients’ deficits and disabilities” (Bartolomeo et al. 2007, p. 2484).
Our aim was to address the issue of involvement of gray versus white matter tissue in spatial neglect. In particular, we were interested in investigating the relationship of white matter injury to the presence of the disorder in a large sample of 140 stroke patients. In theory, this work should help identify if white matter injury is a common predictor of spatial neglect and, if so, which of the fiber bundles typically are involved.
The first step of this approach was a reanalysis of a large imaging dataset of right hemisphere stroke lesions described by Karnath et al. (2004) consisting of a 7-year sample of 140 patients by using a new statistical VLBM protocol. We have recently advocated the use of the Liebermeister measure for binomial data in VLBM studies (Rorden et al. 2007). As this test does not assume fixed marginals, it is more sensitive than the previously used chi-square test (which is overly conservative). The second step of our present approach of white matter fiber tract analysis is the combination of the statistical lesion map resulting from the VLBM procedure with a recently developed set of cytoarchitectonic probabilistic maps of white matter fiber connections (Amunts and Zilles 2001; Zilles et al. 2002; Bürgel et al. 2006). This atlas provides stereotaxic information on the location and variability of cortical areas and white matter fiber tracts in the Montreal Neurological Institute (MNI) reference space (http://www.fz-juelich.de/inb/inb-3/index.php?index=29). In contrast to the reference brain of the Talairach and Tournoux atlas (Talairach and Tournoux 1988) or the MNI single subject or group templates (Evans et al. 1992; Collins et al. 1994; Tzourio-Mazoyer et al. 2002), the probabilistic cytoarchitectonic atlas is based on the analysis of the cytoarchitecture in a sample of 10 different postmortem human brains. By using a modified myelin staining technique, Bürgel et al. (2006) were able to distinguish and map individual tracts at microscopic resolution within the densely packed fibers of the white matter in these 10 human brains. Digitalization and normalization of results derived 3-dimensional, probabilistic maps of 10 different long white matter fiber bundles. The maps illustrate the relative frequency with which a certain fiber tract of the 10 normal human brains was present on an MNI reference brain in a voxel. They thus serve as a measure of intersubject variability of the human white matter fiber bundle anatomy for each voxel of the reference space.
Subjects and Methods
Data were drawn from 140 consecutively admitted stroke patients with circumscribed right hemisphere lesions from a well-defined recruitment area belonging to the University of Tübingen, sampled over a period of 7 years (Karnath et al. 2004). The full details regarding patient characteristics, test criteria, etc., are reported in our previous work (Karnath et al. 2004). In short, following standardized testing for spatial neglect by using the “Letter cancellation” task (Weintraub and Mesulam 1985), the “Bells test” (Gauthier et al. 1989), the “Baking tray task” (Tham and Tegnér 1996), and a copying task (Johannsen and Karnath 2004), the subjects were divided into a group of 78 patients with spatial neglect and 62 control patients who did not show the disorder. Magnetic resonance imaging (MRI) or computerized tomography (Spiral-CT) was carried out in each subject. Lesion mapping was aided by diffusion-weighted imaging for MRI occurring within the first 48 h poststroke and T2-weighted fluid-attenuated inversion recovery sequences when MRI was conducted 48 h or later after the stroke. The mean time between lesion and the MRI was 5.0 days (standard deviation [SD] 5.4). In those subjects who underwent the CT imaging protocol, the mean time since lesion and the CT was 6.7 days (SD 8.4). All lesions were drawn manually (while being blind for the diagnosis of spatial neglect) on axial slices of a T1-weighted template MRI scan from the MNI (http://www.bic.mni.mcgill.ca/cgi/icbm_view) using the MRIcro software package (Rorden and Brett 2000) with a 1 × 1 mm in-plane resolution. This template is approximately oriented to match Talairach space (Talairach and Tournoux 1988) and is distributed with MRIcro. Lesions were mapped onto the slices that correspond to z-coordinates −40, −32, −24, −16, −8, 0, 8, 16, 24, 32, 40, and 50 mm in Talairach space by using the identical or the closest matching axial slices of each individual. The full details regarding imaging protocols etc. are reported in our previous work (Karnath et al. 2004).
Analysis of White Matter Lesion Anatomy
For the present investigation, we used the new nonparametric mapping software that is distributed as part of the MRIcron toolset (Rorden et al. 2007). Specifically, we performed the nonparametric Liebermeister test to identify voxels that when injured predicted the presence of spatial neglect. The resulting statistical map was adjusted for multiple comparisons by using a Bonferroni-corrected P < 0.05 threshold.
Therefore, our initial analysis was based on this statistical map that illustrated all the voxels that predicted the presence of spatial neglect. In a first analysis, we determined the percentage of these voxels that comprised white matter compared with gray matter tissue. We segmented the individual T1-weighted template MRI scan from the MNI (http://www.bic.mni.mcgill.ca/cgi/icbm_view) by using Statistical parametric mapping routines (SPM5; http://www.fil.ion.ucl.ac.uk/spm/). The percentage of overlap of white versus gray matter tissue with the statistical lesion map was determined by using MRIcron software (Rorden et al. 2007).
In order to identify the specific fiber tracts that predict spatial neglect, we segmented the thresholded Liebermeister statistical map using the white matter fiber tracts from the human probabilistic cytoarchitectonic atlas (Bürgel et al. 2006). This atlas is in the same space as the MNI reference brain, with each atlas map illustrating the relative frequency with which a certain fiber tract of 10 normal human brains was present (e.g., a 30% value reflects that the fiber tract was present in that voxel for 3 out of 10 brains). The number of overlapping voxels between the statistical lesion map and the anatomical fiber tract map in the right hemisphere was determined by using the SPM anatomy toolbox of the Jülich atlas (http://www.fz-juelich.de/inb/inb-3//spm_anatomy_toolbox; Eickhoff et al. 2005).
We also conducted a logistic regression that included each white matter fiber tract from the Jülich probabilistic atlas (Bürgel et al. 2006). The extent of injury to each fiber tract was calculated for each individual on a voxel-by-voxel basis, weighting each lesioned voxel by the frequency of the target fiber tract in the atlas. These continuous variables were used to predict the absence or presence of spatial neglect, adding overall lesion volume as a predictor. Note that logistic regression computes the partial correlation for each factor and adding an extra variable (such as overall lesion volume) can increase (if the variable predominantly describes variance that is not accounted for by other variables) or decrease (if the variable predominantly describes variance that is redundant with another variable) statistical significance of the other variables. Finally, we conducted a restricted version of this analysis, where we excluded primary sensory and primary motor tracts (acoustic radiation [AR], corticospinal tract [CT], optic radiation [OR]). Our reasoning was that these fiber bundles are associated with lower level perception and motor control, whereas neglect behavior is often seen as distinctively different from injury that is restricted to such primary processing. On explicit testing, the symptoms of spatial neglect appear to clearly dissociate from the symptoms associated with injury to these primary fiber tracts. Specifically, many neglect patients are not physically blind or deaf, rather they fail to attend and orient toward left-sided visual or auditory information.
Results
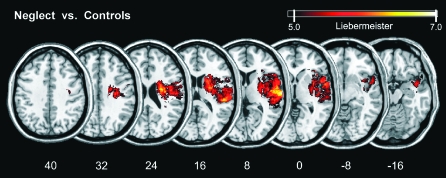
Figure 1 shows all voxels that were—after adjustment for multiple comparisons—significantly more frequently affected in those patients showing spatial neglect than in those without the disorder by using the new Liebermeister approach. In comparison with our previous results obtained with chi-square statistics (Karnath et al. 2004), the present study revealed a larger territory of significant difference. This reflects the overly conservative nature of the original chi-square test, which resulted in fewer voxels surviving the statistical threshold.
Figure 1.
Statistical VLBM analysis comparing the 78 neglect patients with 62 control patients by using the Liebermeister measure (Rorden et al. 2007). Presented are all voxels that were damaged significantly more frequently in neglect patients than in control patients that survived a Bonferroni-corrected alpha level of P < 0.05. No voxels were found that were significantly more likely to be damaged in control patients than in patients with spatial neglect. MNI coordinates of each transverse section are given.
For a first analysis of the white matter lesion anatomy, we determined the percentage to which the statistical lesion map (Fig. 1) affected white compared with gray matter brain tissue. We found that 36.9% of the map overlapped with the white matter portion of the segmented MNI brain, whereas 63.1% of the map overlapped with the gray matter segment. White matter brain tissue thus was less prominently affected than gray matter tissue in those patients showing spatial neglect than in those without the disorder. By using the anatomical parcellation of the MNI single subject brain by Tzourio-Mazoyer et al. (2002) implemented in MRIcron, we found the most statistically significant voxels at MNI coordinates (x 66; y −16; z 8) within the right superior temporal gyrus (STG) and coordinates (x 28; y −7; z 24) within the right periventricular white matter. Regions significantly damaged also encompassed large parts of the right insula, the planum temporale, and operculum (including the opercular portion of the inferior frontal gyrus). The lesion area further extended into the pre- and postcentral gyri, the inferior parietal lobule, and subcortically into the putamen, head of caudate nucleus, as well as external and extreme capsule.
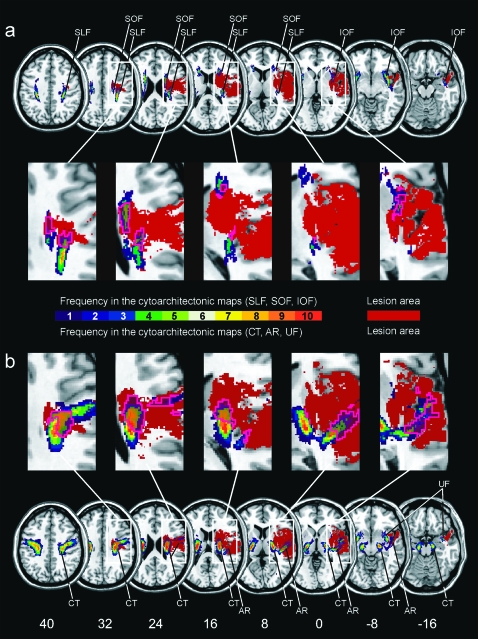
For a closer analysis of the white matter lesion anatomy, we then determined which fiber tracts were affected within the white matter portion of the lesion territory. Figure 2a illustrates the statistical lesion map obtained from the VLBM analysis (Fig. 1) in homogenous brown color. It was superimposed onto the anatomical maps of the white matter fiber tracts from the probabilistic Jülich atlas. Figure 3 gives the quantitative analysis of these data. When we included every single voxel of the anatomical atlas that is, even those voxels in which fiber bundle tissue had been found in only 1 out of the 10 postmortem brains, we revealed that 22.4% of the superior longitudinal fasciculus (SLF), 26.3% of the inferior occipitofrontal fasciculus (IOF), and 39.6% of the superior occipitofrontal fasciculus (SOF) as well as 7.5% of the uncinate fasciculus (UF), 18.4% of the CT, and 46.9% of the AR were located within the territory that was more frequently affected in the group with spatial neglect than in controls (Figs. 2 and 3). There was no overlap between this area and the optic radiation, corpus callosum, fornix, and cingulum from the Jülich atlas. Following the threshold procedure suggested for defining anatomical regions of interest on the basis of the Jülich atlas for functional imaging data (Eickhoff et al. 2005, 2006), we then focused on voxels located within the 30% isocontours of the atlas, that is, those voxels in which a fiber tract was present in at least 3 (30%) of the 10 postmortem brains, to increase the reliability of fiber tract anatomy in the reference map. Under this condition, we revealed that 7.0% of the SLF, 8.2% of the IOF, and 12.7% of the SOF as well as 0.6% of the UF, 9.5% of the CT, and 15.0% of the AR were in the territory identified by the statistically thresholded map.
Figure 2.
Overlap of the statistical lesion map from Figure 1 (in homogenous brown color) with the probabilistic, cytoarchitectonic maps of the white matter fiber tracts from the Jülich atlas (right hemisphere only). The color coding of the atlas from 1 (dark blue, observed in 1 postmortem brain) to 10 (red, overlap in all 10 postmortem brains) represents the absolute frequency for which in each voxel of the atlas a respective fiber tract was present (e.g., a 30% value of a fiber tract in a certain voxel of the reference brain indicates that the fiber tract was present in that voxel in 3 out of 10 postmortem brains). The pink contour demarks the area of the fiber tracts affected by the statistical lesion map. (a) Overlap illustrated for fiber tracts SLF, superior longitudinal fasciculus; IOF, inferior occipitofrontal fasciculus; and SOF, superior occipitofrontal fasciculus. (b) Overlap illustrated for fiber tracts CT, corticospinal tract; AR, acoustic radiation; and UF, uncinate fascicle.
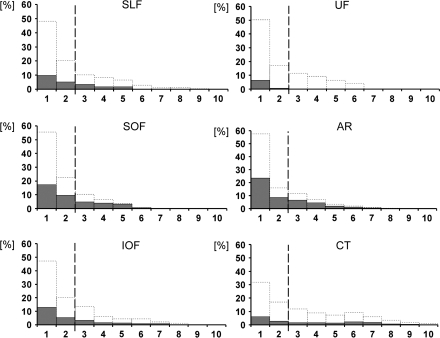
Figure 3.
Quantitative analysis of the data presented in Figure 2. In each of the 6 panels, the area under the dotted lines represents the entire area of a certain anatomical fiber tract from the Jülich atlas (100% of, e.g., the SLF). Along the x-axis, this area is subdivided and plotted according to the percentage with which in each voxel of the atlas the respective fiber tract was present (from 1 to 10). The numbers along the x-axis thus correspond with the color coded illustration of the respective voxel category from Figure 2 (frequency 1–10). The gray bars on top of the dotted bars indicate the percentage of involvement of each of the voxel categories (frequency 1–10) from the respective fiber tract by the statistical map (e.g., a 20% value of a gray bar in category “5” indicates that 20% of all category 5 voxels of that particular fiber tract had been covered by the statistical lesion map). The dashed line indicates the 30% isocontours of the maps from the Jülich atlas. SLF, superior longitudinal fasciculus; IOF, inferior occipitofrontal fasciculus; SOF, superior occipitofrontal fasciculus; CT, corticospinal tract; AR, acoustic radiation; and UF, uncinate fascicle.
The results from the logistic regression are shown in Table 1. When overall lesion volume was taken into account, the CT was the only white matter fiber tract that predicted spatial neglect, surviving adjustment for multiple comparisons by a Bonferroni-corrected P < 0.05 threshold. This model correctly categorized 55 of the 62 patients without neglect and 64 of the 78 patients exhibiting spatial neglect. A correlation matrix for all factors is presented in Table 2. It revealed that lesion volume (r = 0.548) and the CT (r = 0.517) were the strongest individual predictors of spatial neglect. We strongly suspect that the disconnection of the CT has no direct relevance for the presence of spatial neglect (but rather for the frequent additional presence of right-sided hemiparesis in these patients), and we therefore theorize that the association of the CT to spatial neglect observed with the logistic regression reflects its proximity to neighboring cortical and subcortical areas. Note also that the numerical trend was for injury to the cingulum and fornix to be negatively correlated with neglect, suggesting that these regions are distant from the core areas responsible for spatial neglect.
Table 1.
Independent ability to predict spatial neglect as assessed using logistic regression
| Region | P value | P value (partial model) |
| AR | 0.0393 | — |
| Corpus Callosum | 0.9695 | 0.2210 |
| Cingulum | 0.0267 | 0.1207 |
| CT | 0.0039* | — |
| Fornix | 0.8752 | 0.9642 |
| IOF | 0.0077 | 0.0059* |
| OR | 0.635 | — |
| SLF | 0.0161 | 0.7829 |
| SOF | 0.4817 | 0.0506 |
| UF | 0.0133 | 0.0069 |
| Volume | 0.0111 | 0.00001* |
Note: A logistic regression was conducted with the absence or presence of spatial neglect as the dependent variable and the extent of injury to each of the white matter fiber tracts from the Jülich atlas as the independent variables. Overall lesion volume was included as a predictor. Logistic regression assesses partial correlation—measuring whether adding the variable improves the model when all the other variables are already known. Asterisks (*) highlight regions that survived a 5% Bonferroni correction (P < 0.0045 for the 11-factor comparison). We also conducted a partial model where the AR, Corpus Callosum, and OR were excluded (Bonferroni threshold P < 0.00625 for the 8-factor comparison). The results from this partial model are shown in the right column.
Table 2.
Correlation matrix between white matter fiber tracts
| Neglect | AR | CC | Cingulum | CT | Fornix | IOF | OR | SLF | SOF | UF | Volume | |
| Neglect | 1 | 0.477 | 0.208 | −0.114 | 0.517 | −0.054 | 0.421 | 0.01 | 0.445 | 0.465 | 0.379 | 0.548 |
| AR | 0.477 | 1 | 0.147 | −0.122 | 0.507 | −0.036 | 0.771 | 0.001 | 0.518 | 0.567 | 0.618 | 0.787 |
| CC | 0.208 | 0.147 | 1 | 0.531 | 0.263 | 0.628 | 0.137 | 0.694 | 0.258 | 0.303 | 0.159 | 0.479 |
| Cingulum | −0.114 | −0.122 | 0.531 | 1 | −0.077 | 0.718 | −0.164 | 0.612 | −0.155 | −0.135 | −0.137 | 0.051 |
| CT | 0.517 | 0.507 | 0.263 | −0.077 | 1 | −0.001 | 0.634 | −0.12 | 0.892 | 0.811 | 0.589 | 0.716 |
| Fornix | −0.054 | −0.036 | 0.628 | 0.718 | −0.001 | 1 | 0 | 0.748 | −0.028 | −0.011 | 0.04 | 0.159 |
| IOF | 0.421 | 0.771 | 0.137 | −0.164 | 0.634 | 0 | 1 | −0.052 | 0.556 | 0.756 | 0.935 | 0.734 |
| OR | 0.01 | 0.001 | 0.694 | 0.612 | −0.12 | 0.748 | −0.052 | 1 | −0.083 | −0.096 | −0.029 | 0.233 |
| SLF | 0.445 | 0.518 | 0.258 | −0.155 | 0.892 | −0.028 | 0.556 | −0.083 | 1 | 0.687 | 0.493 | 0.668 |
| SOF | 0.465 | 0.567 | 0.303 | −0.135 | 0.811 | −0.011 | 0.756 | −0.096 | 0.687 | 1 | 0.707 | 0.687 |
| UF | 0.379 | 0.618 | 0.159 | −0.137 | 0.589 | 0.04 | 0.935 | −0.029 | 0.493 | 0.707 | 1 | 0.623 |
| Volume | 0.548 | 0.787 | 0.479 | 0.051 | 0.716 | 0.159 | 0.734 | 0.233 | 0.668 | 0.687 | 0.623 | 1 |
Note: Also included is presence of spatial neglect (Neglect) and overall lesion volume (Volume). CC, corpus callosum.
We also conducted a logistic regression that excluded the primary sensory and primary motor fiber bundles (AR, CT, OR). This model included 8 variables (7 fiber bundles and overall lesion volume). The results from this analysis are shown in Table 1. Two variables (IOF and lesion volume) exceeded the Bonferroni threshold of 0.00625 (P < 0.05 corrected for multiple comparisons), with the UF approaching significance. The reason these fiber tracts are not detected in the full model is likely due to their strong correlation with the CT (as shown in Table 2). Despite the reduced number of factors, this model correctly categorized 53 of the 62 patients without neglect and 63 of the 78 patients exhibiting spatial neglect.
Finally, we determined the overall proportion of the lesion territory that overlapped with the white matter fiber tracts identified by our analyses. The CT and the AR were not included due to their obvious involvement in primary motor and acoustic processing. Rather, in this calculation, we focused on those tracts that might be of relevance for spatial neglect in case of its disconnection, that is, the SLF, IOF, SOF, or UF. With the inclusion of every single voxel of the anatomical atlas, that is, even those voxels in which fiber bundle tissue had been found in only 1 out of the 10 postmortem brains, we revealed 10.9% of overlap. In reference to the 30% isocontours of the atlas, we found an overlap of 3.4%. This indicates that between 89.1% and 96.6% of the area, which was significantly more damaged in patients with spatial neglect than in controls, affected neural structures other than the long white matter association fibers SLF, IOF, SOF, and UF, namely predominantly the cortical and subcortical gray matter structures listed above (Fig. 1) as well as the primary motor and primary sensory fiber bundels CT and AR in the white matter.
Discussion
In order to assess the involvement of gray versus white matter tissue in spatial neglect, we conducted a reanalysis of the 140 patients sample described by Karnath et al. (2004). We found that only 36.9% of the territory significantly more affected in patients with spatial neglect than in controls overlapped with white matter tissue. In contrast, 63.1% of this territory affected cortical and subcortical gray matter brain structures. By using the 30% isocontours from the probabilistic cytoarchitectonic atlas provided by Bürgel et al. (2006), that is, the regions where in at least 3 brains from the 10 postmortem brains fiber tract tissue was found histologically, 7.0% of the SLF, 8.2% of the IOF, 12.7% of the SOF, and only 0.6% of the UF overlapped with the territory significantly more affected in patients with spatial neglect than in controls.
The SLF, SOF, and IOF belong to a densely connected perisylvian network in the human right hemisphere composed of the inferior parietal, dorsolateral frontal, superior temporal, and insular cortices (Schmahmann and Pandya 2006; Catani et al. 2007; Schmahmann et al. 2007; Gharabaghi A, Kunath F, Erb M, Grodd W, Tatagiba M, Karnath H-O, unpublished data). The SLF is the major cortical association fiber pathway linking parietal and frontal cortices in both humans and monkeys. It is subdivided into different components (Petrides and Pandya 1984; Makris et al. 2005; Schmahmann et al. 2007). Subcomponent II (SLF II) links the inferior parietal lobule and intraparietal sulcus with the posterior and caudal prefrontal cortex, whereas SLF III connects the rostral inferior parietal lobule with the ventral part of premotor and prefrontal cortex. A further fiber tract stems from the caudal part of the STG, arches around the caudal end of the Sylvian fissure, and extends to the lateral prefrontal cortex along with the SLF II fibers. This latter fiber tract is termed the arcuate fasciculus (AF) (Burdach 1819–1826; Dejerine and Dejerine-Klumpke 1895; Catani et al. 2002, 2005; Makris et al. 2005). Some authors, including those providing the probabilistic white matter fiber tract maps of the Jülich atlas (Bürgel et al. 2006), regarded the AF as a fourth subcomponent of the SLF. Fiber tract “SLF” of the Jülich atlas histologically encompasses subcomponents SLF I–III and the AF (K. Amunts, personal communication).
A fiber bundle situated in close proximity to the SLF II and the AF is the SOF. The SOF was the association fiber tract that was found to be affected most prominently in the present analysis. This long association bundle bidirectionally extends from the inferior parietal lobule and dorsomedial parastriate occipital cortex to caudal, dorsal, and medial frontal lobe areas (Catani et al. 2002; Bürgel et al. 2006; Makris et al. 2007; Schmahmann et al. 2007). Some of its fibers intermingle with SLF II and AF fibers. The IOF runs through the isthmus of the temporal lobe medial to the lower insula and connects ventrolateral frontal cortex with posterior temporal, inferior parietal, and occipital cortices (Nieuwenhuys et al. 1988; Catani et al. 2002; Bürgel et al. 2006).
By using intraoperative electrical stimulation and diffusion tensor imaging (DTI) tractography, Bartolomeo et al. (2007) suggested—in single cases—disconnection of the SLF (Thiebaut de Schotten et al. 2005) and of the IOF (Urbanski et al. 2008) to be associated with spatial neglect. Further, a DTI tractography analysis by He et al. (2007) revealed damage to the SLF and the AF in 5 patients with severe spatial neglect but not in 5 patients with mild neglect. In contrast to these studies, the present investigation used a different technique to investigate the question of whether or not white matter association fibers are predictive of acute spatial neglect. We used a probabilistic cytoarchitectonic atlas based on the histological findings in 10 adult postmortem brains. Further and most importantly, the present study used a large, representative sample of 140 right hemisphere stroke patients.
The majority of voxels where injury predicted spatial neglect compromised gray matter tissue (63.1%), with white matter only accounting for 36.9% of these voxels. However, we found that damage of right perisylvian white matter connections indeed is a typical finding in patients with spatial neglect. The SOF was more prominently affected than the SLF or IOF. However, we did not find a strong predictive relationship between white matter injury and spatial neglect. Overall, the portion of involvement of these fiber bundles was not very high.
Logistic regression analysis identified damage of the CT as the only significant white matter predictor of spatial neglect. Because disconnection of the CT has no known direct relevance for the presence of spatial neglect (but rather for the frequent additional presence of right-sided hemiparesis in these patients), the association of the CT to spatial neglect presumably reflects its proximity to neighboring cortical and subcortical areas. Logistic regression only revealed the IOF as a significant white matter predictor of spatial neglect (with the UF approaching significance) when the primary sensory and primary motor fiber tracts (AR, CT, OR) were excluded from the model. Finally, the percentage of overlap between the statistical lesion map and all the perisylvian network connections (histologically defined via the Jülich atlas) turned out to be small between 3.4% and 10.9%.
It is possible that the histological Jülich atlas (Bürgel et al. 2006) may slightly underestimate the true overlap between the statistical lesion map and the perisylvian white matter network connections. The authors of the atlas cautiously mentioned the possibility that because white matter tracts were mapped on the basis of sections in the coronal plane, fibers that proceed rostrocaudally may have been underestimated. On the other hand, the microscopic mapping used for constructing the Jülich atlas results in a more precise delineation of fiber tracts and enables mapping in some portions of fiber tracts that are beyond the resolution of alternative mapping tools, for example, DTI tractography (Bürgel et al. 2006).
Our findings are in contrast with those of Doricchi and Tomaiuolo (2003), who report that white matter injury strongly predicts spatial neglect. There are clear methodological differences as their population was less acute (mean poststroke interval of 121 days). Therefore, it is possible that white matter involvement may be a marker of those patients who have persistent symptoms. This could be directly studied by conducting an investigation with a large group of patients with chronic injury. The relatively small sample size in the study of Doricchi and Tomaiuolo (2003) makes statistical inference difficult. Although they report Fisher exacts tests that implicated the corticospinal tract, it is unclear how this test was conducted. Specifically, there is no suggestion of controlling for multiple comparisons, the precise region compared (e.g., how was this tract identified) or whether this region was selected post hoc based on the maximum overlap. A further striking difference between this finding and the present observations as well as other work by our group (Karnath et al. 2002) is the high incidence of damage to the putamen in Doricchi and Tomaiuolo's chronic control group who were not experiencing spatial neglect.
The present study cannot decide whether or not spatial neglect should best be interpreted as a “disconnection syndrome” (Watson et al. 1974, 1978, Mesulam and Geschwind 1978; Bartolomeo et al. 2007). Our analyses only demonstrate that the involvement of white matter fiber tracts in the typical lesion territory of patients with spatial neglect is rather small. The overlap between the statistical lesion map and all the perisylvian white matter network connections was between 3.4% and 10.9%. This indicates that between 89.1% and 96.6% of the lesion area affected brain structures other than the perisylvian white matter fiber tracts, namely cortical and subcortical gray matter structures and the primary motor and primary sensory fiber bundles CT and AR. The gray matter structures involved were the superior temporal, inferior parietal, inferior frontal, and insular cortices as well as subcortically the putamen and the caudate nucleus. Hillis et al. (2002) have observed that white matter lesions cause spatial neglect only if the subcortical damage provokes additional malperfusion of cortical gray matter structures in the ipsilesional, right hemisphere. In combination with the present findings, we conclude that damage of gray matter structures appears to be a strong predictor of spatial neglect.
Funding
Bundesministerium für Bildung und Forschung (BMBF-Verbundprojekt “Räumliche Orientierung” 01GW0641), the Deutsche Forschungsgemeinschaft (SFB 550-A4); National Institutes of Health (R01 NS054266); and European Union (PERACT—Marie Curie Early Stage Training MEST-CT-2004-504321).
Acknowledgments
Conflict of Interest: None declared.
References
- Amunts K, Zilles K. Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am. 2001;11:151–169. [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight R, Dronkers N. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Becker E, Karnath H-O. Incidence of visual extinction after left versus right hemispheric stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Bürgel U, Amunts K, Hoemke L, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Burdach KF. Vom Baue und Leben des Gehirns. Leipzig (Germany): Dyk; 1819–1826. [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam M-M, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. [DOI] [PubMed] [Google Scholar]
- Dejerine J, Dejerine-Klumpke AM. Anatomie Des Centres Nerveux. Paris: Rueff et Cie; 1895. [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14:2239–2243. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Fruhmann Berger M, Proß RD, Ilg UJ, Karnath H-O. Deviation of eyes and head in acute cerebral stroke. BMC Neurol. 2006;6:23. doi: 10.1186/1471-2377-6-23. Corrigendum 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits E, Degoankar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Ahonen JP, Dastidar P, Koivisto AM, Laippala P, Vilkki J, Molnár J. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand. 2000;101:195–201. doi: 10.1034/j.1600-0404.2000.101003195.x. [DOI] [PubMed] [Google Scholar]
- Johannsen L, Karnath H-O. How efficient is a simple copying task to diagnose spatial neglect in its chronic phase. J Clin Exp Neuropsychol. 2004;26:251–256. doi: 10.1076/jcen.26.2.251.28085. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Fruhmann Berger M, Küker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion–symptom mapping. J Cogn Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN. The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. Neuroimage. 2007;37:1100–1111. doi: 10.1016/j.neuroimage.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Geschwind N. On the possible role of neocortex and its limbic connections in the process of attention and schizophrenia: clinical cases of inattention in man and experimental anatomy in monkey. J Psychiatr Res. 1978;14:249–259. doi: 10.1016/0022-3956(78)90027-4. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual nelgect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. Berlin (Germany): Springer; 1988. [Google Scholar]
- Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Hemineglect in acute stroke-incidence and prognostic implications: the Copenhagen Stroke Study. Am J Phys Med Rehabil. 1997;76:122–127. doi: 10.1097/00002060-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tham K, Tegnér R. The baking tray task: a test of spatial neglect. Neuropsychol Rehabil. 1996;6:19–25. doi: 10.1080/713755496. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touzé E, Chokron S, Méder J-F, Lévy R, Dubois B, et al. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2008;79:598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Heilman KM, Miller BD, King FA. Neglect after mesencephalic reticular formation lesions. Neurology. 1974;24:294–298. doi: 10.1212/wnl.24.3.294. [DOI] [PubMed] [Google Scholar]
- Watson RT, Miller BD, Heilman KM. Nonsensory neglect. Ann Neurol. 1978;3:505–508. doi: 10.1002/ana.410030609. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam M-M. Mental state assessment of young and elderly adults in behavioral neurology. In: Mesulam M-M, editor. Principles of behavioral neurology. Philadelphia (PA): FA Davis Company; 1985. pp. 71–123. [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptor architecture of the human brain. In: Mazziotta JC, Toga A, editors. Brain mapping: the methods. Amsterdam (The Netherlands): Elsevier; 2002. pp. 573–602. [Google Scholar]