Abstract
Chronic stress exposure has been reported to induce dendritic remodeling in several brain regions, but it is not known whether individual neural circuits show distinct patterns of remodeling. The current study tested the hypothesis that the projections from the infralimbic (IL) area of the medial prefrontal cortex (mPFC) to the basolateral nucleus of the amygdala (BLA), a pathway relevant to stress-related mental illnesses like depression and post-traumatic stress disorder, would have a unique pattern of remodeling in response to chronic stress. The retrograde tracer FastBlue was injected into male rats’ BLA or entorhinal cortex (EC) 1 week prior to 10 days of immobilization stress. After cessation of stress, FastBlue-labeled and unlabeled IL pyaramidal neurons were loaded with fluorescent dye Lucifer Yellow to visualize dendritic arborization and spine density. As has been previously reported, randomly selected (non-FastBlue-labeled) neurons showed stress-induced dendritic retraction in apical dendrites, an effect also seen in EC-projecting neurons. In contrast, BLA-projecting neurons showed no remodeling with stress, suggesting that this pathway may be particularly resilient against the effects of stress. No neurons showed stress-related changes in spine density, contrasting with reports that more dorsal areas of the mPFC show stress-induced decreases in spine density. Such region- and circuit-specificity in response to stress could contribute to the development of stress-related mental illnesses.
Keywords: amygdala, chronic stress, connectivity, infralimbic cortex, neural plasticity, spine density
Introduction
Exposure to stress can precipitate or exacerbate many mental illnesses, most notably major depressive disorder (MDD; Lechin et al. 1996) and post-traumatic stress disorder (PTSD; Turner and Lloyd 2004). Abnormal activity in the medial prefrontal cortex (mPFC) and amygdala is commonly observed in these disorders, such that mPFC activity is suppressed (Liberzon and Phan 2003; Pizzagalli et al. 2004), whereas amygdala activity is increased (Drevets 2003). Moreover, volumetric studies reveal reduced glia and neuron density in the mPFC of suicide victims’ brains (Rajkowska et al. 2001), as well as marked morphological changes in the amygdala of MDD patients (Bouras et al. 2001; Frodl et al. 2003). These structures maintain reciprocal connectivity, comprising a network that governs learning, memory and behavior based on emotionally salient information (Sotres-Bayon et al. 2004). Whereas the amygdala is most commonly recognized for its role in mediating the processing, memory of, and response to, negative stimuli (Hendler et al. 2003; Wright et al. 2003), the mPFC is known to control the inhibition of inappropriate behaviors and thoughts (reviewed in Arnsten 2000), and has been implicated in mediating the suppression of sadness (Levesque et al. 2003). Common symptoms of MDD and PTSD, such as prolonged negative affect, rumination and poor concentration, are consistent with a release of mPFC inhibition of the amygdala.
This relationship has been demonstrated in animal models as well. Although the mPFC sends excitatory projections to the amygdala, in vivo electrical stimulation of the infralimbic (IL) region of the rat mPFC has been shown to result in suppressed amygdala output, likely effected through this afferent's excitation of either gamma-aminobutric acidergic (GABAergic) interneurons in the basolateral nucleus (BLA) or the inhibitory intercalated cells that connect the BLA to the central nucleus of the amygdala (CEA; reviewed in Sotres-Bayon et al. 2004), also see Likhtik et al. 2005). Accordingly, mPFC stimulation results in reduced amygdala response to a conditioned stimulus (Quirk et al. 2003), and stimulation of IL in particular enhances both extinction of conditioned fear and extinction recall (Vidal-Gonzalez et al. 2006). Furthermore, animals with IL lesions demonstrate impaired extinction recall (Lebron et al. 2004). Taken together, these studies identify the mPFC as a powerful regulator of amygdala function. However, the inhibitory nature of this pathway allows for substantial dysfunction in the event that the integrity of this circuit becomes compromised, as with exposure to stress.
Recent work has shown that chronic exposure of rats to stress has robust effects on the branching patterns of apical dendrites in mPFC and amygdala pyramidal cells, causing dendritic retraction in the anterior cingulate (AC), prelimbic (PL), and IL regions of the mPFC (Radley et al. 2004; Izquierdo et al. 2006), but hypertrophy in the amygdala (Vyas et al. 2003). However, this work was performed using randomly chosen neurons, and the effects of stress on the specific neurons that connect the mPFC and BLA are unknown.
The current study tested the hypothesis that neurons in the IL region of the mPFC that project to the BLA would undergo a unique pattern of stress-induced morphological changes when compared with that of randomly chosen neurons or neurons projecting to other cortical areas. We report that with respect to measures of dendritic length and spine density, this pathway appears to be particularly resilient against the effects of chronic stress.
Methods
Animals
Adult Sprague–Dawley rats (males: n = 48), weighing 200–250 g were used in this study. Animals were housed at the Rockefeller University Laboratory Research Center, in a normal 14:10 light:dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rockefeller University and Mount Sinai School of Medicine Institutional Animal Care and Use Committees.
Retrograde Labeling
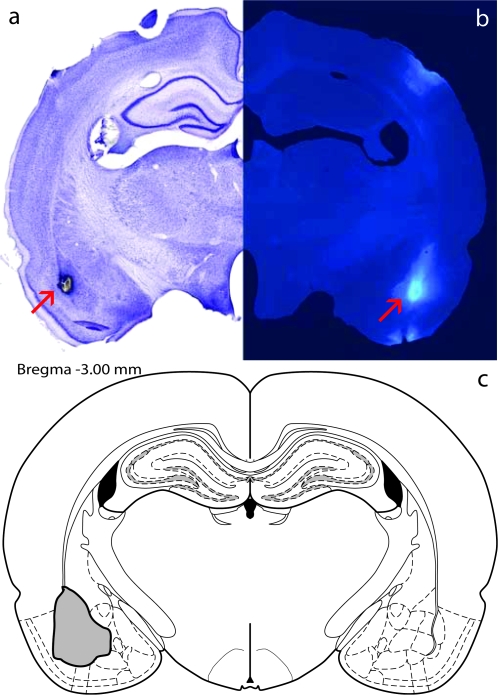
Animals were anesthetized using ketamine (90 mg/kg) and xylazine (4 mg/kg). Each rat was secured in a stereotaxic apparatus, the skull exposed, and bilateral holes drilled above the BLA (AP −3 mm, ML ±5.0 mm, DV −8.0 mm; Paxinos and Watson 1997) or entorhinal cortex (EC: AP −5.8 mm, ML ±6.6 mm, DV −8.0 mm) as a control for possible confounding effects of the tracer itself. A solution of the retrograde tracer Fast Blue (Sigma Aldrich, St Louis, MO; 5%, 200 nL) was deposited through pressure injection using a 1-μl Hamilton microsyringe held by a stereotaxic attachment. The injection was done at a speed of ∼20 nL/min, and the apparatus was left undisturbed for 10 min before syringe removal, to allow for diffusion. The incision was sealed using VetBond, and all animals were given an intramuscular Buprenex injection following surgery and monitored for healthy behavior for several days after surgery. Animals were allowed to recover for 7 days before beginning the stress regimen. Representative syringe placement and Fast Blue diffusion are shown in Figure 1.
Figure 1.
FastBlue injection. Representative syringe placement for retrograde tracer FastBlue injections in the BLA (a), representative Fastblue diffusion into the BLA (b), and schematic of maximal observed diffusion into the BLA (c). Adapted from Paxinos and Watson (2005).
Stress Exposure and Sacrifice
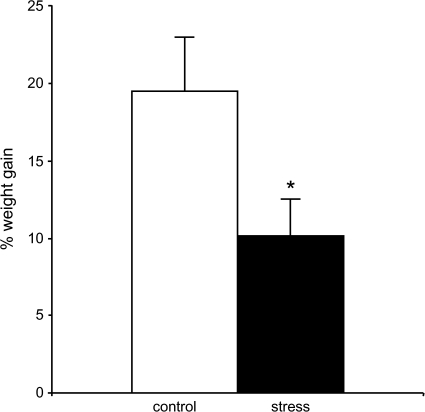
The rats in each “stress” group were placed in plastic rodent immobilization bags for 2 h/day for 10 days, as described in (Vyas et al. 2002), and then returned to their home cages, whereas control animals remained in their home cages in a separate room. This regimen has been shown to produce dendritic remodeling in the hippocampus and amygdala comparable to 21 days of chronic restraint stress (Vyas et al. 2002). Moreover, stressed animals showed significantly less weight gain than controls (P < 0.04), as has been seen with 21 days restraint stress (Fig. 2). Twenty-four hours after cessation of the final stressor, animals were deeply anesthetized with 30% chloral hydrate and perfused transcardially with 1% paraformaldehyde phosphate buffer (pH 7.4), followed by 4% paraformaldehyde and 0.125% glutaraldehyde in phosphate buffer.
Figure 2.
Ten days immobilization stress causes decreased weight gain. Stressed animals gained significantly less weight than control animals over the course of the study. *P < 0.04.
Tissue Preparation, Cell Loading, Reconstruction, and Analysis
After perfusion, the brains were removed and the IL region of the mPFC was dissected (2.0–3.5 mm rostral from Bregma), postfixed, and serial 250-μm-thick sections were collected using a Vibratome (Leica, Vienna, Austria). Sections were mounted on nitrocellulose filter paper and submerged in phosphate buffer.
In order to replicate previous findings and as a way to compare the effects of stress in the IL-BLA pathway to its effects in other IL neurons, brains from each treatment group were assigned to 1 of 3 groups: Random neurons—from brains in which only non-Fast Blue-labeled neurons were loaded; and BLA- or EC-projecting neurons—from brains in which only Fast Blue-labeled neurons were loaded. Random neurons were taken equally from animals who had been infused in the BLA and those who had been infused in the EC. After animals were removed for inaccurate infusion placement and neurons that did not meet the criteria for inclusion in analysis (see below) were removed, the number of cells per group was as follows: random control, n = 21; random stress, n = 20; BLA-projecting control, n = 18; BLA-projecting stress, n = 19; EC-projecting control, n = 17; EC projecting, n = 18.
Fast Blue-labeled neurons were identified using epifluorescence with a UV filter. Both Fast Blue-labeled neurons and “random” neurons were loaded with iontophoretic injections of 5% Lucifer Yellow (Molecular Probes, Eugene, OR), using a DC current of 1–6 nA for 5–10 min, until distal processes are filled with dye and no further loading can be observed. Sections were then mounted and coverslipped in PermaFluor (Thermo Fisher Scientific Inc., Waltham, MA). Neurons were traced and 3-dimensionally reconstructed at 400× using a Zeiss Axiophot 2 microscope equipped with a motorized stage, video camera system and Neurolucida morphometry software (MBF Biosciences, Williston, VT). Criteria for a neuron to be included in analysis were as follows: 1) it must be within layer II/III of the IL region; 2) its dendritic tree must be completely filled; and 3) it must have intact primary to tertiary branches. 3D Sholl analyses were performed on each neuron with NeuroExplorer software (MBF Biosciences). Results were expressed in terms of total apical dendritic length, total branch number on the apical dendrite, and apical dendritic length per radial distance from the soma, in 30-μm increments (Radley et al. 2004) (Sholl 1953). Although FB-labeled neurons in layer V were also observed, only layer II/III neurons were loaded and analyzed, so as to remain consistent with previous studies (Radley et al. 2004).
The method for sampling apical dendritic branches for spine density (i.e., spines per μm dendritic length) was performed as described in (Radley, Rocher, et al. 2006).The selection of a particular branch for optical imaging had to satisfy the following criteria: 1) the entire segment had to fall within a depth of 50 μm, owing to the working distance of the lens; 2) they had to be either parallel or at acute angles to the coronal surface of the section; and 3) they did not show overlap with other branches that would obscure visualization of spines. Segments were selected with a systematic random design at 50, 100, 150, and 200 μm from the soma for digital reconstruction. In total, ∼600 reconstructed dendritic segments were analyzed (∼8 segments/neuron). Dendritic segment and spine reconstructions were performed using a Zeiss 410 confocal laser scanning microscope using a 488-nm excitation wavelength, at a magnification of 100× and a zoom of 5. After gain and offset settings were optimized, segments were digitally reconstructed at 0.1-μm increments, throughout the entire z-axis of the branch. The digitized optical stacks were then deconvolved with AutoDeblur (AutoQuant, Troy, NY) and analyzed for spine number and length using Neuron Studio software (Radley et al. 2008), after which they were manually verified. Values for each branch segment were expressed as spine number/μm. The average dendritic segment was ∼30 μm in length.
Statistical Analysis
Statistical analyses were performed using 2-tailed Student's t-tests to assess possible differences in dendritic length and spine density between stress and control groups for randomly selected, BLA-projecting and EC-projecting neuron sets. The values shown in Figures 2–4 are means ± SEM, calculated from one aggregate (e.g., average) per animal, with variations in the number of neurons per animal taken into consideration. A mixed model for repeated measures ANOVA with Bonferroni post hoc was used to test the effect of stress on dendritic length across the apical dendritic trees (Sholl analysis). The statistical significance level was set at α = 0.05.
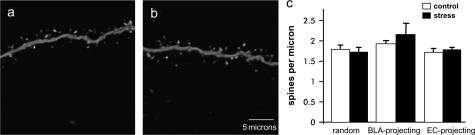
Figure 4.
Stress does not alter spine density in the IL. Representative randomly selected neuron apical dendrite segments from control (a) and stressed (b) animals. Neither randomly selected neurons nor BLA-projecting nor EC-projecting neurons showed any significant change in spine density with stress.
Results
Stress Causes Dendritic Remodeling in Randomly Selected and EC-Projecting Neurons, but not BLA-Projecting Neurons
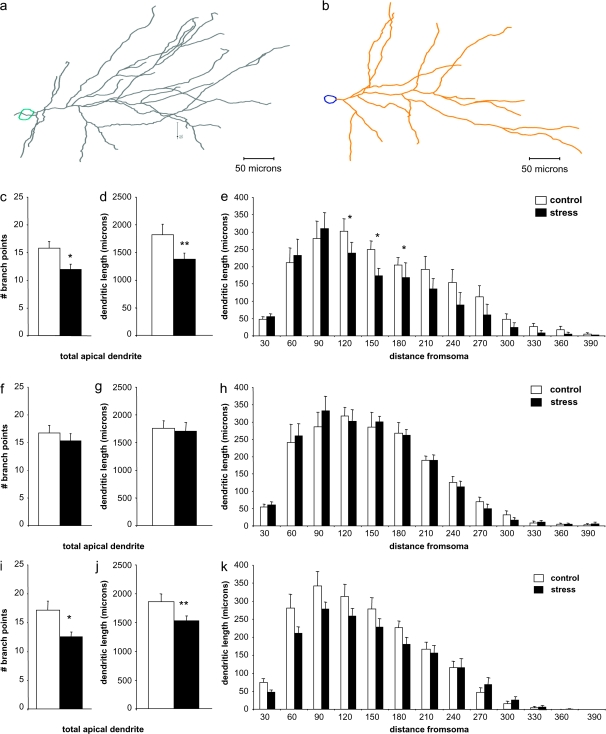
Representative Neurolucida tracings of randomly selected IL neurons are shown from control and stressed groups in Figure 3a,b, respectively. Stressed rats showed dendritic retraction in randomly selected IL neurons, with approximately 25% fewer branch points in stressed rats (15.3 ± 1.2 vs. 11.6 ± 0.9, P = 0.01; Fig. 3c), and total apical dendrite length approximately 23% less in stressed rats than in control rats (1842.9 ± 118.8 vs. 1413.5 ± 100.8 μm, P < 0.01; Fig. 3d). For the Sholl analysis, a 2-way ANOVA with repeated measures revealed significant overall effects of stress (F1,29 = 16.94, P < 0.0001) and distance from soma (F1,29 = 47.12, P < 0.0001), but no overall interaction. However, Bonferroni post hoc tests revealed that this change was due to retraction at intermediate dendritic levels, between 120 and 180 μm from the soma (P = 0.04, 0.01, and 0.02 for the 120-, 150-, and 180-μm intersection points, respectively; Fig. 3e). In contrast, neurons projecting to the BLA showed no stress-related differences in branch point number (16.7 ± 1.4 vs. 15.3 ± 1.3, P = 0.46; Fig. 3f) or overall dendritic length (1748.8 ± 97.2 vs. 1703.2 ± 106.9 μm, P = 0.42; Fig. 3g), nor were there any differences in dendritic length at any distance from the soma (Fig. 3h). EC-projecting neurons showed an almost 27% reduction in branch points in stressed rats (16.8 ± 1.6 vs. 12.3 ± 0.8, P = 0.01, Fig. 3i), and approximately 18% reduction in total apical dendritic length (1846.8 ± 136.4 vs. 1520.7 ± 80.7, P < 0.04; Fig. 3j). Sholl analysis did not reveal significant localization of remodeling in these neurons, but there appeared to be a trend toward more marked retraction in proximal dendrites (Fig. 3k).
Figure 3.
Randomly selected neurons and EC-projecting neurons, but not BLA-projecting neurons, show retraction with stress. Representative randomly selected neuron apical dendrite Neurolucida tracings from control (a) and stressed (b) animals. In rats exposed to stress, apical dendrites in randomly selected IL layer II/III pyramidal neurons show reduced number of branch points (c) and overall dendritic length (d) when compared with control rats. Sholl analysis suggests that these changes are accounted for by retraction in intermediate dendrites, approximately 120–180 μm from the soma (e). Apical dendrites in amygdala-projecting IL layer II/III pyramidal neurons show no change in branch points (f) or in overall dendritic length (g) when compared with control rats. Moreover, there were no group differences at any distance from the soma (h). Apical dendrites in EC-projecting layer II/III neurons showed significantly reduced number of branch points (i) and overall dendritic length (j), but no significant changes at individual distances from the soma (k). *P < 0.05; **P < 0.01.
Stress Does Not Induce Changes in Spine Density in IL Neurons
Representative dendrite segment images from randomly selected neuron control and stress groups are shown in Figure 4a,b, respectively. In contrast to reports of the AC and PL regions of the mPFC in which 16% reduction in spine densities have been reported (Radley, Rocher, et al. 2006), no significant changes in IL apical dendrite spine density were observed in stressed rats in randomly selected, BLA-projecting or EC-projecting neurons (Fig. 4c).
Discussion
The current study is the first report of circuit-specific effects of stress on neuronal morphology, demonstrating that neurons may respond differently to stress according to their efferent target. It was found that while randomly selected IL pyramidal neurons, which likely primarily target other cortical areas (Takagishi and Chiba 1991), and neurons that project to the EC showed stress-induced retraction of the apical dendritic arbor, IL neurons that project to the BLA did not show apical dendritic remodeling with stress, suggesting that this pathway is particularly resilient against the effects of stress.
Research to date suggests that the effects of repeated stress on neuronal morphology in the brain are region-specific; whereas repeated stress can cause dendritic retraction in the AC, PL (Radley et al. 2004), IL (Wellman 2001), and hippocampus (McEwen 2001), it has been shown to cause dendritic expansion in the orbitofrontal cortex (Liston et al. 2006), and amygdala (Vyas et al. 2003). Our data extend such region-specific findings to the level of circuit specificity within a single region. This seemingly “negative” finding is likely not an artifact of the neurons containing Fast Blue, since FastBlue-labeled IL neurons that project to the EC show stress-related remodeling similar to that of randomly selected neurons.
This is also the first report of the effects of stress on spine density in the IL. In contrast to studies of other mPFC regions that have demonstrated stress-induced spine loss (Seib and Wellman 2003; Radley, Rocher, et al. 2006), no stress-induced changes in spine density were observed in random, BLA-projecting or EC-projecting neurons. The possibility remains that like those in the PL and AC, spines in the IL may undergo stress-induced changes in shape (Radley et al. 2008); this question will be addressed in future studies. Still, the disparity between the effects of stress in the IL and in the PL is consistent with a growing body of literature suggesting that the IL and PL are functionally distinct. First, there is anatomical evidence indicating that the IL and PL make discrete connections with other brain regions (Vertes 2004). Additionally, it has been demonstrated that the IL and PL have diverging effects on an animal's expression of extinguished conditioned fear; although microstimulation of the IL promotes extinction-like behavior, the same stimulation of PL can increase fear expression (Vidal-Gonzalez et al. 2006). Finally, it has also been found that ablation of either the PL or IL produces opposite effects on stress-induced activation of the paraventricular hypothalamic nucleus (Radley, Arias, et al. 2006), suggesting that these 2 regions may play distinct roles in modulating the stress response. Whether the effects of stress on PL neurons are circuit-specific as in the IL will be the subject of future studies.
Functional Implications of Circuit-Specific Stress Effects
As described above, the IL–BLA connections are well-defined in the rat brain, with substantial excitatory projections from the IL synapsing in the BLA, although there is some debate as to whether these afferents primarily target GABAergic cells in the BLA that project to the CEA, or target the inhibitory intercalated cells between the BLA and CEA (Sotres-Bayon et al. 2004). Regardless of the target, the effect on amygdala is the same and results in an inhibition of CEA output (Quirk et al. 2003; Rosenkranz et al. 2003). Behaviorally, activity in this pathway is most commonly associated with recall of conditioned fear extinction. This process involves the suppression of a fear response to a stimulus that has previously been learned to be associated with an aversive event, and then extinguished through repeated presentation of the stimulus without the aversive event. IL stimulation enhances this behavior (Vidal-Gonzalez et al. 2006), whereas IL lesioning attenuates it (Lebron et al. 2004). The IL does not appear to mediate the acquisition or extinction of conditioned fear, suggesting a very specific role in the maintenance and regulation of appropriate learned emotional responses. The effects of stress on this function have not been thoroughly explored, although it has been reported that exposure to chronic stress can impair the recall of extinction (Miracle et al. 2006). In light of the present data, however, this would appear to be due to effects on the IL–BLA pathway other than general dendritic remodeling.
Given its role and apparent dysfunction in stress-related mental illnesses, that the IL–BLA pathway would be especially resilient against, rather than vulnerable to, the effects of stress is somewhat surprising. As the stress regimen used here reliably induced dendritic remodeling in randomly selected and EC-projecting neurons and caused an attenuation of weight gain commonly seen in chronically stressed rats, it is unlikely that the rats’ experience simply was not “stressful” enough to cause dendritic remodeling in this subset of neurons. In humans, although MDD is among the most common of mental illnesses and is highly correlated with exposure to stress (Turner and Lloyd 2004), most people experience many stressful events without developing a stress-related disorder. It is commonly thought that the transition from “normal” effects of stress to clinical pathologies relies on an interaction between environmental and genetic factors. Thus, the mPFC-amygdala pathway may be, in most individuals, especially protected from the detrimental effects of stress, but vulnerable to stress when predisposing factors are present. Another intriguing possibility is that if BLA-projecting neurons are selectively spared from stress-induced remodeling, this pathway may then be primed for increased input, and possible overstimulation, which could lead to dysfunction. This hypothesis will be investigated in future studies.
Funding
National Institutes of Health grants (MH58911) to J.H.M., P.R.H., and B.S.M., and (MH075506) to R.M.S.
Acknowledgments
We would like to thank William Janssen, Deena Goldwater, Bridget Wicinski, Melinda Miller, and Gwendolyn Wood for contributing their knowledge and technical assistance. Conflict of Interest: None declared.
References
- Arnsten AFT. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Lechin F, Van der Dijs B, Benaim M. Stress versus depression. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:899–950. doi: 10.1016/0278-5846(96)00075-9. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1997. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2005. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Sholl D. Dendritic organization of the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]