Abstract
Background:
Both hereditary and sporadic forms of chronic pancreatitis are associated with an increased risk of developing pancreatic ductal adenocarcinoma (PDA). Inflammation has been identified as a significant factor in the development of solid tumour malignancies. We have recently shown that thymoquinone (Tq), the major constituent of Nigella sativa oil extract, induced apoptosis and inhibited proliferation in PDA cells. Tq also increased p21 WAF1 expression, inhibited histone deacetylase (HDAC) activity, and induced histone hyperacetylation. HDAC inhibitors have been shown to ameliorate inflammation-associated cancer. In this study, we evaluated the anti-inflammatory potential of Tq in PDA cells in comparison with that of a specific HDAC inhibitor, trichostatin A (TSA).
Methods:
PDA cells were treated with or without Tq (25–75 µM), with or without pre-treatment of tumour necrosis factor (TNF)-α (25 ng/ml). The effect of Tq on the expression of different proinflammatory cytokines and chemokines was analysed by real-time polymerase chain reaction (PCR). Luciferase-labelled promoter studies evaluated the effect of Tq on the transcription of monocyte chemoattractant protein-1 (MCP-1) and nuclear factor-κB (NF-κB). The effect of Tq on the constitutive and TNF-α-induced activation and nuclear translocation of NF-κB was examined by ELISA and immunohistochemistry.
Results:
Tq dose- and time-dependently significantly reduced PDA cell synthesis of MCP-1, TNF-α, interleukin (IL)-1β and Cox-2. At 24 h, Tq almost completely abolished the expression of these cytokines, whereas TSA had a less dramatic effect. Tq, but not TSA, significantly and dose-dependently reduced the intrinsic activity of the MCP-1 promoter. Tq also inhibited the constitutive and TNF-α-mediated activation of NF-κB in PDA cells and reduced the transport of NF-κB from the cytosol to the nucleus.
Conclusions:
Our data demonstrate previously undescribed anti-inflammatory activities of Tq in PDA cells, which are paralleled by inhibition of NF-κB. Tq as a novel inhibitor of proinflammatory pathways provides a promising strategy that combines anti-inflammatory and proapoptotic modes of action.
Keywords: pancreatic cancer, inflammation, Nigella sative, thymoquinone
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the most lethal cancers of the gastrointestinal tract, with a death to incidence ratio of 0.99.1 The poor prognosis of PDA is attributable to its tendency for late presentation, aggressive local invasion, early metastasis and resistance to chemotherapy.2 The only curative treatment is surgical resection; however, only 10–15% of patients will have localized disease at presentation.3 Retrospective studies show a rising incidence of pancreatic tumours and mortalities.4–7 This, inaddition to its poor prognosis, stresses the need to elucidate the mechanisms underlying pancreatic carcinogenesis and to find new treatments.
Several studies have observed an increased incidence of PDA in patients with chronic pancreatitis,8–11 and all inflammatory cytokines in chronic pancreatitis have been linked to pancreatic carcinogenesis. Familial chronic pancreatitis, which accounts for <1% of all cases of pancreatitis, is also associated with increased risk of PDA,12,13 especially with longer duration of the disease process.14 In human PDA and in animal models that recapitulate the disease progression, an intense fibro-inflammatory reaction composed of stromal and immune cells accompanies the progression from normal histology to PDA.15–17
Studies on mouse models of inflammation-associated cancer have implicated the transcriptional factor nuclear factor-κB (NF-κB) and the proinflammatory mediator tumour necrosis factor-α (TNF-α) in cancer progression.18,19 The NF-κB pathway may have dual actions in tumour promotion, firstly, by preventing the death of cells with malignant potential, and, secondly, by stimulating the production of proinflammatory cytokines in inflammatory cells in the tumour mass.18 Constitutive activation of NF-κB has been observed in a number of different PDA cell lines,20,21 but not in non-tumorigenic pancreatic epithelial cells.22 Activation of NF-κB has also been observed in animal models of pancreatic cancer21 and in human pancreatic tissue.22 NF-κB activation may also contribute to the characteristic resistance of pancreatic tumour cells to the apoptotic effect of chemotherapeutic agents.23,24 Thus, inhibiting the activation of NF-κB could be a useful adjunct in the medical management of the disease.
Herbal therapies are commonly used for the prevention and treatment of cancer, although we have little understanding of their molecular and cellular bases of action. Nigella sativa, or the black cumin seed, is a herb used in traditional medicine in many Middle Eastern and Asian countries to treat a broad array of diseases. Thymoquinone (Tq) (2-isopropyl-5-methyl-1,4-benzoquinone), the most abundant constituent of the seed oil extract, has been shown to have anti-neoplastic activities in different types of cancer.25–28 Tq also has cytoprotective effects that are mainly mediated through its antioxidant and anti-inflammatory activities.29 Despite Tq's interesting potential as an anti-neoplastic agent, its anti-inflammatory effects in PDA cells have not been evaluated.
We have recently shown that Tq reduces PDA cell growth and promotes apoptosis through increasing the Bax-Bcl-2 ratio, activating capase-3, and inhibiting histone deacetylase (HDAC) activity and transcription.30 In this study, we evaluated the anti-inflammatory potential of Tq in PDA cells in comparison with a specific HDAC inhibitor, trichostatin A (TSA).
Materials and methods
Cell culture and treatments
HS766T PDA cells, kindly provided by Dr Scott Kern, Johns Hopkins University School of Medicine, Baltimore, MD, USA, were used for all the experiments. MiaPaca and AspC-1 cells, which were purchased from the American Type Culture Collection (Manassas, VA, USA), were used in some experiments to confirm the results. Cells were cultured at 1 × 104 to near confluence in six-well plates and maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal bovine serum in a humid atmosphere of 5% CO2 : 95% air. A purified preparation of Tq (Fisher) was dissolved in methanol and serial concentrations were prepared. Controls were treated with vehicle only. For all experiments, cell lines were treated with or without Tq (25–75 µM) or the HDAC inhibitor, TSA (300 µM). In some experiments, TNF-α (25 ng/ml) was added 30 min before adding Tq or TSA, to elicit inflammation. Tq and TSA were obtained from Sigma-Aldrich, Inc. (St Louis, MO, USA) and TNF-α was purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
RNA extraction and real-time reverse transcription polymerase chain reaction
Total RNA was isolated using Trizol reagent (Life Technologies, Inc., Gaithersburg, MD, USA). RNAs were quantified and input amounts were optimized for each amplicon. Primers and probes were designed with the help of Primer Express software (Applied Biosystems, Inc., Foster City, CA, USA). Primers and probes were selected for the following cytokines and chemokines: TNF-α; interleukin-β (IL-1β); cyclooxiygenase-2 (Cox-2); IL-8, and monocyte chemoattractant protein-1 (MCP-1). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as the housekeeping gene. cDNA was prepared, diluted and subjected to real-time polymerase chain reaction (PCR) using TaqMan technology (7500 Sequence Detector; Applied Biosystems, Inc.). Probes were labelled with a reporter and a quencher. Each sample was analysed in at least two independent assays with duplicate samples and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The relative mRNA levels were presented as unit values of 2∧[CT (GAPDH)–CT (gene of interest)], where CT is the threshold cycle value defined as the fractional cycle number at which the target fluorescent signal passes a fixed threshold above baseline.
Transient transfection and promoter studies
To evaluate the effect of Tq on MCP-1 and NF-κB transcription, we used the MCP-1 gene promoter (GenBank™ accession no. AF079313) and NF-κB gene promoter (GenBank™ accession no. in progress) in luciferase expression vector pGL3 basic (Promega, Madison, WI, USA), kindly provided by Dr Decio Ezirik, Free University of Brussels, Brussels, Belgium.31 HS766T cells were seeded into 24-well culture plates (105). At ∼80% confluence they were co-transfected by TransFast reagent (Promega) and 0.5 µg of pGL3 vectors containing the luciferase-labelled MCP-1 or NF-κB promoters and 0.1 µg of green fluorescence protein (GFP) as transfection control. Two hours later, serum-containing medium was overlaid and the cells were incubated for an additional 24 h. The cells were then incubated with serum-free medium for 18 h, after which Tq (25–75 µM) was added for 3 h. In some experiments, cells were pre-treated with TNF-α (25 ng/ml) for 30 min before adding Tq or TSA. Luciferase activities were assayed with the Dual-Luciferase Reporter Assay System (Promega Corp.) in a Veritas Microplate Luminometer (Turner Designs, Sunnyvale, CA, USA). Transfection efficiency was normalized using the total protein concentration of the cell lysates. The results for Tq-treated cells were expressed as a fold-induction of the luciferase activity of the same construct in control condition, taking a control (no treatment) value as 1.
NF-κB activation assay
NF-κB activation was analysed using quantification of nuclear translocation with the NF-κB/p65 ActivELISA kit to detect the active form of the p65 subunit (Imgenex Corp, San Diego, CA, USA). Briefly, whole-cell extracts were prepared from 5 × 105 HS766T cells that were subjected to TNF-α (25 ng/ml) for 30 min, followed by a 1-h incubation with Tq (75 µM) or TSA (300 µM). Cytoplasmic and nuclear extracts were prepared according to the manufacturer's instructions. Briefly, the cytoplasmic fraction was collected in the supernatant of the whole-cell lysates after centrifugation for 30 s at 28 487 gin a cold microcentrifuge. Nuclear fraction was collected in the supernatant of the nuclear pellet after its resuspension in 100 µl nuclear lysis buffer, incubation at 4 °C for 30 min, and centrifugation in a microcentrifuge at 28 487 g for 10 min at 4 °C. Nuclear fractions were subjected to ELISA (enzyme-linked immunosorbent assay) using specific, anti-NF-κB antibodies, according to the manufacturer's instructions. Absorbance was read at a 405-nm wavelength using a Synergy HT multi-detection microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Immunohistochemical staining of PDA cells
HS766T cells were grown in chamber slides (lab-tech, NUNC; Thermo Fisher Scientific, Inc., Rochester, NY, USA). To study the localization of the p65 NF-κB subunit, cells were treated with TNF-α (25 ng/ml) for 30 min before adding Tq (75 µM) or TSA (300 µM) for 1 h, after which they were washed with Hanks balanced salt solution, pH 7.4, at 37 °C after aspiration of the culture medium. Cells were then fixed in 4% formaldehyde in phosphate buffered saline (PBS) at room temperature for 10 min before being treated with 0.1% Triton X-100 for 30 s to permeabilize nuclear membranes. After blocking non-specific reaction with normal donkey serum, the cells were incubated overnight with anti-rabbit p65 IgG (400 ng/ml) at 4 °C. Cells were then washed three times for 5 min each with PBS and incubated for 30 min at room temperature with biotinylated goat anti-rabbit IgG, diluted 1 : 200 in PBS (Vector Laboratories, Inc., Burlingame, CA, USA), as the secondary antibody. 3,3′-diaminobenzidine tetrahydrochloride chromogenic substrate (Vector Laboratories, Inc.) was used according to the manufacturer's protocol to visualize the chromogenic reaction. Cells were rinsed three times for 5 min each with PBS, counterstained with haematoxylin, mounted on glass slides and viewed by light microscopy.
Statistical analysis
All experiments were performed between four and six times. Data were analysed for statistical significance by anova with post-hoc Student's t-test analysis. Data are presented as mean ± standard error of the mean (SEM). Continuous normally distributed variables were analysed by Student's t-test. These analyses were performed with the assistance of a computer program (jmp 5; SAS Software Corp., Cary, NC, USA). Differences were considered significant at P≤ 0.05.
Results
Tq dose-dependently reduces the expression of TNF-α and MCP-1
Real-time reverse transcriptase (RT)-PCR was used to analyse the effect of different doses of Tq (25–75 µM) on expression levels of TNF-α and MCP-1 mRNA in HS766T cells. Cells were treated with Tq for 6 h. As Fig. 1A shows, Tq significantly reduced the expression in both genes (P < 0.05 at 50 µM and P < 0.02 at 75 µM, respectively).
Figure 1.
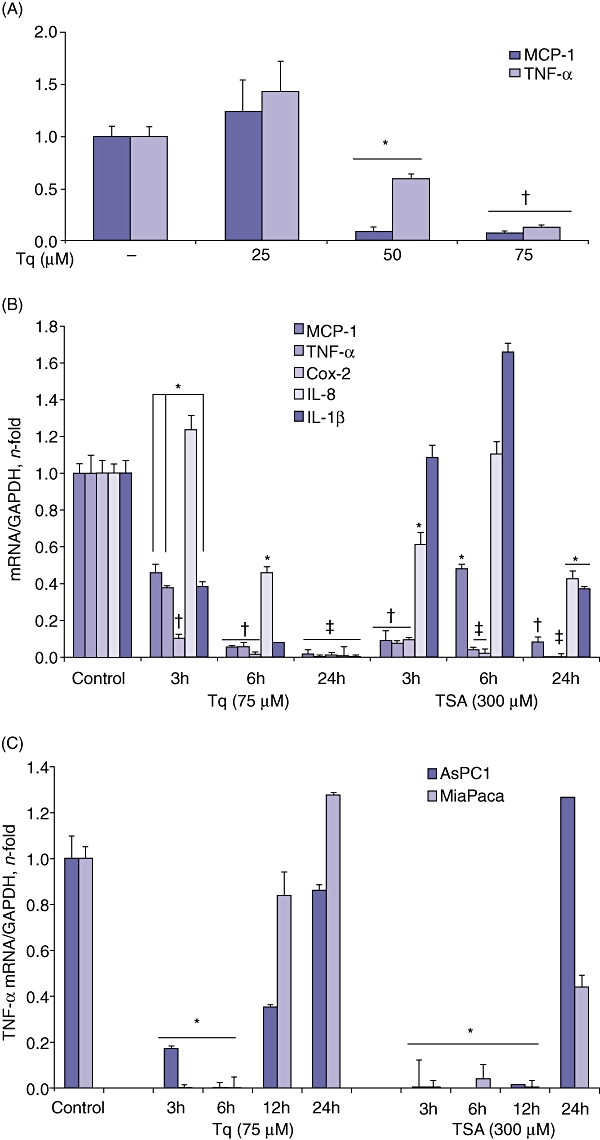
(A) Tq dose-dependently reduces the expression of TNF-α and MCP-1. Real-time polymerase chain reaction (PCR) of HS766T cells after incubation with Tq (25–75 µM) for 6 h. Tq dose-dependently reduces the expression of TNF-α and MCP-1. Data are expressed as mean ± standard error of the mean. Each experiment was performed three times and repeated three times for reproducibility. *P < 0.05, †P < 0.02 vs. untreated control cells using one-way repeated anova with subsequent all pairwise comparison procedure by Student's t-test. (B) Tq time-dependently reduces the expression of different proinflammatory cytokines and chemokines. HS766T cells were incubated with Tq (75 µM) or TSA (300 µM) for 3 h, 6 h and 24 h. At 24 h, Tq almost completely abolished the expression of MCP-1, TNF-α, Cox-2, IL-8 and IL-1β, whereas TSA had a less significant effect. *P < 0.05, †P < 0.005, ‡P < 0.002 vs. untreated control cells using one-way repeated anova with subsequent all pairwise comparison by Student's t-test. (C) Tq reduces the expression of TNF-α in AsPC-1 and MiaPaca cells. Cells were subjected to real-time PCR after treatment with Tq (75 µM) or TSA (300 µM) for 3 h, 6 h, 12 h and 24 h. *P < 0.05 vs. untreated control cells using one-way repeated anova with subsequent all pairwise comparison by Student's t-test
Tq significantly reduces the expression of different proinflammatory cytokines and chemokines in HS766T cells
Next, we analysed the effect of Tq on the expression levels of proinflammatory cytokines and chemokines in HS766T cells and compared these with the effects of TSA at different time-points. Cells were treated with Tq (75 µM) and TSA (300 µM) for 3 h, 6 h and 24 h. With the exception of IL-8, levels of which seem to initially increase after 3 h of Tq treatment, the remaining cytokines showed significant reductions (MCP-1 and TNF-α[P < 0.05]; Cox-2 [P < 0.02]). After 24 h, Tq almost completely abolished the transcription of all examined cytokines and chemokines (Fig. 1A). TSA was variably effective in reducing the expression of the cytokines. At 24 h, however, it significantly reduced expression of MCP-1 (P < 0.005), TNF-α and Cox-2 (P < 0.002), but had less significant effects on IL-8 and IL-1β (P < 0.05).
Tq reduces the expression of TNF-α in AsPC-1 and MiaPaca cells
To test the sensitivity of other PDA cells to Tq, we evaluated its effect on the expression of TNF-α in AsPC-1 and MiaPaca cells. Cells were treated with Tq (75 µM) and TSA (300 µM) for 3 h, 6 h, 12 h and 24 h. Figure 1C shows that both cell lines were sensitive to Tq and TSA at 3–6 h, when TNF-α mRNA was undetectable. Interestingly, at 12 h, TNF-α mRNA levels started to increase, especially in the Tq-treated cells. TNF-α expression was increased at 24 h with Tq and TSA treatment, suggesting that PDA cells may vary in their sensitivity to Tq and that a different treatment regimen should be adopted with each cell line to assure the consistent reduction of TNF-α levels.
Tq dose-dependently and significantly reduces the endogenous activity of MCP-1 promoter
MCP-1, a protein from the C-C chemokine subfamily, is expressed in human PDA and in some PDA cell lines.32 The potential contribution of MCP-1 to PDA progression is of major interest because MCP-1 production correlated with the levels of macrophage content in transplanted tumours in vivo.33,34 To evaluate the effect of Tq on MCP-1 transcription, HS766T cells were transfected with luciferase-labelled MCP-1 promoters and treated with Tq (25, 50, 75 µM) and TSA (50, 150, 300 µM) for 3 h. As seen in Fig. 2, Tq dose-dependently reduced the luciferase activity of the MCP-1 promoter. TSA did not have a significant effect on the endogenous activity of the MCP-1 promoter (Fig. 2).
Figure 2.
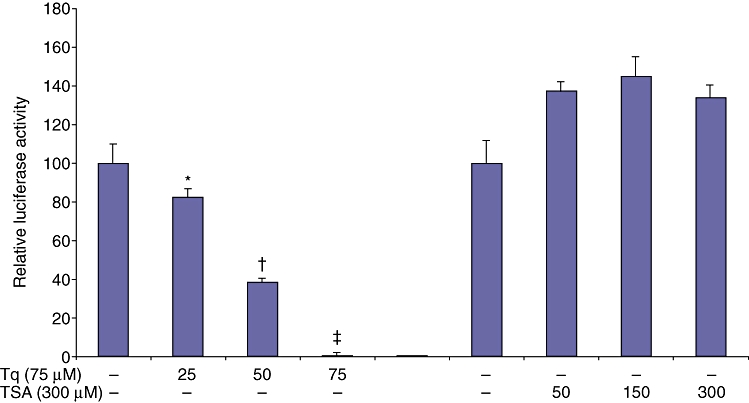
Tq dose-dependently reduces the intrinsic activity of MCP-1 promoter. HS766T cells were transfected with luciferase-labelled MCP-1 promoter. Relative luciferase activity was calculated after deduction of the activity levels with pGL3 vector alone. Results represent mean ± standard error of the mean of triplicate determinations. All experiments were repeated at least three times to confirm reproducibility. (*P < 0.05, †P < 0.005, ‡P < 0.002 vs. control by Student's t-test)
Tq time-dependently reduces the constitutive and TNF-α-induced activation of NF-κB in HS766T and MiaPaca cells
Activation of the transcription factor NF-κB is an essential step for activating the transcription of different cytokines and chemokines examined. We investigated whether Tq mediates its cytokine/chemokine-regulatory effect through inhibition of the constitutive and/or TNF-α-induced NF-κB activation. We evaluated p65 nuclear translocation in HS766T cells after their treatment with or without TNF-α by two independent assays: p65 NF-κB activity assay, and nuclear immunolocalization. HS766T cells were treated with Tq (75 µM) and TSA (300 µM) for 30 min, 1 h and 2 h (Fig. 3A). To analyse the effect of Tq on the TNF-α-mediated activation of NF-κB, cells were pre-treated with TNF-α (25 ng/ml) for 30 min, and then treated with Tq (75 µM) or TSA (300 µM) for 1 h. Cells were analysed for the presence of active forms of NF-κB p65 using the ActivELISA kit (Imgenex Corp.). The anti-p65 antibody-coated plate captures free p65 and the amount of active p65 is detected by colorimetric detection at OD 405 after adding a second anti-p65 antibody followed by alkaline phosphatase-conjugated secondary antibody. As early as after 30 min, Tq alone reduced the constitutive activity of NF-κB in HS766T cells by ∼1.7-fold, compared with a 1.5-fold reduction by TSA (Fig. 3A) (P < 0.05). TNF-α increased the activation of NF-κB 1.54-fold, an effect that was reduced 2.72-fold by Tq (75 µM) (P < 0.005). TSA had similar effects (Fig. 3A).
Figure 3.
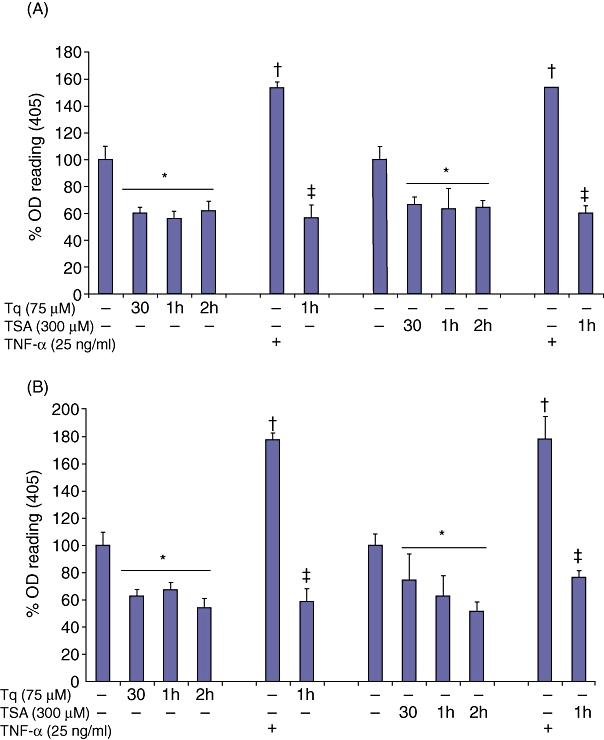
Tq inhibits the constitutive and TNF-α-mediated activation of NF-κB. Lysates from (A) HS766T cells and (B) MiaPaca cells treated with Tq (75 µM) and TSA (300 µM) with or without pre-treatment with TNF-α (25 ng/ml) were analysed for the presence of the active forms of NF-κB p65 using the ActivELISA kit. Both Tq and TSA demonstrated significant downregulation of the constitutive and TNF-α-mediated NF-κB p65 activation. Values are expressed as mean ± standard error of the mean of three experiments. *P≤ 0.05 vs. untreated control values; †P < 0.02 vs. control untreated values; ‡P < 0.005 vs. TNF-α-treated values, using one-way repeated anova with subsequent all pairwise comparison by Student's t-test
In MiaPaca cells, Tq alone reduced the constitutive activity of NF-κB by ∼1.8-fold, compared with 1.6-fold by TSA (Fig. 3B). TNF-α increased the activation of NF-κB 1.78-fold, an effect that was reduced more than three-fold by Tq (75 µM) (P < 0.002). TSA had a lesser but still significant effect whereby it reduced the activation of NF-κB 2.3-fold (Fig. 3B) (P < 0.005). These findings suggest that one mechanism by which Tq reduces inflammation is the inhibition of the constitutive and TNF-α-mediated activation of NF-κB.
Tq reduces the TNF-α-mediated nuclear translocation of NF-κB
Immunohistochemical staining with anti-rabbit p65 IgG was used to analyse the nuclear translocation of NF-κB after pre-treatment with TNF-α and subsequent treatment with Tq or TSA. The number of clearly stained nuclei in 10 fields was averaged, and the data were calculated as the percentage of nuclear staining/total number of nuclei. In the control untreated cells, a diffuse cytoplasmic staining was observed, whereas cells treated with TNF-α had a clear nuclear staining, indicating nuclear translocation of p65. Adding Tq to the cells prevented the TNF-α-induced nuclear translocation of p65 in HS766T cells more significantly than did TSA (P < 0.02 and P < 0.05, respectively) (Fig. 4A) (control 8 ± 0.8 stained nuclei/105 nuclei; TNF-α 94 ± 21 stained nuclei/105 nuclei; TNF-α+ Tq 12 ± 0.4 stained nuclei/105 nuclei; TNF-α+ TSA 34 ± 15 stained nuclei/105).
Figure 4.
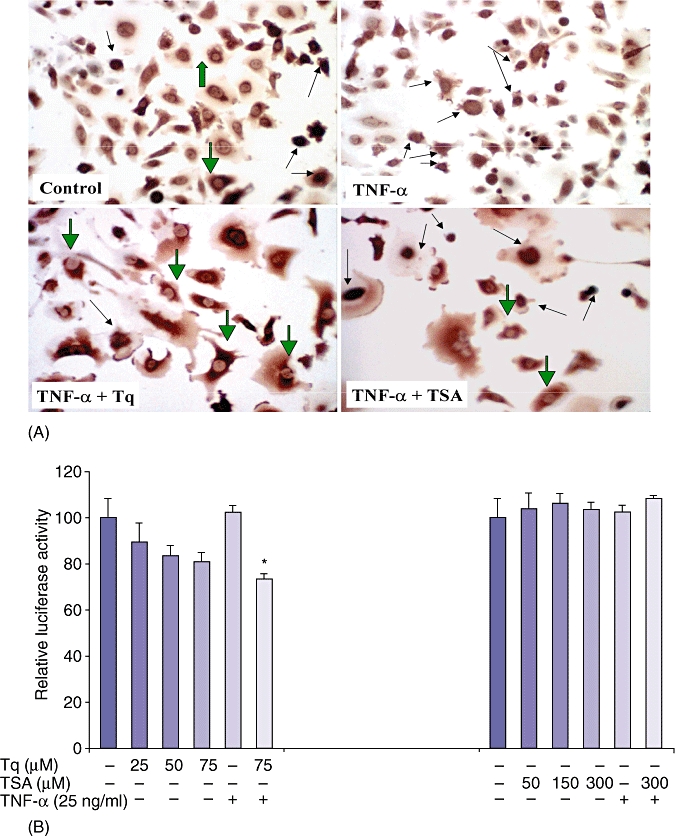
Tq reduces the TNF-α-mediated nuclear translocation of NF-κB. (A) Immunohistochemical staining for p65 NF-κB subunit shows mainly cytoplasmic staining in the control cells (large green arrows). Control cells treated with TNF-α show intense nuclear staining (black arrows), whereas Tq- and, to a lesser extent, TSA-treated cells show inhibition of the TNF-α-induced nuclear translocation in most of the cells (green arrows), and few cells retain their nuclear staining (small black arrows). (B) Tq dose-dependently reduces the constitutive and TNF-α-induced activation of NF-κB promoter. HS766T cells were transfected with luciferase-labelled NF-κB promoter. Relative luciferase activity was calculated after deduction of the activity levels with pGL3 vector alone. Results represent mean ± standard error of the mean of triplicate determinations. All experiments were repeated at least three times to confirm reproducibility. (*P < 0.05, vs. untreated control cells by Student's t-test)
Tq dose-dependently reduces the constitutive and TNF-α-induced activation of NF-κB promoter
To further elucidate the effect of Tq on NF-κB, we investigated whether Tq has a direct effect on NF-κB transcription. HS766T cells were transfected with luciferase-labelled NF-κB promoter and then treated with Tq (25–75 µM) or TSA (50–300 µM) for 3 h. As seen in Fig. 4B, there was a dose-dependent decrease in endogenous NF-κB promoter activation as well as a significant (P < 0.05) decrease in cells pre-treated with TNF-α after Tq treatment. TSA had no effect on the endogenous or TNF-α-induced activation of the NF-κB promoter. These data suggest that Tq not only regulates the activity of NF-κB, but also regulates its transcription.
Discussion
Herbs as alternative cancer therapies have attracted a great deal of recent attention as a result of their low toxicity and costs. In this study, we investigated the potential anti-inflammatory effects of Tq, the major constituent of the Middle Eastern herb Nigella sativa, in PDA cells. We show that Tq has strong anti-inflammatory activities in several PDA cell lines. We demonstrate that Tq mediates its effects through reducing the activity and transcription of NF-κB. Evidence for the anti-neoplastic activities of Tq has been reported, with various observations ranging from the activation of genes that promote apoptosis to the activation of proapoptotic pathways in several types of cancer.30,35 Until now, however, there have been no reports analysing the anti-inflammatory potential of Tq in PDA cells. Thus, the present study is the first to analyse the anti-inflammatory effects of Tq in PDA cells.
TNF-α plays an essential role in many inflammatory conditions. In accordance with its overwhelming importance in inflammatory processes, several lines of evidence point to a tumour-promoting function of TNF-α. Studies have shown that overexpression of TNF-α confers invasive properties in a cell type-dependent manner in nude mice.36–38 A crucial role of TNF-α in the initiation of skin tumours and hepatic carcinogenesis is evident from studies of TNF-α and TNF receptor-1 knockout mice.39–41 A variety of tumour-promoting effects of TNF-α have been shown in vitro and include protection against physiologic and pharmacologic apoptosis inducers, induction of angiogenic factors, enhancement of tumour cell motility, activation of oncogenic pathways, and triggering of epithelial to mesenchymal transition.42 Our in vitro data show that Tq significantly reduced mRNA expression of multiple proinflammatory cytokines and chemokines, mainly TNF-α (Fig. 1A–C).
Several investigations have provided recent evidence for the potential contribution of MCP-1 to cancer progression.32,34 MCP-1 produced by pancreatic tumour cells could promote the recruitment of monocytes or macrophages. Immune cells or other stromal cells within the tumour release inflammatory cytokines, including TNF-α and IL-1β, which, in turn, increase MCP-1 production and amplify monocyte recruitment.42 Our data here show that in HS766T cells, Tq potently reduced MCP-1 mRNAexpression levels, time- and dose-dependently (Fig. 1A–B). TSA had variable and less significant effects. This may be explained by the direct effect of Tq on MCP-1 promoter, where it reduced its intrinsic activity (Fig. 2). Studies to elucidate the exact mechanism through which Tq reduces the activity of MCP-1 promoter are ongoing in our laboratory.
The NF-κB pathway is regularly and robustly activated by TNF-α and mediates many of the pro-tumoral effects of TNF-α. As treating cells with Tq inhibited the transcription of many inflammatory cytokines, such as IL-1β, IL-8, and chemokines, such as MCP-1, we studied the transcription factor NF-κB as a possible target for Tq-mediated effects. NF-κB comprises a collection of dimers composed of various combinations of members of the Rel family. Five mammalian Rel proteins have been identified: p50, p52, c-Rel, p65 (RelA) and RelB. NF-κB is sequestered in the cytoplasm by binding to inhibitor protein κ Bα (IκBα). Following cytokine exposure, IκBα is phosphorylated, ubiquitinated and degraded by the proteasomal complex exposing the NF-κB nuclear recognition site to translocate to the nucleus and bind κB consensus sequences in promoter regions of numerous proinflammatory genes such as TNF-α.43 NF-κB activation has been reported in PDA cell lines and primary tumour samples.44,45 Furthermore, increased TNF-α levels were found in patients suffering from pancreatic cancer.46 Our data demonstrate, for the first time, that Tq inhibits the constitutive and TNF-α-induced activation of NF-κB (Fig. 3A, B) and inhibits the translocation of NF-κB to the nucleus in PDA cells (Fig. 4A). These data are consistent with recent data published by Sethi et al.,47 which showed similar effects of Tq in myeloid and embryonic kidney cells.
Most interestingly, Tq inhibited the transcription of NF-κB through reducing its promoter activity (Fig. 4B), indicating that Tq has a dual inhibitory effect, the first through inhibiting the NF-κB signalling pathway and the second through inhibiting its transcription. The details of the mechanism by which Tq downregulates NF-κB promoter activity are still unclear. Tq may induce binding of transcription factors to the promoter or a suppressor region. Alternatively, Tq may induce changes in the secondary and tertiary structures of the promoter. Studies addressing transcription factor binding are ongoing in our laboratory.
The potent anti-inflammatory effect of Tq on TNF-α and other inflammatory mediators is promising for its potential as a preventive and therapeutic strategy in PDA. Clinical trials with TNF-α-blocking agents are currently underway in patients with solid cancers. Thus far, these trials show that TNF-α blockers are well tolerated, which is in good accordance with experience of anti-TNF-α treatment in patients with chronic inflammatory diseases.48 Therefore, the results presented in our study strongly suggest that surgical resection of PDA combined with a Tq therapy represents a promising novel therapeutic option. Corresponding adjuvant clinical trials should therefore be pursued.
Acknowledgments
The authors wish to thank Dr Decio Eizirik, Free University, Brussels, for providing the MCP-1 and NF-κB promoter constructs.
This study was funded by the American Cancer Society.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JP, Eibl G, Reber HA, Hines OJ. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer. 2003;2:12. doi: 10.1186/1476-4598-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warshaw AL, Gu ZY, Wittenberg J, Waltman AC. Preoperative staging and assessment of respectability of pancreatic cancer. Arch Surg. 1990;125:230–233. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- 4.Rohan T, McMichael AZ. Alimentary tract cancer mortality in Australia, 1908–1978. An epidemiological appraisal. Med J Aust. 1981;1:232–235. [PubMed] [Google Scholar]
- 5.Imaizumi Y. Longitudinal Gompertzian analysis of mortality from pancreatic cancer in Japan, 1955–1993. Mech Ageing Dev. 1996;90:163–181. doi: 10.1016/0047-6374(96)01760-5. [DOI] [PubMed] [Google Scholar]
- 6.Levi F, Decarli A, La Vecchia C. Trends in cancer mortality in Switzerland, 1951–1984. Rev Epidemiol Sante Publique. 1988;36:15–25. [PubMed] [Google Scholar]
- 7.Lilliemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: state-of-the-art care. CA Cancer J Clin. 2000;50:241–268. doi: 10.3322/canjclin.50.4.241. [DOI] [PubMed] [Google Scholar]
- 8.Rocca G, Gaia E, Iuliano R. Increased incidence of cancer in chronic pancreatitis. J Clin Gastroenterol. 1987;9:175–179. doi: 10.1097/00004836-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki M. Chronic pancreatitis in Japan: epidemiology, prognosis, diagnostic criteria, and future problems. J Gastroenterol. 2003;38:315–326. doi: 10.1007/s005350300058. [DOI] [PubMed] [Google Scholar]
- 10.Maisonnneuve P, Lowenfels AB. Chronic pancreatitis and pancreatic cancer. Dig Dis. 2002;20:32–37. doi: 10.1159/000063165. [DOI] [PubMed] [Google Scholar]
- 11.Queneau PE, Adessi GL, Thibault P. Early detection of pancreatic cancer in patients with chronic pancreatitis; diagnostic utility of a Kras point mutation in the pancreatic juice. Am J Gastroenterol. 2001;96:700–704. doi: 10.1111/j.1572-0241.2001.03608.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowenfels AB, Maisonnneuve P, Dimagnao EP, Elitsur T, Gates LK, Perrault J, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;90:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 13.Ghadrian P, Liu G, Gallinger S, Schmocker B, Paradis A, Lal G, et al. Risk of pancreatic cancer among individuals with a family history of cancer of the pancreas. Int J Cancer. 2002;97:807–810. doi: 10.1002/ijc.10123. [DOI] [PubMed] [Google Scholar]
- 14.Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and the risk of pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:1433–1437. doi: 10.1111/j.1749-6632.1999.tb09524.x. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Pre-invasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 16.Leach SD. Mouse models of pancreatic cancer: the fur is finally flying! Cancer Cell. 2004;5:7–11. doi: 10.1016/s1535-6108(03)00337-4. [DOI] [PubMed] [Google Scholar]
- 17.Pikarksy E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 18.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Chandler NM, Canete JJ, Callery MP. Increased expression of NF-κB subunits in human pancreatic cancer cells. J Surg Res. 2004;118:9–14. doi: 10.1016/S0022-4804(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 20.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-KB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 22.Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, et al. Function of nuclear factor κB in pancreatic cancer metastases. Clin Cancer Res. 2003;9:345–354. [PubMed] [Google Scholar]
- 23.Fahy BN, Schliemann MG, Virudachalam S, Bold JG. Inhibition of AKT abrogates chemotherapy-induced NF-κB survival mechanisms: implications for therapy in pancreatic cancer. J Am Coll Surg. 2004;198:591–599. doi: 10.1016/j.jamcollsurg.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse M, Folsch UR, et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 25.Salomi NJ, Nair SC, Panikkar KR. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- 26.Al-Majed AA, Al-Omar FA, Nagi MN. Neuroprotective effects of thymoquinone against transient forebrain ischaemia in the rat hippocampus. Eur J Pharmacol. 2006;543:40–47. doi: 10.1016/j.ejphar.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Salomi NJ, Nair SC, Jayawardhanan KK, Varghese CD, Panikkar KR. Antitumour principles from Nigella sativa seeds. Cancer Lett. 1992;31:41–46. doi: 10.1016/0304-3835(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 28.Worthen D, Ghosheh O, Crooks P. The in vitro anti-tumour activity of some crude and purified components of black seed Nigella sativa L. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
- 29.Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41:283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 30.Arafat HA, Chipitsyna G, Gong Q, Davis M, Zaaqoq A, Laffer B, et al. The Nigella sativa seed extract, thymoquinone, triggers apoptotic cell death in human pancreatic cancer cells. Dig Dis. 2008;15:309–315. [Google Scholar]
- 31.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 32.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- 33.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 34.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–1734. [PMC free article] [PubMed] [Google Scholar]
- 35.Gahli-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 36.Malik ST, Naylor MS, East N, Oliff A, Balkwill FR. Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur J Cancer. 1990;26:1031–1034. doi: 10.1016/0277-5379(90)90044-t. [DOI] [PubMed] [Google Scholar]
- 37.Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel DN. Enhancement of experimental metastasis by tumour necrosis factor. J Exp Med. 1993;177:1391–1398. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orosz P, Kruger A, Hubbe M, Ruschoff J, Von Hoegen P, Mannel DN. Promotion of experimental liver metastasis by tumour necrosis factor. Int J Cancer. 1995;60:867–871. doi: 10.1002/ijc.2910600624. [DOI] [PubMed] [Google Scholar]
- 39.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumour necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 40.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-α receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23:1902–1910. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 41.Knight B, Yeoh GC, Husk KL, Ly T, Abraham LJ, Yu C, et al. Impaired preneoplastic changes and liver tumour formation in tumour necrosis factor receptor type 1 knockout mice. J Exp Med. 2000;192:1809–1818. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-α as a tumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin AS. The nf-kappa-b and i-kappa-b proteins – new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 44.Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumour necrosis factor-α and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–1358. [PubMed] [Google Scholar]
- 45.Sclabas GM, Fujioka S, Schmidt C, Evans DB, Chiao PJ. NF-κB in pancreatic cancer. Int J Gastrointest Cancer. 2003;33:15–26. doi: 10.1385/IJGC:33:1:15. [DOI] [PubMed] [Google Scholar]
- 46.Ariapart P, Bergstedt-Lindqvist S, van Harmelen V, Permert J, Wang F, Lundkvist I. Resection of pancreatic cancer normalizes the preoperative increase of tumour necrosis factor-α gene expression. Pancreatology. 2002;2:491–494. doi: 10.1159/000064719. [DOI] [PubMed] [Google Scholar]
- 47.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 48.Balkwill F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]


