Abstract
Background:
The optimal management of acute pancreatitis remains controversial and current treatment protocols vary in degrees of medical and surgical management. Our group has previously shown in population-based studies that high-volume (HV) hospitals have lower rates of in-hospital mortality after pancreatectomy. We sought to examine if a similar mortality benefit exists for patients admitted with acute pancreatitis.
Methods:
Using the Nationwide Inpatient Sample (NIS), we examined discharge records for all adult admissions during 1998–2006 with a primary diagnosis of acute pancreatitis of any aetiology. Unique hospital identifiers were used to divide hospital volumes into equal thirds based on the number of admissions for acute pancreatitis per year (lowest tertile [low volume, LV]≤64 admissions/year; medium tertile [medium volume, MV] 65–117 admissions/year; highest tertile [high volume, HV]≥118 admissions/year). Covariates included patient demographics, hospital characteristics and patient co-morbidities using the Elixhauser index. Adjusted mortality represented the primary outcome measure and adjusted length of stay (LOS) and total charges were considered secondary measures.
Results:
There were 416 489 primary admissions for acute pancreatitis during the study period. In-hospital mortality for the cohort amounted to 1.6% (n= 6446). Hospital admissions for acute pancreatitis increased over the study period (P < 0.0001). High-volume hospitals tended to be large (82%), urban (99%) teaching (59%) centres (P < 0.0001), which cared for patients with more co-morbidities (35.9% of patients at HV hospitals vs. 29.1% at LV hospitals had at least three co-morbidities; P < 0.0001). Low-volume centres appeared more likely to perform pancreatic procedures than HV hospitals (odds ratio [OR] 1.50, 95% confidence interval [CI] 1.32–1.70). Patients at HV hospitals had a lower likelihood of a prolonged adjusted LOS compared with those at LV (OR 0.75, 95% CI 0.71–0.79) or MV (OR 0.82, 95% CI 0.79–0.85) hospitals. After adjusting for patient and hospital factors, there was an in-hospital mortality benefit associated with being treated at an HV centre (OR 0.70, 95% CI 0.63–0.77). The decision to operate on a given patient did not alter the mortality benefit of the HV hospital.
Conclusions:
Rates of admissions for acute pancreatitis in the USA are increasing. High annual hospital volume of acute pancreatitis cases confers a shorter LOS, lower adjusted mortality and a lower likelihood of pancreatic procedure for patients admitted with acute pancreatitis. Although HV hospitals were less likely than MV or LV centres to perform pancreatic procedures, the role of surgery remains unclear. Further studies should examine other possible reasons for this mortality benefit, such as the availability of specialists, the quality of critical care facilities and the timing of operative intervention.
Keywords: acute pancreatitis, Nationwide Inpatient Sample, volume, Elixhauser morbidity
Introduction
Hospital volume has been associated with improved patient outcomes in several past studies.1–4 These studies, however, mostly dealt with complex surgical procedures and cancer resections. Our group has previously shown that high-volume (HV) hospitals have better outcomes after pancreatectomy and liver resection.4,5 Few, if any, studies have shown the potential benefits of HV treatment in medical diagnoses such as acute pancreatitis. One study examining hospital volume and acute liver failure failed to show a mortality benefit at HV hospitals,6 whereas another, examining inflammatory bowel disease (IBD), showed an outcome benefit in surgical IBD patients only.7 This may reflect a lack of definable outcomes or failure to attribute differences in care to a specific intervention.
Given the rising incidence and high cost of acute pancreatitis, and the lack of studies examining volume–outcome benefits in medical diagnoses, the identification of a volume–outcome relationship in acute pancreatitis may have important implications for future policy and the referral of complicated cases. We sought to examine the relationship between hospital admissions and improved outcomes in the care of patients with acute pancreatitis on a national level.
Materials and methods
We used the Nationwide Inpatient Sample (NIS) for the years 1998–2006 to extract data for all patients presenting with a primary diagnosis of acute pancreatitis (ICD-9-CM 577.0). Our methods were as previously described.4,8 The NIS is the largest national, all-payer hospital inpatient care database in the USA. It is supported by the Healthcare Cost and Utilization Project (HCUP) and contains all-payer discharge information for 100% of patient discharges from participating hospitals. Data exist for approximately seven million hospital discharges per year from a stratified sample of 20% of non-federal US community hospitals in participating states, including academic and specialty hospitals. The NIS contains hospital-level information obtained from a direct link to the American Hospital Association's (AHA) annual survey of hospitals, which includes hospital type (teaching, non-teaching) and geographic region (northeast, west, south, midwest as defined by the US Census Bureau). Each record in the NIS represents a single hospital discharge and includes a unique identifier.
The study was reviewed by the University of Massachusetts Institutional Review Board (IRB) as appropriate for exemption from IRB oversight as no personal identifiers were used in the registry data.
Study population
Diagnosis and procedures were identified by the Clinical Modification of the International Classification of Diseases, 9th Revision (ICD-9-CM) diagnostic and procedural codes. All patients with a primary diagnosis of acute pancreatitis (ICD-9-CM 577.0) were identified. Patients under the age of 18 years were excluded from the dataset.
Hospital volume
Unique hospital identifiers were used to determine the number of cases of acute pancreatitis seen at each individual hospital. Hospital identifiers remain the same throughout the different years of the NIS and are linked to hospital characteristics via the AHA annual survey as described above. Each record in the NIS is considered as a single unit assigned to a specific hospital. As a result of NIS sampling, it is possible for a hospital to be included in a particular year and not included the following year. Additionally, a hospital's volume may change yearly. Therefore, hospital volume was calculated on an annual basis. Extrapolation of the dataset using institutional weighting was not performed. Hospital volume was divided into equal thirds based on the number of cases of acute pancreatitis admitted per year (lowest tertile [low volume, LV]≤64 admissions/year; middle tertile [medium volume, MV] 65–117 admissions/year; highest tertile [high volume, HV]≥118 admissions/year). All data were analysed by volume tertile.
Variables
Patient demographics and hospital characteristics were captured within the NIS. Age was maintained as a continuous variable. Race was divided into White, Black, Hispanic or Other, which included, but was not limited to, Asians, Pacific Islanders and Native Americans. Income was categorized in four quartiles. Income bracket was a categorical variable derived from the median household income in the patient's residential zip code. For 1998–2002, these quartiles were defined based on 1999 demographics such that the maximum of the first quartile was 150% of the poverty level, and the national median income represented the boundary between the second and third quartiles. For 2003–2006, the quartiles were adjusted annually to divide patients equally, so that the national median income again represented the boundary between the second and third quartiles. Payer type was divided into four groups: Medicare, Medicaid, private insurance, and other. Hospital size was reported in tertiles, as small, medium and large. The cut-off for each tertile differed depending on the region, location (rural/urban) and teaching status of the hospital, so that hospitals were divided approximately equally. Hospital region, location and teaching status were also examined individually.
For purposes of risk adjustment, co-existing co-morbidity was compiled to create an Elixhauser co-morbidity index.9 This index identifies 29 disease entities that are considered true co-morbid diseases associated with adverse outcomes in hospitalized patients. Patients were given a score of 0, 1, 2 or ≥3 based on the number of co-morbidities.
Outcomes
The primary endpoint examined in this study was in-hospital mortality. Mortality was defined as death from any cause prior to discharge. Secondary outcome measures included prolonged length of stay (LOS) and total hospital charges. Prolonged LOS was defined as any stay that was greater than that of the 90th percentile of the whole cohort, which was 8 days. A categorical value was then assigned according to whether or not the hospital stay exceeded that of the 90th percentile. This captured complicated cases and long hospitalizations and reflected atypical discharge patterns.
In order to examine if access to and performance of pancreatic procedures affected outcomes, several pancreatic procedures were identified (Table 1). A dichotomous variable was created to convey any pancreatic procedure performed and was subsequently analysed as both an outcome and a covariate.
Table 1.
Pancreatic procedures and corresponding ICD-9-CM codes
| Pancreatic procedure | ICD-9-CM code |
|---|---|
| Endoscopic retrograde cholangiopancreatography | 51.10 |
| Pancreatotomy | 52.0 |
| Excision of pancreatic lesion | 52.2 |
| Marsupialization of pancreatic cyst | 52.3 |
| Internal drainage of pancreatic cyst | 52.4 |
| Partial pancreatectomy | 52.5 |
| Total pancreatectomy | 52.6 |
| Radical pancreaticoduodenectomy | 52.7 |
Statistical analysis
Data were analysed using sas Version 9.1 (SAS Institute, Inc., Cary, NC, USA). Categorical variables and outcomes were tested for statistical significance with chi-square analysis. Continuous variables and outcomes were tested using anova. Temporal trends were assessed using the Cochrane–Armitage trend test. Statistical significance was defined by P < 0.05.
Univariate predictor variables with a P < 0.10 were included in the multivariate logistic regression analysis. Logistic regression was used to analyse categorical outcomes while adjusting for covariates. Covariates included age, gender, race, insurance type, high income bracket, co-morbidities and hospital characteristics. The performance of a pancreatic procedure was analysed as both a covariate for mortality and an individual outcome. A Hosmer–Lemeshow goodness-of-fit test was performed to confirm the final models. All results in the regression model were represented by an odds ratio (OR) and 95% confidence interval (CI). All regression models were performed separately.
Results
During 1998–2006, a total of 416 489 discharges with a primary diagnosis of acute pancreatitis were captured in the NIS. The number of cases of acute pancreatitis increased with each successive year in this time period, from 36 510 cases in 1998 to 51 895 cases in 2006 (Fig. 1) (P < 0.0001).
Figure 1.
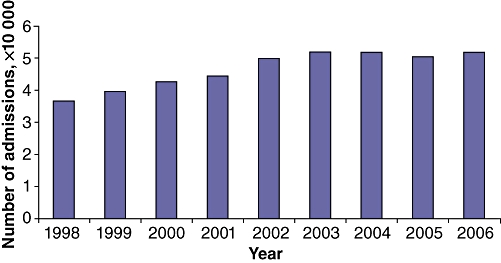
Number of admissions for acute pancreatitis in the USA by year (total n= 416 489)
Demographic characteristics of patients admitted with acute pancreatitis are shown in Table 2, broken down into the three volume groups. When compared with the MV and LV hospitals, HV hospitals tended to be large (82.2% vs. 56.1% vs. 32.7%, respectively), urban (98.9% vs. 89.1% vs. 58.6%, respectively), teaching (58.5% vs. 36.7% vs. 13.8%, respectively) centres (P < 0.0001). High-volume hospitals treated Black patients more commonly than either MV or LV hospitals (22.1% vs. 19.0% vs. 13.7%, respectively). High-volume hospitals also treated more patients with three or more co-morbidities (35.9% vs. 33.4% vs. 29.1%, respectively).
Table 2.
Patient demographics and hospital characteristics of admissions with a primary diagnosis of acute pancreatitis
| Demographic | Low-volume centre (n= 137 497) | Medium-volume centre (n= 139 624) | High-volume centre (n= 139 368) | P-value |
|---|---|---|---|---|
| Female gender | 50.0% | 49.5% | 49.1% | 0.0001 |
| Race | <0.0001 | |||
| White | 72.3% | 64.1% | 59.7% | |
| Black | 13.7% | 19.0% | 22.1% | |
| Hispanic | 9.1% | 12.1% | 14.3% | |
| Other | 4.9% | 4.8% | 3.9% | |
| Primary payer | <0.0001 | |||
| Medicare | 36.1% | 33.2% | 30.7% | |
| Medicaid | 14.1% | 13.9% | 14.4% | |
| Private insurance | 34.4% | 37.7% | 37.3% | |
| Other | 15.5% | 15.3% | 17.7% | |
| High income bracket | 19.7% | 30.0% | 28.1% | <0.0001 |
| Elixhauser co-morbidity score | <0.0001 | |||
| 0 | 19.6% | 17.3% | 16.1% | |
| 1 | 26.4% | 24.3% | 23.2% | |
| 2 | 24.9% | 25.0% | 24.9% | |
| ≥3 | 29.1% | 33.4% | 35.9% | |
| Teaching hospital | 13.8% | 36.7% | 58.5% | <0.0001 |
| Urban hospital | 58.6% | 89.1% | 98.9% | <0.0001 |
| Hospital size by beds | <0.0001 | |||
| Small | 30.6% | 10.6% | 1.4% | |
| Medium | 36.7% | 33.4% | 16.4% | |
| Large | 32.7% | 56.1% | 82.2% | |
| Hospital region | <0.0001 | |||
| Northeast | 18.0% | 16.7% | 14.5% | |
| Midwest | 25.2% | 19.8% | 19.0% | |
| South | 38.3% | 44.9% | 46.7% | |
| West | 18.4% | 18.7% | 19.8% |
Unadjusted, univariate outcomes are shown in Table 3. The overall in-hospital mortality for the cohort was 1.6% (n= 6446). There was a significant mortality difference according to volume among the providers of care for acute pancreatitis (P < 0.0001). Patients in HV and MV hospitals seemed to have a slightly longer LOS than those in LV hospitals, with 12.0% of patients at HV hospitals experiencing a prolonged LOS vs. 8.9% of patients at LV hospitals. Additionally, on univariate analysis, HV and MV hospitals had higher mean total charges ($24 100 at HV hospitals) compared with LV hospitals ($16 600). Overall, only 0.93% of patients in the overall cohort underwent a pancreatic procedure during the inpatient stay. The most commonly performed pancreatic procedures were endoscopic retrograde cholangiopancreatography (ERCP) (n= 18 196) and pancreatotomy (n= 1627). Prior to any adjustment, a larger percentage of patients at HV hospitals underwent a pancreatic procedure than at MV or LV hospitals (1.19% vs. 1.04% vs. 0.54%, respectively).
Table 3.
Patient outcomes for admissions with a primary diagnosis of acute pancreatitis
| Outcome | Low-volume centre | Medium-volume centre | High-volume centre | P-value |
|---|---|---|---|---|
| Mortality | 1.4% | 1.7% | 1.5% | <0.0001 |
| Length of stay (median), days | 5.3 (4) | 6.0 (4) | 6.1 (4) | <0.0001 |
| Prolonged length of stay | 8.9% | 11.7% | 12.0% | <0.0001 |
| Total charges | $16 600 | $23 600 | $24 100 | <0.0001 |
| Any pancreatic procedure | 0.54% | 1.04% | 1.19% | <0.0001 |
Results of the multivariate analysis for in-hospital mortality using logistic regression are shown in Table 4. Factors associated with higher odds of mortality included increasing age (OR 1.04, 95% CI 1.04–1.05), having a single co-morbidity (OR 1.72, 95% CI 1.48–1.99), being treated at a teaching hospital (OR 1.43, 95% CI 1.34–1.53), being treated at an urban hospital (OR 1.50, 95% CI 1.35–1.53), and being treated at a large hospital (OR 1.61, 95% CI 1.45–1.80). After adjusting for covariates, there was an independent in-hospital mortality benefit for patients treated at HV hospitals compared with LV hospitals (OR 0.70, 95% CI 0.63–0.77) and HV hospitals compared with MV hospitals (OR 0.77, 95% CI 0.72–0.83). Similar analysis was performed for the outcome of prolonged LOS. High-volume hospitals showed a significantly lower likelihood of prolonged LOS compared with LV hospitals (OR 0.77, 95% CI 0.74–0.80) and MV hospitals (OR 0.83, 95% CI 0.81–0.86).
Table 4.
Odds ratios of mortality in 416 489 admissions with a primary diagnosis of acute pancreatitis
| Factor | Odds ratio | 95% confidence interval |
|---|---|---|
| Age | 1.04 | 1.04–1.05 |
| Female gender | 0.74 | 0.69–0.78 |
| Race | ||
| White | Reference | |
| Black | 0.80 | 0.73–0.87 |
| Hispanic | 0.85 | 0.76–0.94 |
| Other | 0.82 | 0.71–0.96 |
| Primary payer | ||
| Medicare | Reference | |
| Medicaid | 1.02 | 0.91–1.15 |
| Private insurance | 0.83 | 0.76–0.91 |
| Other | 0.75 | 0.66–0.86 |
| Elixhauser co-morbidity score | ||
| 0 | Reference | |
| 1 | 1.72 | 1.48–1.99 |
| 2 | 2.57 | 2.24–2.96 |
| ≥3 | 3.56 | 3.11–4.08 |
| Teaching hospital | 1.43 | 1.34–1.53 |
| Urban hospital | 1.50 | 1.35–1.53 |
| Hospital size by beds | ||
| Small | Reference | |
| Medium | 1.26 | 1.13–1.40 |
| Large | 1.61 | 1.45–1.80 |
| Hospital volume | ||
| Low | Reference | |
| Medium | 0.90 | 0.83–0.98 |
| High | 0.70 | 0.63–0.77 |
Multivariate logistic regression adjusting for covariates was also performed with the performance of any pancreatic procedure as an outcome variable. Low-volume centres appeared to be more likely to perform pancreatic procedures than HV hospitals (OR 1.50, 95% CI 1.32–1.70). As ERCP was the most common procedure performed, we then used multivariate logistic regression to examine the performance of ERCP alone and other pancreatic procedures separately. Low-volume centres were independently more likely to perform ERCP (OR 1.22, 95% CI 1.15–1.29) and more likely to perform a pancreatic procedure other than ERCP (OR 1.50, 95% CI 1.32–1.72). When the performance of any pancreatic procedure was added as a covariate to the multivariate logistic regression for in-hospital mortality, the mortality benefit associated with HV hospitals did not change.
Discussion
In this study of the NIS, we confirmed that annual admissions for acute pancreatitis are increasing in the USA. We found that patients admitted with acute pancreatitis have better outcomes when admitted to hospitals with high volumes of acute pancreatitis cases. Significant demographic differences exist between HV hospitals and MV and LV hospitals, at both the patient and hospital levels. After adjusting for these differences using multivariate logistic regression, care at an HV hospital resulted in a significant in-hospital mortality benefit compared with care at an MV or LV hospital. Care at an HV hospital also reduced the likelihood of a prolonged LOS. After adjusting for covariates in a multivariate logistic regression, LV centres were found to be more likely to perform pancreatic procedures than HV centres. The performance of these procedures did not alter the mortality benefit associated with HV hospitals. Univariate analysis of total charges seemed to indicate that HV centres spend more per case than LV centres.
Previous studies have shown that the incidence of acute pancreatitis has increased in the USA and internationally.10–13 Our study of the NIS confirms these temporal trends and extends the existing data on acute pancreatitis by analysing outcomes based on hospital volume and assessing the impact on patient outcomes of pancreatic procedures. Increasing numbers of admissions will have significant impact on future health care expenditures and resources. Optimizing both the standard of care and the utilization of resources should be important priorities in future policy planning.
We have shown a clear and important relationship between hospital volume and outcomes in acute pancreatitis. Birkmeyer et al. previously showed that high hospital volume was associated with various benefits in relation to surgical procedures, including heart and lung surgery and abdominal cancer resections.1–3 Birkmeyer et al. went on to show the benefits associated with individual surgeon volume.14 However, few, if any, studies have examined the hospital volume benefit as it relates to a medical diagnosis. Outcome benefits attributable to high hospital volume have been shown for acute myocardial infarction,15 HIV/AIDS,16 and surgical IBD patients.7 For acute pancreatitis, our results show a clear, risk-adjusted outcome benefit at HV hospitals that holds true over a significant period of time.
The initial results of our univariate analysis of outcomes indicated that HV hospitals had an overall low mortality rate of 1.5%, a higher percentage of patients with a prolonged LOS, and a higher percentage of patients undergoing pancreatic procedures compared with LV centres. However, the significant demographic differences at both the patient and hospital level between the different hospital volume groups made it necessary to attempt to adjust for these differences using multivariate analysis. A previous study examining hospital volume effects on acute liver failure did not show a mortality benefit at HV hospitals, but did show that higher-volume hospitals tended to treat sicker patients.7 Our results also indicate that HV hospitals treat a higher percentage of Black and Hispanic patients, and a higher proportion of patients with multiple co-morbidities. These disparities can result in confounding, which may explain the findings of our initial univariate analysis.
The hospital volume benefit in outcomes seen in surgical procedures makes clinical sense. Surgical specialization,17 multidisciplinary care,18 and surgeon experience19 have been shown to independently improve patient outcomes after surgery. Higher-volume hospitals may be more likely to offer such services. A previous study showed disparities in access to liver resection among different hospital volume groups, with higher-volume hospitals providing more procedures.20 Given that acute pancreatitis may require both medical and surgical care, we speculated that access to pancreatic procedures may explain the volume benefit seen at HV hospitals. Initial univariate analysis of outcomes (Table 3) indicates that a higher percentage of patients underwent a pancreatic procedure at HV hospitals than at LV or MV hospitals. However, these results are difficult to interpret because of the demographic differences between patient populations at HV, MV and LV centres. After adjusting for patient and hospital factors using multivariate analysis, LV hospitals are found to be actually more likely to perform pancreatic procedures than HV hospitals. Furthermore, the performance of pancreatic procedures did not alter the mortality benefit associated with care at HV centres. This is an interesting finding because it indicates that LV hospitals, despite treating fewer Black and Hispanic patients, and patients with fewer co-morbidities, are seemingly more likely to perform pancreatic procedures, despite a higher likelihood of in-hospital mortality overall.
The true mechanism of the volume benefit in our study remains unclear and is most likely multifactorial. Perhaps the availability of quality intensive care and gastroenterological specialists, as well as access to advanced diagnostic and therapeutic procedures, contribute to improved outcomes at HV hospitals. Intensive care specialists running intensive care units have been previously associated with improved outcomes in critically ill patients.21 Higher volumes may also lead to the development of streamlined treatment protocols and processes of care, resulting in improved outcomes based on the overall experience of the health care team.
Several limitations of this study must be considered. This was a retrospective study and has the associated constraints as a result of the level of NIS data. The main outcome measure of this study was in-hospital mortality. This may reflect a lower mortality rate compared with studies using 30-day mortality rates as most patients were probably discharged from hospital prior to the potential death (if applicable). Our study used population-based data with only limited information on patient and treatment factors, which limits our evaluation of medical factors such as admission laboratory tests (lactate dehydrogenase [LDH], white blood cell count, glucose, aspartate aminotransferase [AST]) and treatment over the first 48 hours (mechanical ventilation, antibiotic use, etc.). Although the NIS represents the largest all-payer database of hospital discharge records in the USA, there is no guarantee that our cohort is representative of local demographics and medical practices, which may vary by state and community.
To our knowledge, this is the first study to show a significant outcome benefit for acute pancreatitis at HV hospitals. The large sample size and high number of covariates examined indicate that our findings are, indeed, real. Few past studies have shown such a volume benefit in a medical diagnosis.
Given the rising incidence and high treatment cost of acute pancreatitis in the USA, our study suggests that referral of complicated cases of acute pancreatitis to HV centres should be considered at the policy level. Future studies should endeavour to elucidate compelling reasons for this outcome benefit, such as the availability of critical care facilities and specialists, and the true role of operative intervention.
Conflicts of interest
None declared.
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 4.Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: surgeon or centre? J Gastrointest Surg. 2008;12:1709–1716. doi: 10.1007/s11605-008-0627-3. [DOI] [PubMed] [Google Scholar]
- 5.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, McGinley EL, Saeian K. Effect of hospital volume and teaching status on outcomes of acute liver failure. Liver Transpl. 2008;14:1347–1356. doi: 10.1002/lt.21519. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan AN, McGinley EL, Binion DG. Does it matter where you are hospitalized for inflammatory bowel disease? A nationwide analysis of hospital volume. Am J Gastroenterol. 2008;103:2789–2798. doi: 10.1111/j.1572-0241.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 8.Csikesz NG, Simons JP, Tseng JF, Shah SA. Surgical specialization and operative mortality in hepato-pancreatico-biliary (HPB) surgery. J Gastrointest Surg. 2008;12:1534–1539. doi: 10.1007/s11605-008-0566-z. [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Co-morbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 11.Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas. 2006;33:336–344. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- 12.Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA., Jr. Increasing United States hospital admissions for acute pancreatitis, 1998–2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Spanier BW, Dijkgraaf MG, Bruno MJ. Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur J Gastroenterol Hepatol. 2008;20:653–658. doi: 10.1097/MEG.0b013e3282f52f83. [DOI] [PubMed] [Google Scholar]
- 14.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 15.Thiemann DR, Coresh J, Oetgen WJ, Powe NR. The association between hospital volume and survival after acute myocardial infarction in elderly patients. N Engl J Med. 1999;340:1640–1648. doi: 10.1056/NEJM199905273402106. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham WE, Tisnado DM, Lui HH, Nakazono TT, Carlisle DM. The effect of hospital experience on mortality among patients hospitalized with acquired immunodeficiency syndrome in California. Am J Med. 1999;107:137–143. doi: 10.1016/s0002-9343(99)00195-3. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery. 2001;130:415–422. doi: 10.1067/msy.2001.117139. [DOI] [PubMed] [Google Scholar]
- 18.Makary MA, Sexton JB, Freischlag JA, Millman EA, Pryor D, Holzmueller C, et al. Patient safety in surgery. Ann Surg. 2006;243:628–632. doi: 10.1097/01.sla.0000216410.74062.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarborough JE, Pietrobon R, Clary BM, Marroquin CE, Bennett KM, Kuo PC, et al. Regionalization of hepatic resections is associated with increasing disparities among some patient populations in use of high-volume providers. J Am Coll Surg. 2008;207:831–838. doi: 10.1016/j.jamcollsurg.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]


