Abstract
Background:
A number of prognostically relevant clinicopathological variables have been proposed for pancreatic neuroendocrine neoplasms. However, a standardized prognostication system has yet to be established for patients undergoing potentially curative tumour resection.
Methods:
We examined a prospectively maintained, single-institution database to identify patients who underwent potentially curative resection of non-metastatic primary pancreatic neuroendocrine neoplasms. Patient, operative and pathological characteristics were analysed to identify variables associated with disease-specific and disease-free survival.
Results:
Between 1991 and 2007, 43 patients met inclusion criteria. After a median follow-up of 68 months, 5-year disease-specific survival was 94% and 5-year disease-free survival was 72%. Tumours sized ≥5 cm and vascular invasion were associated with worse disease-specific survival. Tumours sized ≥5 cm, nodal metastases, positive resection margins and perineural invasion were associated with worse disease-free survival. A scoring system consisting of tumour size ≥5 cm, histological grade, nodal metastases and resection margin positivity (SGNM) permitted stratification of disease-specific (P= 0.006) and disease-free (P= 0.0004) survival. This proposed scoring system demonstrated excellent discrimination of individual disease-specific and disease-free survival outcomes as reflected by concordance indices of 0.814 and 0.794, respectively.
Conclusions:
A simple scoring system utilizing tumour size, histological grade, nodal metastases and resection margin status can be used to stratify outcomes in patients undergoing resection of primary pancreatic neuroendocrine neoplasms.
Keywords: neuroendocrine, pancreas, surgery, prognostication
Introduction
Pancreatic neuroendocrine neoplasms are rare tumours with an annual incidence of one to five per 100 000.1,2 Perhaps as a result of increased awareness and improved diagnostic capabilities, their prevalence has increased substantially over the last 25 years.3 Although usually indolent, the biological behaviour of pancreatic neuroendocrine neoplasms ranges from benign to frankly malignant. However, classic criteria that define malignant behaviour, such as local invasion and presence of lymph node or distant metastases, have not always correlated with risk of recurrence and death.4
A number of clinical and pathological parameters have been used to classify these neoplasms for prognostication and to define criteria for surgical therapy. Incorporation of staging variables (size, lymph node and distant metastases) and histological variables (presence of necrosis, secretory function, mitotic index,proliferative index, perineural invasion, vascular invasion) have resulted in accurate, but sometimes complex, classification schemes.4,5 Most of these have divided pancreatic neuroendocrine neoplasms into well- and poorly differentiated neoplasms; high-grade neuroendocrine carcinoma appears to be a separate disease entity with a poor prognosis regardless of treatment.6
Previous analyses of resected pancreatic neuroendocrine neoplasms have included patients with metastatic and locally advanced tumours, or have excluded patients with hormone-secreting tumours.4,5,7–10 This heterogeneity is likely to render their findings less applicable to patients with resectable, localized disease. In this study, we analysed the individual, operative and pathological characteristics of patients with non-metastatic pancreatic neuroendocrine neoplasms undergoing potentially curative tumour resection. Our aim was to create a simplified, reproducible prognostication system to stratify this patient population according to risk for disease recurrence and death.
Materials and methods
Patients who underwent potentially curative resection of primary pancreatic neuroendocrine neoplasms at the University of Wisconsin School of Medicine and Public Health were identified from a prospectively maintained database after institutional review board approval. Potentially curative resection was defined as surgical removal of all macroscopic disease (R0 or R1 resection); patients undergoing repeat tumour resections and those with evidence of distant metastatic disease and/or locally advanced unresectable disease were excluded from analysis. Tumour location was determined preoperatively with computed tomography, magnetic resonance imaging and somatostatin receptor scintigraphy as deemed necessary by the treating physicians. Additional therapeutic modalities including chemotherapy and somatostatin analogue therapy were used at the discretion of treating physicians. Demographic and operative data were collected, and all pathological specimens were re-examined by a single pathologist (AGL) to confirm the diagnosis of pancreatic neuroendocrine neoplasm and to analyse histological characteristics. These included tumour size, location, perineural and vascular invasion, mitotic rate, necrosis, status of surgical margin and lymph node metastases. The lymph node ratio (LNR), defined as the ratio of malignant to total lymph nodes resected, was calculated for each patient. Neoplasms were classified as low grade (≤2 mitoses per 50 high-power fields and no necrosis) or intermediate grade (>2 mitoses per 50 high-power fields and/or focal necrosis) according to previously defined criteria.4 Patients were followed clinically and with tumour markers and cross-sectional imaging as deemed necessary. Medical record review and patient contact were performed to obtain information on survival and disease status. Disease recurrence was defined by radiographic evidence of new tumours. Overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS) were calculated using the Kaplan–Meier method. Univariate analysis was performed with the log-rank test for categorical variables and with Cox regression analysis for continuous variables. Statistical significance was defined as P < 0.05. All variables found to have P < 0.1 by univariate analysis for either DSS or DFS were combined into potential three- or four-variable scoring systems in which one point was assigned for the presence of each potentially prognostic variable. Depending on the presence of each variable, scores lay in the range of 0–3 for three-variable systems and 0–4 for four-variable systems. The association between each possible scoring system combination and DSS and DFS was evaluated using the log-rank test. The predictive ability of the optimal prognostic scoring system to discriminate DSS and DFS outcomes was quantified using the Harrell's concordance index (c-index).11 The c-index is calculated as the probability that, for each randomly generated pair of patients from a dataset, the patient with the favourable prognostic score survived longer than the patient with the unfavourable prognostic score. A prognostication system with a c-index of 1 indicates that the prognostic system predicted the correct outcome in all cases; a c-index of 0 implies that the incorrect outcome was predicted in all cases, and a c-index of 0.5 means that the system correctly predicted the outcome 50% of the time (in effect, demonstrating no predictive ability). Non-informative pairs (e.g. pairs for whom survival was equal or in which the survivor had a shorter follow-up interval) were not included in the calculation of the c-index.
Results
Patient characteristics
A total of 43 patients underwent potentially curative tumour resection of non-metastatic pancreatic neuroendocrine neoplasms between 1991 and 2007. The majority of patients were female and most tumours were localized in the pancreatic body and tail (Table 1).
Table 1.
Summary of demographic, histological and operative characteristics of 43 patients undergoing potentially curative resection of localized primary pancreatic neuroendocrine neoplasms
| Variable | Mean ± SD (% or range) |
|---|---|
| Age, years | 53.4 ± 15.6 (24–81) |
| Female | 29 (67%) |
| Operation | |
| Pancreaticoduodenectomy | 15 (35%) |
| Distal pancreatectomy | 23 (53%) |
| Enucleation | 5 (12%) |
| Maximal tumour size, cm | 4.3 ± 3.3 (0.5–17.0) |
| Tumour number | 1.2 ± 0.4 (1–3) |
| Multifocal disease | 6 (14%) |
| Microscopic margin positivity | 7 (18%) |
| Nodal metastases | 18 (42%) |
| Number of nodes resected | 7.2 ± 7.7 (0–38) |
| Number of positive nodes | 1.1 ± 2.4 (0–11) |
| Lymph node ratio | 0.14 ± 0.24 (0–1) |
| Vascular invasion | 4 (10%) |
| Perineural invasion | 6 (14%) |
| Grade | |
| Low | 19 (44%) |
| Intermediate | 24 (56%) |
| 60-day mortality | 0 (0%) |
| Length of stay, days | 8.4 ± 4.5 (3–28) |
| Median length of stay, days | 7 |
| Median follow-up of survivors, months | 68 |
SD, standard deviation
Perioperative characteristics
Median postoperative length of hospital stay was 7 days (range 3–28 days). The majority of patients underwent formal pancreatic resections for tumour extirpation. Most patients underwent R0 resections and no perioperative mortalities were encountered (Table 1).
Histological characteristics
Nineteen low-grade and 24 intermediate-grade neoplasms were identified in this patient population. Thirty-seven patients had single tumours and six patients had two or three tumours resected. The mean size of the largest tumours resected was 43 mm (range 5–170 mm). Vascular, perineural and lymphatic invasion were seen in four, six and eight patients, respectively. Eighteen patients had lymph node metastases. The mean number of lymph nodes resected was seven (range 0–38) (Table 1).
Outcomes
After a median follow-up of 68 months, 5-year OS, DSS and DFS were 91%, 94% and 72%, respectively (Fig. 1). Median OS, DSS and DFS were not reached. Three patients had local or regional recurrence and four were diagnosed with distant recurrence during follow-up. Four of these seven patients had undergone R0 resections. Median time to recurrence was 24 months (range 4–96 months) and median survival after recurrence was 49 months. The onset of disease recurrence was associated with worse overall survival (P= 0.012).
Figure 1.
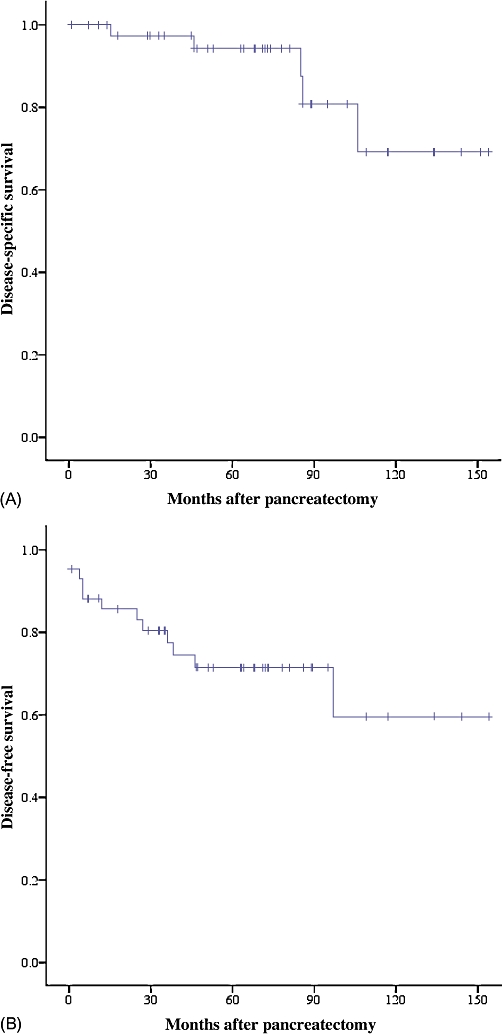
Kaplan–Meier estimates of survival in 43 patients undergoing potentially curative resection of primary pancreatic neuroendocrine neoplasms. (A) Disease-specific survival (n= 43); (B) disease-free survival (n= 43)
Prognostic factors
On univariate analysis, variables associated with worse DSS were size ≥5 cm (P= 0.041) and lymphovascular invasion (P= 0.044). Multivariate analysis was not undertaken because of the limited sample size and number of events. Variables associated with worse DFS on univariate analysis were tumour size ≥5 cm (P= 0.032), positive resection margin (P < 0.0001), lymph node metastasis (P= 0.007) and perineural invasion (P= 0.014) (Tables 2 and 3).
Table 2.
Demographic, histological and operative variables associated with disease-specific survival in 43 patients undergoing potentially curative resection of localized primary pancreatic neuroendocrine neoplasms
| Variable | Number of patients | Median DSS, months | P-value (univariate) | Median DFS, months | P-value (univariate) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 14 | NR | 97 | ||
| Female | 29 | NR | 0.457 | NR | 0.487 |
| Age | |||||
| <50 years | 16 | NR | NR | ||
| ≥50 years | 27 | NR | 0.896 | 97 | 0.400 |
| Grade | |||||
| Low | 19 | NR | NR | ||
| Intermediate | 24 | NR | 0.244 | 97 | 0.091 |
| Tumour size | |||||
| <5 cm | 29 | NR | NR | ||
| ≥5 cm | 14 | 106 | 0.041 | 97 | 0.032 |
| Tumour multifocality | |||||
| Absent | 37 | NR | NR | ||
| Present | 6 | 86 | 0.724 | 97 | 0.318 |
| Margin | |||||
| Negative | 36 | NR | NR | ||
| Positive | 7 | 106 | 0.074 | 25 | <0.001 |
| Nodal status | |||||
| Negative | 25 | NR | NR | ||
| Positive | 18 | NR | 0.486 | 46 | 0.007 |
| Vascular invasion | |||||
| Absent | 39 | NR | NR | ||
| Present | 4 | NR | 0.038 | 38 | 0.349 |
| Perineural invasion | |||||
| Absent | 37 | NR | NR | ||
| Present | 6 | NR | 0.422 | 25 | 0.014 |
Statistically significant values are shown in bold
DFS, disease-free survival; DSS, disease-specific survival; NR, not reached
Table 3.
Disease-specific and disease-free survival in 43 patients with resected pancreatic neuroendocrine neoplasms
| SGNM score | n | 5-year DSS, % | 5-year DFS, % |
|---|---|---|---|
| 0 | 8 | 100 | 100 |
| 1 | 17 | 100 | 86 |
| 2 | 11 | 89 | 60 |
| 3 | 4 | 89 | 25 |
| 4 | 3 | 67 | 33 |
SGNM, tumour size, grade, lymph node status, resection margin status; DFS, disease-free survival; DSS, disease-specific survival
Scoring system
The variables of tumour size ≥5 cm, intermediate histological grade, positive margin, nodal metastases, vascular invasion and perineural invasion were analysed as three- or four-variable scoring systems. A scoring system giving one point each for tumour size ≥5 cm, intermediate histological grade, presence of lymph node metastases and resection margin positivity (SGNM) was found to have the strongest association with DSS and DFS. To permit optimal discrimination of outcomes, this scoring system was used to divide patients into two groups (with scores of 0–1 and 2–4) for stratification of DSS outcomes and into four groups (with scores of 0, 1, 2 and ≥3) for stratification of DFS outcomes. Increasing SGNM score correlated with DSS (P= 0.006) and DFS (P= 0.0004). The c-index of the DSS scoring system was 0.814 (95% confidence interval [CI] 0.727–0.902); the c-index of the DFS scoring system was 0.797 (95% CI 0.677–0.911) (Tables 2–5; Figs 2 and 3).
Table 5.
Disease-free survival based on SGNM score in 43 patients undergoing potentially curative resection of primary pancreatic neuroendocrine neoplasms
| SGNM score | n | 5-year DFS, % | P-value |
|---|---|---|---|
| 0 | 18 | 100 | 0.004 |
| 1 | 17 | 86 | |
| 2 | 11 | 60 | |
| 3–4 | 7 | 14 |
Statistically significant values are shown in bold
SGNM, tumour size, grade, lymph node status, resection margin status; DFS, disease-free survival
Figure 2.
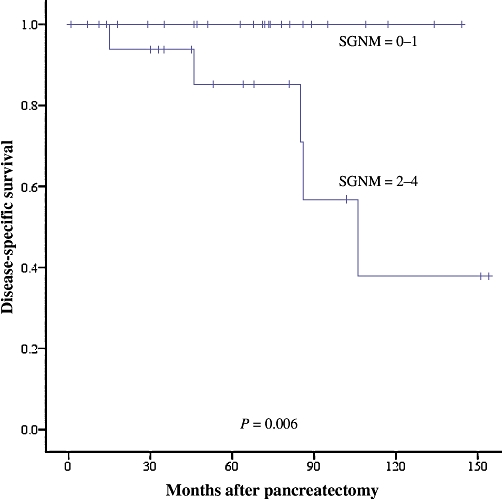
Disease-specific survival according to SGNM score in 43 patients undergoing potentially curative resection of primary pancreatic neuroendocrine neoplasms (P= 0.006). SGNM, size, grade, lymph node status, resection margin status
Figure 3.
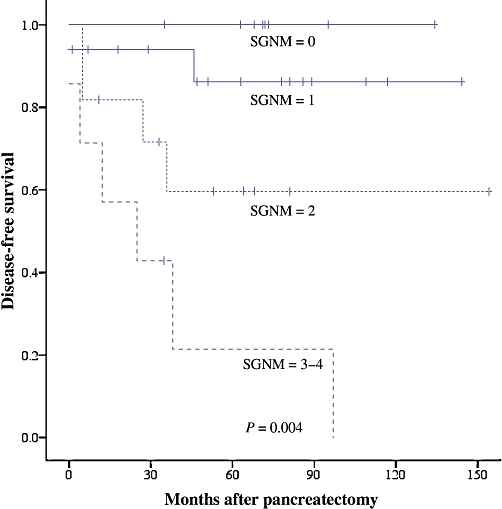
Disease-free survival according to SGNM score in 43 patients undergoing potentially curative resection of primary pancreatic neuroendocrine neoplasms (P= 0.004). SGNM, size, grade, lymph node status, resection margin status
Table 4.
Disease-specific survival based on SGNM score in 43 patients undergoing potentially curative resection of primary pancreatic neuroendocrine neoplasms
| SGNM score | n | 5-year DSS, % | P-value |
|---|---|---|---|
| 0–1 | 25 | 100 | 0.006 |
| 2–4 | 18 | 86 |
Statistically significant values are shown in bold
SGNM, tumour size, grade, lymph node status, resection margin status; DSS, disease-specific survival
Discussion
Optimal treatment guidelines for pancreatic neuroendocrine neoplasms are obfuscated by their relative rarity and indolence. It is evident that patients with localized neoplasms are more likely to undergo attempted resection and have significantly better prognoses than patients with locally advanced or metastatic neoplasms.12,13 Most studies that have attempted to define prognostic criteria for pancreatic neuroendocrine neoplasms have included patients with localized and metastatic neoplasms; as a result, their findings may have limited applicability for patients with localized disease.4,5,13–15 The World Health Organization system divides pancreatic neuroendocrine neoplasms into well-differentiated neuroendocrine tumours, well-differentiated neuroendocrine tumours of uncertain behaviour, well-differentiated pancreatic neuroendocrine carcinomas and poorly differentiated neuroendocrine carcinomas.16,17 This system incorporates the presence of metastases, gross invasion, tumour diameter, angio-invasion, mitoses and proliferative index. It has been criticized for being based more on expert opinion than on published data,5 and has limited utility for the subset of patients undergoing complete gross resection of pancreatic tumours. Bilimoria et al.12 used the National Cancer Database to validate the American Joint Committee on Cancer (AJCC) classification system for pancreatic adenocarcinomas for prognostication of pancreatic neuroendocrine neoplasms. They reported that tumour size and the presence of lymph node and distant metastases can be used to reliably stratify operatively managed and non-operatively managed pancreatic neuroendocrine patients according to 5- and 10-year survival.12 Similarly, the European Neuroendocrine Tumour Society and Rindi et al. examined the use of the tumor node metastasis (TNM) system for all gastroenteropancreatic neuroendocrine tumours.8,18 In the present study, we focused on patients undergoing resection of localized pancreatic neuroendocrine neoplasms and determined that tumour size, histological grade, lymph node metastases and resection margin status can be combined in a simplified prognostication system (SGNM) that correlates with risk for disease recurrence (P= 0.0004) and death (P= 0.0006). This scoring system demonstrates a strong predictive ability to discriminate DSS and DFS outcomes as measured by its c-indices of 0.797 and 0.814, respectively. These quantitative measures of predictive ability compare very favourably with c-indices that have been calculated for novel prognostic nomograms developed to predict individual outcomes for patients with pancreatic adenocarcinoma (0.64),19 hepatocellular carcinoma (0.74),20 extremity sarcoma (0.77)21 and gastric adenocarcinoma (0.80).22
This SGNM scoring system is more effective in discriminating differential outcomes for disease recurrence than for disease-related mortality. This probably reflects the paucity of patients who died over the course of follow-up in our study. Indeed, after a median follow-up of 68 months, 5-year OS and DSS rates were 91% and 94%, respectively. By contrast, our own recent analysis of patients undergoing resection of hepatic neuroendocrine metastases identified a 5-year OS of 65%.23 In previous studies that have grouped patients with localized and advanced or metastatic pancreatic neuroendocrine neoplasms together, 5-year survival has ranged between 49% and 93%.2,4,5,10,14,15,24
We observed that tumour size >5 cm correlated with DSS and DFS on univariate analysis (P= 0.041 and P= 0.032, respectively). Other authors have similarly reported that tumour size correlates with survival on univariate, but not multivariate, analysis.4,5,15
The prognostic relevance of tumour necrosis and mitotic rate were previously validated by Hochwald et al., who used these histological factors to classify pancreatic neuroendocrine neoplasms into low and intermediate grades that correlate with survival.4,25 These same criteria have also been shown to be prognostically important for resected hepatic neuroendocrine neoplasms.23 In the present study, the association between histological grade (using these same criteria) and disease recurrence neared but did not reach statistical significance (P= 0.091). However, inclusion of histological grade in our proposed classification scheme permitted more accurate prognostication of DSS (P= 0.123 without grade, P= 0.006 with grade) and better discrimination of differences in DFS (data not shown). The presence of vascular invasion and that of perineural invasion were independently associated with worse DSS and DFS, respectively; however, inclusion of these pathological parameters did not improve the ability of the scoring system to discriminate expected survival or recurrence outcomes (data not shown).
We observed a significant correlation between nodal metastases and DFS, and including this parameter into our scoring system enhanced prognostication of both DSS and DFS (data not shown). Lymph node ratio (defined as the ratio of positive lymph nodes to the total number of lymph nodes examined) was also prognostically relevant, but its inclusion did not improve the prognostic strength of our scoring system over that seen with nodal status alone (data not shown). In a study of 29 patients with resected pancreatic neuroendocrine neoplasms, Sarmiento et al. reported a statistically significant association between lymph node metastases and OS.24 As stated above, the pancreatic adenocarcinoma AJCC TNM staging system, which incorporates the presence of nodal metastases, has also been found to have some utility for pancreatic neuroendocrine neoplasms.8,12,18 Previous analysis has demonstrated that the presence of lymph node metastases does not correlate with tumour size;4 this biological independence may explain its utility as an independent variable in our proposed SGNM scoring system.
We observed a strong association between positive resection margins and worse DFS (P < 0.001), whereas the association between positive resection margins and DSS neared but did not reach statistical significance (P= 0.074). Few other studies have examined the prognostic impact of this variable. In a study of 60 patients with resected pancreatic neuroendocrine neoplasms with and without distant metastases, Phan et al. found margin status to be the most powerful predictor of survival; the impact of margin status on survival of patients without metastases was not reported.2 In a recent study of patients undergoing partial hepatectomy for hepatic neuroendocrine neoplasms, margin status was also associated with survival.23
Our study is clearly limited by its relatively small sample size, its reliance on a single-institution database, and the retrospective nature of its data collection. As a result, our proposed SGNM scoring system will require validation either prospectively or with larger datasets. Perhaps as a result of the small number of deaths in our series (despite its 68-month follow-up), the scoring system appears to have more utility for the prediction of recurrence than death. Importantly, the onset of disease recurrence portended an adverse outcome, as recurrence was associated with decreased survival (P= 0.012). Given the prolonged survival that is to be expected in patients undergoing resection of pancreatic neuroendocrine neoplasms, further discrimination of differences in DSS will probably require a larger sample size and longer follow-up. We did not examine the prognostic value of tumour secretory function because the majority of previous studies have not shown an association.4,14,26,27 Although some authors have reported that functional tumours have better prognoses than non-functional neoplasms,26,28,29 this finding probably reflects symptoms produced by hormone secretion, which lead to earlier diagnosis and smaller tumour size at the time of resection.2,4,24 Finally, we were unable to control for utilization of postoperative oncological therapies in this retrospective analysis. However, in light of the paucity of effective adjuvant therapies available for patients with resected pancreatic neuroendocrine neoplasms,30 it is unlikely that the use of such treatments would have significantly altered the course of post-resection survival.
In conclusion, we propose a simple SGNM scoring system that utilizes the reproducible and readily evaluable parameters of tumour size ≥5 cm, histological grade, nodal metastases and resection margin status to stratify patients who are undergoing potentially curative resection of pancreatic neuroendocrine neoplasms according to their risk for recurrence and death.
Conflicts of interest
None declared.
References
- 1.Alexakis N, Neoptolemos JP. Pancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2008;22:183–205. doi: 10.1016/j.bpg.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumours: review of 125 patients. J Gastrointest Surg. 1998;2:473–482. doi: 10.1016/S1091-255X(98)80039-5. [DOI] [PubMed] [Google Scholar]
- 3.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumours. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 4.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, et al. Prognostic factors in pancreatic neuroendocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt AM, Anlauf M, Rousson V, Schmid S, Kofler A, Riniker F, et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumours. Am J Surg Pathol. 2007;31:1677–1682. doi: 10.1097/PAS.0b013e31805f675d. [DOI] [PubMed] [Google Scholar]
- 6.Nasser H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of Vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588–594. doi: 10.1097/01.pas.0000157974.05397.4f. [DOI] [PubMed] [Google Scholar]
- 7.Van Eeden S, Quaedvlieg PF, Taal BG, Offerhaus GJ, Lamers CB, Van Velthuysen ML. Classification of low-grade neuroendocrine tumours of midgut and unknown origin. Hum Pathol. 2002;33:1126–1132. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 8.Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumours. Cancer. 2008;13:256–265. doi: 10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 9.Solcia E, Rindi G, Paolotti D, La Rosa S, Capella C, Fiocca R. Clinicopathological profile as a basis for classification of the endocrine tumours of the gastroenteropancreatic tract. Ann Oncol. 1999;10(Suppl 2):9–15. doi: 10.1093/annonc/10.suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 10.Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumours. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluation assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Merkow RP, Tomlinson JS, Stewart AK, Ko CY, et al. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumours. J Am Coll Surg. 2007;205:558–563. doi: 10.1016/j.jamcollsurg.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Solorzano CC, Lee JE, Pisters PW, Vauthey JN, Ayers GD, Jean ME, et al. Non-functioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 14.Jarufe NP, Coldham C, Orug T, Mayer AD, Mirza DF, Buckels JA, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157–162. doi: 10.1159/000087148. [DOI] [PubMed] [Google Scholar]
- 15.La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, et al. Prognostic criteria in non-functioning pancreatic endocrine tumours. Virchows Arch. 1996;429:323–333. doi: 10.1007/BF00198436. [DOI] [PubMed] [Google Scholar]
- 16.Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 17.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumours: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 18.Rindi G, Villanacci V, Ubiali A. Lamberts SWJ, Doglioti L. Advances in Oncology. Bristol: Bioscientifica; 2002. Classification of neuroendocrine phenotype in cancer; pp. 31–42. [Google Scholar]
- 19.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho CS, Gonen M, Jarnagin WR, D'Angelica MI, Blumgart LH, DeMatteo RP. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281–291. doi: 10.1016/j.jamcollsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 22.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 23.Cho CS, Labow DM, Tang L, Klimstra DS, Loeffler AG, Leverson GE, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer. 2008;113:126–134. doi: 10.1002/cncr.23523. [DOI] [PubMed] [Google Scholar]
- 24.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumours of the pancreas: longterm survival analysis. World J Surg. 2002;26:1267–1271. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 25.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 26.White TJ, Edney JA, Thompson JS, Karrer FW, Moor BJ. Is there a prognostic difference between functional and non-functional islet cell tumours? Am J Surg. 1994;168:627–630. doi: 10.1016/s0002-9610(05)80134-5. [DOI] [PubMed] [Google Scholar]
- 27.Lo CY, van Heerden JA, Thompson GB, Grant CS, Söreide JA, Harmsen WS. Islet cell carcinoma of the pancreas. World J Surg. 1996;20:878–883. doi: 10.1007/s002689900134. [DOI] [PubMed] [Google Scholar]
- 28.Kent RB, Van Heerden JA, Weiland LH. Non-functioning islet cell tumours. Ann Surg. 1981;193:185–190. doi: 10.1097/00000658-198102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckhauser FE, Cheung PS, Vinik AJ, Strodel WE, Lloyd RV, Thompson NW. Non-functioning malignant neuroendocrine tumours of the pancreas. Surgery. 1986;100:978–988. [PubMed] [Google Scholar]
- 30.Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother. 2008;9:2617–2626. doi: 10.1517/14656566.9.15.2617. [DOI] [PubMed] [Google Scholar]


