Abstract
Background:
In patients with hilar cholangiocarcinoma, ipsilateral en bloc hepatic resection improves survival but is associated with increased morbidity. Preoperative biliary drainage of the future liver remnant (FLR) and contralateral portal vein embolization (PVE) may improve perioperative outcome, but their routine use is controversial. This study analyses the impact of FLR volume and preoperative biliary drainage on postoperative hepatic insufficiency and mortality rates.
Methods:
Patients who underwent hepatic resection and for whom adequate imaging data for FLR calculation were available were identified retrospectively. Patient demographic, operative and perioperative data were recorded and analysed. The volume of the FLR was calculated based on the total liver volume and the volume of the resection that was actually performed using semi-automated contouring of the liver on preoperative helical acquired scans. In patients subjected to preoperative biliary drainage, the preoperative imaging was reviewed to determine if the FLR had been decompressed. Hepatic insufficiency was defined as a postoperative rise in bilirubin of 5 mg/dl above the preoperative level that persisted for >5 days postoperatively. Operative mortality was defined as death related to the operation, whenever it occurred.
Results:
Sixty patients were identified who underwent hepatic resection between 1997 and 2007 and for whom imaging data were available for analysis. During this period, preoperative biliary drainage of the FLR was used selectively and PVE was used in only one patient. The mean age of the patients was 64 ± 11.6 years and 68% were male. The median length of stay was 14 days and the overall morbidity and mortality were 53% and 10%, respectively. Preoperative FLR volume was a predictor of hepatic insufficiency and death (P= 0.03). A total of 65% of patients had an FLR volume ≥30% (39/60) of the total volume. No patient in this group had hepatic insufficiency, but there were two operative deaths (5%), both occurring in patients who underwent preoperative biliary drainage. By contrast, in the group with FLR < 30% (21/60, 35%), hepatic insufficiency was seen in five patients and operative mortality in four patients, and were strongly associated with lack of preoperative biliary drainage of the FLR (P= 0.009). Patients with an FLR ≥ 30% were more likely to have radiographic evidence of ipsilateral lobar atrophy and hypertrophy of the FLR (46.2% vs. 9.5% in patients with FLR < 30%; P= 0.004).
Conclusions:
In patients undergoing liver resection for hilar cholangiocarcinoma, FLR volume of < 30% of total liver volume is associated with increased risk for hepatic insufficiency and death. Preoperative biliary drainage of the FLR appears to improve outcome if the predicted volume is < 30%. However, in patients with FLR ≥ 30%, preoperative biliary drainage does not appear to improve perioperative outcome and, as many of these patients have hypertrophy of the FLR, PVE is likely to offer little benefit.
Keywords: hilar cholangiocarcinoma, klatskin tumor, jaundice, biliary drainage
Introduction
There have been several studies advocating ipsilateral en bloc partial hepatectomy in the treatment of hilar cholangiocarcinoma to increase the rate of negative histologic margins and improve survival.1–9 However, extended liver resection in this setting is associated with significant risk for postoperative hepatic insufficiency and other complications. Most retrospective large series reveal mortality rates of 0–20% and morbidity rates of 14–67%.2,5,6,8,10–19 In an attempt to improve perioperative outcome, many centres have advocated extensive preoperative biliary drainage and ipsilateral portal vein embolization (PVE) (embolization of the hemi-liver to be resected) in order to improve the function of the future liver remnant (FLR).
The role of preoperative biliary drainage prior to liver resection for hilar cholangiocarcinoma has been debated for many years. Recent review articles in HPB have offered conflicting views supporting routine biliary drainage20 and selective preoperative biliary drainage.21 However, there are no prospective randomized studies analysing the utility of preoperative biliary drainage prior to extended liver resection for hilar cholangiocarcinoma. Indeed, all the studies that show no benefit to preoperative biliary drainage have primarily involved patients undergoing biliary drainage for periampullary malignancy without concomitant liver resection.22–26
In the management of hilar cholangiocarcinoma, many Asian centres have advocated routine extensive biliary drainage prior to operation.3,6,17,20,27 This approach appears to have been primarily based on experimental studies showing the deleterious immunologic effects of cholestatic jaundice and increased susceptibility to endotoxaemia that are partially reversed after biliary drainage procedures.28,29 Recent clinical studies from several Japanese centres have shown generally low mortality rates when utilizing a strategy of preoperative biliary drainage, PVE (for right-sided and extended left-sided resections) and major hepatobiliary resection.3,8 However, many Western centres have been more selective in their utilization of biliary drainage. The rationale against routine drainage has involved the increased risk for infectious complications with endoscopic or percutaneous drainage catheters and the risk for tumour seeding associated with percutaneous biliary drainage.30,31 A retrospective case comparison by Cherqui et al. revealed no differences in mortality or recovery of hepatic synthetic function between patients who did or did not undergo preoperative biliary drainage.32 A recent review article by the same group has advocated selective utilization of preoperative biliary drainage only in patients with cholangitis, longstanding jaundice, poor nutrition and a liver remnant volume of <40% of total volume.21
The purpose of this study was to evaluate the impact of preoperative biliary drainage, stratified by FLR volume, on postoperative hepatic dysfunction and mortality in patients undergoing major liver resection for proximal biliary cancer. Given that many patients with hilar cholangiocarcinoma have hypertrophy of the FLR, which is primarily caused by ipsilateral portal vein involvement and is likely to be protective against postoperative liver failure, our goal was to determine if biliary drainage could be targeted at the subgroup of patients with no hypertrophy of the FLR.
Materials and methods
From a prospective database, we identified all patients with a pathologic diagnosis of hilar cholangiocarcinoma who underwent en bloc partial hepatectomy as part of their treatment at Memorial Sloan Kettering Cancer Center (MSKCC) during 1991–2007. We then reviewed all preoperative imaging studies available to determine which patients had preoperative computed tomography (CT) or magnetic resonance imaging (MRI) scans that were sufficient for volumetric analysis. Patients with imaging prior to 1997 and those who presented with scans from other institutions were excluded as these images were not suitable for calculation of FLR volume. The current study therefore includes 60 patients with hilar cholangiocarcinoma who underwent liver resection and for whom preoperative imaging was adequate for volumetric analysis. Some of these patients have been included in previous studies from this institution.1,11
All imaging data (helical CT or MRI) were transferred to an independent workstation for assessment. Retrospectively, total liver volume and the volume of liver resected were calculated as previously described.33 Total liver volume and volume of resection were determined by semi-automated contouring of the liver on preoperative CT or MRI scans. This was performed on serial transverse scans at either 0.25-cm or 0.5-cm intervals, including the entire liver. On each slice the total liver was outlined and the sum of the slices calculated by integrated software techniques using the density threshold. This was repeated for the volume of the liver to be resected (based on the actual resection that had been performed). The difference between total liver volume and resected volume was the volume of liver remaining, or the FLR. The FLR was determined for each patient.
All patients in this study underwent ipsilateral en bloc partial hepatectomy for a potentially curative resection utilizing a standardized approach, as previously described.1,11,34,35 Full exploration was performed to exclude any evidence of metastatic disease. Exposure of the biliary confluence and assessment for vascular involvement were accomplished by early transection of the common bile duct at the level of the duodenum with reflection superiorly. En bloc partial hepatectomy was performed in the entire extrahepatic biliary system (supraduodenal bile duct and gall bladder) and included a subhilar lymphadenectomy (clearance of all lymph nodes within the hepatoduodenal ligament to the level of the common hepatic artery). Caudate lobectomy was performed routinely for tumours involving the left hepatic duct and in any patient in whom it was considered necessary to achieve complete tumour clearance. Histologic assessment of resection margins was performed intraoperatively and additional tissue was resected, if feasible, when residual microscopic disease was suspected on frozen-section histology. Resection and reconstruction of major vascular structures were performed when necessary to achieve tumour clearance for disease that was otherwise resectable. Roux-en-Y biliary–enteric reconstruction was performed to a segment of jejunum approximately 70 cm in length.
Patient demographic, operative and perioperative data were recorded and analysed (Table 1). The hepatic resections performed included right or left hepatectomy or extended right or extended left hepatectomy. Other operative variables analysed were need for portal vein resection with reconstruction and need for concomitant pancreaticoduodenectomy. Preoperative imaging was reviewed to determine whether the patient had evidence of lobar atrophy of the involved lobe with hypertrophy of the remnant liver. Hepatic lobar atrophy was considered to be present if cross-sectional imaging demonstrated a small, often hypoperfused lobe, with crowding of dilated intrahepatic ducts, as previously described.1 Specific attention was paid to preoperative biliary drainage and the data analysed included the method of drainage (endoscopic, percutaneous or operative), the portion of liver drained, and the preoperative serum bilirubin level. In addition, post-drain placement scans were reviewed in detail by the study radiologist (LS) to determine the efficacy of the procedure. In the event of preoperative biliary drainage, persistent dilatation of the intrahepatic bile ducts in the FLR was taken as evidence of inadequate decompression.
Table 1.
Demographics and operative data
| Variable | Number |
|---|---|
| Demographics | |
| Median age, years | 65 (range 35–87) |
| Male/female, n | 41/19 |
| Preoperative biliary stent, n | 49 (82%) |
| Preoperative liver remnant drained, n | 31 (52%) |
| Portal vein embolization, n | 1 (2%) |
| Mean ± SD preoperative serum bilirubin, mg/dl | 3.9 ± 5.6 |
| Operation | |
| Right hepatectomy, n | 5 |
| Left hepatectomy, n | 19 |
| Extended right hepatectomy, n | 30 |
| Extended left hepatectomy, n | 6 |
| Caudate resection, n | 29 |
| Portal vein resection, n | 5 |
| Whipple, n | 1 |
SD, standard deviation
Postoperative complications were recorded and graded on a scale of 0 (no complications) to 5 (perioperative mortality), as previously described.36 Postoperative hepatic insufficiency was defined by a rise in bilirubin >5 mg/dl that persisted for >5 days postoperatively. Operative mortality was defined as death during the index hospitalization or within 90 days of surgery. Patients were stratified into two groups according to whether FLR volume was <30% or ≥30% of total liver volume. Statistical calculations were performed using spss Version 17 (SPSS, Inc., Chicago, IL, USA). Continuous variables were compared using Student's t-test (two-tailed) and categorical variables with chi-squared test. Logistic regression was used to determine independent predictors of outcome, using death and hepatic dysfunction as the dependent variable. P-values < 0.05 were considered significant. Numeric data are presented as median values and/or mean values ± one standard deviation.
Results
Demographics and operative results
From January 1991 until October 2007, 106 patients underwent hepatic resection for treatment of hilar cholangiocarcinoma at MSKCC. For 60 (57%) of these patients, cross-sectional imaging studies from which volumetric analysis could be performed were available. Patients included 19 women (32%) and 41 men (68%), with a mean age of 64 ± 11.6 years (median 65 years, range 35–87 years). A total of 82% (49/60) of the patients underwent a preoperative biliary drainage procedure. Only one patient underwent PVE prior to resection. The mean preoperative bilirubin level was 3.9 ± 5.6 mg/dl (median 1.8 mg/dl, range 0.3–22.7 mg/dl).
Resectional procedures consisted of excision of the extrahepatic biliary tree, subhilar lymphadenectomy and en bloc partial hepatectomy in all patients. The hepatic resections performed were as follows: extended right hepatectomy (n= 30); right hepatectomy (n= 6); extended left hepatectomy (n= 5), and left hepatectomy (n= 19). En bloc caudate resection was performed in 29 patients (48.3%). The caudate resections were performed routinely in all cases of left and extended left hepatectomies. In addition, five patients submitting to extended right hepatectomy underwent concomitant caudate resection. Other procedures performed included portal vein resection with reconstruction in five patients and concomitant pancreaticoduodenectomy in one patient.
Mean hospital length of stay was 14.3 ± 9.8 days from the date of resection. Major complications (grade 3 or higher, including perioperative deaths) occurred in 32 patients (53%). Hepatic insufficiency (defined as an increase in postoperative bilirubin level >5 mg/dl that persisted for >5 days) was seen in five patients (8%). The overall mortality rate was 10% (six patients).
Volumetric analysis
Of the 60 imaging studies with which volumetric analysis was performed, 31 (52%) comprised MRI examinations and the remaining 29 (48%) consisted of CT scans. The median percentage of liver remaining (FLR) for all patients was 34.8%. Twenty-one patients had an FLR volume of < 30% and 12 had an FLR volume of <25%. Twenty patients had ipsilateral lobar atrophy with hypertrophy of the FLR. As expected, the presence of atrophy/hypertrophy strongly correlated with FLR volume. Of the 21 patients with FLR < 30%, only two had atrophy/hypertrophy identified on preoperative imaging, compared with 18 of 39 (46.2%) with an FLR ≥ 30% (P= 0.004), including 65% (15/23) of those with an FLR > 50%.
Biliary drainage
Of the 60 patients who underwent resection, 82% (49/60) underwent preoperative biliary drainage, including 100% (21/21) of patients with a calculated FLR volume of <30% (Figure 1). However, there was significant variation in both the method of drainage and its efficacy in decompressing the FLR. Percutaneous transhepatic biliary drainage was performed in 33 of 49 patients (67%). Of the remaining 16 patients, 14 had endoscopic biliary drainage only, one had a segment 3 bypass and another had an operatively placed drainage catheter; the latter two procedures were performed prior to referral. However, despite the high proportion of patients submitting to preoperative biliary drainage, only 63% (31/49) of drains were positioned to decompress the FLR, whereas the remaining 18 patients underwent drainage of the ipsilateral liver (i.e. the hemi-liver that was resected). Most of these patients presented having already undergone a biliary drainage procedure and, although serum bilirubin levels normalized in most patients, review of the post-drainage scans revealed persistently dilated ducts in the remnant liver.
Figure 1.
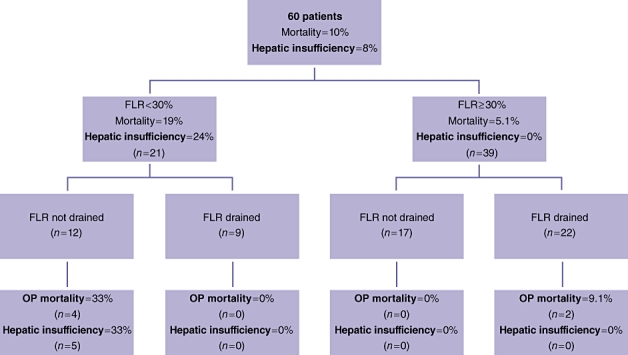
Outcomes in patients stratified by future liver remnant volume and adequacy of biliary decompression, FLR, future liver remnant; OP, operative
Perioperative results
Several preoperative variables were analysed to determine their relationships to postoperative hepatic insufficiency and death. Future liver remnant volume, preoperative drainage of the FLR, estimated blood loss and need for packed red blood cell (PRBC) transfusion were strongly associated with postoperative hepatic insufficiency and death on univariate analysis (Table 2). However, on multivariate analysis, only the FLR volume and need for PRBC transfusion reached statistical significance. Preoperative serum bilirubin level, preoperative albumin level, age and gender were not found to be predictive of perioperative outcome. Likewise, whether a patient underwent any preoperative biliary drainage procedure was not associated with postoperative hepatic insufficiency and death; however, there was some correlation between perioperative outcome and drainage of the FLR (P= 0.08 for postoperative hepatic insufficiency and death on multivariate analysis). A significant correlation between these two variables was highly dependent on whether the FLR was <30%, >30% or = 30% of total liver volume (see below). The impact of PVE on outcome could not be assessed directly as only one patient in this study underwent this procedure.
Table 2.
Univariate and multivariate analysis of preoperative predictors of hepatic insufficiency and death
| Variable | Univariate analysis P-value | Multivariate analysis P-value |
|---|---|---|
| Age | 0.53 | – |
| Gender | 0.73 | – |
| Preoperative albumin | 0.19 | – |
| Preoperative bilirubin | 0.24 | – |
| Any biliary drainage procedure | 0.10 | – |
| Drainage of future liver remnant | 0.019 | 0.08 |
| Future liver remnant volume | 0.012 | 0.019 |
| Estimated blood loss | 0.047 | 0.64 |
| Packed red blood cell transfusion | 0.002 | 0.008 |
FLR volume < 30% and biliary drainage of the FLR
An FLR < 30% was the post-potent predictor of postoperative hepatic dysfunction and death (P < 0.001). Twenty-one patients had a predicted FLR volume of <30%. Of these 21 patients, nine patients underwent drainage of the FLR and 12 did not. In the latter group, absence of preoperative FLR biliary drainage was strongly associated with postoperative hepatic insufficiency and death (P= 0.009), with four perioperative deaths and five patients developing postoperative hepatic dysfunction from which they eventually recovered. In all four deaths, hepatic dysfunction was seen early in the postoperative course. None of the nine patients who underwent adequate drainage of the FLR died or developed hepatic insufficiency (Figure 1).
FLR volume ≥ 30% and biliary drainage of the FLR
Thirty-nine patients had an FLR volume ≥ 30%. The perioperative outcome in this group was markedly better, with only two perioperative deaths and no cases of hepatic insufficiency (Figure 1). The two deaths occurred in patients who underwent preoperative biliary drainage of the liver remnant, although this association did not reach statistical significance; of the 17 patients with FLR ≥ 30% and no biliary drainage, none developed hepatic insufficiency and none died perioperatively. It is of note that the two deaths seen in this cohort, both in patients who underwent preoperative biliary drainage, were caused by portal vein thrombosis and intraoperative hypotension.
Discussion
The practice of preoperative biliary drainage prior to extended liver resection for hilar cholangiocarcinoma is widely variable. There have been no prospective randomized studies thatspecifically compare results in patients who have undergone preoperative biliary drainage prior to extended liver resection with results in patients who have not. Several centres have shown good results with the routine utilization of preoperative biliary drainage and PVE.3,8 Another group has demonstrated improved outcomes after modifying its management of patients with hilar cholangiocarcinoma to include a strategy of percutaneous biliary drainage instead of endoscopic drainage, preoperative PVE prior to right hepatectomy, routine caudate lobectomy and radical lymphadenectomy.37 However, the impact of each of these techniques on outcome is unclear. Furthermore, few studies take into account the incremental increase in cost, length of hospital stay and morbidity related to these procedures.
Efforts to identify those patients most likely to benefit from preoperative biliary drainage have been few, with most studies using drainage in all cases. Several studies do not support the routine use of biliary drainage prior to extended liver resection for hilar cholangiocarcinoma. Cherqui et al. conducted a case-control study comparing morbidity and mortality after liver resection in undrained jaundice patients and matched patients without biliary obstruction.32 This study found no difference in liver failure and mortality after liver resection in jaundiced and non-jaundiced patients. However, it did find that transfusion requirements and morbidity, specifically bile leaks and subphrenic abscesses, were significantly higher in patients with obstructive jaundice. Additionally, a study from MSKCC demonstrated increased infectious complications in patients submitted to biliary drainage.30
At MSKCC, patients frequently present having already undergone a variety of drainage procedures, often endoscopic, with varying degrees of success in terms of biliary decompression. In many cases, additional procedures are required in order to correct problems related to the initial drainage attempts. By contrast, some patients present without having undergone any prior biliary procedures. Over the time period of the current study, the authors used a policy of selective biliary drainage prior to major hepatectomy and preoperative PVE was not routinely utilized. The primary aim of this study was to determine which patients benefit most from preoperative biliary drainage.
Prior studies have shown that successful drainage of merely 30% of the liver in a patient with obstructive jaundice will normalize bilirubin level.38,39 In patients with hilar cholangiocarcinoma, isolation of the right and left biliary systems is common, and it is therefore possible to relieve jaundice by draining only one side of the liver, provided the drain is not placed in an atrophic lobe. Indeed, in the present study, the success rate of biliary drainage in normalizing serum bilirubin was very high. However, despite a normal serum bilirubin level, several patients had drainage of only the ipsilateral liver, with persistent dilatation of the intrahepatic ducts in the FLR, and the lack of a relationship between preoperative serum bilirubin level and perioperative outcome probably reflects this. In the present analysis, the only preoperative variable that independently predicted hepatic insufficiency and death was liver remnant volume; preoperative biliary drainage, in and of itself, was not a significant factor, which again is likely to be related to whether or not the FLR had been decompressed. Indeed, when the data were further analysed, adequate biliary decompression of the FLR (endoscopic, percutaneous or surgical) began to assume some association with perioperative hepatic dysfunction and mortality.
The true impact of preoperative biliary drainage emerged only when patients were stratified by FLR volume. In the group with FLR < 30%, the rate of hepatic insufficiency and death was 75% when biliary decompression of the FLR had not been performed, which is in striking contrast to the 0% rate in patients in whom adequate drainage had been achieved. However, a beneficial effect of preoperative biliary drainage was not seen in patients with a calculated FLR ≥ 30%. In this cohort, there were no cases of hepatic insufficiency and only two deaths, both of which occurred in patients who underwent drainage of the FLR. These observations would suggest that an adequate FLR, even in the face of persistent biliary obstruction, possesses sufficient hepatic functional reserve to support an extended resection, whereas a marginal FLR requires biliary decompression in order to enhance postoperative function. The strong association between FLR volume and clinical evidence of FLR hypertrophy further reinforces this conclusion. The findings also argue strongly for a selective approach to preoperative biliary drainage.
The role of preoperative PVE was not specifically addressed by this study and, as it was used in only one patient, a potential beneficial impact cannot be determined. However, given the favourable perioperative results observed in patients with an FLR volume ≥ 30%, which was also strongly correlated with FLR hypertrophy, preoperative PVE would be likely to offer little improvement in this group. However, patients with an FLR volume of < 30% might well derive some advantage from preoperative PVE, although how much this intervention would add, beyond the observed beneficial impact of biliary drainage alone, remains an open question.
In conclusion, for patients undergoing ipsilateral en bloc hepatic resection for hilar cholangiocarcinoma, an FLR volume of < 30% appears to be a strong indication for preoperative biliary drainage, provided the liver remnant is adequately decompressed. Whether preoperative PVE would further improve perioperative outcome is unknown, although the results of the present study would suggest a limited role. By contrast, patients with an FLR volume ≥ 30% appear to derive very little benefit from preoperative biliary drainage. In the latter group, preoperative PVE would likewise seem to offer little in terms of reducing postoperative liver-related morbidity and mortality.
Conflicts of interest
None declared.
References
- 1.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blungart LH. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. discussion 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128–134. doi: 10.1007/s005340050166. [DOI] [PubMed] [Google Scholar]
- 5.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 7.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. discussion 523–525. [DOI] [PubMed] [Google Scholar]
- 8.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhards MF, van Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma – a single centre experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–712. doi: 10.1097/01.sla.0000160817.94472.fd. discussion 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 14.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Okaya T, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg. 1999;189:575–583. doi: 10.1016/s1072-7515(99)00219-7. [DOI] [PubMed] [Google Scholar]
- 17.Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148–155. doi: 10.1016/j.surg.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabata M, Kawarada Y, Yokoi H, Higashiguchi T, Isaji S. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:148–154. doi: 10.1007/s005340050169. [DOI] [PubMed] [Google Scholar]
- 20.Nagino M, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Kondo S, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:25–30. doi: 10.1007/s00534-007-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent A, Tayar C, Cherqui D. Cholangiocarcinoma: preoperative biliary drainage (Con) HPB (Oxford) 2008;10:126–129. doi: 10.1080/13651820802007472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries-Jones R, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896–899. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- 23.Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–1198. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 24.McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH, et al. Preoperative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371–375. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- 25.Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP., Jr Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–553. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985;97:641–648. [PubMed] [Google Scholar]
- 27.Maguchi H, Takahashi K, Katanuma A, Osanai M, Nakahara K, Matuzaki S, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:441–446. doi: 10.1007/s00534-006-1192-3. [DOI] [PubMed] [Google Scholar]
- 28.Diamond T, Dolan S, Thompson RL, Rowlands BJ. Development and reversal of endotoxaemia and endotoxin-related death in obstructive jaundice. Surgery. 1990;108:370–374. discussion 374–375. [PubMed] [Google Scholar]
- 29.Roughneen PT, Gouma DJ, Kulkarni AD, Fanslow WF, Rowlands BJ. Impaired specific cell-mediated immunity in experimental biliary obstruction and its reversibility by internal biliary drainage. J Surg Res. 1986;41:113–125. doi: 10.1016/0022-4804(86)90016-8. [DOI] [PubMed] [Google Scholar]
- 30.Hochwald SN, Burke EC, Jarnagin WR, Fong Y, Blumgart LH. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. doi: 10.1001/archsurg.134.3.261. [DOI] [PubMed] [Google Scholar]
- 31.Sakata J, Shirai Y, Wakai T, Nomura T, Sakata E, Hatakeyama K. Catheter tract implantation metastases associated with percutaneous biliary drainage for extrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:7024–7027. doi: 10.3748/wjg.v11.i44.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 33.Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 34.Blumgart LH, Benjamin IS. Liver resection for bile duct cancer. Surg Clin North Am. 1989;69:323–337. doi: 10.1016/s0039-6109(16)44789-4. [DOI] [PubMed] [Google Scholar]
- 35.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RC, II, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg. 2002;235:803–813. doi: 10.1097/00000658-200206000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488–1494. doi: 10.1002/bjs.5482. [DOI] [PubMed] [Google Scholar]
- 38.Baer HU, Rhyner M, Stain SC, Glauser PW, Dennison AR, Maddern GJ, et al. The effect of communication between the right and left liver on the outcome of surgical drainage for jaundice due to malignant obstruction at the hilus of the liver. HPB Surg. 1994;8:27–31. doi: 10.1155/1994/17262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanapa P. Recovery patterns of liver function after complete and partial surgical biliary decompression. Am J Surg. 1996;171:230–234. doi: 10.1016/S0002-9610(97)89554-2. [DOI] [PubMed] [Google Scholar]


