Abstract
Siglecs (sialic acid-binding, immunoglobulin [Ig]-like lectins) are a family of single-pass transmembrane cell surface proteins found predominantly on leukocytes. Their unique structural characteristics include an N-terminal carbohydrate-binding (“lectin”) domain that binds sialic acid, followed by a variable number of Ig-like domains, hence these structures are a subset of the Ig gene superfamily. Another unique feature of Siglecs is that most, but not all, possess so-called immunoreceptor tyrosine-based inhibitory motifs (“ITIMs”) in their cytoplasmic domains, suggesting that these molecules function in an inhibitory capacity. Siglec-8, the eighth member identified at the time, was discovered as part of an effort initiated almost a decade ago to identify novel human eosinophil and mast cell proteins. Since that time, its selective expression on human eosinophils and mast cells has been confirmed. On eosinophils, Siglec-8 engagement results in apoptosis, whereas on mast cells, inhibition of FcεRI-dependent mediator release, without apoptosis, is seen. It has subsequently been determined that the closest functional paralog in the mouse is Siglec-F, selectively expressed by eosinophils but not expressed on mast cells. Despite only modest homology, both Siglec-8 and Siglec-F preferentially recognize a sulfated glycan ligand closely related to sialyl Lewis X, a common ligand for the selectin family of adhesion molecules. Murine experiments in normal, Siglec-F-deficient mice and hypereosinophilic mice have resulted in similar conclusions that Siglec-F, like Siglec-8, plays a distinctive and important role in regulating eosinophil accumulation and survival in vivo. Given the resurgent interest in eosinophil-directed therapies for a variety of disorders, plus its unique additional ability to also target the mast cell, therapies focusing on Siglec-8 could some day prove to be a useful adjunct to our current armamentarium for the treatment of asthma, allergies and related disorders where overproduction and overactivity of eosinophils and mast cells is occurring.
Introduction
Acute and chronic allergic inflammatory responses share a number of characteristic biochemical and cellular features. Many of these features resemble those found in non-allergic diseases such as nasal polyposis and eosinophilic esophagitis where evidence of selective accumulation and activation of mast cells and eosinophils is also seen [1, 2]. From a pathophysiologic standpoint, processes selectively regulating eosinophil and mast cell-related activities, including hematopoiesis and cell trafficking, accumulation, survival, activation and apoptosis, contribute to their inflammatory activities [3, 4]. Strategies designed to interrupt each of these events are being used as therapeutic agents or are being developed, and range from small molecule receptor antagonists to biologicals capable of neutralizing IgE, cytokines and other proteins [5]. Despite some unanswered questions [6-8], work continues to focus on the central role of the eosinophil and the mast cell in a number of allergic, hematologic and inflammatory conditions. Efforts to complete the description of the eosinophil surface phenotype and understand unique IgE receptor signaling cascades are quite advanced [4, 9], and such work should lead to the discovery of cell type-specific markers for identification of mast cells and eosinophils as well as the development of agents designed to selectively antagonize the biology of these cells. Monoclonal antibodies such as omalizumab [7] and mepolizumab [10], reslizumab [11] and MEDI-563 [12] are recent examples of such efforts, and additional ongoing targets that are selective for eosinophils and mast cells include the chemokine receptor CCR3 and the prostaglandin D2 receptor CRTh2 [5].
Another therapeutic approach focuses on activation of inhibitory or death receptors on cells by using agonist antibodies or small molecules for their selective engagement and subsequent downregulation of cell function and/or survival. For example, FAS, TGF-β and corticosteroids are effective in inducing eosinophil apoptosis and serve to counteract survival signals encountered by these same cells such as IL-5, GM-CSF and others [4], yet they lack cell specificity and the degree of safety one would want for use in a chronic disease such as asthma. For the mast cell, targeting IgE and its receptor has been shown to be clinically effective and at least in murine systems, regulation of IgE binding may alter mast cell survival [13]. The ability to develop antibodies and small molecules that could engage inhibitory receptors such as FcγRII (CD32) might also result in inhibitory responses, especially if selectively targeted to mast cells [14].
Experiments dating back a decade or longer to identify novel surface markers selectively expressed on eosinophils and mast cells were undertaken with the hope that this would lead to the development of new cell type-specific targets for therapy. This review will discuss the effort that led to the discovery of Siglec-8, and its closest function mouse paralog, Siglec-F. After a brief review of the Siglec receptor family, Siglec-8 and Siglec-F function on human eosinophils, and Siglec-8 function on mast cells, will be covered, followed by a discussion of their shared glycan ligands and known in vivo biology.
Siglecs
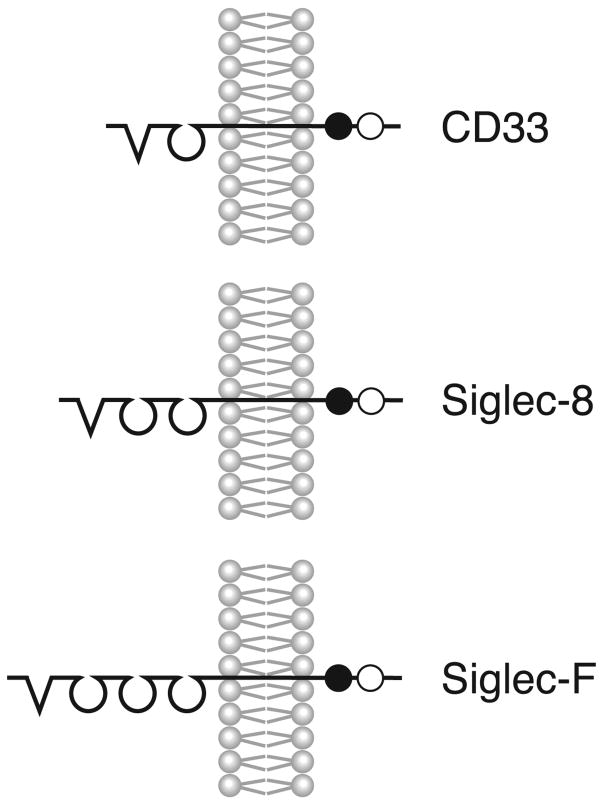
The term Siglecs, which stands for sialic acid-binding immunoglobulin-like lectins (lectins being structures that bind carbohydrates), was coined in 1998 [15] to describe a subset of the immunoglobulin (Ig) gene superfamily that differed from other Ig members mainly at the outermost and innermost ends of the molecule. By definition, the domain of Siglecs found at the most membrane-distal extracellular location (N-terminus) uniquely binds different forms of sialic acid, while the membrane-proximal cytoplasmic domains usually, but not always, contain certain conserved amino acid sequences found in other groups of inhibitory receptors that are referred to as immunoreceptor tyrosine-based inhibitory motifs, or ITIMs. Siglecs are also considered Type I, or single pass, transmembrane surface proteins, which is not to be confused with the fact that Siglecs also belong to the I-type or immunoglobulin-like lectin family. There are now 14 known human Siglecs, and each possesses a characteristic number of Ig-like domains varying in number from two to seventeen, but most commonly two to four as do those of the CD33 (Siglec-3) subfamily including Siglec-8 and Siglec-F (see Figure 1). For detailed recent general reviews on Siglecs, the reader is referred to other recent publications [16-22].
Figure 1.
Structural characteristics of human Siglec-3 (CD33), human Siglec-8 and mouse Siglec-F. V-shaped N-terminal structures indicate the arginine-containing V-set domains with lectin activity; these are then followed by varying numbers of C2-type Ig repeat domains. For the cytoplasmic portions of each Siglec, the closed and open circles represent ITIM and ITIM-like motifs, respectively.
Discovery and characteristic features of Siglec-8
Siglec-8 was originally discovered from a human eosinophil cDNA library prepared from a patient of this author with hypereosinophilic syndrome. A leading technology at that time involved purifying mRNA and then focusing on mRNA that contained so-called expressed sequence tags, which meant it was a subset of mRNA that contained a unique sequence targeting the mRNA to ribosomes, and hence was destined for translation into protein. This material could be isolated, amplified and then randomly sequenced. Using this approach in collaborations with industry collaborators, a total of about 10,000 such sequenced eosinophil mRNAs were entered into a database and then searched for homology to known mRNA sequences as well as ones that had been described but had unknown function. In this eosinophil cDNA library was an mRNA sequence predicting for a protein that had homology to CD33, also known as Siglec-3, which was found predominantly on myelomonocytic cells and used as a marker and target for certain hematopoietic malignancies [23, 24]. Additional investigation revealed that indeed this mRNA encoded for a mature protein designated by us as SAF-2 (for sialoadhesin family member 2), but also reported by another laboratory using the same starting eosinophil material and ultimately named Siglec-8, as the eighth known member of the Siglec family at the time [25, 26]. Additional investigations by a third laboratory led to the discovery of an alternatively spliced form, which is now known to be the predominant form in which there are two putative inhibitory motifs in the cytoplasmic domain [27, 28]. Additional independent work using a comparative transcriptome approach has confirmed the highly selective nature of Siglec-8 expression by eosinophils [29]. When monoclonal antibodies to the extracellular region of Siglec-8 were generated, prominent and selective expression of Siglec-8 on human eosinophils was confirmed [25, 26], but, somewhat surprisingly, Siglec-8 was also found to be expressed on human mast cells and to a weak but consistent degree on human basophils [25].
Siglec-8 contains two ITIM-like cytoplasmic domains conserved in other inhibitory and immune receptors (Figure 1). Receptors with these structures after ligation or crosslinking typically recruit inhibitory phosphatases such as src-homology domain containing tyrosine phosphatase-1 (SHP-1), which are felt to mediate a variety of downstream effects including inhibition of proliferation, inhibition of secretion, and even cell death [20]. It is interesting to note that unlike other cell surface receptors and activation markers normally used to distinguish eosinophils from neutrophils and other cells, such as CCR3, CD9 and CD49d, Siglec-8 appears to be a very late terminal differentiation antigen and once expressed surface levels remain extremely stable [3, 25, 30]. Evidence for this includes the fact that flow cytometric evaluation of blood and bronchoalveolar lavage eosinophils show similar levels of Siglec-8 surface expression (unpublished observations). Activation of eosinophils in vitro with a variety of substances known to induce eosinophil activation and resulting in up-regulation and down-regulation of other cell surface markers fail to affect Siglec-8 surface protein expression [25]. To date, none of the eosinophilic cell lines, including those derived from HL60 and others, express Siglec-8, further evidence of the concept that this is a late differentiation antigen ([25] and unpublished observations]. However, nothing is known about regulation of Siglec-8 expression at the genetic level, nor has there been any reports regarding the promoter region of Siglec-8. The gene itself is located on chromosome 19q13 in a region associated with many other CD33-related Siglecs [31], suggesting that Siglec-8 evolved through gene duplication. Ongoing studies [32] are exploring the possibility that single nucleotide polymorphisms in and around the Siglec-8 gene might influence expression and/or function of the molecule and thus be associated with eosinophilic or allergic disease phenotypes.
Function of Siglec-8 on human eosinophils and mast cells
When initially exploring function of Siglec-8, one of the limitations in the field of Siglec biology was a lack of knowledge regarding natural carbohydrate-containing glycan ligands. To obviate this, most laboratories resorted to the use of antibodies as artificial ligands. Our initial approach to studying Siglec-8 function has followed this same paradigm. Incubation of human eosinophils under Siglec-8 antibody crosslinking conditions resulted in pronounced cell death that was mediated through apoptosis rather than necrosis [33]. Mechanistic studies implicated both caspases and reactive oxygen species generation resulting in mitochondrial injury in this cell death, and a paradigm emerged that engagement of Siglec-8 activates the apoptotic pathway involving generation of reactive oxygen species leading to downstream mitochondrial dysfunction and caspase cleavage before apoptotic death ensues [34, 35].
One of the initially confusing observations regarding Siglec-8-induced cell death was that unlike most other eosinophil death pathways that can be overridden by counterbalanced survival signals such as those provided by the cytokines IL-5 and GM-CSF, Siglec-8-induced death was enhanced by these cytokines in that cells would die even more readily with even less of a Siglec-8 engagement signal [33]. These results were subsequently confirmed in eosinophils primed in vivo following allergen bronchoprovocation, and primed cells no longer used caspases in the apoptosis process, instead relying exclusively on reactive oxygen species generation and mitochondrial injury [36]. Overall, these data suggest that activated eosinophils might be particularly susceptible to pharmacologic approaches that engage Siglec-8 [22].
As mentioned above, Siglec-8 was originally discovered based on efforts to discover new eosinophil surface proteins, but it quickly became clear that mast cells also express Siglec-8 [25]. Subsequent studies using in vitro methods to generate mast cells from CD34+ progenitors showed that Siglec-8 is not expressed on early precursors but appears on the cell surface at about the same time as do other mast cell markers such as intracellular histamine and surface FcεRI [37]. Additional work showed, for reasons that are still not entirely clear, that mast cells do not undergo apoptosis when Siglec-8 is crosslinked, nor does this enhance apoptosis when cells are forced to undergo apoptosis through other pathways [38, 39]. Instead, crosslinking of Siglec-8 results in profound inhibition of IgE receptor-triggered histamine and prostaglandin D2 release, but interestingly not the release of other newly formed mediators such as cytokines [38]. Based on parallel studies with both human mast cells and Siglec-8-transfected RBL cells, crosslinking of Siglec-8 inhibits IgE receptor-induced calcium flux responses [38], reminiscent of other mast cell responses that are inhibited by engagement of ITIM-containing surface receptors [14, 40]. Transfected cells were used to show the requirement of the ITIM domain by showing that the introduction of a point mutation into the membrane proximal ITIM tyrosine residue resulted in complete elimination of this inhibitory biology [38], as has been observed for Siglec-7 and Siglec-9 in transfected cells [41, 42]. Ongoing work is beginning to explore Siglec biology on human basophils but these cells tend to express higher levels of Siglecs other than Siglec-8; indeed, mast cells most prominently express Siglec-6 and basophils prominently express Siglec-5 and others, and it is therefore possible that other approaches involving Siglecs may prove to be useful strategies for inhibiting biological responses of these cells too [21, 43-45].
While our studies using monoclonal antibodies to Siglec-8 were ongoing, another group discovered that intravenous immunoglobulin (IVIG) preparations that are used commercially contain autoantibodies to Siglec-8 at a high enough titer so as to also induce eosinophil apoptosis in vitro, especially in cytokine-primed cells [46]. The in vivo relevance of this concept remains somewhat controversial, as recent reports would suggest that administration of IVIG does not routinely lead to an acute eosinopenia [47, 48], but these preparations of IVIG were not specifically tested for their titers of Siglec-8 antibody, which can vary from lot to lot and brand to brand (unpublished observations). Nevertheless, it is tempting to speculate that the varied efficacy of IVIG used to treat patients with hypereosinophilic and mast cell-associated disorders could be due to differences in titers of anti-Siglec-8 antibodies, as indeed there have been favorable reports of the use of IVIG in conditions such as chronic urticaria and Churg-Strauss syndrome [49, 50].
Ligands for Siglec-8
By definition, Siglecs bind sialic acid, but it turns out that the conformation of these sialic acids are extremely critical in determining which sialylated structures bind to which Siglecs. In general, CD33-related Siglecs, including Siglec-8, tend to recognize α2,3-linked sialic acid, while other Siglecs prefer α2,6-linked or α2,8-linked sialic acid [16, 20]. A major advance in the field of glycobiology devoted to understanding glycan-lectin binding occurred with the development of glycan binding arrays. One of the best examples of this is the glycan array developed by the NIH-funded Consortium for Functional Glycomics (http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml) whereby hundreds of carbohydrate structures have been covalently coupled to a slide in a way that allows rapid screening of carbohydrate binding of suspected lectins [51-53]. Using this approach, we discovered that Siglec-8 very specifically recognizes 6′ sulfated sialyl Lewis X (6′-sulfo-sLex or NeuAcα2-3Galβ1-4(Fucα1-3)(6-O-sulfo)GlcNAc) [54]. Sialyl Lewis X, a known ligand for E-, P- and L-selectin [55], does not bind Siglec-8 but instead absolutely requires the presence of the sulfate on the 6 position of the galactose residue. Despite this subtle difference, there are very few structures screened by the Consortium for Functional Glycomics that selectively recognize 6′-sulfo-sLex, although Siglec-7 and a few other lectins did bind to varying degrees. In preliminary studies, incubation of whole human blood with a polyacrylamide polymer decorated with 6′-sulfo-sLex resulted in binding to eosinophils that was Siglec-8-dependent. In contrast, no detectable binding to any other leukocyte subtype was detected, including monocytes and lymphocytes that express Siglec-7 [56]. Efforts are underway to determine where this glycan might be expressed in tissues in vivo, and which enzymes might be required for its synthesis, and the exact nature of the glycoprotein(s) or glycolipid(s) that display this complex sulfated sugar (see below).
Expression and function of Siglec-F
With the discovery of Siglec-8, efforts were initiated to discover its counterpart in the mouse, where systems would be more amenable to in vivo manipulation for exploring its biology. There are only about half as many Siglecs in mice compared to humans [21] (note that by nomenclature agreement, those unique to the mouse are lettered rather than numbered [15]), and based on structural homology alone, there was no clear mouse ortholog of Siglec-8. Therefore, different strategies were needed to determine which Siglecs were most prominently expressed by mouse eosinophils. Using IL-5 transgenic mice and Northern blotting, Siglec-E, -F and -G were all expressed at the mRNA level in mouse eosinophils, but patterns of expression were markedly increased in IL-5 transgenic mice only for Siglec-F and Siglec-G [57]. Ultimately, it was not until antibodies were generated that this issue was completely resolved, and in it is now clear that Siglec-G is expressed on B lymphocytes [58] while Siglec-F is most prominently expressed by mouse eosinophils [59-61]. This was somewhat unexpected based on sequence homology alone, since Siglec-F more closely resembles human Siglec-5, which is not expressed by human eosinophils [57, 62]. Other differences were subsequently found among patterns of cellular surface expression of Siglec-F and Siglec-8. Siglec-F is not expressed on mouse mast cells and instead is expressed on a wider range of cells including alveolar macrophages and at very low levels on T cells and neutrophils, none of which in humans express Siglec-8; and unlike Siglec-8, surface levels of Siglec-F on eosinophils and other cells increase during allergic inflammatory responses [21, 62-64]. Based on these original reports, Siglec-F has emerged as a reliable distinguishing marker of eosinophils among granulocytes in the mouse [65-69]. Despite these structural and cellular differences, it is remarkable that Siglec-F, like Siglec-8, has evolved to selectively recognize 6′-sulfo-sLex [61]. Therefore, because of its preferential expression on eosinophils and preference for binding the same ligand, Siglec-F and Siglec-8 are best thought of as functionally convergent paralogs. Subsequent studies by Zhang et al. successfully deleted the Siglec-F gene from mice [63]. While these mice are phenotypically normal, when put into allergen sensitization and provocation models of asthma they display a more pronounced bone marrow, blood and tissue eosinophilia due to reduced rates of apoptosis; changes in airways hyperreactivity did not reach statistical significance [63]. Studies of normal mice that have undergone allergen sensitization and provocation revealed that administration of a Siglec-F antibody markedly reduced circulating eosinophil numbers but did not affect airways hyperreactivity [70]. Other studies revealed that systemic administration of two different types of Siglec-F antibodies led to profound depletion of circulating and tissue eosinophils [71]. These models included normal mice, IL-5 transgenic mice and a mouse model of hypereosinophilic syndrome/chronic eosinophilic leukemia. The effect of the Siglec-F antibody was specific in that no change in mast cell or alveolar macrophage numbers were seen, and no changes in blood counts other than eosinophil numbers was seen in any of the hypereosinophilia models or even in normal mice [71]. It appears that the reduction in eosinophil numbers in vivo was due to apoptosis based on ex vivo studies with blood samples from Siglec-F-treated mice, as well as studies in which eosinophils from IL-5 transgenic mice were exposed to Siglec-F antibody in vitro and characteristic changes indicative of apoptosis were seen [71]. So while not a perfect replica of the human Siglec-8 expression pattern, these mice data do provide some proof of concept that targeting an eosinophil-selective Siglec can have profound effects on eosinophil numbers in both blood and tissues.
Finally, besides its effect on eosinophil survival, studies looking at cell surface trafficking of Siglec-F after binding antibody or artificial glycan ligands revealed that Siglec-F undergoes internalization via a process that, like apoptosis, is dependent on the cytoplasmic ITIM domain [72]. These investigators also demonstrated that Siglec-F endocytosis differed from pathways involved in Siglec-2 (CD22) internalization in that Siglec-F internalization was independent of clathrin and dynamin and resulted in its movement into lysosomes. Whether this is true of Siglec-8, and whether this influences its biology in eosinophils, is not known.
Tissue ligands for Siglec-8 and Siglec-F
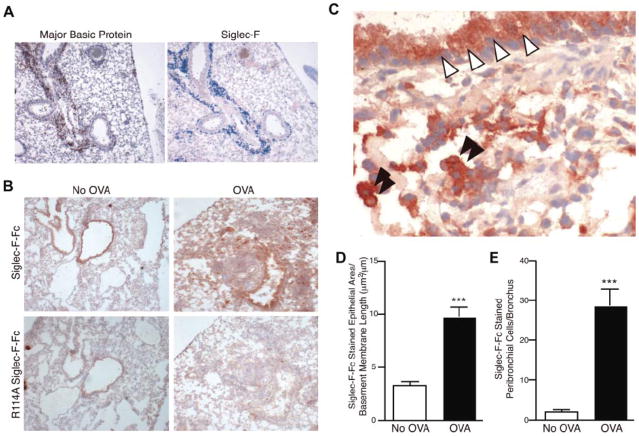
The exact biochemical identity of natural tissue ligands for Siglec-8 and Siglec-F remains unknown. However, immunohistochemical approaches to find glycan ligands in mouse lung utilizing Siglec-F Ig fusion protein [63, 73] and Siglec-8 Ig fusion protein [73] (which, as mentioned above should both attach to 6′-sulfo-sLex) found constitutive and selective binding to airway epithelial cells, and binding to epithelium and lung mononuclear cells increased following OVA sensitization and challenge ([63] and see Figure 2). Based on this, along with the occurrence of sulfated sLex-like structures made by airway cells, bronchial (and perhaps mononuclear cell) mucins have been suggested as candidate ligands for Siglec-F and Siglec-8 [63]. Indeed, a recent report documented the ability of mucins to engage other Siglecs on monocytes and induce apoptosis [74]. Given the endogenous and inducible expression of Siglec-8 and Siglec-F ligands on airway epithelium, it is possible that eosinophils entering the airway may engage such ligands and have their tissue lifespan reduced via apoptosis, and as such this could represent a novel pathway for selectively clearing the airway of unwanted eosinophils. Further studies are needed to fully characterize these endogenous ligands.
Figure 2.
Siglec-F and sialylated Siglec-F ligands are up-regulated upon OVA challenge. (A) Serial sections of frozen lung from WT OVA-sensitized and -challenged mice were stained with antibodies against MBP (left panel, reddish brown color is positive) or Siglec-F (right panel, blue color is positive). Only the inflamed lungs were positive, as shown. (B) Recombinant soluble Siglec-F-Fc was used to probe for Siglec-F ligands in the lungs from OVA-sensitized and -challenged (OVA) or OVA-sensitized and PBS-challenged (No OVA) mice. Positive staining appears a dark reddish-brown color. The arginine-mutated R114A Siglec-F-Fc was used as a negative control, as it is deficient in sialylated ligand binding. Results shown are typical of n = 4 for each group and representative of 2 experiments. (C) Higher-magnification photomicrograph of an OVA-sensitized and -challenged lung section, probed with Siglec-F-Fc. Bronchiolar cells of the lung epithelia (white arrowheads) and mononuclear cells in the lung parenchyma (black arrowheads) were positive for Siglec-F ligands. For panels A-C, a 10x/0.25 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them. (D) Surface area of the Siglec-F ligand–positive bronchiolar epithelia. Mouse lungs were immunostained with Siglec-F-Fc and the area of bronchial epithelial Siglec-F-Fc immunostaining was quantitated by image analysis, with results expressed in squared micrometer/micrometer length of the basement membrane of the bronchus. WT mice challenged with OVA had a significant increase in levels of Siglec-F-Fc epithelial immunostaining compared with control non–OVA-challenged WT mice. (E) Mouse lungs were immunostained with Siglec-F-Fc, and the number of positive peribronchial cells quantitated was by image analysis. WT mice challenged with OVA had a significant increase in the numbers of peribronchial Siglec-F-Fc–positive cells compared with control non–OVA-challenged WT mice. (D-E) ***P < .001. Each error bar represents the standard error of the mean (SEM). Reproduced with permission from [63].
Indeed, work is underway to purify and chemically characterize ligands of Siglec-F and Siglec-8. Also underway are studies to determine whether enzymes required for synthesis of 6′-sulfo-sLex co-localize to the epithelium. These enzymes would include keratin sulfate galactose 6-O sulfotransferase (KSGal6ST), also referred to as carbohydrate sulfotransferase 1 (CHST-1), which would be required to introduce the sulfate residue on the galactose, and α2,3-sialyltransferases that add the terminal sialic acid to the galactose. Ongoing studies using immunohistochemistry, RT-PCR and mice deficient in each specific enzyme should help determine their tissue localization, expression levels and functional contribution under normal and inflammatory conditions.
Conclusions
Engagement of Siglec-8 and its closest functional paralog in the mouse, Siglec-F, on eosinophils in vitro and in vivo result in profound and selective apoptosis. Although Siglec-F is not found on mouse mast cells, on human mast cells Siglec-8 ligation in vitro results in inhibition of degranulation. Given the more unique combination of selective anti-eosinophil and anti-mast cell mediator release biology, targeting a molecule like Siglec-8 could provide therapeutic advantages for conditions such as asthma beyond those seen by targeting just the eosinophil or the mast cell alone. Theoretically, pharmacological strategies that could be used to exploit this may include IVIG, monoclonal antibodies or glycomimetics based on 6′-sulfo-sLex that act as artificial glycan ligands. Hopefully such efforts will also some day help to further our understanding of the role of the eosinophil and the mast cell in a variety of diseases including asthma.
Footnotes
This work was supported by grants AI41472 and AI72265 from the National Institutes of Health, as well as the Human Immunology grant program of the Dana Foundation. Dr. Bochner also received support as a Cosner Scholar in Translational Research from Johns Hopkins University. Dr. Bochner is a co-author on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, under a licensing agreement between GlaxoSmithKline and the Johns Hopkins University, Dr. Bochner may be entitled to a share of royalties received by the University on the potential sales of such products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Marone G, Triggiani M, Genovese A, Paulis AD. Role of human mast cells and basophils in bronchial asthma. Adv Immunol. 2005;88:97–160. doi: 10.1016/S0065-2776(05)88004-6. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Reviews. 2001;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin North Am. 2007;27:357–75. doi: 10.1016/j.iac.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet. 2008;372:1073–87. doi: 10.1016/S0140-6736(08)61449-X. [DOI] [PubMed] [Google Scholar]
- 6.Bochner BS. Verdict in the case of therapies versus eosinophils: the jury is still out. J Allergy Clin Immunol. 2004;113:3–9. doi: 10.1016/j.jaci.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti-IgE for chronic asthma in adults and children. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003559.pub3. CD003559. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan SD, Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008;63:670–84. doi: 10.1111/j.1398-9995.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 9.Rivera J, Fierro NA, Olivera A, Suzuki R. New Insights on Mast Cell Activation via the High Affinity Receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 11.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Reed J, Kolbeck R, Molfino N, Kozhich A, Humbles A, Erjefalt J, et al. MEDI-563, a humanized anti-IL-5Rα antibody with enhanced effector function, induces reversible blood eosinopenia in mild asthmatics. J Allergy Clin Immunol. 2008;121:S47. (abstr) [Google Scholar]
- 13.Sly LM, Kalesnikoff J, Lam V, Wong D, Song C, Omeis S, et al. IgE-induced mast cell survival requires the prolonged generation of reactive oxygen species. J Immunol. 2008;181:3850–60. doi: 10.4049/jimmunol.181.6.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxon A, Kepley C, Zhang K. “Accentuate the negative, eliminate the positive”: engineering allergy therapeutics to block allergic reactivity through negative signaling. J Allergy Clin Immunol. 2008;121:320–5. doi: 10.1016/j.jaci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v–vi. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 16.Varki A, Angata T. Siglecs - the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 17.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–9. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 18.Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr Opin Struct Biol. 2002;12:609–15. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–7. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 21.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. The Year in Immunology 2008. 2008 doi: 10.1196/annals.1443.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Pawankar R, Holgate S, Rosenwasser LJ, editors. Allergy Frontiers: From Epigenetics to Future Perspectives. Tokyo: Springer-Verlag; 2008. in press. [Google Scholar]
- 23.Andrews RG, Torok-Storb B, Bernstein ID. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983;62:124–32. [PubMed] [Google Scholar]
- 24.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521–34. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 25.Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 26.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 27.Foussias G, Yousef GM, Diamandis EP. Molecular characterization of a Siglec-8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec-8 gene. Biochem Biophys Res Commun. 2000;278:775–81. doi: 10.1006/bbrc.2000.3866. [DOI] [PubMed] [Google Scholar]
- 28.Aizawa H, Plitt J, Bochner BS. Human eosinophils express two siglec-8 splice variants. J Allergy Clin Immunol. 2002;109:176. doi: 10.1067/mai.2002.120550. [DOI] [PubMed] [Google Scholar]
- 29.Liu SM, Xavier R, Good KL, Chtanova T, Newton R, Sisavanh M, et al. Immune cell transcriptome datasets reveal novel leukocyte subset-specific genes and genes associated with allergic processes. J Allergy Clin Immunol. 2006;118:496–503. doi: 10.1016/j.jaci.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Nutku E, Aizawa H, Tachimoto H, Hudson SA, Bochner BS. Expression and function of Siglec-8 isoforms in human eosinophils, basophils and mast cells. In: Bienenstock J, Ring J, Togias AG, editors. Allergy Frontiers and Futures, Proceedings of the 24th Symposium of the Collegium Internationale Allergologicum. Cambridge Massachusetts: Hogrefe and Huber; 2004. pp. 130–2. [Google Scholar]
- 31.Yousef GM, Ordon MH, Foussias G, Diamandis EP. Genomic organization of the siglec gene locus on chromosome 19q13.4 and cloning of two new siglec pseudogenes. Gene. 2002;286:259–70. doi: 10.1016/s0378-1119(02)00432-8. [DOI] [PubMed] [Google Scholar]
- 32.Grant AV, Bochner BS, Guo JP, Araujo MI, Ponte EV, Cruz AA, et al. A genetic variant in the promoter of the Siglec8 gene is associated with wheeze in a Brazilian population. J Allergy Clin Immunol. 2007;119:S175. (abstr) [Google Scholar]
- 33.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 34.Nutku E, Aizawa H, Hudson SA, Bochner BS. Function of Siglec-8 on human eosinophils. Clin Exp Allergy Reviews. 2004;4(suppl 2):76–81. [Google Scholar]
- 35.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–24. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 36.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokoi H, Myers A, Matsumoto K, Crocker PR, Saito H, Bochner BS. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006;61:769–76. doi: 10.1111/j.1398-9995.2006.01133.x. [DOI] [PubMed] [Google Scholar]
- 38.Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Yokoi H, Hudson SA, Bovin N, Schnaar RL, Bochner BS. Surface expression, inhibitory function and candidate ligand for Siglec-8 on human mast cells. Allergy Clin Immunol Int: J World Allergy Org. 2008 2:23–6. [Google Scholar]
- 40.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 41.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–9. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 42.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–25. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 43.Ghannadan M, Hauswirth AW, Schernthaner GH, Muller MR, Klepetko W, Schatzl G, et al. Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol. 2002;127:299–307. doi: 10.1159/000057747. [DOI] [PubMed] [Google Scholar]
- 44.Florian S, Sonneck K, Czerny M, Hennersdorf F, Hauswirth AW, Buhring HJ, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–62. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 45.Hauswirth AW, Florian S, Schernthaner GH, Krauth MT, Sonneck K, Sperr WR, et al. Expression of cell surface antigens on mast cells: mast cell phenotyping. Methods Mol Biol. 2005;315:77–90. doi: 10.1385/1-59259-967-2:077. [DOI] [PubMed] [Google Scholar]
- 46.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–11. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Kuo HC, Yang KD, Liang CD, Bong CN, Yu HR, Wang L, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. 2007;18:354–9. doi: 10.1111/j.1399-3038.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuo HC, Wang CL, Wang L, Yu HR, Yang KD. Patient characteristics and intravenous immunoglobulin product may affect eosinophils in Kawasaki disease. Pediatr Allergy Immunol. 2008;19:184–5. doi: 10.1111/j.1399-3038.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell BF, Barr RM, Black AK, Francis DM, Kermani F, Niimi N, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138:101–6. doi: 10.1046/j.1365-2133.1998.02033.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsurikisawa N, Taniguchi M, Saito H, Himeno H, Ishibashi A, Suzuki S, et al. Treatment of Churg-Strauss syndrome with high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2004;92:80–7. doi: 10.1016/S1081-1206(10)61714-0. [DOI] [PubMed] [Google Scholar]
- 51.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rapoport EM, Pazynina GV, Sablina MA, Crocker PR, Bovin NV. Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 2006;71:496–504. doi: 10.1134/s0006297906050051. [DOI] [PubMed] [Google Scholar]
- 53.Campanero-Rhodes MA, Childs RA, Kiso M, Komba S, Le Narvor C, Warren J, et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun. 2006;344:1141–6. doi: 10.1016/j.bbrc.2006.03.223. [DOI] [PubMed] [Google Scholar]
- 54.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 55.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, et al. E-selectin receptors on human leukocytes. Blood. 2008 doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudson SA, Bovin N, Crocker PR, Bochner BS. Polymers containing 6′-sulfated sialyl Lewis X (6′-su-sLex) selectively engage Siglec-8 on human eosinophils. J Allergy Clin Immunol. 2009 (abstract in press) [Google Scholar]
- 57.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–30. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 59.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J Biol Chem. 2001;276:45128–36. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 61.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–35. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 62.Guo JP, Nutku E, Yokoi H, Schnaar R, Zimmermann N, Bochner BS. Siglec-8 and Siglec-F: inhibitory receptors on eosinophils, basophils and mast cells. Allergy Clin Immunol Inter - J World Allergy Org. 2007;19:54–9. [Google Scholar]
- 63.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–44. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 66.Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 2007;179:4766–74. doi: 10.4049/jimmunol.179.7.4766. [DOI] [PubMed] [Google Scholar]
- 67.Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797–802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 68.Dyer KD, Czapiga M, Foster B, Foster PS, Kang EM, Lappas CM, et al. Eosinophils from lineage-ablated Delta dblGATA bone marrow progenitors: the dblGATA enhancer in the promoter of GATA-1 is not essential for differentiation ex vivo. J Immunol. 2007;179:1693–9. doi: 10.4049/jimmunol.179.3.1693. [DOI] [PubMed] [Google Scholar]
- 69.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–9. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kearley J, Jones C, McMillan SJ, Cromie K, Crocker PR, Lloyd CM. Anti-Siglec-F antibody treatment during allergen-induced airway inflammation reduces eosinophil numbers but has no effect on airway hyperreactivity in vivo. Am J Respir Crit Care Med. 2007;175:A690. (abstr) [Google Scholar]
- 71.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–63. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, et al. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo J, Myers A, Choi O, Lee H, Zhu Z, Hudson S, et al. Ligands for Siglec-8 and Siglec-F: binding characteristics and tissue distribution. J Allergy Clin Immunol. 2007;119:S299. (abstr) [Google Scholar]
- 74.Ishida A, Ohta M, Toda M, Murata T, Usui T, Akita K, et al. Mucin-induced apoptosis of monocyte-derived dendritic cells during maturation. Proteomics. 2008;8:3342–9. doi: 10.1002/pmic.200800039. [DOI] [PubMed] [Google Scholar]