Abstract
Nicotine is the principle addictive agent delivered via cigarette smoking. The addictive activity of nicotine is due to potent interactions with nicotinic acetylcholine receptors (nAChRs) on neurons in the reinforcement and reward circuits of the brain. Beyond its addictive actions, nicotine is thought to have positive effects on performance in working memory and short-term attention-related tasks. The brain areas involved in such behaviors are part of an extensive cortico-limbic network that includes relays between prefrontal cortex (PFC) and cingulate cortex (CC), hippocampus, amygdala, ventral tegmental area (VTA) and the nucleus accumbens (nAcc). Nicotine activates a broad array of nAChRs subtypes that can be targeted to pre- as well as peri- and post-synaptic locations in these areas. Thereby, nicotine not only excites different types of neurons, but it also perturbs baseline neuronal communication, alters synaptic properties and modulates synaptic plasticity.
In this review we focus on recent findings on nicotinic modulation of cortical circuits and their targets fields, which show that acute and transient activation of nicotinic receptors in cortico-limbic circuits triggers a series of events that affects cognitive performance in a long lasting manner. Understanding how nicotine induces long-term changes in synapses and alters plasticity in the cortico-limbic circuits is essential to determining how these areas interact in decoding fundamental aspects of cognition and reward.
Keywords: Nicotine, Cognition, Limbic, Acetylcholine, Synaptic plasticity
1. Introduction
Activation of nicotinic receptors regulates neuronal excitability and modulates synaptic transmission via direct mechanisms and by indirect mechanisms. Direct effects include activation of nicotine-gated cationic channels causing local depolarization. Indirect pathways involve the activation of nicotine-gated channels that are highly conductive to Ca2+. Activation of such channels elicit Ca2+ dependent signaling cascades, additional Ca2+ release from internal stores and/or exocytosis. The nAChRs expressed in cortico-limbic circuits include nAChR channel subtypes of varying subunit composition that differ in how they contribute to tuning synaptic plasticity by virtue of their distinct biophysical properties, pharmacological characteristics and differential targeting to specific somato-dendritic and axonal domains. This review attempts to fuse the results of recent studies of both the cellular and circuit mechanisms of nAChR activation to better understand how nicotine modifies cortico-limbic circuits underlying cognitive functions.
The stars of our discussion of cortico-limbic circuits are the layer 5 cortical pyramidal neurons and a selected subset of their many limbic targets, specifically the VTA, ventral hippocampus, amygdala and nAcc. Recent studies within the prefrontal cortex and at sites of prefrontal cortical projections have provided important new insights into the synaptic mechanisms by which nAChRs modulate the activity of cortico-limbic relays. Both inputs to and outputs from layer 5 cortical neurons and synapses within each of the aforementioned target fields are modulated by nAChR activation. The final outcome of nicotinic modulation at the synaptic, circuit and, ultimately, behavioral level, depends critically on the nAChR expression pattern, nAChR localization and nAChR subunit composition. These factors collaborate in nicotine’s ability to cause long-term changes in the rules that govern synaptic plasticity in cortico-limbic circuits thereby altering cognitive performance and reward.
1.1. Subunit composition and localization of nicotinic acetylcholine receptors in cortico-limbic circuits
To date, a total of 12 genes encoding neuronal nAChRs subunits have been identified in an array of vertebrates and invertebrates (for review see [1]). Nine α-type subunits (α2-α10 encoded by CHRNA2-10) and 3 non-α-type or β-type subunits (β2-β4; CHRNB2-4) have been cloned and sequenced from multiple species [1,2, reviewed in 3, 4-7]. The subunit composition of nAChRs varies depending on the brain region (for review see [1,7,8,9-12]). It is thought that the neuronal nAChRs assemble in a manner analogous to the muscle homolog, and hence are pentameric complexes of 2 α and 3 non-α-type subunits. However, several studies indicate that nAChR subunit stoichiometry may be more complicated, including pentamers of between 3 and 5 α-type subunits. Likewise, as acetylcholine (ACh) and other ligands bind at the α-γ and α-δ interface of muscle nAChRs, nicotinic ligands are proposed to bind at the interface of α and non-α subunits in neuronal nAChRs, although direct evidence for this idea is lacking. In any case, binding of the agonist is transduced into the gating of the receptors ion channel pore that is permeable to multiple cationic species (Na+, K+, Ca2+) and even large organic cations such as tetraethylammonium (TEA) (for review see [13]). The relative permeability for specific cations depends on the subunit composition of the nAChRs complex. The α7-containing nAChRs (written as α7*), historically identified by their binding of α-bungarotoxin (αBgTx), are the champions of Ca2+ permeable nAChRs [14-17]. Heterologous expression of homomeric α7* nAChRs reveals fractional Ca2+ currents of 6-12% which is comparable to estimates of Ca2+ permeability of N-methyl-d-aspartate (NMDA) receptors [16,18,19] and considerably greater than that of heteromeric (αβ)* nAChRs, (2-5%) [20].
The majority of the CNS nAChRs are relatively high-affinity nicotine-binding sites, that include α4 and β2 type subunits (aka α4β2* nAChRs); the αBgTx-binding (i.e. α7* nAChRs) are the next most abundant (reviewed in [7]). Homomeric, α7* nAChRs, expressed in heterologous systems are the best studied of the α7-containing nAChRs and are renowned for their relatively low agonist affinity and rapid desensitization kinetics in the presence of 100 μM ACh or higher. More recent work, especially by Papke and colleagues, have emphasized the multiple gating modes of α7* nAChRs noting in particular the activation of a non-desensitizing small amplitude current by ACh concentrations of 20 μM or lower [21,22]. Considering that ACh and choline, both activators of α7-containing receptors are present in cerebrospinal fluid (CSF), it is possible that under normal physiological conditions, there is a tonic activation of α7* nAChRs. Higher concentrations of agonist might decrease the net contribution of α7* nAChRs, thereby decreasing ligand-gated calcium influx, preventing cytotoxicity [22].
There is increasing evidence that native α7* nAChRs may contain non-α7* nAChR subunits [23-27, e.g. see, 28]. Studies of such heteromeric α7* nAChRs in neurons in vivo and in vitro indicate different pharmacological properties and desensitization kinetics compared to the homomeric α7 nAChRs [28,29].
The least abundant nAChR subunits in the CNS are α9 and α10 which, unlike most, are expressed in non-neural tissues and organs, (e.g. bone marrow, nasal epithelium, and embryonic blood cells) as well as in innervated sensory epithelia and ganglia [30,31]. β3, α5 and α6, once considered orphan subunits, have now all been identified as components of native nAChRs in several brain regions. Their inclusion in nAChR complexes have been shown to confer important differences in agonist and antagonist profile, ion permeation, Ca2+-permeability, rates of desensitization and, most recently, to influence axonal and dendritic targeting of nAChRs [25,32-35].
Anatomical, electrophysiological and neurochemical studies have long established that α4β2* nAChRs and α7* nAChRs are the most abundant neuronal nAChRs and are present in the corticolimbic circuits upon which this review focuses. Key examples include studies demonstrating pre- and post-synaptic α7* or α4β2* nAChRs in hippocampus [36-39], PFC [40]; somatosensory cortex [41], and the VTA [42]; (for reviews see: [7,43]). Binding studies revealed nAChR-binding sites over the length of axons and provided evidence for anterograde transport of nAChRs to synaptic terminals [44-47].
A rather elegant study quantified the locations of α7* nAChRs at synapses in the VTA [42]. The vast majority of α7* nAChRs were situated at pre- and peri-synaptic sites, with only ∼10% located within the active zone or near a post-synaptic density. α7* nAChRs were found on vesicular glutamate transporter (vGluT) positive terminals that were devoid of vesicular cholinesterase transporter (VChat) staining, consistent with their localization on glutamatergic, not cholinergic, terminals. One of the main glutamatergic inputs to the VTA arises from the PFC: the presence of α7* nAChRs on these pre-synaptic terminals supports a cholinergic mechanism for regulating glutamatergic input to the VTA dopaminergic (DA) neurons [42].
In contrast to α7* nAChRs in VTA, the majority of which appear to be associated with pre-synaptic glutamatergic terminals, a wide variety of non-α7* nAChRs are expressed by the intrinsic DA and γ-aminobutyric acid (GABA) neurons within the VTA per se [42,48-55]. Most VTA GABAergic neurons express nAChRs that contain α4 and β2 subunits, and are blocked by dihydro-β-erythroidine (DHβE) [52]. VTA dopamine neurons express at least three pharmacologically distinguishable nAChRs, only one of which is α7* nAChRs. [50,54-56].
The composition of nAChRs that are expressed by the VTA and targeted to their dopaminergic projections to the nAcc are probably the best defined in subunit composition of any native nAChR population [53,57,58]. Pharmacological and gene deletion studies have revealed that these nAChR complexes include α3, α4, α5, α6 and β2 β3 and β4 subunits in various combinations [53,57,58]. A simplifying factor in the morass of nAChR subtypes detected in the nAcc is that the preponderance of nicotinic effects are due to activation of pre-synaptic nAChRs that modulate the dopaminergic vs. glutamatergic inputs. That is, the nAcc medium spiny neurons (MSN) themselves express little, if any, nAChRs.
The lack of α7* nAChRs on pre-synaptic dopaminergic inputs from VTA to nAcc contrasts with strong evidence for both α7* and non-α7* nAChRs on convergent glutamatergic projections from the PFC and/or hippocampus [42,47]. In fact the differential targeting of αβ* nAChRs to dopaminergic inputs to nAcc vs. α7* nAChRs to glutamatergic pre-synaptic sites is likely of key importance for the varied and activity dependent effects of nicotine on the dopaminergic drive to tonically active vs phasic firing nAcc neurons [59-61]. Overall, it is the distinctions amongst the various nAChR subtypes in the cortico-limbic circuits as described above that underlie the pleiotropic effects of nicotine on attention-related behaviors [54]. In the sustained presence of 100-500 nM nicotine as experienced by smokers plus with the varied levels of released ACh, the differential activation and desensitization kinetics of nAChR subtypes are likely to be strong determinants of exactly how the local circuitry is tuned [52,56,62]. Of course there is still the complicating contribution of pre- and post-synaptic muscarinic AChRs in cortical limbic circuits, an important reminder that one must be circumspect in comparing the effects of nicotine vs. ACh in dissecting the mechanisms of cholinergic modulation of circuit activity.
Details of the localization and subunit composition of nAChRs in prefrontal cortex have also come into increasing resolution in recent years. In fact, one of the first reports of pre-synaptic nAChRs governing glutamatergic transmission in the CNS was conducted in frontal cortex [63-65]. The prefrontal cortex receives glutamatergic inputs from the medial dorsal nucleus of the thalamus [66]. These thalamo-cortical projections are excited by nicotine and give rise to a strong increase in glutamatergic inputs to layer 5 as well as layer 6 pyramidal neurons [65,67,68]. Application of low levels of nicotine induces glutamate release in a manner that is largely dependent on a superthreshold activity (i.e. tetrodotoxin (TTX) sensitive) and on the presence of intact thalamico-cortical projections [65]. The profile of activation of thalamo-cortical inputs by low concentrations of agonist is consistent with an important role of α2β4* nAChRs as is the loss of nicotinic modulation at thalamo-cortical inputs in β2* nAChRs knock-out (KO) mice [65].
Support for modulatory effects of pre-synaptic nAChRs activation in the PFC comes from a variety of approaches including electrophysiological recordings and assay of release from isolated nerve terminals [40,69,70]. A recent study testing the relative contribution of β2* nAChRs vs. α7* nAChRs on glutamatergic synaptosomes from PFC [69] demonstrated that both α7* and non-α7* nAChRs appear to be important although each modulates excitatory amino acid (EAA) release via distinct mechanisms. α7* nAChRs are predominantly found on ryanodine positive terminals and activation of these channels leads to calcium-induced calcium release (CICR). The involvement of CICR that is coupled to pre-synaptic extracellular signal-regulated kinase (ERK2) activation and synapsin-1 phosphorylation provides a cellular mechanism for pre-synaptic facilitation in response to α7* nAChR activation. On the other hand, activation of non-α7* nAChRs increases release via recruitment of voltage-gated calcium channels (VGCCs), accounting for early observations that nAChR modulation of glutamatergic transmission in PFC is due, at least in part, to a TTX-sensitive mechanism. The participation of both α7* and non-α7* nAChRs (which are likely α4β2* nAChRs) in the facilitation of [3H]d-aspartate release by distinct activity and Ca2+-dependent mechanisms underscores the importance of recognizing that multiple nAChR signaling pathways converge to fine tune cortical circuits. Obviously, the presence of cholinergic input to the PFC and the regulation of local ACh release is a key to the modulatory role of cholinergic circuits in these regions. Considerable mystery vis a vis mechanism remains: it is not clear which of the various glutamatergic inputs to layer 5 pyramidal neurons express α7* nAChRs and/or non-α7* nAChRs, but direct nAChR gating of the layer 5 pyramidal neurons per se does not play an important role in nAChR modulation of PFC circuits. Thus, direct application of nicotine to layer 5 pyramidal neurons does not elicit detectable current, and single-cell PCR analysis of layer 5 pyramidal neurons did not detect mRNAs for even the most abundant nAChR subunits α4, β2 and α7 [68]. In contrast, specific interneuron populations in PFC layer 5 do express nAChRs. Regular spiking non-pyramidal interneurons (RSNP) as well as low-threshold spiking interneurons (LTS) show inward currents upon direct nicotine application, and they express mRNA for α4, β2 and α7 subunits [68].
A different picture arises for the location of nAChRs in layer 6 of prefrontal cortex. In rat, pyramidal neurons in this layer do express nAChRs and show prominent inward currents upon ACh application [67]. Specifically pyramidal neurons that project to the thalamus are excited by nicotine and pharmacological evidence suggests that these neurons express nAChRs that contain the α5 subunit. Whether layer 6 interneurons express nAChRs is unclear at this point, although glutamatergic inputs to fast spiking interneurons is greatly facilitated by nAChR stimulation [67]. Taken together, nAChRs are expressed in both output layers of the PFC, layer 5 and 6, but their distribution among pyramidal neurons and interneurons differs for the two layers. It will be important to learn how simultaneous activation of these receptors alters the total output of the PFC.
1.2. Nicotinic modulation of synaptic plasticity in selected cortico-limbic circuits
During initial phases of exposure to drugs of abuse and the acquisition of addiction, neuronal networks in brain areas involved in reward, such as the VTA, nAcc and PFC, undergo strong adaptations that lead to behavioral sensitization [71]. Lesioning dorsomedial PFC prevented the expression of cocaine sensitization in rats [72,73] and blocking dopamine receptors specifically in medial PFC prevented behavioral as well as neurochemical sensitization to cocaine [74]. Recently, it has become clear that nicotine, like other drugs of abuse, has lasting effects on cortico-limbic circuits and synapses [47,51,52,75-79].
Several recent studies have revealed that the effects of nicotine or acetylcholine on synaptic connections within neuronal networks can outlast nAChR stimulation and desensitization [47,75,80]. Long-term modulation of glutamatergic and GABAergic synaptic connections adds another level of complexity to nicotinic modulation of neuronal networks. In the following sections we consider evidence for longer lasting effects of nicotinic modulation in selected cortico-limbic regions.
1.2.1. Nicotinic modulation of synaptic plasticity in prefrontal cortex
Synaptic plasticity is critically important for cognitive function, and in particular, synaptic plasticity in the PFC has been directly associated with attention and working memory [81]. The relative timing of action potentials in pre- and post-synaptic neurons has a profound impact on the induction of long-term potentiation (LTP) or depression (LTD). When a pre-synaptic spike precedes a post-synaptic spike within a short time window of several tens of milliseconds, LTP is induced. The reverse order of spike timing results in LTD [82,83]. In mouse PFC, nicotine strongly affects this timing-dependent synaptic plasticity, which is called spike-timing-dependent plasticity (STDP). Stimulation of nicotinic AChRs in PFC modify STDP induced by pairing stimulation of the excitatory inputs to PFC layer 5 pyramidal neurons with post-synaptic spikes elicited 5 ms after each synaptic response [68]. This coordinated stimulation induced robust LTP; however, when the same stimulus paradigm was applied in the presence of nicotine concentrations experienced by smokers, LTP was eliminated and a depression of the excitatory inputs to these cells was observed. Which nAChRs on what neurons are responsible for this effect? As discussed above, mouse PFC layer 5 pyramidal neurons do not express nicotinic receptors themselves. Rather, the nAChRs involved in the nicotinic modulation of LTP induction increase inhibitory GABAergic inputs to the pyramidal cells, as the nicotinic modulation of plasticity was abolished by inhibitors of GABA type A(GABAA) receptors. As described above, LTS and RSNP GABAergic interneurons found in the PFC layer 5 express nAChR subunits on their soma that activate these neurons when nicotine is present. FS interneurons are excited indirectly by nAChRs that increase glutamatergic excitation of those cells. Thus, nicotine exposure enhances inhibitory input to the layer 5 pyramidal neurons through both direct and indirect excitation of inhibitory GABA interneurons (Fig. 1).
Fig. 1.

Model of nicotinic modulation of layer 5 pyramidal neuron in the PFC
In layer 5 of the mouse PFC, nicotinic AChRs are expressed on thalamocortical projections (T: yellow ovals on presynaptic profiles) on the cell bodies of low-threshold-spiking GABAergic cells (LTS) and by the regular-spiking-non-pyramidal cells (RSNP). FS cells do not express nAChRs. The glutamatergic inputs from T are increased by nicotine application eliciting an increased excitatory drive to the pyramidal neurons (Pyr), the LTS and the FS components. In addition RSNP cells are directly depolarized by nicotine due to activation of somato-dendritic nAChRs expressed by these cells, without alteration of the excitatory input. Thus, in the PFC nAChR activation results in an increased excitatory drive to the LTS neurons due to both direct activation of somato-dendritic nAChRs and to the indirect enhancement of glutamatergic input to LTS via activation of presynaptic nAChRs. Increased activation of LTS results in increased inhibitory tone from the LTS to layer 5 pyramidal neurons, such that overall effect translates into a net inhibition of the pyramidal cells activity.
Studies in other cortical areas indicate that increases in post-synaptic calcium concentration are critical for the induction of synaptic plasticity [84-86]. Using two-photon imaging of intracellular calcium levels, it was found that action potentials that propagated from the soma into the dendrites of layer 5 pyramidal cells elicited increases in dendritic calcium concentration. Nicotine enhanced the GABA input to the same dendrites, resulting in less calcium entry, likely due to failure of action potential back-propagation from the soma. Thus, nicotine suppresses post-synaptic calcium changes, thereby altering the conditions necessary for synaptic potentiation. Burst-like stimulation of the pyramidal cell in the presence of nicotine could restore post-synaptic calcium to concentrations comparable to those seen in the absence of nicotine, as well as the STDP, indicating that strong post-synaptic stimulation could overcome the nicotinic modulation [68,87].
It is somewhat counter intuitive that nicotine in PFC decreases the likelihood of LTP induction, given that nAChRs are generally excitatory and their activation in other brain areas has been shown to enhance LTP [36,51,88]. The activation of distributed nAChRs provides the PFC neuronal network with a wide range of computational possibilities, but the functional consequences of this modulation are hard to predict from these data alone. Nicotine alters the rules for synaptic plasticity resulting from timed pre-synaptic and post-synaptic activity and increases LTP threshold by reducing dendritic calcium signals. As such, the function of the medial PFC network will most likely change in the presence of nicotine. Most likely, distal apical dendrites of layer 5 pyramidal neurons in superficial layers will be more quantitatively affected by the nicotinic mechanisms we found to block STDP than the synapses that are located closer to the cell body. By reducing dendritic action potential propagation in apical dendrites, nicotine hampers communication between cell body and distal synapses in layer 5 pyramidal neurons. This potentially could strongly affect information processing in the neuronal network of the medial PFC as a whole, and will alter the output of the PFC. At the same time, increased activity in pyramidal neurons restores the conditions for STDP to occur. The presence of nicotine and increased threshold for STDP could reduce cognitive performance in healthy naive rodents [89]. Alternatively, since PFC neuronal activity could be increased during PFC-based cognitive behavior, nicotine may provide conditions under which signal-to-noise ratio in PFC information processing is enhanced, thereby improving cognitive performance [89,90]. It is possible that enhancing signal-to-noise for phasic activity within the PFC, rather than simply increasing excitability, could be an effective mechanism for cognition-enhancing drugs.
1.2.2. Critical periods of nicotinic modulation of synaptic plasticity in cortico-limbic circuits
The effects of nicotine exposure and/or the effects of disruption of nicotinic signaling during early critical periods have been demonstrated most clearly in studies of projections to and from cortex and thalamus (see [91,92] for recent reviews). Nicotine exposure early on alters the development of neuronal ensembles that subserve the transmission of information between primary sensory cortical areas and their corresponding thalamic nuclei (reviewed in[92,93]). Studies in the auditory system demonstrate an essential role for nAChRs in the refinement of thalamo-cortical projections and implicate α4β2* nAChrs in the gating of auditory transmission [80,94]. Studies using nAChR subunit selective KO mice demonstrate distinct roles for both α7* nAChRs and β2* nAChRs in the development of thalamo-cortical and cortico-thalamic projections, respectively (and see [92] for review, [95]). The observation that selective knock out during early critical periods can dramatically alter performance in a passive avoidance task in the adult underscores the key role of normal nAChR “tone” in neural development. In particularly elegant studies Berg and colleagues have demonstrated an essential role of early nicotinic signaling in the developmental switch of GABAergic transmission from excitatory to inhibitory [96].
1.2.3. Nicotinic modulation of synaptic plasticity in VTA
The PFC extends glutamatergic projections into numerous regions associated with the (meso)limbic system; Layer 5 pyramidal neurons of the infra limbic (IL) and prelimbic (PL) main projection sites include: the ventral striatum (∼25%), lateral hypothalamus (∼25%), basal lateral amygdala (BLA; 8%) and the VTA(4%), The latter input arises primarily from layer 5 PFC. About 40% of the neurons in layer 6 project to the medial thalamus [97].
Nicotinic AChRs located in the VTA are critically involved in nicotine addiction (see [98] for recent review). Indeed, in rats, intra-VTA infusions of nicotine increase DA concentration in the nAcc [99], an effect thought to mediate the reinforcing properties of most addictive drugs [100]. Also, intra-VTA infusion of nicotinic antagonists blocks the effect of systemic nicotine injections on accumbal DA release [99] and disrupt nicotine self-administration [101]. Electrophysiological experiments demonstrate that nicotine is able to increase the firing rate of DA neurons both in vitro and in vivo [50,102,103]. Although actions on nAChRs located on GABAergic interneurons or glutamatergic terminals in the VTA, or on pedunculopontine neurons, have been proposed to contribute to these effects [52,104,105], somato-dendritic nAChRs expressed by VTA DA neurons remain good candidates for the primary reinforcing action of nicotine.
Glutamatergic transmission onto DA neurons is enhanced by activation of pre-synaptic nAChRs [42,51]. Interestingly, cholinergic synaptic terminals were not in close vicinity to glutamatergic terminals expressing α7-containing nAChR, consistent with a “volume” mode of cholinergic signaling [42,106,107]. When nicotine arrives in the VTA, it stimulates glutamatergic terminals as well as dopamine neurons, thereby favoring conditions of pre- and post-synaptic paired activation and a Hebbian type of synaptic plasticity. Nicotine-induced pairing resulted in LTP of glutamatergic inputs [51]. Nicotine also induced LTP in vivo measured as an increase in AMPA/NMDA receptor ratio [76]. Together these findings suggest that synaptic plasticity in the VTA may be induced after smoking a single cigarette and most likely underlies the persistent effects of the drug on dopamine release in the nAcc and PFC.
The α7* nAChRs involved in this mechanism are not desensitized significantly by low nicotine concentrations associated with tobacco smoking [52,56]. However, the non-α7* nAChRs on GABAergic neurons undergo rapid desensitization within minutes after the start of nicotine exposure, and as a consequence, reduced the inhibitory input to the dopamine neurons [52,56]. Desensitization of GABAergic neuron nAChRs not only prevents further activation by nicotine, but it also precludes the contribution of these receptors to endogenous cholinergic transmission [52]. Thereby, VTA dopamine neurons are disinhibited by desensitization of non-α7* nAChRs [52]. Despite their rapid desensitization properties, it was shown by using genetically engineered mice lacking β2 subunits [103] or expressing α4 subunits hypersensitive to nicotine[108] that these subunits are very important for nicotine addiction.
The dopaminergic projections from the VTA to the nAcc are studded with multiple subtypes of nAChRs that modulate accumbens dopamine release. Indeed, the dopaminergic nerve terminals in the striatum have as many as five different nAChR subtypes (α4β2, α4α5β2, α4α6β2β3, α6β2β3 and α6β2) with different pharmacological properties. Although this elaborate array of nAChRs in accumbens is consistent with fine-tuned control of striatal dopamine release the role of these receptors in encoding nicotine addiction is less clear (reviewed in [109]).
1.2.4. Nicotinic modulation of cortico-accumbens circuits
The nucleus accumbens comprise GABAergic medium spiny and aspiny neurons and a small population of cholinergic interneurons. Apart from input of intrinsic GABAergic interneurons, the MSN is regulated by glutamatergic inputs from the PFC, hippocampus and the amygdala and dopaminergic neurons from the VTA. Add to this the unknown, but likely significant modulation of MSN activity by tonic levels of ACh throughout the striatum. Studies of cholinergic influences on striatal activity emphasize the contribution of muscarinic AChRs, rather than nAChRs [110,111].
The multiple relays projecting to the nAcc encode a wide range of sensory context for integration by the striatum and, ultimately, for the output of motivated behaviors. Such inputs convey emotional salience of positive and negative valence (VTA, amygdala), executive function (PFC), and novelty relative to prior experience (ventral hippocampus/subiculum). Electrophysiological studies in nAcc demonstrate that the glutamatergic projections from PFC and ventral hippocampus are important in the regulatory excitatory tone of the output of MSN [112-116].
The tuning of activity and the extent of glutamatergic input to the nAcc from PFC vs. hippocampus and amygdala are important in both the filtering and detection of inputs. The convergence of these inputs in the nAcc is thought to be a synaptic substrate of information gating [114]. Of particular importance vis a vis the role of this gating circuit in attention and working memory deficits is recent work of Grace and colleagues recording in vivo from rat ventral hippocampus, nAcc and PFC in concert [128]. These studies reveal that the initiation of ventral hippocampus drive of the nAcc requires activity from PFC. Thus, at low levels of activation the ventral hippocampus regulates nAcc activity in concert with a “permissive action” of the PFC. Interestingly the latter influence appears to be indirect, requiring the activation of D2 dopamine receptors. The level of ventral hippocampus drive is also critical: if ventral hippocampus input is sufficient to induce LTP, PFC activity is no longer required for ventral hippocampus-nAcc activation. Since both the amount of dopamine release and the extent of glutamatergic transmission in the nAcc are strongly regulated by nAChRs, it is not surprising that nicotinic actions in nAcc are state dependent and complex [128].
Indeed the effects of nicotine administration on nAcc firing as recorded in vitro and in vivo are far from straightforward ([59-61,117], and for review see [118]). Recent in vivo recordings in our lab examined the effects of nicotine on ventral hippocampus to nAcc circuits in genetically modified mice testing for the degree of coherence between hippocampus and nAcc firing before and after nicotine administration. Preliminary findings indicate that the pattern of phasic bursting activity in the nAcc and the degree of hippocampus-nAcc coherence are strongly affected by nicotine and that the altered patterns of activity can persist long after the removal of nicotine from the system (unpublished: M. Nason and LWR).
To address the mechanism(s) that might underlie sustained effects of nicotine at ventral hippocampus to nAcc synapses, we moved to a gene chimeric, ventral hippocampus microslice plus wild type (WT) nAcc co-culture [47]. These studies document that in WT-WT ventral hippocampus to nAcc circuits there is strong pre-synaptic nAChR-mediated control on glutamatergic transmission. Although both α7* and non-α7* nAChRs participate in nicotine-induced synaptic facilitation at ventral hippocampus to nAcc synapses, it is the α7* nAChRs that mediate the long lasting potentiation of ventral hippocampus activity [47]. Thus selective blockade of pre-synaptic α7* nAChRs or studies of chimeric cultures of α7* KO ventral hippocampus with WT nAcc neurons reveal only a transient facilitation of glutamate release. Something about α7* nAChRs converts the transient effects of nicotine into long lasting changes in synaptic transmission (see discussion below and [47]).
1.2.5. Nicotinic modulation of synaptic plasticity in hippocampal and cortico-amygdala circuits
Pairing of nicotine with activity potently modulates transmission and alters the probability of LTP in hippocampus and at cortico-amygdala synapses as well. In the hippocampus, nicotine by itself induces LTP at a minority of the glutamatergic synapses. However, nicotine paired with a subthreshold induction protocol induces synaptic plasticity at the majority of glutamatergic synapses [36,88,119]. Activation of post-synaptic nAChRs on CA1 pyramidal neurons can boost short-term plasticity into LTP in Schaffer collateral synapses [36]. Activating nAChRs on interneurons that synapse on pyramidal neurons can prevent LTP in glutamatergic synapses [36]. Thus, timing and localization of nAChR activity in the hippocampus can determine whether LTP will occur or not. These different types of nicotinic modulation of LTP may contribute to the well-known effects of nicotine on learning and memory. Since CA1 pyramidal neurons in the ventral hippocampus project to the PFC [120], modulation of synaptic plasticity in the CA1 area by nicotine may also participate in the effects of nicotine on attention performance.
Nicotine has long been known to modulate activity in the amygdala [121] and prenatal exposure to nicotine has been strongly linked with difficulties in emotional behaviors later in life [92]. Cholinergic neurons from the basal forebrain send projections to several nuclei in the amygdala with the lateral and basolateral nuclei receiving particularly strong inputs [122,123]. Modulation of cortico-amygdala input by ACh influences BLA output to other limbic structures, including the nAcc, thereby altering emotional salience of learned experiences and reinforcement of motivated behaviors (for reviews see [124,125]). Recent studies demonstrate that the effects of nicotine exposure on amygdala circuits far outlast the time of nicotine administration [75,126]. Even a single trial, low-dose, exposure to nicotine can elicit lasting facilitation of cortical inputs to the BLA [75]. Pharmacological studies in WT and nAChR subunit KO mice reveal that activation of pre-synaptic α7* and non-α7* nAChRs, facilitates glutamatergic transmission in an activity-dependent manner. With low-frequency stimulation of cortical inputs nicotine elicits robust facilitation of transmission at most cortico-BLA synapses and synaptic strength remains elevated for 15-30 min after nicotine washout. Most striking is the observation that nicotine reduces the threshold for activation of long-term potentiation of cortico-BLA synapses evoked by patterned (theta burst) stimulation [75].
1.3. What are the mechanisms by which nicotine alters the code of synaptic plasticity?
At this point, we hope to have convinced you that the diversity of nAChRs is not a perverse fluke of molecular redundancy, but rather a rich substrate for synaptic tuning. There is little doubt that the distinct biophysical (Ca2+ permeability, kinetics) and pharmacological properties (affinity, desensitization) as well as the cellular localization of nAChRs are important determinants of how cholinergic tone regulates circuit activity. Three key features should be emphasized. First, despite the high agonist affinity of some nAChRs and the rapid rates of desensitization of others, neither receptor occupancy nor the probability of opening are “maxed out” at baseline ACh concentrations or with a cigarette or two added to the soup. Second, there are likely multiple mechanisms that are involved in turning a transient increase in nAChR activation to long-term alterations in synaptic plasticity. That is, yes, the effects of nicotine at individual synapses, in complex circuits and on behavior are long lasting. Third, there are critical windows of nAChR activation where too much or too little can be catastrophic (for elegant examples see [92,95,96]). We conclude with a discussion of possible mechanisms that might underlie some of the long-term effects of nicotinic AChR activation and a brief summary of potential disease relevance.
1.3.1. Role of calcium signaling in nAChR-stimulated transmitter release
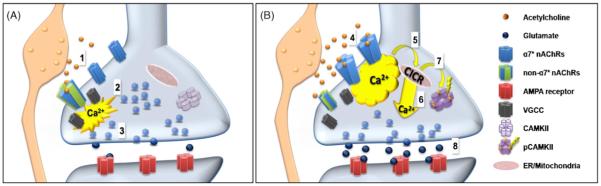
From the above discussion it is clear that despite the high affinity of β2* nAChRs for nicotine and the inactivation that occurs with an IC50∼1-60 nm [62], the concentrations of nicotine due to smoking (100-500 nM) cause little desensitization of α7* nAChRs (IC50∼7 μM) [36,51,127]. Furthermore, the targeting of both of these types of receptors to axons and axon terminals establishes an ideal impedance ratio. Thus, a little activation goes a long way. Gating of αβ* (or non-α7*) nAChRs leads to local depolarization and activation of voltage gated Ca2+ channels (VGCC) [69]. Gating of α7* nAChRs at low agonist concentrations results in a slow non-desensitizing, influx of Ca2+ directly through these high Ca2+ permeability channels. With the rise in Ca2+, more Ca2+ may be released from intracellular Ca2+-stores (CICR) [129,130]. Phasic activation Ca2+ signaling can lead in turn to stable activation of pre-synaptic Ca2+/calmodulin kinase (CAMKII), perhaps supporting the 1-10 s bursts of multiquantal release as described at mossy fiber CA3 synapses. Long-term stable changes in probability of release (10 min to 1 h) by nicotine, as well as sustained changes in synaptic transmission appear to require coordinate pre- and post-synaptic activity that can be boosted by nAChR activation [51,52,68,75]. In initial studies examining changes in Ca2+ signaling directly in axonal projections from PFC and ventral hippocampus microslices, we find that application of nicotine elicits an immediate rise in internal calcium at 2-5 μm “hotspots” along the axons. This initial response is followed by a further increase in Ca2+ at these sites after 2-3 min which persists with oscillatory periods of 1-2 min for at least 30 min after nicotine application (unpublished data: MM, HM and LWR). The long lasting Ca2+ signaling is blocked by antagonists of α7* nAChRs and is not seen in preparations from α7 KO animals. Recent studies from other labs also stress the differences in the contribution of β2* nAChRs compared to α7* nAChRs on glutamate release from PFC synaptosomes. α7* nAChRs appear to link into downstream signaling cascades that allow longer term and different profiles of Ca2+ oscillations [69]. ERK1/2 is increased after activation of α7* nAChRs with peak values after 1.5 to 3 minutes. This correlates with an increase in phoshporylation of synapsin-1 which increases the size of the ready releasable pool. Fig. 2 illustrates the convergence of these findings in a model, which is of course, only a model. Nonetheless from the cellular to the circuit level studies, there is increasing evidence that nAChR can (somehow) exert profound and long term changes in synaptic plasticity.
Fig. 2.
Nicotinic receptors regulate presynaptic gain via both α7*n AChRs and non-α7* nAChRs
Presynaptic α7* and non-α7* nAChRs modulate glutamate release via two different mechanisms. Activation of non-α7* nAChRs (A-1) cause depolarization which activates voltage gated calcium channels (VGCC) (2), and elicits fusion and exocytosis (3). (B) Release of higher concentrations of ACh or addition of nicotine activates low affinity α7* nAChRs (4) Upon their activation (5) calcium enters through the channels which can lead to further increases in Ca2+ via mobilization from internal Ca2+ stores (calciuminduced calcium release (CICR)); 6) The high levels of calcium allow for phosphorylation of Ca2+/calmodulin kinase (pCAMKII) (7) which may contribute to the sustained phase of elevated glutamate release (8).
2. Summary
Recent studies emphasize new and previously unappreciated aspects of nAChR signaling in the CNS. The effects can be long lasting, appear in critical periods during development and, if altered, may result in lifelong changes in behaviors subject to cholinergic modulation. Indeed, studies of several CNS disorders have implicated a role for cholinergic circuits in the normal functioning and maintenance of central circuits underlying attention, cognitive performance, motivation and memory. The role of cholinergic signaling in the brain may be quite complex, dependent on oscillatory signaling at synapses and in circuits. Rather than turning systems on or off, it appears that cholinergic tuning involves changes in the balance between inhibitory and excitatory inputs. In this manner, nAChRs participate in more subtle but crucial aspects of modulating synaptic plasticity.
References
- [1].Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53(4):447–56. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- [2].Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors halpha 2beta 2, halpha 2beta 4, halpha 3beta 2, halpha 3beta 4, halpha 4beta 2, halpha 4beta 4 and halpha 7 expressed in xenopus oocytes. J Pharmacol Exp Ther. 1997;280(1):346–56. [PubMed] [Google Scholar]
- [3].Changeux JP, Edelstein SJ. Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr Opin Neurobiol. 2001;11(3):369–77. doi: 10.1016/s0959-4388(00)00221-x. [DOI] [PubMed] [Google Scholar]
- [4].Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function, in reviews of physiology. Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- [5].Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74(6):363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [6].Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27(9):482–91. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [7].Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- [8].Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [H-3] dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301(2):651–60. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- [9].McGehee DS. Nicotinic receptors and hippocampal synaptic plasticity... it’s all in the timing. Trends Neurosci. 2002;25(4):171. doi: 10.1016/s0166-2236(00)02127-5. [DOI] [PubMed] [Google Scholar]
- [10].Wonnacott S, Sidhpura N, Balfour DJK. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5(1):53. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [11].Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: relevance to nicotine addiction. Biochem Pharmacol. 2008;75(1):323–33. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004:109–20. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- [13].Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35(1):1. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- [14].Wonnacott S, Harrison R, Lunt G. Immunological cross-reactivity between the alpha-bungarotoxin-binding component from rat brain and nicotinic acetylcholine receptor. J Neuroimmunol. 1982;3(1):1–13. doi: 10.1016/0165-5728(82)90013-3. [DOI] [PubMed] [Google Scholar]
- [15].Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5(5):1307–15. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25(6):317. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- [17].Fucile S, Renzi M, Lauro C, Limatola C, Ciotti T, Eusebi F. Nicotinic cholinergic stimulation promotes survival and reduces motility of cultured rat cerebellar granule cells. Neuroscience. 2004;127(1):53–61. doi: 10.1016/j.neuroscience.2004.04.017. [DOI] [PubMed] [Google Scholar]
- [18].McGehee DS, Heath MJS, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by Presynaptic receptors. Science. 1995;269(5231):1692–6. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- [19].Rogers M, Colquhoun LM, Patrick JW, Dani JA. Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. J Neurophysiol. 1997;77(3):1407–17. doi: 10.1152/jn.1997.77.3.1407. [DOI] [PubMed] [Google Scholar]
- [20].Haghighi AP, Cooper E. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine alpha 3beta 4 and alpha 4beta 2 receptors. J Neurosci. 2000;20(2):529–41. doi: 10.1523/JNEUROSCI.20-02-00529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Papke RL. Estimation of both the potency and efficacy of [alpha]7 nAChR agonists from single-concentration responses. Life Sci. 2006;78(24):2812–9. doi: 10.1016/j.lfs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [22].Papke RL, Meyer E, Nutter T, Uteshev VV. Alpha 7 receptor-selective agonists and modes of alpha 7 receptor activation. Eur J Pharmacol. 2000;393(13):179–95. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- [23].El-Hajj RA, McKay SB, McKay DB. Pharmacological and immunological identification of native [alpha]7 nicotinic receptors: evidence for homomeric and heteromeric [alpha]7 receptors. Life Sci. 2007;81(16):1317–22. doi: 10.1016/j.lfs.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol (Lond) 1997;504(3):603–10. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of [alpha]5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380(6572):347–51. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- [26].Yu CR, Role LW. Functional contribution of the alpha 5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol (Lond) 1998;509(3):667–81. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Girod R, et al. Heteromeric complexes of alpha 5 and/or alpha 7 subunits—effects of calcium and potential role in nicotine-induced presynaptic facilitation. Molecular and functional diversity of ion channels and receptors. 1999;868:578–90. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- [28].Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, et al. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540(2):425–34. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yu CR, Role LW. Functional contribution of the alpha 7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol (Lond) 1998;509(3):651–65. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha 10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA. 2001;98(6):3501–6. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. [Alpha]9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79(4):705–15. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- [32].Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in {alpha}4{beta}2* nicotinic receptors. Mol Pharmacol. 2008;74(1):132–43. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- [33].Gerzanich V, Wang F, Kuryatov A, Lindstrom J. Alpha 5 subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286(1):311–20. [PubMed] [Google Scholar]
- [34].Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human [alpha]6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39(13):2570–90. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- [35].Fischer H, Orr-Urtreger A, Role LW, Huck S. Selective deletion of the {alpha}5 subunit differentially affects somatic-dendritic versus axonally targeted nicotinic ACh receptors in mouse. J Physiol (Lond) 2005;563(1):119–37. doi: 10.1113/jphysiol.2004.075788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31(1):131–41. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- [37].Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, et al. Ultrastructural distribution of the {alpha}7 nicotinic acetylcholine receptor subunit rat hippocampus. J Neurosci. 2001;21(20):7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ge SY, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25(26):6084–91. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha 7 and alpha 4 beta 2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86(6):3043–55. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- [40].Lubin M, Erisir A, Aoki C. Ultrastructural immunolocalization of the {alpha}7 nAChR subunit in guinea pig medial prefrontal cortex. Ann N Y Acad Sci. 1999;868(1):628–32. doi: 10.1111/j.1749-6632.1999.tb11337.x. [DOI] [PubMed] [Google Scholar]
- [41].Levy RB, Aoki C. Alpha 7 Nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and -negative excitatory synapses in rat sensory cortex. J Neurosci. 2002;22(12):5001–15. doi: 10.1523/JNEUROSCI.22-12-05001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jones IW, Wonnacott S. Precise localization of {alpha}7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24(50):11244–52. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nashmi R, Lester H. CNS localization of neuronal nicotinic receptors. J Mol Neurosci. 2006;30(1):181–4. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- [44].Hancock ML, Canetta SE, Role LW, Talmage DA. Presynaptic type III neuregulin1-ErbB signaling targets {alpha}7 nicotinic acetylcholine receptors to axons. J Cell Biol. 2008;181(3):511–21. doi: 10.1083/jcb.200710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20(2):92. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- [46].Clarke PB, Hamill GS, Nadi NS, Jacobowitz DM, Pert A. H-nicotine- and 125I-alpha-bungarotoxin-labeled nicotinic receptors in the interpeduncular nucleus of rats. II. Effects of habenular deafferentation. J Comp Neurol. 1986;251(3):407–13. doi: 10.1002/cne.902510311. [DOI] [PubMed] [Google Scholar]
- [47].Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of {alpha}7 nicotinic acetylcholine receptors. J Neurosci. 2008;28(37):9111–6. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology. 2006;184(34):292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- [49].Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9(13):3097–101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- [50].Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390(6658):401–4. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- [51].Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27(2):349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- [52].Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- [53].Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of alpha 6-Containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22(4):1208–17. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11(1):60–9. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21(5):1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23(8):3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65(6):1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- [58].Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal {alpha}6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by {beta}3 subunit gene deletion. Mol Pharmacol. 2005;67(6):2007–15. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- [59].Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7(6):581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- [60].Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7(6):583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- [61].Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28(7):1672–81. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons but not pyramidal cells. J Neurosci. 1998;18(4):1187–95. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gioanni Y, Rougeot C, Clarke PBS, Lepouse C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11(1):18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- [64].Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993;56(1):23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- [65].Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28(2):216–25. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- [66].Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- [67].Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28(35):8756–64. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54(1):73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [69].Dickinson JA, Kew JNC, Wonnacott S. Presynaptic {alpha}7 and {beta}2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74(2):348–59. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- [70].Wang BW, Liao WN, Chang CT, Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. 2006;59(8):491–501. doi: 10.1002/syn.20267. [DOI] [PubMed] [Google Scholar]
- [71].Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(23):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- [72].Yoshikawa T, Watanabe A, Shibuya H, Toru M. Involvement of the fimbria fornix in the initiation but not in the expression of methamphetamineinduced sensitization. Pharmacol Biochem Behav. 1993;45(3):691–5. doi: 10.1016/0091-3057(93)90526-y. [DOI] [PubMed] [Google Scholar]
- [73].Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82(4):1103–14. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- [74].Beyer CE, Steketee JD. Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex. 2002;12(5):526–35. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- [75].Jiang L, Role LW. facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J Neurophysiol. 2008;99(4):1988–99. doi: 10.1152/jn.00933.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- [77].Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- [78].Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4(12):1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- [79].Girod R, Role LW. Long-lasting enhancement of glutamatergic synaptic transmission by acetylcholine contrasts with response adaptation after exposure to low-level nicotine. J Neurosci. 2001;21(14):5182–90. doi: 10.1523/JNEUROSCI.21-14-05182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10(9):1168–75. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- [81].Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10(4):438–46. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [82].Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275(5297):213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- [83].Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18(24):10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Koester HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci USA. 1998;95(16):9596–601. doi: 10.1073/pnas.95.16.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275(5297):209–13. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- [86].Sjöström PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002;12(3):305–14. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- [87].McGehee DS. Nicotine and synaptic plasticity in prefrontal cortex. Sci STKE. 2007;2007(399):e44. doi: 10.1126/stke.3992007pe44. [DOI] [PubMed] [Google Scholar]
- [88].Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846(1):137–43. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- [89].Day M, Pan JB, Buckley MJ, Cronin E, Hollingsworth PR, Hirst WD, et al. Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem Pharmacol. 2007;73(8):1123–34. doi: 10.1016/j.bcp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [90].Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138(34):266–74. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- [91].Clarke PB. Nicotinic modulation of thalamocortical neurotransmission. Prog Brain Res. 2004;145:253–60. doi: 10.1016/S0079-6123(03)45017-6. [DOI] [PubMed] [Google Scholar]
- [92].Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: Modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(1):254–62. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Metherate R, Hsieh CY. Synaptic mechanisms and cholinergic regulation in auditory cortex. Prog Brain Res. 2004;145:143–56. doi: 10.1016/s0079-6123(03)45010-3. [DOI] [PubMed] [Google Scholar]
- [94].Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotineinduced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20(16):6106–16. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG, et al. Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci. 2003;23(9):3837–43. doi: 10.1523/JNEUROSCI.23-09-03837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314(5805):1610–3. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- [97].Gabbott P, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492(2):145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- [98].Markou A. Review. Neurobiology of nicotine dependence. Phil Trans R Soc B: Biol Sci. 2008;363(1507):3159–68. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75(6):348–52. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- [100].Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653(12):278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- [102].Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128(3):351–8. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- [103].Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the [beta]2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- [104].Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res. 2000;113(12):97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- [105].Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 2001;158(2):190–7. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- [106].Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53(5):603–25. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- [107].Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20(4):142–50. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- [108].Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- [109].Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74(8):1235–46. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lovinger DM, Partridge JG, Tang K-C. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann N Y Acad Sci. 2003;1003(1):226–40. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- [111].Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50(3):443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [112].Goto Y, O’Donnell P. Altered prefrontal cortex-nucleus accumbens information processing in a developmental animal model of schizophrenia. Ann N Y Acad Sci. 2003;1003(1):398–401. doi: 10.1196/annals.1300.035. [DOI] [PubMed] [Google Scholar]
- [113].Goto Y, O’Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001;21(12):4498–504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Rev. 2000;31(23):330–41. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- [115].O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15(5 I):3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].West AR, Floresco SB, Charara ALI, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003(1):53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- [117].Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- [118].Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47(1):699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- [119].Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551(Pt 2):539–50. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313(4):574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- [121].Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat-brain.1. Projections to the Limbic telencephalon. Brain Res Bull. 1984;13(6):751–84. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- [122].Changeux JP, Bertrand D, Corringer P-J, Dehaene S, Edelstein S, Lena C, et al. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Rev. 1998;26(23):198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- [123].Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138(34):217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- [124].McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- [125].Power AE. Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80(3):178–93. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- [126].Huang Y-Y, Kandel ER, Levine A. Chronic nicotine exposure induces a long-lasting and pathway-specific facilitation of LTP in the amygdala. Learn Mem. 2008;15(8):603–10. doi: 10.1101/lm.975308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70(4):439–46. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- [128].Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28(39):9797–805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sharma G, Grybko M, Vijayaraghavan S. Action Potential-Independent. Nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci. 2008;28(10):2563–75. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38(6):929–39. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]