Abstract
Brain slice preparations are well-established models for a wide spectrum of in vitro investigations in the neuroscience discipline. However, these investigations are limited to acute preparations or thin organotypic culture preparations due to the lack of a successful method that allows culturing of thick organotypic brain slices. Thick brain slice cultures suffer necrosis due to ischemia deep in the tissue resulting from a destroyed circulatory system and subsequent diffusion-limited supply of nutrients and oxygen. Although thin organotypic brain slice cultures can be successfully cultured using a well established roller tube method (a monolayer organotypic culture) (Gahwiler B H, 1981) or a membrane insert method (up to 1–4 cell layers, <150μm)(Stoppini L et al., 1991), these methods fail to support thick tissue preparations. A few perfusion methods (using submerged or interface/microfluidic chambers) have been reported to enhance the longevity (up to few hours) of acute slice preparations (up to 600μm thick) (Hass H L et al., 1979; Nicoll R A and Alger B E, 1981; Passeraub P A et al., 2003). Here, we report a unique interstitial microfluidic perfusion technique to culture thick (700μm) organotypic brain slices. The design of the custom-made micro-perfusion chamber facilitates laminar, interstitial perfusion of oxygenated nutrient medium throughout the tissue thickness with concomitant removal of depleted medium and catabolites. We examined the utility of this perfusion method to enhance the viability of the thick organotypic brain slice cultures after 2 days and 5 days in vitro (DIV). We investigated the range of amenable flow rates that enhance the viability of 700μm thick organotypic brain slices compared to the unperfused control cultures. Our perfusion method allows up to 84.6% viability (P<0.01) and up to 700μm thickness, even after 5 DIV. Our results also confirm that these cultures are functionally active and have their in vivo cytoarchitecture preserved. Prolonged viability of thick organotypic brain slice cultures will benefit scientists investigating network properties of intact organotypic neuronal networks in a reliable and repeatable manner.
Introduction
Brain researchers use a variety of experimental models and experimental techniques to study brain function at various levels of complexity – from the molecular, to systems, to the behavioral level. In neuroscience and neuroengineering research, cell and tissue cultures constitute well-established and accepted in vitro models. Compared to in vivo experiments, in vitro preparations offer the advantages of being well-defined and providing better control of input/output variables; maintenance of constant temperature, pH, O2 concentration over the course of an experiment; and better accessibility for physical, chemical or electrical manipulation; and elimination of irrelevant peripheral factors. In vitro models also permit simultaneous use of advanced, noninvasive techniques such as multiphoton imaging, multi-site multielectrode recording and pharmacological studies (Bliss T V and Lomo T, 1973; Collin C et al., 1997; Potter S M et al., 2004). Although many in vitro studies use networks of dissociated cultured neurons, slice cultures are more accurate in representing the in vivo-like cyto-architecture, stereotypic organization of functional units and local structural pathways of the brain. With more veridical network circuitry, cultured slice studies of network communication, encoding and processing might be more applicable to intact animals.
In the history of fundamental and applied brain research, slice preparations have played a vital role in revealing the function of different parts of the brain, and have been used to study mechanisms of learning and memory. Most of our understanding of synaptic plasticity and mechanisms of long-term potentiation of synapses is attributed to the original studies using acute or cultured hippocampal and cortical slices (Bliss T V and Lomo T, 1973). Recently, several groups reported studies using thick (>400 μm) cortical, hippocampal or co-culture slice preparations (Baker R E et al., 2006; Bindokas V P et al., 1998; Klapstein G J and Colmers W F, 1997; Lim C et al., 1998; MacLean J et al., 2006). However, due to the lack of a reliable method to culture thick brain slices, these studies were confined to acute slice preparations allowing only up to a few hours of continuous and reliable analysis (Bindokas V P et al., 1998; Klapstein G J and Colmers W F, 1997; Lim C et al., 1998; MacLean J et al., 2006; Baker R E et al., 2006).
Very little is known about the long-term network properties of information processing in the structural and functional units of the brain. Although, current culturing methods allow only 1–4 cell thick organotypic cultures with satisfactory longer term viability (Simoni A D and Yu L M, 2006), organotypic brain slice cultures offer an opportunity to study in vitro, a wide range of phenomena including neurogenesis (Raineteau O et al., 2004), synaptogenesis (Nikononko I and al., 2003), regeneration (Linke R et al., 1995), protein expression (Ehrengruber M U and al., 1999; Kakegawa W et al., 2004; Lundstrom K and al., 2001), and responses to physical trauma (Krassioukov AV et al., 2002). Thick organotypic brain slice cultures represent an advanced in vitro model for neuroscience research that requires larger portions of intact laterally and tangentially interacting stereotypic pathways within one area, or across different regions of the brain. Unfortunately, it has been a challenge to culture such thick nervous tissue for extended periods of time over which reliable studies can be completed. It was suggested in previous studies (Stoppini L, 1991) that the metabolic decay of the tissue is due to a limited supply of media and oxygen and poor waste removal. This is more pronounced in thick slices where the problem of inadequate, diffusion-limited mass transport is exacerbated (Stoppini L et al., 1991). We hypothesized that a convection-based interstitial perfusion method that provides flow of oxygenated nutrient medium through the entire thickness of the slice would satisfy the cellular metabolic needs at a sufficiently high volume flow rate to result in enhanced culture viability. Specifically, in vivo-like nutrient supply could be restored when the convective exchange rate exceeds the rate of depletion of nutrients and oxygen through the entire thickness of the tissue. Recent work demonstrates the usefulness of three-dimensional (3D), convective intercellular perfusion to enhance the viability of high density three dimensional engineered neuronal constructs (Cullen D K et al., 2007). Presently, most of the brain slice studies are either time-constrained to a few minutes or hours (acute slice preparations) (Baker R E et al., 2006; Bindokas V P et al., 1998; Klapstein G J and Colmers W F, 1997; Lim C et al., 1998; MacLean J et al., 2006), or are confined to thin slice cultures (flattening to <150μm) (Baker R E et al., 2006; Bindokas V P et al., 1998; Klapstein G J and Colmers W F, 1997; Lim C et al., 1998; MacLean J et al., 2006). Perfusion methods have been developed to maintain the health of acute slice preparations for a few hours using custom made or commercially available perfusion chambers. These can broadly be divided into two categories: submerged-type and interface-type chambers (Hass H L et al., 1979; Nicoll R A and Alger B E, 1981; Passeraub P A et al., 2003). In submerged chambers, the tissue slice is entirely submerged in the culture medium and perfused using oxygenated medium (Nicoll R A and Alger B E, 1981; Shi W X and Bunney B S, 1990; Zbicz K L and Weight F F, 1985). In interface-type chambers the tissue rests on a mesh or a porous membrane at the interface between a reservoir of medium or a channel of perfusate below the mesh, and a humidified atmosphere of gases above the mesh (Hass H L et al., 1979; Li C L and McIlwain H, 1957; Reynaud J C et al., 1995). An extensively used variation of the interface-type chamber is the Haas chamber (Hass H L et al., 1979), where the tissue rests on a nylon mesh through which the bathing fluid is delivered by capillary action. However, the interstitial mass transport through the preparations cultured in both types of the chamber still remains diffusion- and/or capillary action-limited. For example, in submerged tissue perfusion the interstitial nutrient and gas concentration does not equilibrate with the medium surrounding the tissue. Instead, concentration gradients arise from the medium-exposed surfaces of the tissue towards the tissue interior. Concentration gradients are also present in preparations cultured in interface-type chambers, where only one side of the tissue is exposed to the open channel flow of perfusate below the mesh, through which the tissue wicks the medium. Hence, the lack of forced interstitial perfusion limits the tissue viability. Microfabricated and biocompatible devices analogous to the Haas chamber were proposed (Passeraub P A et al., 2003) to circulate the artificial cerebrospinal fluid among micropillars supporting the tissue. Besides these, there are other slice culturing methods: one involves alternating media and oxygen environment changes in a roller-tube (Gahwiler B H, 1988), while the other is a membrane-insert culturing method (Stoppini L, 1991) (Figure 1a–f). Notably, molecular diffusion and/or capillary transport is the dominating mode of nutrient and oxygen supply in all of the above mentioned methods. Although these methods temporarily improve the viability of acute slice preparations, diffusion barriers in oxygen and nutrient delivery to deeper layers of the tissue pose an upper limit on the longevity and/or the thickness of the preparation that can be reproducibly grown in vitro (Albertson T. E., 1998). In this investigation, we report a unique convective-flow based interstitial perfusion method to successfully culture 700 μm thick organotypic brain slices with enhanced viability. This perfusion system was realized using a custom-made biocompatible microfluidic chamber, which enables continuous infusion of the equilibrated nutrient medium with concurrent, mass-equilibrated withdrawal of depleted medium and waste, by way of a syringe pump carrying opposing syringes on a single drive (Vukasinovic J and A, 2006) (Figure 1g,h). This perfusion technique maintained viable 700μm thick organotypic brain slice cultures 5 DIV with unprecedented thickness maintenance. Slice cultures preserved their organotypic organization and were functionally active. The range of non-invasive perfusion rates that augment the viability of slice cultures was also established. This culturing method could be used to enhance the viability of other thick organotypic slice preparations as well. Maintenance of long-term thick organotypic brain slices cultures will enable high-fidelity brain research in vitro, e.g., using multiple layers of cortex in tangential slices with intact lateral connectivity.
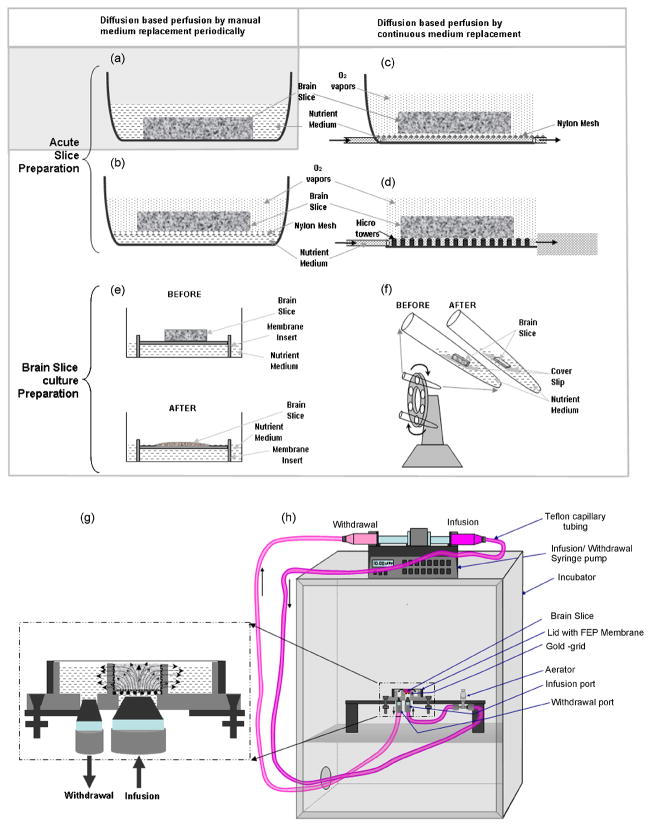
Figure 1.
A comparison of various brain slice (acute or organotypic cultures) maintenance methods. (a) A submerged-type method for nutrient delivery to the acute slice preparation. (b–f) Interface-type methods. (b) The brain slice is supported on a nylon mesh at the interface between the nutrient medium and moisturized atmosphere. The nutrient medium diffuses from the bottom while the oxygen diffuses from the top of the acute slice. (c) Haas-type method. The brain slice is maintained at the interface of nutrient medium and moisturized oxygen, with the nylon mesh resting on the bottom of the dish and continuous wicking of the nutrient medium through the mesh. (d) A microfabricated variant of the Haas-type chamber. The nylon mesh is replaced by an array of microfabricated pillars. The acute slice rests on the top of the micropillars at the interface between micropillar-confined flow of perfusate in a channel underneath the slice, and humidified atmosphere above the slice. This method could successfully be used to maintain viable (up to 600μm) thick acute brain slice preparations for a few hours, due to enhanced nutrient delivery as a result of perfusion. (e) A classical roller-tube method to culture organotypic brain slices for days. The brain slice is adhered to a cover slip using plasma/thrombin clot and is kept at an interface of nutrient medium and air in a test tube. The test tubes are placed on a rotating holder at ~12 r.p.h., inclined at 10° to alternatively provide nutrients and oxygen. This method is successful only for thin brain slice cultures. The slices originally cut at ~ 250 μm thinned-down to 1–3 cell layers thickness in a couple of days. (f) Another interface method to culture organotypic brain slices. In this method, the brain slices are held at nutrient-air interface using a permeable membrane. This method is simple, however, it also supports only thin brain slices, which thin down from 450 μm to ~ 150 μm in a couple of days. (g) Our convection-enhanced interstitial nutrient and oxygen delivery method. The microfabricated device supports thick brain slices and allows 3D perfusion of oxygenated nutrient medium from the bottom of the tissue that is anchored to an electron microscopy grid, serving as a porous substrate. The depleted medium is withdrawn peripherally at the same flow rate, to maintain continuous perfusion over long time periods up to days. Throughout the experiment, the chamber is covered with a gas-permeable FEP membrane attached to the Teflon lid, to allow gaseous exchange from the top of the tissue, prevent medium evaporation, maintain desired pH and osmolarity, and prevent microbial infections. (h) A cartoon of the fluidic set-up to culture thick brain slices. Continuous infusion of fresh medium and withdrawal of depleted medium is enabled by a single syringe pump carrying opposing syringes on a single drive. The infusion line contains an aerator and a check-valve to equilibrate the nutrient medium with the incubator environment prior to injection, and to enable pulsation-free infusion. The entire set-up is kept in the incubator set at 5% CO2, 9% O2, 65% Relative Humidity and 35°C temperature.
Methods
Brain Slice Preparation
The brains were obtained from the c57BL/6J and B6.Cg.Tg (Thy1-EYFP)16Jrs/J mice (The Jackson Laboratory, ME) from postnatal day 11–16. The mice were anesthetized and decapitated according to approved NIH protocols and regulations. To obtain healthy slice cultures with high viability, it is critical to complete all the steps quickly (< 7–8 minutes; from brain harvest to tissue mounting in the microperfusion chamber) before starting the perfusion. The brains were extracted from the skull within 1 minute and kept in the ice-cold nutrient medium for 1 minute to reduce metabolic activity of the tissue and to facilitate smooth slicing. Multiple 700 μm cortical slices were obtained using a McIlwain tissue chopper. These were cut into 3mm diameter discs using a sterile biopsy tool to fit snugly in the infusion chamber. The tissue slices were kept in the ice-cold oxygenated nutrient medium until mounted onto the chamber.
The nutrient medium consisted of 50% OptiMEM (Gibco, Invitrogen, Inc.), 25% Hanks Balanced Salt Solution (HBSS) (Gibco, Invitrogen, Inc.), 25% Equine Serum, 500 μl of 0.5 mM Glutamax, and 0.45 g of D-glucose/100ml of medium. The pH of the medium was maintained between 7.2 – 7.4 by equilibration with a 5% CO2 atmosphere. The medium was sterilized by filtering it with a 0.2 μm filter in a sterile hood and stored in a sterile glass bottle with a custom fabricated lid fitted with a 12.5-μm thick fluorinated ethylene-propylene (FEP) membrane (Potter S. M. and DeMarse T. B., 2001). The non-porous FEP membrane is significantly permeable to gases and relatively impermeable to water vapor, thus allowing the nutrient medium to equilibrate with the incubator environment, which was regulated at 9% O2, 5% CO2, 65% relative humidity and 35°C temperature (Brewer G. J. and C.W., 1989). This medium was used for both perfusion and slice harvesting.
Tissue adhesion to the interior of the infusion chamber is critical to the success and repeatability of forced interstitial perfusion, and ultimately to tissue viability. Poor adhesion facilitates the formation of domains of low resistance to fluid flow. This causes a nonuniform supply of nutrients through the tissue thickness, thus reducing convective nutrient delivery to the deeper tissue layers. A specific procedure was developed to ensure relatively quick and reliable tissue adherence to the interior walls of the infusion chamber and the gold-grid substrate. After assembling the fluidic system, the gold grid and the internal walls of the infusion chamber were coated with 20 μl of laminin (1 mg/ml) 30–40 minutes prior to tissue plating. As described above, the brain slices were cut to the exact size of the infusion chamber using a circular biopsy tissue cutter. Upon plating, a Teflon lid holding a taut FEP membrane (Potter S P and DeMarse T B, 2001) was fitted on the top of the culture chamber and perfusion was initiated. For the infusion rates that are ≤30μl/hr, the laminin coating and the taut FEP membrane placed directly above the tissue were sufficient to keep the tissue in place and encourage its adhesion to the interior of the infusion chamber.
Microperfusion chamber design, fabrication, and flow diagnostics
The microperfusion chamber (Vukasinovic J and A, 2006) comprises an inner infusion (culture) chamber; an outer withdrawal chamber; and an inlet and an outlet port (Figure 2a,b). A gold electron microscopy grid (1GG300 PELCO®) was used as the substrate to seat the tissue and deliver the nutrient medium into the infusion chamber via the inlet port. The grid measures 3 mm in diameter and has 40% open area for fluid flow through ~584 microopenings (54μm × 54μm). The infusion (culture) chamber (Figure 2c) is in direct fluidic communication with the withdrawal chamber by way of 150-μm wide microchannels in the cylindrical enclosure bounding the culture chamber. Microchannels start mid-height through the enclosure and extend to the top of the chamber (Figure 2a). The infusion chamber (Figure 2c) measures 3.5 mm in diameter and 700 μm in height. A Teflon lid fitted with a selectively permeable FEP membrane encapsulates the device (Figure 2d). The FEP membrane equilibrates the sample with the incubator environment, maintains asepsis and reduces evaporation (Potter S M and DeMarse T B, 2001).
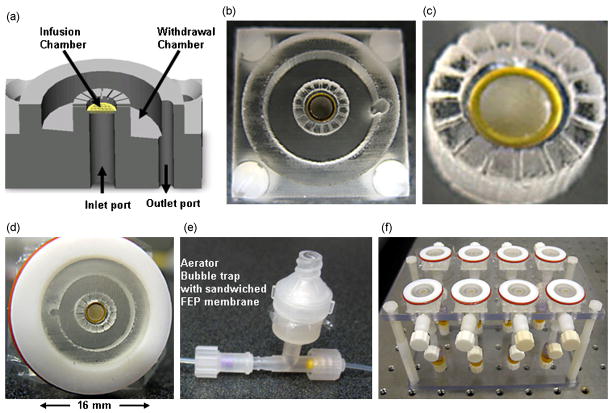
Figure 2.
The microperfusion chamber. (a) A 3D drawing of the device, showing an inner (culture) chamber, an outer (withdrawal) chamber, and inlet and outlet ports. (b) Device fabricated in PDMS. (c) Magnified view of the culture chamber. A porous substrate (gold grid) at the bottom of the chamber delivers the nutrient medium to the tissue. Tissue is sealed to the chamber via an adhesive layer of laminin. The cylindrical enclosure contains 350μm deep microchannels starting mid-height through the culture chamber to facilitate the outflow of media from the culture into the withdrawal chamber. (d) A microperfusion chamber encapsulated by a semi-permeable membrane in a Teflon lid. A substantially gas permeable, and substantially water and water vapor impermeable (fluorinated ethylene-propylene, FEP) membrane is stretched over the Teflon lid using O-rings. The membrane maintains asepsis, facilitates gas exchange, prevents tissue desiccation, and equilibrates the medium contained within the culture chamber. (e) An in-line aerator/bubble trap placed in the infusion circuit. It is a two-piece device sandwiching an FEP membrane. This device serves to equilibrate the medium with the incubator environment just prior to injection into the culture chamber. It also removes gas bubbles from the medium and damps fluctuations in liquid delivery. (f) A custom fabricated Plexiglas stand to support a plurality of perfusion chambers for parallel experimentation. This stand eases the sterile transfer of experimental set-up from the laminar flow-hood into the incubator.
The infusion circuit contains an aseptic aerator/bubble trap and a check valve. An FEP membrane is sandwiched between the upper and the lower parts of the aerator assembly (Figure 2e). Together the aerator and the check valve ensure uninterrupted and smooth injection of the oxygenated medium into the culture chamber. In this low-pressure system, the aerator damps the pulsations in nutrient delivery caused by the stepper motor of the syringe pump. The check valve prevents reverse flow through the aerator and the culture chamber. The aerator also equilibrates the nutrient medium (prior to its injection into the culture chamber) with the incubator environment and outgases air bubbles. This ensures that a sufficient amount of oxygen is provided to the tissue from the top (through the FEP membrane) and from the bottom, via injected medium. 3D solid object printing (McDonald J et al., 2002) was used to produce disposable molds for the replica molding of perfusion chambers in biocompatible, polydimethylsiloxane (PDMS) rubber. The gold grid perfusion substrate is attached to the bottom of the culture chamber, using a thin-layer of contact-sealing PDMS prepolymer/catalyst mixture (Sylgard 184, Dow Corning), to yield a watertight bond upon heat curing. All devices were tested and steam-autoclaved prior to experiments.
To experimentally validate the induced flow within the culture chamber (without the tissue), we used microscopic particle image velocimetry (μ-PIV), as explained elsewhere (Cullen D K et al., 2007; Wereley S T and Meinhart C D, 2004). To do so, the chamber and fluidic setup was filled with suspension of 500-nm fluorescent particle tracers in water. A 3D convection within the culture chamber was induced by an array of microjets emanating from the porous grid. Flow downstream from the grid, and peripheral perfusate extraction through microchannels is depicted in Figure 3A. A small culturing volume (about 7μl) allows rapid exchange of perfusate (~40 exchanges/hr for the flow validation here), and reduces the amount of used medium.
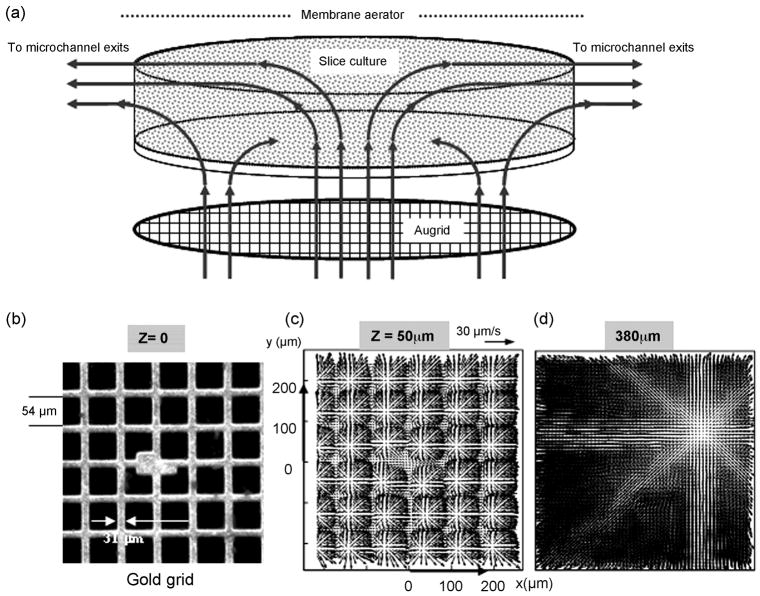
Figure 3.
Schematic of the induced flow within the culture chamber. (a) Measurement domain and (b) measured velocity distributions within the culture chamber. (c–d) Measurements were taken in several planes that are parallel to the porous grid perfusion substrate and normal to the axes of microjets emanating from about 584 square openings of a gold mesh. The elevation, Z, is measured from the perfusion substrate. (b) The field of view includes the central portion of the gold screen (450 × 457μm) with an array of about 6 × 6 nozzles in focus. The nominal volume flow rate is 5 μl/min, with microjet ejection velocity of about 50 μm/s and a nozzle based Reynolds number of 0.003. (c) Immediately downstream from the perfusion substrate, flow is characterized by the radial spreading of submerged, laminar microjets normal to their axes. Upon discharge from the micronozzles, microjets broaden, i.e. spread out radially. Momentum exchange between the jets and the lower momentum medium within the chamber causes this broadening with concomitant reduction in jet streamwise velocity (along their axes). A continuing reduction in jet streamwise momentum causes turning from their nominally vertical trajectories. Adjoining microjets begin to interact, lose coherence and merge. (d) Starting mid-height through the chamber, peripheral fluid withdrawal creates substantial 3D convection that drives the merging jet outflows towards the exit microchannels.
Experimental set-up and culture perfusion
Culture perfusion was set up using the custom microfluidic chamber, stiff FEP microtubing (500 μm internal diameter), and a syringe pump (KD Scientific 260) that enables continuous infusion of nutrients into the culture chamber and simultaneous mass-equilibrated withdrawal of perfusate from the withdrawal chamber (Figure 1h). Except for the pump, all components of the perfusion system were steam-autoclaved prior to use.
A custom Plexiglas stand was built to hold securely a number of perfusion chambers and their respective aerators in an upright position (Figure 2f). The stand also facilitated the transfer of setups from the sterile hood environment into the incubator upon tissue plating and encapsulation. Both the infusion and the withdrawal tubes were attached to medium-filled syringes using luer-lock couplings. To ensure that slices were submerged prior to the start of experiments, the withdrawal of medium was delayed with respect to infusion (during set-up) until the withdrawal chambers were fully submerged.
Viability assay, image acquisition, and data analysis
Tissue viability was assessed by a live/dead assay using cell-permeant (Hoechst) and cell-impermeant (propidium iodide) fluorescent nuclear stains (Invitrogen). The cultures were labeled with 20 μl of propidium iodide and Hoescht in 200 μl of nutrient medium, after 2 or 5 days in vitro. The cultures were incubated with fluorescent labels for 30–40 minutes prior to imaging. The flow was stopped and the infusion and withdrawal ports of the chamber were sealed with biocompatible plugs to avoid evaporation of the nutrient medium during imaging. Multiple z-stack images of dead and living nuclei in each tissue slice were collected using a 20X (NA 0.5) achroplan water immersion objective lens (Zeiss, Inc.) in two separate detector channels of the Zeiss 510NLO microscope, with simultaneous two-photon excitation of both fluorophores at 800 nm. Each tissue slice was imaged at 4 or more randomly chosen locations across its diameter. Slice viability (n = 3–12 slices per culture type or condition) was assessed by counting of dead and live nuclei using ImageJ software routines. Image stacks were processed for background noise reduction to enhance the contrast of the nuclei. Data are presented as mean ± SEM unless otherwise noted. Statistical significance of the data was estimated using one way analysis of variance (ANOVA) (Figures 4, 5) and two-way ANOVA (Figures 6) followed by Tukey’s pair-wise comparison using Sigmastat software (Systat Software, Inc.). The results were considered significant for p<0.05.
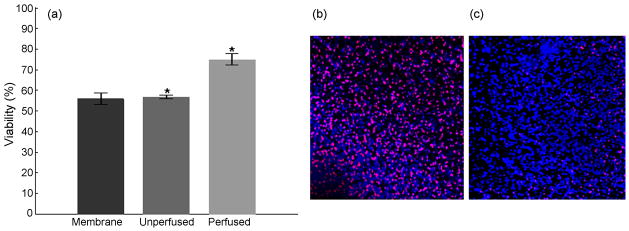
Figure 4.
Interstitial perfusion enhances viability of the culture. (a) Perfusion at 20μl/hr for 48 hours increased the viability of the cultures by 31.7%, compared to unperfused sister cultures, and to 30.7% compared to membrane insert controls. (p<0.01). A generalized linear model ANOVA followed by a Tukey’s multiple comparison test was used to evaluate statistical significance of the data. (b) Representative z-projection of unperfused culture (c) Representative z-projection of perfused culture. The cell death (red) is widespread in the unperfused cultures compared to perfused cultures. Images are ~440 μm across.
Figure 5.
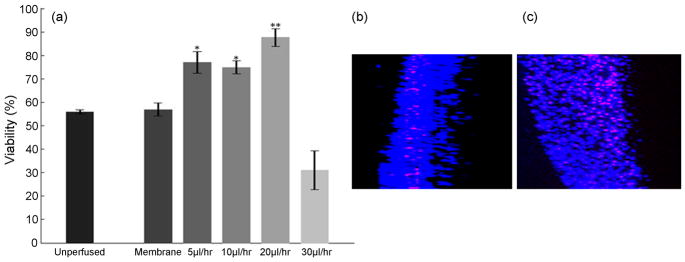
Evaluation of the useful range of perfusion rates. (a) Viability of cultures after 48 hrs of perfusion at chosen flow rates. The flow rate of 20μl/hr is optimal to maximize the viability. A generalized linear model ANOVA followed by a Tukey’s multiple comparison test was used to evaluate statistical significance. ** indicates p<0.01 compared to membrane and unperfused controls. * indicates p<0.05 compared to membrane and unperfused controls. (b,c) A partial view through the slice thickness (extracted from side view rendered with 3D projection) from unperfused and perfused cultures respectively at a flow rate of 30 μl/hr. Unperfused cultures are thinner, with the cell death prominently organized in layers with obviously higher cell density. The perfused tissue shows widespread cell death around the microchannels formed in the tissue due to excessive flow rate applied.
Figure 6.
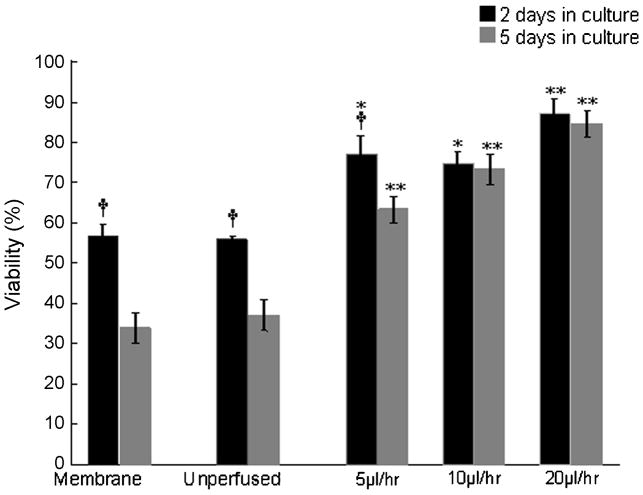
Assessment of long-term viability of the organotypic cultures. Cultures were perfused at particular flow rates for 2 and 5 DIV. Although at lower rates the viability of perfused cultures decreases from 2 days to 5 DIV, the decrease in viability over time is insignificant at higher flow rates, corroborating that 20 μl/hr is an optimal flow rate to maintain > 86% long-term viability of initially 700 μm thick organotypic brain slice cultures. By contrast, the reduction in viability of the unperfused cultures and membrane insert controls is notable from 2 to 5 DIV. A two-way ANOVA test followed by Tukey’s multiple comparison test was used to evaluate statistical significance. (†) indicates p<0.01 significant change in viability from 2DIV to 5DIV for the same culture condition (i.e., perfused at a given flow rate, unperfused cultures, membrane controls). (*) and (**) indicate p<0.05 and p<0.01 respectively, for perfused culture viability, compared to that of unperfused and membrane-insert control cultures at a given time point (2DIV or 5 DIV).
Tissue Fixing and Haematoxylin and Eosin (H&E) Staining
To measure the slice thickness and examine cellular morphology across the slice thickness, cultures were fixed and sliced along their thickness into 20–50 μm slices. The tissue was fixed in 2% paraformaldehyde prepared in 0.5X PBS, with its pH and osmolarity adjusted to that of the nutrient medium that is supplied to the cultures. Adjustments of these parameters were crucial to prevent changes in tissue thickness during fixation. This was ensured by measurements of fixed acute tissue slices of various thicknesses. The fixed slices were stained with H&E stain using the supplied protocol and were mounted on gelatin-coated slides for imaging. Tissue thickness and cellular morphology was examined using bright field microscopy.
Functional Activity Recording
The Axoclamp electrophysiology station was modified to adopt a 50 μm diameter stainless steel wire electrode and a ground electrode (SS31605, World precision instruments, Inc). The wire electrode was firmly held using a holder attached to an xyz stage (Sutter Instruments) that allowed precise positioning of the electrode to facilitate probing of the tissue at various sites and depths. Spontaneous activity was measured relative to a ground electrode using commercial hardware and software (Axon Instruments Digidata 1320A 16 bit data acquisition system, and A. M. Systems Differential AC Amplifier model 1700) at a 10 kHz sampling rate. When there was little or no recorded spontaneous activity, the culture was treated with 35°C 120 mM KCl solution to record chemically evoked activity.
Results
Perfusion augments the viability of thick brain slices
It is generally believed that thick brain slice cultures suffer necrosis due to unmet metabolic needs of the cells in the deeper tissue layers as a result of diffusion-limited mass transport of nutrients and oxygen from the surface of the tissue towards its interior. To establish whether forced interstitial perfusion augments tissue viability beyond the diffusion-limited range, 700μm thick slices of mouse cortex measuring 3 mm in diameter were continuously perfused for 2 days with equilibrated nutrient medium at a flow rate of ~3 culture volume exchanges per hour (20μl/hr). Two types of diffusion-limited control cultures were also used: the first were cultured in unperfused, but otherwise identical custom, perfusion chambers (“unperfused sister cultures”) while the second (“standard controls”) used the static membrane interface method (Stoppini L, 1991). All cultures were kept in the same sterile incubator under identical environmental conditions throughout the experiment. Slice viability was assessed with multiphoton imaging of the samples labeled with nuclear fluorescent probes (live/dead assay; see Methods for details). The perfused cultures demonstrated significantly better viability than both types of controls (p<0.01; Figure 4a). Additionally, z-projections of the image stacks of non-perfused and perfused organotypic brain slice cultures clearly show more pronounced and widespread cell degeneration in unperfused cultures compared to the cultures supported with active forced-convection based interstitial mass-transport (propidium iodide-labeled red nuclei, Figure 4b,c). This corroborates that passive diffusion-limited mass transport of nutrients and oxygen was not sufficient to support the metabolic requirements of the densely packed cells in unperfused brain slices.
Viability of thick brain slices depends on perfusion rates
Brain tissue is composed of various types of cells having diverse shapes with mechanically very delicate structures like dendrites and spines. While increasing interstitial flow rates of equilibrated medium would be expected to provide a higher concentration of nutritive substances and oxygen throughout the tissue, raising the flow rate beyond a certain threshold could ultimately cause structural and/or functional tissue damage. The difference in normal stresses along the cell body due to the interstitial pressure gradient that is induced by forced convection may cause cellular contraction in the direction of the gradient (resulting in cytoskeletal injury, changes to cytoarchitecture and global deformation of the slice). Viscous shear stresses induced by the fluid motion that are exerted on the cellular membranes could also be damaging. Therefore, an optimal medium exchange rate needs to be established that is sufficiently high to obtain satisfactory tissue viability, but still low enough not to harm the cultures over the long term.
We hypothesize that with the increase in flow rate, the tissue viability monotonically increases until an optimal flow rate is reached and then may begin to decrease. We examined the range of useful perfusion rates that enhance culture viability without mechanical damage to the tissue. Experiments were conducted at continuous infusion rates of 5, 10, 20, 30 μl/hr, with concomitant removal of depleted perfusate at the same flow rate. Our results indicate that the survival of the cultures significantly depends on the flow rates (P<0.01). While for flow rates ≤ 20 μl/hr, the tissue viability increased gradually with a gradual increase in flow rate; the higher flow rates were found to be detrimental to the tissue. The perfusion rates of 5 μl/hr and 10 μl/hr enhance tissue viability compared to two sets of unperfused controls by more than 26% and 36% respectively (Figure 5a, Table 3). At 20 μl/hr or about 3 culture volume exchanges per hour, slices exhibit greater than 84% viability compared to ~54% viability of control cultures, this represents >30% viability enhancement compared to the unperfused sister cultures. These results suggest that a flow rate that corresponds to ~3 volume-exchanges per hour is optimal (Table 1). Higher exchange rates, beyond 3 exchanges per hour, were found to be deleterious to the tissue and the observed viability was lower than that of control cultures. The reduction in viability at higher exchange rates may be due to depletion of substances secreted by the cells to regulate their microenvironment, such as neurotrophins; partial or complete detachment of the tissue from the perfusion substrate; and/or to injuries associated with high pressure gradients and fluid shear stress.
Table 3.
Viability as a function of flow rate and time
| Flow rate | % Increased Viability compared to unperfused control | % Increased Viability compared to membrane insert based control | |||
|---|---|---|---|---|---|
| (μl/hr) | Exchanges/hour | 2DIV | 5DIV | 2DIV | 5DIV |
| 5 | 0.71 | 21.2 | 26.3 | 20.2 | 29.5 |
| 10 | 1.41 | 19.0 | 36.2 | 18 | 39.4 |
| 20 | 2.85 | 31.7 | 47.6 | 30.7 | 50.8 |
| 30 | 4.28 | −24.8 | - | − 25.8 | - |
Table 1.
Perfusion parameters
| μl/hr | μl/day | Ml/day | Number of medium exchanges/hr | Number of medium exchanges/day |
|---|---|---|---|---|
| 5 | 120 | 0.12 | 0.7 | 17.1 |
| 10 | 240 | 0.24 | 1.4 | 34.3 |
| 20 | 480 | 0.48 | 2.8 | 68.6 |
| 30 | 720 | 0.72 | 4.3 | 102.9 |
Pressure measurements were taken at various flow rates using a Microtector differential pressure gauge (Dwyer Instruments) (Table 2). A significant decrease in a measured pressure difference across the tissue signifies either a formation of non-interstitial paths of lower resistance around the tissue (adhesion failure), or, the formation of interstitial paths of lower resistance to fluid flow, due to tissue damage. Inspection and imaging of the tissue following the pressure measurements revealed the actual cause. The z-stack images show mechanical damage to the tissue at various places across the culture in the form of small channels formed at higher (>20 μl/hr) flow rates (Figure 5b, c). The formation of interstitial fluidic paths of low resistance diminishes the uniformity of tissue perfusion and ultimately results in lower viability.
Table 2.
Pressure Measurements
| FLOW RATES (μl/hr) | PRESSURE (dynes/cm2) |
|---|---|
| 10 | 33.6 |
| 15 | 38.6 |
| 20 | 46.1 |
| 30 | 48.6 |
Optimal flow rates maintain long-term enhanced viability
The effect of various flow rates on the tissue viability was assessed and compared at two time points, after 2 days and after 5 days of continuous perfusion. We hypothesized that at an optimal flow rate, the level of viability would be maintained better over time, compared to unperfused cultures. The unperfused sister cultures show 55.9 % viability after 2DIV and 37% after 5DIV, which translates to a decrease of 18.9% in viability level from 2 DIV to 5 DIV respectively. Similarly, the membrane based cultures showed a decrease of 23.1% viability from 2 DIV (56.9%) to 5 DIV (33.8%).
In the perfused cultures, after 2 DIV the viability levels of cultures at 5, 10 and 20 μl/hr are 77.1%, 74.9%, 87.6% respectively, while the corresponding viability after 5 DIV was found to be 63.3%, 73.2% and 84.6%. This corresponds to a decline of 13.8%, 1.7% and 3.0% viability levels from 2 DIV to 5 DIV at 5, 10 and 20 μl/hr flow rates respectively (Tables 3, 4). These results show that using our perfusion method to supply oxygenated medium throughout the tissue thickness, the decrease in viability from 2 DIV to 5 DIV becomes negligible at some flow rates (Figure 6).
Table 4.
Decease in viability over time at various perfusion rates
| % Viability | ||||
|---|---|---|---|---|
| Flow rate (μl/hr) | Exchanges/hour | 2DIV | 5DIV | % Decreased in Viability from 2 DIV to 5 DIV |
| Membrane-based unperfused cultures | 56.9 | 33.8 | 23.1 | |
| Unperfused sister cultures | 55.9 | 37.0 | 18.9 | |
| 5 | 0.71 | 77.1 | 63.3 | 13.8 |
| 10 | 1.41 | 74.9 | 73.2 | 1.7 |
| 20 | 2.85 | 87.6 | 84.6 | 3.0 |
| 30 | 4.28 | 31.1 | - | - |
Qualitative characterization of thick brain slices
a. Organotypic organization and thickness of the slice cultures
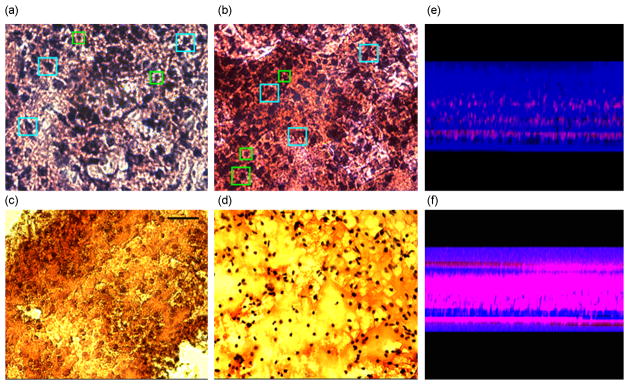
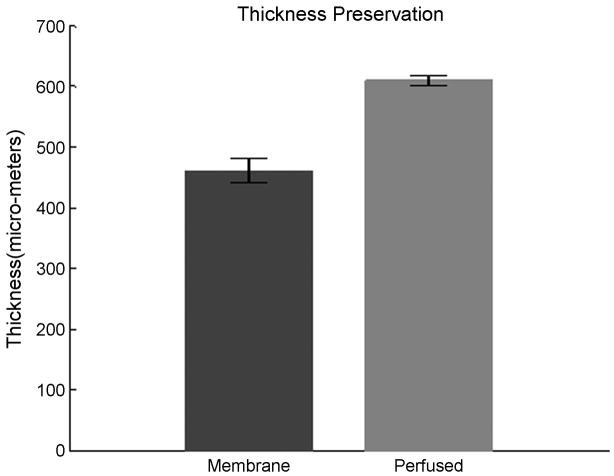
We next investigated the morphology of the tissue in order to determine its organotypic organization. The perfused and unperfused tissues were fixed in 2% paraformaldehyde solution in 0.5X PBS (isotonic – see Methods). The fixed tissue was then cut into 20μm or 50μm thick transverse sections to image the morphology throughout the slice thickness. These tissue sections were stained with haematoxylin and eosin (H&E) stain and imaged with bright-field microscopy (Figure 7(a–d)). The images demonstrate that the overall structure, cell sizes and shapes of the perfused slices are commensurate with those of the fresh tissue (baseline) slices that were fixed immediately after cutting and stained identically, while the unperfused slices showed impaired cell health and poor overall structure. The perfused cultures and baseline tissue showed comparable cell densities, cell types, and cell sizes along with a complex network of dendritic arborizations. In unperfused controls, the health of the cells appeared compromised, with sparse dendritic network and comparatively smaller soma size throughout the culture thickness. This was notably worse in the middle of the cultures. These features were even more degraded in membrane-insert controls where the cell size and morphology were substantially different from those of the baseline tissue, and the dendritic network was not visible. In both sets of unperfused controls, the identification of the cell type based on their characteristic morphology was not feasible. Figure 7(a–d) shows representative photomicrographs of cultures labeled with H & E stain. In unperfused cultures, cell death occurs from the center outwards. In perfused-slices, moderate death may be confined to some layers of the culture, in the direction of nutrient reduction along the flow direction (Figures 5e–f). Interestingly, the average thickness of the perfused slices was significantly maintained compared to membrane-insert based cultures (Figure 8).
Figure 7.
Qualitative morphology assessment at 5 DIV. (a) Baseline* tissue showing common cell types found in cortex, for example, pyramidal cells (cyan squares), star-shaped astrocytes (green squares), etc. (b) Perfused slices after 5DIV show similar cell densities and cell shapes with distinguishable characteristic cell types.. (c) Unperfused control slice cultures after 5 DIV demonstrate lower cell density approximately in the middle layers of the tissue. (d) Unperfused membrane insert cultures** exhibit compromised health with reduced cell size and cell density. The field of view of each image is 666 μm by 500 μm. (* Baseline: Freshly cut tissue that was fixed and stained to assess its viability and morphology at cellular level in the beginning of the experiment. ** membrane insert cultures means cultures as described by Stoppini, 1991).Representative partial thickness view of (e) perfused and (f) unperfused tissue showing significantly reduced cell death (red color) in perfused cultures compared to unperfused ones (live/dead assay).
Figure 8.
The maintenance of culture thickness after 5 DIV. Perfused slices exhibit significantly enhanced thickness compared to the standard membrane insert controls. Slices were 700μm thick when plated.
b. Functional activity of the cultured slices
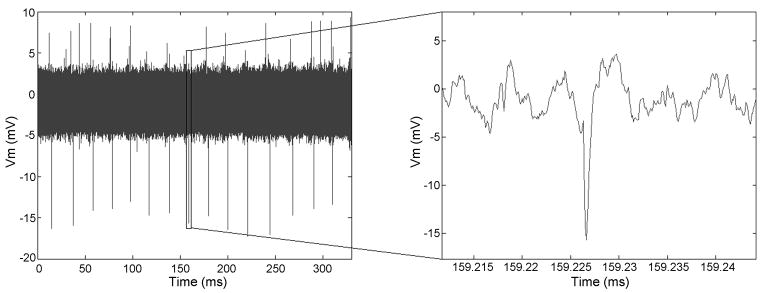
To assess the usefulness of perfused slice cultures for electrophysiological studies, we recorded their functional activity at 5 DIV. The majority of the cultures showed activity in terms of spiking spontaneously or after depolarizing them chemically by adding 120 mM KCl solution to the bath. Notably, activity was observed in most slices in the deeper layers of the tissue – approximately half way through the thickness. A representative recording of chemically evoked action potentials from a 5 DIV culture are shown in Figure 9.
Figure 9.
Representative extracellular activity recordings from a perfused slice after 5 DIV. Either spontaneous or chemically evoked activity could be recorded in 75% of the cultured slices. The amplitudes of recorded activity traces depend on the relative position of electrode to the firing neurons. The figure shows chemically evoked activity.
Discussion and conclusions
In this study, we successfully cultured viable 700μm thick organotypic cortical slices using a unique three dimensional interstitial perfusion method to perfuse oxygenated nutrient medium through the tissue thickness. Densely packed cells in a brain slice along with intertwined delicate networks of dendrites, spines, synapses and extracellular matrix demand careful handling and a well-controlled physiological environment to preserve their long-term viability. Several factors can affect the maintenance of healthy organotypic brain slices in culture. These include tissue harvesting, composition of nutrient medium, pH, osmolarity, temperature, humidity level, oxygen level, removal of metabolic waste and dead cell debris, intracellular signaling causing programmed cell death, etc. Diffusion-based interstitial supply of oxygenated medium poses an upper limit on the thickness and longevity of traditional cultured brain slice preparations (Simoni A D, 2006). Our perfusion method restores the functional circulatory system by maintenance of vital parameters such as continuous interstitial supply of nutrients and oxygen along with concomitant removal of catabolites and depleted nutrient medium, and maintenance of pH, humidity, and temperature at physiologically relevant levels (Brewer G. J. and Cotman C.W., 1989).
Our gas-permeable and biocompatible microfluidic chamber and the associated fluidic circuit are designed to provide sufficient nutrient and oxygen concentration throughout the tissue thickness, while maintaining the culture in a physiologically relevant controlled environment. Successful implementation of forced convection-based 3D interstitial mass transport demands reliable tissue adhesion to the perfusion substrate and the inner walls of the culture chamber, to prevent the formation of non-interstitial paths of low resistance for fluid flow. In the absence of any such paths, the infused medium is constrained to flow interstitially through the tissue thickness before exiting to the withdrawal chamber. A reliable tissue adhesion method is achieved by coating the infusion chambers with laminin followed by 30 minute incubation, and by cutting the brain slices in the form of circular discs of appropriate diameter to fit snugly and cover the entire volume of the laminin-coated culture chambers. Adhesion of the circular slice disc is further encouraged by an FEP membrane, stretched tightly across a Teflon lid and in contact with the tissue, to prevent the slice from floating when infusion is initiated and the slice is just starting to adhere to the internal walls of the culture chamber. Our results indicate that forced interstitial convection of oxygenated nutrient medium through the tissue enhances its viability compared to diffusion-based mass-transport in two types of control cultures. Although, initially there is a linear increase in the viability of cultures with increasing infusion rates (Figure 5), at higher rates (> 20μl/hr) the viability begins to deteriorate. When the nutritive medium is forced interstitially by means of laminar convection, the magnitude of pressure (normal stress), shear stress, pressure gradient and shear stress gradient may be injurious to the cells. Both normal and shear stresses are directly related to the magnitude of the interstitial flow rate. For example, for a steady, laminar flow of medium through a uniform, round capillary (Hagen-Poiseuille flow) the highest fluid shear stress appears at the capillary wall, τw = 32μ Q/πD3, where τw is the wall shear stress, μ dynamic viscosity of the medium, Q volume flow rate through the capillary, and, D the internal diameter of the capillary (Munson B R et al., 2005). Accordingly, the pressure drop (difference in normal stress) equals Δp = 128μL Q/πD4, where L is the length of the capillary. Assuming that the diameter of the capillary equals that of an interstitial clearance, with the capillary length equivalent to the length of flow path across the tissue, the ratio of pressure drop to shear stress Δp/τw = 4 L/D is expected to be ≫1. Hence, in addition to shear, normal stress gradients may also be damaging to the culture although cellular susceptibility to these mechanisms could vary. Furthermore, forcing the medium interstitially through a substrate-bound slice weakens the strength of the tissue-to-substrate adhesion layer. This is particularly important in thicker slices where the pressure below the tissue becomes sufficiently high to cause its partial or full detachment. Therefore, the limiting factor in tissue thickness, cultured using forced convection interstitial perfusion (FCIP), may not be normal stresses and shear stresses causing injuries, but rather, the failure of the adhesion layer. To ease the stipulation on adhesion-promoting coatings, and to facilitate the implementation of the FCIP, we propose to design chambers with reversed flow for our future applications, that is, to introduce the medium laterally and withdraw depleted medium and catabolites below the culture. This may enable us to determine the upper limits on the thickness of slices cultured using FCIP (due to flow-induced tissue damage, rather than adhesion failure). In addition, vacuum (rather than pressure) below the slice, facilitates initial slice positioning and adhesion to the interior of the coated culture chamber. In the absence of tissue detachment from the substrate, at high flow rates, injuries caused by microjets emanating from the perfusion source facilitate the formation of channels of lower resistance to interstitial fluid flow and disruption of interstitial perfusion. As the volume flow rate increases and the optimal flow rate is approached the difference in viability (reduction in viability) from 2 to 5 DIV becomes negligible. Approximately 3 culture volume exchanges per hour correspond to an optimal flow rate resulting in over 84% viability after 5DIV (Figure 5). Once the flow rate was optimized to satisfy the metabolic demands of the entire slice through its full thickness without causing any injuries, the viability from 2 to 5 DIV did not deviate significantly (remained preserved). It is expected that unless the slices of explanted tissue are expected to “mature” like dissociated monolayer cultures, their metabolic demands, once met, should not vary considerably over the longer term in culture. We suggest testing of longer term viability experiments as future work. In these experiments, the nutrient medium fed to the slice culture may not be optimal to support all cells/cell types equally, thus may result in more pronounced drop in viability in certain tissue layers than in others. Thus, it may be important to optimize the culture medium to obtain even better and longer-term viability of the cultures.
It is noteworthy that not all of the cell death can be attributed to inadequate interstitial nutrient and gas availability because the peripheral cell layers are injured during slicing and will not recover. We hypothesize that the viability of the unperfused cultures decreased dramatically after 5 DIV compared to perfused slices for two reasons: first, the insufficient nutrient and oxygen supply to starved layers, and second, dying cells injured during the slice cutting process release harmful chemicals that could diffuse into the neighboring layers and trigger programmed cell death (Kim J and al., 2002; Kovacs R and al., 2002). Such triggered death could be an additional reason for more pronounced cell death in non-perfused than in perfused cultures, due to constant wash-out of harmful chemicals from perfused slices. In non-perfused cultures, toxic chemicals secreted by dying cells may spread to the adjacent layers and cause their degeneration. While our perfusion method was designed to address both causes of cell death, it may not be equally efficient in eliminating them because some layers of the slice may be more sensitive to toxic components than others. Excessive spontaneous and synchronous firing of neurons can cause cell death that was mitigated by blocking NMDA channels with high Mg2+ in the nutrient medium (Pozzo Miller L D et al., 1994). Using our perfusion method, Mg2+ and any other agents could be quickly supplied to enhance the viability of thick brain slices further. Optimization of culture conditions by changing the composition of the interstitial bathing medium, and a more thorough characterization of the organotypic tissue organization using immuno-staining, are suggested as future work. Our studies also show that perfused slices are generally thicker than unperfused controls. Both perfusion and tissue adhesion to the interior surfaces of the culture chamber may have played an important role in the maintenance of thickness. Perfusion limits the tissue collapse due to metabolic decay of certain layers, while the culture chamber prevents lateral tissue spreading. (Note that in static membrane-insert or roller-tube cultures, tissue spreads out rapidly in the absence of adequate structural support.) Hence, 700 μm-thick slices thinned down by only 80–100μm, resulting in approximately 600μm thickness after 5 days of in vitro perfusion. This thinning of perfused slices could be partly ascribed to wash out of dead or slowly dying cells that were injured initially during slicing. We were also able to record spontaneous or chemically evoked activity from most of the perfused cultures (Figure 9). Signal thresholds are electrode-specific (noise is tied to electrode size and material) and depend on cell-to-electrode distance and variability of culture condition among other things. The absence of spontaneous or evoked electrical activity in some of the cultures may be attributed to two causes: either the single wire electrode might not have come in contact with a firing neuron or the culture may have been injured by the electrode during the recording. Electrode properties such as conductivity and surface area may be the limiting factor in recording relatively weak extracellular signals from the cells. A more comprehensive examination of viability warrants further studies using multielectrode arrays (Dong H W and Buonomano D V, 2005; Egert U et al., 2002; Egert U et al., 2006; Wagenaar D A et al., 2006). Our interstitial perfusion technique could easily be used with commercially available porous multielectrode arrays (pMEAs) that have microfluidic perforations for perfusion of nutrient medium, along with the ability to stimulate and record from thick sections of explanted tissue (Egert U et al, 2006; Jahnsen et al, 1999). pMEA perforations may be used to withdraw depleted medium below the tissue while supplying fresh medium to the tissue laterally to avert tissue floating. This flow configuration also facilitates culture positioning and ensures close contact between electrodes and the culture, thus improving the signal to noise ratio (SNR) of extracellular recordings and lowering stimulation amplitudes to prevent electrolysis. Perforations on pMEAs comprise approximately 28% of the culture-occupied area.
Our findings suggest that viable thick organotypic slices could be cultured using our perfusion method and used for long-term morphological studies with multiphoton microscopy. They could also become a useful in vitro model for electrophysiology and pharmacological studies. Our culturing technique could be used to culture thicker organotypic slices from other species or parts of the brain that are traditionally cultured using the membrane insert method, such as cerebellum (Ghoumari A M and al., 2003), striatum (Becq H et al., 1999), spinal cord (Oishi Y et al., 2004), olfactory epithelium (Gong Q et al., 1996), thalamus and cortex (Dong H W and Buonomano D V, 2005; Letinic K et al., 2002). These thick organotypic cultures may benefit a wide spectrum of neuroscience investigations including learning and memory (De Simoni A et al., 2003; Engert F and Bonhoeffer T, 1999; Galimberti I and al., 2006; Nagerl U V et al., 2004), development (Gong Q et al., 1996; Nikononko I and al., 2003; Oishi Y et al., 2004; Raineteau O et al., 2004), traumatic brain injury (LaPlaca M C and Thibault L E, 1997; Oishi Y et al., 2004; Stoppini L et al., 1991), regeneration (Oishi Y et al., 2004), the effects of pharmacological agents on network properties, ischemia studies (Perez Velazquez J L et al., 1997), and so on. Flexibility in design of perfusion chambers, and their facile fabrication approach, allow changing of various dimensions to accommodate culturing of organotypic co-cultures of different shapes and thicknesses. However, each time any of these parameters is changed, the flow rate would need to be re-optimized.
Acknowledgments
This work was supported by the National Institute of Health Bioengineering Research Partnership grant EB00786. We thank Michele LaPlaca, Ravi Bellamkonda, Richard Nichols, Kacy Cullen, and Zenas Chao for their useful discussions and inputs.
References
- Albertson TELGS. Neuropharmachology methods in epilepsy research. CRC Press LLC; Boca Raton, FL, USA: 1998. [Google Scholar]
- Baker RE, Corner MA, Pelt J. Spontaneous neuronal discharge patterns in developing organotypic mega-co-cultures of neonatal rat cerevral cortex. Brain Res. 2006;1101:29 – 35. doi: 10.1016/j.brainres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Becq H, Bosler O, Geffard M, Enjalbert A, Herman JP. Anatomical and functional reconstruction of the nigrostraital system in vitro: selective innervation of the straitum of dopaminergic neurons. J Neuroscience Res. 1999;58:553 – 66. doi: 10.1002/(sici)1097-4547(19991115)58:4<553::aid-jnr8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Lee CC, Colmers WF, Miller RJ. Changes in mitochondrial function resulting from synaptic activity in the rat hippocampal slice. J Neurosci. 1998;18:4570 – 87. doi: 10.1523/JNEUROSCI.18-12-04570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology. 1973;232:331– 56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Cotman WC. Survival and growth of hippocampal neurons in defined medium at low density – advantages of a sandwich culture technique or low oxygen. Brain Res. 1989;494:65 – 74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. Journal of Neurophysiology. 1997;77:1614 – 23. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Vukasinovic J, Glezer A, LaPlaca MC. Microfluidic engineered high cell density three dimensional neural cultures. Journal of Neural Engineering. 2007 doi: 10.1088/1741-2560/4/2/015. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. Journal of Physiology. 2003;550:135 – 47. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Buonomano DV. A technique for repeated recordings in cortical organotypic slices. J Neurosci Methods. 2005;146:69 – 75. doi: 10.1016/j.jneumeth.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Egert U, Heck D, Aertsen A. 2-dimensional monitoring of spiking networks in acute brain slices. Exp Brain Res. 2002;142:268 – 74. doi: 10.1007/s00221-001-0932-5. [DOI] [PubMed] [Google Scholar]
- Egert U, Okujeni S, Nisch W, Boven KH, Rudorf R, Gottschlich NAS. Optimized oxygen availability and signal-to-noise ratio in brain slice recordings with perforated microelectrode arrays. 5th International meeting on Subtrate-integrated Micro Electrode Arrays Reutlingen; 2006. [Google Scholar]
- Ehrengruber MU, et al. Recombinant Semliki Forest virus and Sindbis virus efficiently efficiently infect neurons in hippocampal slices cultures. Proceedings of National Academy of Sciences, USA. 1999;96:7041 – 6. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66 – 70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic cultures of neural tissue. Trends Neuroscience. 1988;11:484 – 9. doi: 10.1016/0166-2236(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. Journal of Neuroscience Methods. 1981;4:329 – 42. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Galimberti I, et al. Long-term rearrangements of hippocampa mossy fiber terminal connectivity in the adult regulated by experience. Neuron. 2006;50:749 – 63. doi: 10.1016/j.neuron.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, et al. Mifepristone (RU486) protects Purkinje cells from cell death in organotypic slice cultures of postnatal rat and mouse cerebellum. Proceedings of National Academy of Sciences, USA. 2003;100:7953 – 8. doi: 10.1073/pnas.1332667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Liu WL, Srodon M, Foster TD, Shipley MT. Olfactory epithelial organotypic slice cultures: a useful tool for investigating olfactory neural development. Int J Dev Neurosci. 1996;14:841 – 52. doi: 10.1016/s0736-5748(96)00056-1. [DOI] [PubMed] [Google Scholar]
- Hass HL, Schaerer B, Vosmansky M. A simple perfusion chamber for study of nervous tissue slices in vitro. J Neurosci Methods. 1979;1:323 – 5. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Jahnson H, et al. Coupling of organotypic brain slices cultures to silicon-based arrays of electrodes. Methods. 1999;18:160–72. doi: 10.1006/meth.1999.0769. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur J Neuroscience. 2004;20:101 – 10. doi: 10.1111/j.1460-9568.2004.03461.x. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. Cytoskeleton disruption causes apoptotic deneration of dentate granule cells in hippocampal slice cultures. Neuropharmacology. 2002;42:1109 – 18. doi: 10.1016/s0028-3908(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol. 1997;78:1651 – 61. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- Kovacs R, et al. Free radical-mediated cell damage after experimental status epilepticus in hippocampal slice cultures. J Neurophysiol. 2002;88:2909 – 18. doi: 10.1152/jn.00149.2002. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Ackery A, Schwartz G, Adamchik Y, Liu YMGF. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res Brain Res Protoc. 2002;10:60 – 8. doi: 10.1016/s1385-299x(02)00180-0. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Thibault LE. An in vitro traumatic injury model to examine the response of neurons to a hydrodynamically-induced deformation. Ann Biomed Eng. 1997;25:665 – 77. doi: 10.1007/BF02684844. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neucortex. Nature. 2002;417:645 – 9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Li CL, McIlwain H. Maintenance of resting potentials in slices of mammalian cerebreal cortex and other tissues in vitro. J Physiol - London. 1957;139:178 – 90. doi: 10.1113/jphysiol.1957.sp005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Blume HW, Madsen JR, Saper CB. Connections of the hippocampal formation in humans: I. The mossy fiber pathway. The Journal of Comparative Neurology. 1998;385:325 – 51. doi: 10.1002/(sici)1096-9861(19970901)385:3<325::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Linke R, Heimrich B, Frotscher M. Axonal regeneration of identified septohippocampal projection neurons in vitro. Neuroscience. 1995;68:1 – 4. doi: 10.1016/0306-4522(95)00174-h. [DOI] [PubMed] [Google Scholar]
- Lundstrom K, et al. Semliki Forest virus vectors: efficient vehicles for in vitro and in vivo gene delivery. FEBS Lett. 2001;504:99 – 103. doi: 10.1016/s0014-5793(01)02707-7. [DOI] [PubMed] [Google Scholar]
- MacLean J, Fenstermaker V, Watson BO, Yuste R. A visual thalamocortical slice. Nat Methods. 2006;3:129 – 34. doi: 10.1038/nmeth849. [DOI] [PubMed] [Google Scholar]
- McDonald J, Chabinyc M, Metallo S, Anderson J, Stroock AGW. Prototyping of microfluidic devices in poly(dimethylsiloxane) using solid-object printing. Analytical Chemistry. 2002;74:1537–45. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- Munson BR, Young DFaHOT. Fundamentals of Fluid Mechanics. 5. John Wiley & Sons, Inc; 2005. [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759 – 67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981;4:153 – 6. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Nikononko I, et al. Integrins are invloved in synaptogenesis, cell speading, and adhesion in the postnatal brain. Brain Res Dev Brain Res. 2003;140:185 – 94. doi: 10.1016/s0165-3806(02)00590-4. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Baratta J, Robertson RTOS. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: effects of age and neurotrophic factors. J Neurotrauma. 2004;21:339 – 56. doi: 10.1089/089771504322972121. [DOI] [PubMed] [Google Scholar]
- Passeraub PA, Almeida AC, V TN. Design, microfabrication and characterization of a microfluidic chamber for the perfusion of brain tissue slices. Journal of Biomedical Devices. 2003;5:147–55. [Google Scholar]
- Perez Velazquez JL, Frantseva MV, Carlen PL. In vitro ischemia promotes glutamate-mediated free radical generation and intracellular calcium accumulation in hippocampal pyramidal neurons. J Neuroscience. 1997;17:9085 – 94. doi: 10.1523/JNEUROSCI.17-23-09085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SM, Booth MC, Brumfield JR, Passaro PA, Rambani K, Towal BR. Combining time-lapse optical microscopy and multielectrode arrays to study learning in vitro. Microscopy and Microanalysis. 2004;10:1238–9. [Google Scholar]
- Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110:17 – 24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Pozzo Miller LD, Mahanty NK, Connor JA, Landis DMD. Spontaneous pyramydal cell death in organotypic slice cultures from rat hippocampus is prevented by glutamate receptor antagonists. Neuroscience. 1994;63:471–87. doi: 10.1016/0306-4522(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Rietschin L, Gradwohl G, Guillemot F, Gahwiler BH. Neurogenesis in hippocampal slice cultures. Molecular Cell Neuroscience. 2004;26:241 – 50. doi: 10.1016/j.mcn.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Reynaud JC, Martini F, Chatel C, Buclin M, Raggenbass M, Puizillout JJ. A new interface chamber for the study of mammalian nervous tissue slices. J Neurosci Methods. 1995;58:203 – 8. doi: 10.1016/0165-0270(94)00177-i. [DOI] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. A small volume chamber for electrical recording from submerged brain slices and a pulse-free medium supply using peristalic pump. J Neurosci Methods. 1990;35:235 – 40. doi: 10.1016/0165-0270(90)90129-4. [DOI] [PubMed] [Google Scholar]
- Simoni AD, Yu LM. Preparation of organotypic hippocampal slice cultures: interface method. Nature Protocols. 2006;1:1439–45. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of neural tissue. Journal of Neuroscience Methods. 1991;37:173 – 82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Vukasinovic JAG. A microperfusion chamber for neuronal cultures. Proceedings of the 2006 ASME Summer Bioengineering Confernece; 2006. [Google Scholar]
- Wagenaar DA, Pine J, Potter SM. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neuroscience. 2006;7:11. doi: 10.1186/1471-2202-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wereley ST, Meinhart CD. Micron resolution particle image velocimetry. In: Breuer K, editor. Diagnostic techniques in microfluidics. Springer Verlag; New York: 2004. [Google Scholar]
- Zbicz KL, Weight FF. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985;53:1038 – 58. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]