Abstract
The hormonal regulation of sexual behavior has been the topic of study for over 50 years and yet controversies persist regarding the importance of early versus late events and the identity of the critical neural and cellular substrates. We have taken a mechanistic approach toward the masculinizing actions of the gonadal steroid estradiol, as a means to understand how organization of the neuroarchitechture during a perinatal sensitive period exerts enduring influences on adult behavior. We have identified important roles for prostaglandins, FAK and paxillin, PI3 kinase and glutamate, and determined that cell-to-cell signaling is a critical component of the early organizational events. We have further determined that the mechanisms mediating different components of sexual behavior are distinct and regionally specific. The multitude of mechanisms by which the steroid estradiol, exerts divergent effects on the developing nervous system provides for a multitude of phenotypes which can vary significantly both within and between the sexes.
Keywords: Preoptic area, Hypothalamus, Lordosis, Prostaglandins, Glutamate, Estradiol, Testosterone, Development, Sex differences, Sexual behavior
Introduction
It has been 50 years since the publication of the iconic Phoenix, Goy, Gerall and Young (Phoenix et al., 1959) manuscript proposing the Organizational / Activational Hypothesis of hormone effects on sex differences in reproductive behavior and now it is the focus of this celebratory special issue of Hormones and Behavior. Fifty years is either a long time or the blink-of-an-eye for the study of a single research topic, depending on your point of view. In this time, the dogma that has emerged is, simply put, that developmental exposure to gonadal steroids acts on the brain to organize the neural substrate which is then selectively activated in the adult to induce expression of sex specific behavior. This elegant synthesis effectively explained a collection of disparate data and provided a framework against which future work could be tested. Evidence in support of the essential truths of the hypothesis have steadily accumulated over the intervening 50 years, but evidence challenging or refuting the hypothesis has piled up in an equally compelling fashion. The majority of published reports have focused on hormonal manipulations and quantification of behavioral endpoints. The approach of this laboratory has been a detailed exploration of the cellular mechanisms of organizational steroid effects as a means for understanding how early hormone action could persist into adulthood and thereby regulate behavior.
Brief historical overview
There are several seminal events that are built on the original Phoenix report that have influenced the course of the field ever since. The original animal model, the guinea pig, is a species with a small litter size, long gestation and precocial young. The fact that the seminal organizational events occurred prenatally both confounded an investigation by the hormones of pregnancy and precluded the ability to manipulate males and females independently. The laboratory rat soon replaced the guinea pig as the dominant species for study due to the large litter size, short gestation and the observation that the sensitive period for sexual differentiation began prenatally but extended well into the postnatal period. That estradiol is the masculinizing steroid in rodents was codified by the Aromatization Hypothesis, which reconciled the divergent observations that exogenous administration of estradiol was as or more effective than testosterone at masculinizing behavior, that the critical brain regions expressed estrogen receptors and that the synthetic enzyme, estradiol synthase, or aromatase, was highly active in the neonatal brain. An additional critical piece of the puzzle was determining that the steroid binding globulin, alpha-fetoprotein, preferentially binds estrogens in rodents, thereby allowing testosterone to freely enter cells where it is converted to estradiol (see for review (McCarthy, 2008). Decades later did it become apparent that androgens mediate sexual differentiation of the primate brain (see for review (Wallen, 2005), but this does not undermine the utility of the rodent model unless the cellular mechanisms of estradiol and androgens to mediate masculinization are different. Determining if this is true depends first on determining the mechanisms in rodents before attempting a more focused approach in primates.
At the time of elucidation of the Aromatization Hypothesis it was self evident that the brain controlled behavior and was therefore the “hormone responsive tissue” referred to by Phoenix and colleagues, who had limited their hypothesis to behavior. Despite overwhelming confidence that the brain was responsible, there was surprisingly little evidence for sex differences in this structure. There were early hints of hormonally-mediated changes in dendritic profiles (Pfaff, 1966; Raisman and Field, 1971), but the magnitude of the differences were small and of unknown significance. It was the discovery of spectacular sex differences in the song control nuclei of birds (see for review (Arnold et al., 1996) that prompted a re-examination of the rodent brain and led directly to the discovery of the sexually dimorphic nucleus of the preoptic area (Gorski et al., 1980). This small group of Nissle dense cells located in the central heart of the medial preoptic nucleus is 5–7 times larger in males than females and has proved valuable both for establishing mechanisms by which the brain is organized and as a bell weather of estradiol-mediated masculinization. The realization that subregions of the brain could be substantially larger in one sex led to the discovery of many more volumetric sex differences in the mammalian brain (Guillamon and Segovia, 1996; Byne, 1998; Simerly, 2002), including humans (Swaab and Fliers, 1985; Allen et al., 1989; Allen and Gorski, 1990, 1992) The first insight into the mechanism establishing the sex difference in SDN size came from the spinal cord, in particular the spinal nucleus of the bulbocavernosus, or SNB, which houses motor neurons innervating the penis. Not surprisingly this nucleus is substantially larger in males, and Arnold and colleagues established that this is because the motor neurons in the female die early in development (Nordeen et al., 1985). The principle that males and females start with the same cohort of cells but they preferentially die in one sex or the other has now been generalized to several reproductively relevant brain nuclei (see for review (Forger, 2006), most notably the SDN (Davis et al., 1996), and the AVPV (Murakami and Arai, 1989).
While it is highly likely that size matters, there are many brain regions critical to the control of reproduction that are not noticeably larger in one sex. In the 1980s, after the extensive attention given to SDN, was a re-examination of the potential for synaptic differences. Exhaustive and conclusive studies by Arai and colleagues, most notably Matsumoto, demonstrated profound differences between males and females in the frequency of dendritic spine versus somatic synapses in the arcuate nucleus (Matsumoto and Arai, 1980), ventromedial nucleus (Matsumoto and Arai, 1986) and amygdala (Nishizuka and Arai, 1981). Their use of EM to identify and quantify the different types of synapses left no room for doubt that the synaptic profile was differentially weighted towards excitatory spine synapses or inhibitory somatic synapses in one sex or the other in specific brain regions. Moreover, the work of Matsumoto and Arai demonstrated unambiguously the ability of steroid hormones to permanently organize this sex difference in synaptic patterning. What remained to be determined was the cellular mechanism behind the hormone action.
Mechanisms mediating masculinization of sexual behavior
Masculinization in this context is a steroid-hormone driven developmental process that organizes the neural substrate so that it is conducive to being activated by androgens in adulthood for the expression of male sex behavior directed at sexually receptive females. It is useful to divide male sexual behavior into an appetitive component, meaning the motivation to seek out and pursue receptive females, and a consummatory component, the physical act of copulation with a female, which in male rodents is quantified as mounts, thrusts and ejaculations. Appropriate expression of both components of male sexual behavior requires a functioning medial preoptic area (POA) and it is reasonable to assume that this is a principle site of organizational actions of steroids to masculinize behavior. This is also the brain region housing the SDN and much attention was given to the notion that the larger nucleus in males is critical for male behavior (De Jonge et al., 1989; Jarzab et al., 1990; Houtsmuller et al., 1994), an idea proved false by some (Todd et al., 2005), but still persisted by others (Jeong et al., 2008). There is some evidence that the SDN may be part of a network that suppresses female sexual behavior (Hennessey et al., 1986), and therefore a substrate for defeminization, or that its function may be to direct sexual preference (Baum, 2006). Regardless, in addition to the SDN there is a marked sex difference in the morphology of neuronal dendrites and protoplasmic astrocytes of the medial POA (Amateau and McCarthy, 2002a,b). Beginning in the first few days of life, male rats have a higher density of spine synapses per unit dendrite, and male astrocytes have longer and more frequently branching processes. The POA neuronal dendrites of male Macaques also have a higher density of spines and this is observed prior to puberty (Ayoub et al., 1983), confirming that the neuronal architecture of primate brains is organized in a sex specific manner and suggesting that regardless of which hormone mediates the effect (i.e. estradiol versus testosterone), the endpoint is the same.
Estrogen receptors are members of a superfamily of transcription factor receptors that interact directly with DNA to either promote or repress gene expression. Estrogen receptors also reside in the membrane where they interact directly with various protein kinases to directly activate signal transduction pathways. This duality of action provides for a startling level of complexity in potential steroid effects. The same hormone can directly impact the expression of multiple genes, which can initiate a cascading profile of secondarily activated genes, while at the same time exerting rapid membrane initiated classic signal transduction pathways (see for review McCarthy 2008). Given this complexity, it was not terribly surprising that no one effector pathway had been identified to mediate estradiol-induced masculinization of sex behavior. Indeed, many had quite justifiably concluded that instead of activating one gene 100-fold, it was more likely that estradiol activated ten genes 10-fold, or even 100 genes 1-fold or less. For many years the empirical data supported this view. Almost all the major neurotransmitter systems had been implicated as important to normal masculinization, including noradrenaline (Beyer and Feder, 1987), acetycholine (Dohler et al., 1991), dopamine (Hull et al., 1998), serotonin (Ladosky and Gaziri, 1970) and even the amino acid GABA (Davis et al., 2000). However, the data implicating each system was restricted to demonstrating that if neurotransmission was disrupted, so was masculinization. What was missing was the ability to induce masculinization in females in the absence of exogenous steroid administration, in other words to substitute for the steroid by administering the downstream cellular process activated by the steroid.
This goal was achieved with the surprising discovery that the agent of change in this case was not a traditional neurotransmitter at all, but was instead a lipid membrane derived molecule, prostaglandin E2 (PGE2). Prostaglandins are derived from arachidonic acid following cyclinization by the cyclooxygenase enzymes, COX-1 and COX-2 (Kaufmann et al., 1997). Usually associated with inflammation, PGE2 is emerging as an important modulator of neural activity both by indirect effects on glia (Bezzi et al., 1998; Sanzgiri et al., 1999), and directly on neurons (Rage et al., 1997; Kasai and Mizumura, 2001; McCullough et al., 2004). In the mature POA, PGE2 is a potent stimulator of LHRH release and plays an essential role in maturation of the pulse generator at puberty (Ma et al., 1997). This observation prompted a talented graduate student, Stuart Amateau, to ask whether PGE2 might also play a role in the developing POA, and more specifically in the process of masculinization. Through a series of studies, Amateau established that during the sensitive period for sexual differentiation, activation of estrogen receptors in the POA induces expression of COX-2 (and COX-1), which leads to a 7-fold increase in PGE2, but not the other prostanoids (Amateau and McCarthy, 2004). PGE2 induces the formation of neuronal dendritic spines via a mechanism that involves at least in part, the release of glutamate and the activation of AMPA, but not NMDA, glutamate receptors (Amateau and McCarthy, 2002a). Research by others reveals a calcium dependent release of glutamate from astrocytes following stimulation by PGE2 (Bezzi et al., 1998), and astrocytes of the POA are responsive to estradiol and sexually dimorphic (Amateau and McCarthy, 2002b). This has led us to propose a working model in which PGE2 released from estradiol-responsive neurons in the male promotes glutamate release from astrocytes which in turn promotes the induction and/or maturation of dendritic spines. Treating females with either estradiol or PGE2 results in a male-like pattern of dendritic spines, and blocking COX-1 and COX-2 activation in males produces a female-like level of spines. Confirmation that this is truly an organizational effect in the classic sense is confirmed by maintenance of the induced changes well into adulthood (Amateau and McCarthy, 2002a, 2004; Wright et al., 2008).
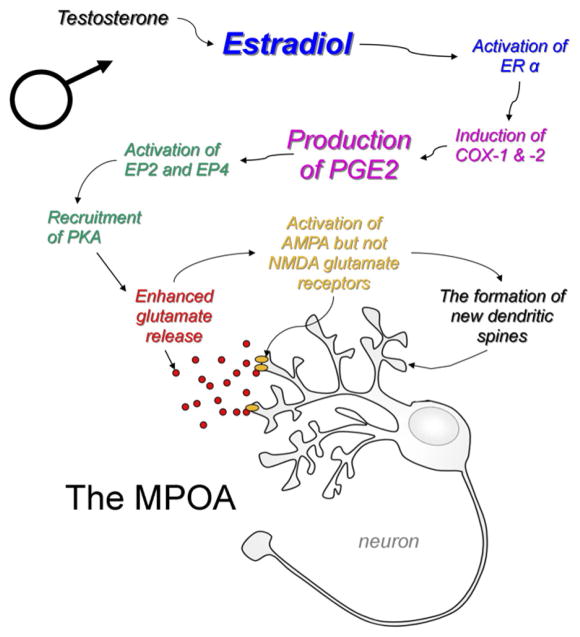
Prostaglandins act via multiple receptors but for PGE2 the four principle ones are EP1–4. Each receptor has a different affinity for PGE2 and a unique signal transduction profile, yet the degree of cross-talk and overlap between the receptors is substantial (see for review (Regan, 2003). There are no detectable sex differences in the mRNA levels for each receptor during the sensitive period for sexual differentiation or after (Burks et al., 2007), and it appears instead that it is sex differences in the COX enzymes that drive the process of masculinization (Amateau and McCarthy, 2004). All four receptors for PGE2 are expressed in the neonatal POA, and all four have been implicated as contributing to PGE2-induced masculinization (Burks et al., 2007), but it does not seem that all four are necessary. Convergent evidence generated by Christopher Wright implicates EP2 and EP4 as the dominant receptors involved (Wright et al., 2008). Activation of either EP2 or EP4 increases protein kinase A (PKA) signaling, and recent findings indicate that disrupting PKA neonatally blocks masculinization (Wright and McCarthy, unpublished observation – Fig. 1). The activation of PKA may also explain the apparently positively reinforcing effects of PGE2. As little as a single intracerebroventricular injection of PGE2 to newborn females is sufficient to induce full masculinization of dendritic spine density and adult sexual behavior (Wright et al., 2008). The activation of PKA following binding of PGE2 to EP2 or EP4 is capable of inducing more PGE2 production by up regulating the synthesis and activity of the COX enzymes via a GSK mediated pathway (Regan, 2003). This positive feed forward effect of PGE2 likely involves both COX-1 and COX-2, which can be found in neurons, astrocytes and microglia. Thus distinguishing the precise cellular site of estradiol action in this system is challenging and may ultimately prove to be unimportant as the role for cell-to-cell communication emerges as a critical component of the process of masculinization.
Fig. 1.
Estradiol induces prostaglandin E2 (PGE2) synthesis to promote dendritic spine formation and sexual differentiation in the POA. Estradiol aromatized from testicular androgens binds to ER alpha and induces transcription of the COX-1 and COX-2 genes, the rate limiting enzymes in PGE2 synthesis. PGE2 activates multiple receptors, the most important being EP2 and EP4, both of which are linked to activation of Protein Kinase A (PKA). Through mechanisms that remain poorly understood, PKA enhances the actions of glutamate at AMPA, but not NMDA receptors, and the formation of new dendritic spines and the organization of a higher density of spine synapses on male POA neurons compared to female.
The next frontier in understanding PGE2-induced masculinization is to place the findings in the POA into the context of the entire circuitry controlling male and female sexual behavior. The increase in dendritic spines on POA neurons must reflect an increase in afferent input, but from where is unknown. Does the increase in dendritic spines reflect more afferent inputs from the same source or the establishment of a new source of input? There are multiple behaviorally relevant sources of input to the POA, including projections from the amygdala relevant to olfactory stimuli, important reciprocal connections with the ventromedial nucleus (VMN) of the hypothalamus, a critical brain region in the control of female sexual behavior, and thalamic nuclei that convey information about genital stimulation (Simerly, 2002). We currently have no information as to whether any or all of these regions are contributing to the increased excitatory input to the male POA.
Mechanisms mediating defeminization and feminization of sexual behavior
Defeminization is the active developmental process whereby the capacity to express female sexual behavior in adulthood is lost. It has been known for some time that this is a separate physiological process from masculinization (Vreeburg et al., 1977), and it was recently established that the mechanism of defeminization is entirely independent of PGE2 action (Todd et al., 2005). As with the gonad and gonadal duct systems, the female phenotype is the default, and for a male to develop properly, the female phenotype must be repressed or removed. That this is required for the reproductive organs seems clear, but why it occurs in the brain is a mystery. Given that sexual behavior must be activated in adulthood by sex-typic hormones, the need to alter the neuronal substrate controlling female sex behavior in males is not obvious.
Regardless of why it occurs, advances into the mechanism of defeminization have been made. Male mice with a null mutation for the beta isoform of the estrogen receptor, known as BERKOs, have incomplete defeminization but normal masculinization (Kudwa et al., 2005), suggesting a differential role for each receptor subtype in mice. In rats, early work suggested a distinction between the relative importance of androgens versus estrogens in defeminization versus masculinization, respectively (Vreeburg et al., 1977), but it is clear that defeminization can be induced by estradiol just as effectively (Todd et al., 2005). An important limitation in studies of defeminization is the lack of an identifiable neuroanatomical substrate. The POA is the critical brain region controlling male sexual behavior, but as noted above, the SDN-POA may act to suppress female sexual behavior and therefore serve as a substrate for defeminization. The VMN of the hypothalamus is the essential region for expression of female sexual behavior and it is also possible that this nucleus is a substrate for defeminization, either alone or in connection with the SDN.
Exploration of the VMN as a potential site subject to defeminization begins with a search for sex differences in the neuroanatomy. Similar to the POA, male VMN neurons have more dendritic spine synapses than females (Schwarz et al., 2008). However, unlike the POA, the greater number of spines in males is not due to an increased spine density per unit dendrite but is instead the result of a more complex dendritic tree in males. Male VMN dendrites are significantly longer and branch more frequently than those of females (Mong et al., 1999). Neurite growth and branching is a complex process that involves intrinsic and extrinsic signals, including interaction with the substrate as well as other cells. Membrane to membrane signaling via the ephrins (Penzes et al., 2003; Gamble et al., 2005) and membrane to substrate signaling via the integrins (Robles and Gomez, 2006) are viable targets for hormone action but have not been investigated to-date.
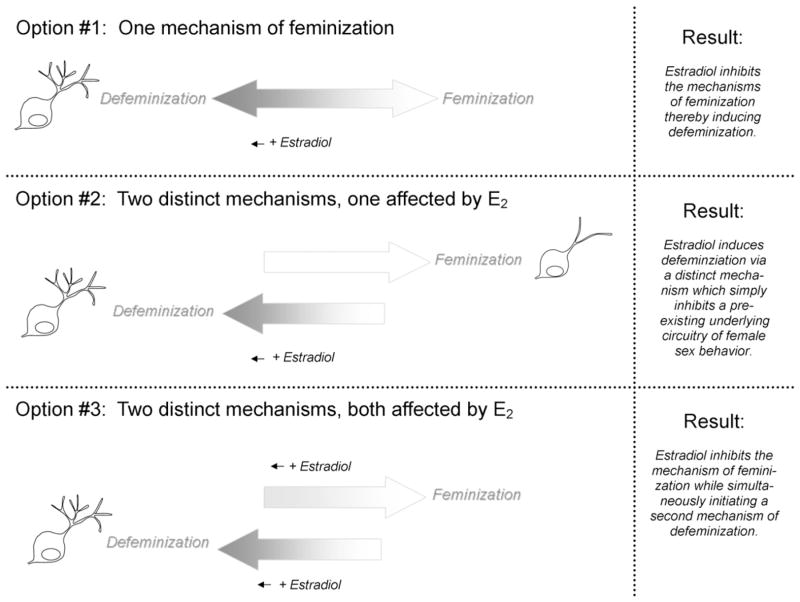
An additional group of signaling molecules of interest is focal adhesion kinase (FAK) and its associated proteins, including paxillin (Ren et al., 2004). These molecules were first of interest in the study of metastatic cancers but have since been found to be integral to neuronal growth and branching (Yang et al., 2003; Rico et al., 2004; Robles and Gomez, 2006). In a search for sex differences in signaling molecules, Debra Speert found FAK and paxillin were both significantly higher in neonatal female hypothalamus and were down regulated by estradiol (Speert et al., 2007). The decrease in FAK and paxillin induced by estradiol was correlated with an increase in neurite complexity, suggesting a plausible mechanism establishing the longer and more frequently branching dendrites of males. The observation that estradiol decreases FAK and paxillin is unusual since most, if not all, other molecules implicated in sexual differentiation involve an increase in expression in response to estrogens or androgens. Given that defeminization is the active removal of the capacity for female sexual behavior, it is plausible that suppression of the endogenously high levels of FAK and paxillin by estradiol is a mechanism to achieve that end. In this instance, higher levels of FAK and paxillin in females could be a component of the default process of feminization. Distinguishing between the two possibilities may be difficult. If defeminization is simply the suppression of mechanisms inducing feminization, then they are two sides of the same coin and cannot be separated. Conversely, if defeminization were a process that simply overrides feminization, they would indeed be two separable processes (Fig. 2). In both scenarios the behavioral endpoint would be the same, the loss of the capacity to respond to adult activational steroids with expression of female sexual behavior, but in the first instance the mechanistic process of feminization would be blocked, whereas in the second instance the cellular events of feminization would occur but would be negated by the cellular events of defeminization (Fig. 2). The only hope for resolving the conundrum is to identify the cellular mechanisms of defeminization and feminization.
Fig. 2.
Feminization versus defeminization. We propose three theoretical conceptualizations of feminization versus defeminization. The first option is that the two processes are inseparable and estradiol-induced defeminization erases feminization. A prediction of this option would be the inability to recover the capacity for female sex behavior in adulthood. The second option proposes distinct mechanisms for defeminization and feminization, both of which occur but the former is induced by estradiol and suppresses the latter which is independent of estrogen action during development. An example would be the formation of a larger SDN in order to suppress the female sexual behavior neural template. The third option involves two separate mechanisms induced by estradiol, one to suppress the process of feminization and the other to initiate defeminization. The suppression of FAK and paxillin and the induction of glutamate release by estradiol in the developing VMN would fit this model.
The development of the testes and the ovary provides an apt analogy in that both begin from an undifferentiated gonadal anlage which will by default become an ovary but will differentiate into a testis following expression of a single gene, SRY (Harley and Goodfellow, 1994). The undifferentiated gonad has two tissue types and the one, the cortex, develops in the female, and the other, the medulla, develops in the male. This primordium is bipotential and thus producing male characteristics necessarily reduces female characters. Thus for testis development there is a single critical event that provides a wedge point for exploration (Sekido and Lovell-Badge, 2009). The current state of understanding of how the testis develops reflects this advantage as it is highly detailed and advanced and far exceeds that of the ovary, about which much less is known (Loffler and Koopman, 2002). This is likely only a matter of time, however, given that the ovary is a complex well-characterized endocrine organ about which experimental predictions can be made. In the brain, we do not even know what a female brain is, other than it is not male. Thus elucidating the mechanism(s) of feminization is a daunting challenge.
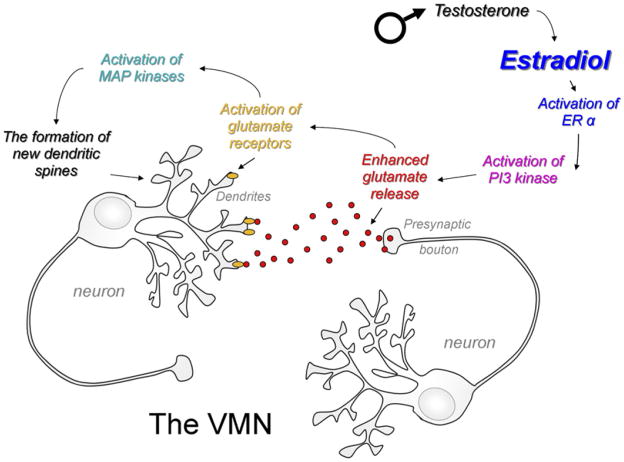
Returning to the question of defeminization and the plausible assumption that a good place to begin looking is the VMN of the mediobasal hypothalamus, a surprising role for non-genomic actions of estradiol has recently emerged. As discussed above, steroid receptors are members of a transcription factor super-family and their principle action is at the genome. However, steroid receptors can also interact with and initiate signal transduction pathways originating at the membrane (see for review McCarthy 2008). These effects tend to be rapid and transient. Therefore, when considering steroid-induced sexual differentiation of the brain it seemed unlikely that membrane-initiated events would be an important contributor. But the facts have proved otherwise. Jaclyn Schwarz has demonstrated that in immature VMN neurons estradiol binds to its cogent receptor and rapidly promotes the release of glutamate from presynaptic terminals, which in turn activates postsynaptic NMDA and AMPA receptors, leading to calcium influx, activation of MAP kinase and promotion of dendritic spine formation (Schwarz et al., 2008). The enhanced glutamate release requires estradiol-induced activation of PI3 Kinase, a kinase involved in a wide range of cellular functions, and this occurs as rapidly as 1 h after steroid exposure. Neither the activation of PI3 Kinase nor the enhancement of glutamate release by estradiol requires protein synthesis, but they do require the estrogen receptor since the effects are blocked by pretreatment with the ER antagonist, ICI 182,780. In a series of elegant experiments involving latrotoxin-induced glutamate release, Jaclyn Schwarz further demonstrated that construction and maintenance of new spines in the postsynaptic cell does require protein synthesis, but not the activation of ER (Schwarz et al., 2008). Thus there is an essential requirement for cell-to-cell communication for establishment of sexually dimorphic synaptic patterning in the VMN (Fig. 3). This is similar to the POA where cell-to-cell communication is also critical, and yet different in that there is no apparent role for astrocytes in the VMN but there is in the POA.
Fig. 3.
Estradiol enhances glutamate release to promote cell-to-cell communication and sexual differentiation in the VMN. In the developing male VMN dendrites are longer and branch more frequently than in the females, resulting in a greater number of dendritic spines. This permanent organizational effect begins with the nongenomic activation of PI3 kinase by ligand-activated ER alpha. The increased released glutamate binds to receptors on the post-synaptic neuron, inducing activation of MAP kinase, protein synthesis and the formation of new dendritic spines (Schwarz et al., 2008).
The observation that estradiol promotes glutamate release to induce dendritic spines in the hypothalamus predicts that neonatal glutamate administration would substitute for estradiol in inducing defeminization. Conversely, blocking glutamate transmission neonatally should disrupt estradiol-induced defeminization. Both of these predictions have been proven true when assessed behaviorally or anatomically (Schwarz et al., 2008; Schwarz and McCarthy, 2008). However, there is also a positive effect of glutamate receptor activation on organization of male sexual behavior, suggesting a functional connection between the cellular events of masculinization and defeminization.
Why so many mechanisms?
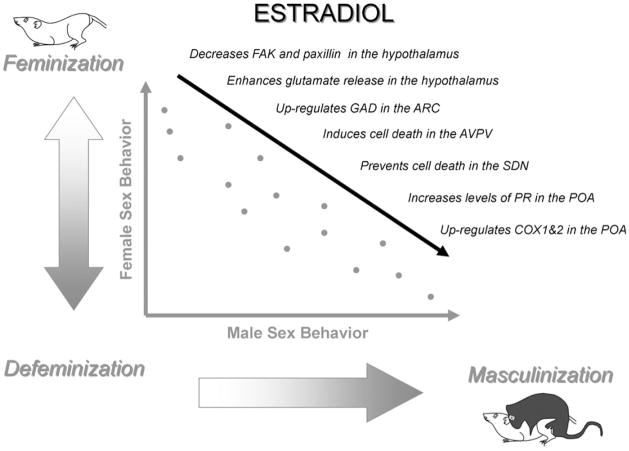
Given that there appears to be a functional connection between the cellular processes of masculinization and defeminization, it is reasonable to ask, why they are not achieved by the same cellular mechanism. If normal male development requires a 2-step process in which the male phenotype is induced and the female phenotype is erased, it would seem much more efficient to simply achieve both endpoints with one mechanism. In other words, the process of masculinization would effectively erase the female neural substrate. That this is not the case is most definitively demonstrated by the behavior of animals in which masculinization has been prevented or induced via the prostaglandin pathways. Males that were treated neonatally with a COX1 and 2 inhibitor show very low levels of masculine sex behavior as adults, and if the same animals are treated with a hormonal milieu conducive to female sexual receptivity they show no response. Thus these animals are essentially asexual as adults. Conversely, females masculinized by PGE2 as neonates respond perfectly well as adults when treated with sequential estradiol and progesterone and exhibit normal female sexual receptivity (Todd et al., 2005). Thus it is apparent that PGE2 mediated masculinization is entirely distinct from defeminization, but why? What is the advantage of having an entirely separate mechanism for defeminization? Is there a cost to retaining the capacity to show female sexual behavior when a male would never see the appropriate hormonal milieu anyway? This question remains unanswered, but when exploring the role of glutamate in defeminization we found some animals with both incomplete masculinization and incomplete defeminization. This resulted in animals with a phenotype somewhere in between normal males and females and revealed the capacity for a far greater variability in behavioral responding than would be possible if a single mechanism mediated both processes (Fig. 4). The potential for variability is even more pronounced when one considers that during the same developmental time frame estradiol is preventing cell death in the SDN (Arai et al., 1996), inducing cell death in the anteroperiventricular (AVPV) nucleus (Murakami and Arai, 1989; Forger et al., 2004), increasing BDNF levels in the hippocampus (Solum and Handa, 2002), inducing PR in the POA (Quadros et al., 2002), increasing GABA and it’s rate-limiting enzyme, GAD, in the arcuate nucleus (Mong et al., 2002) and numerous others including some that may not yet have been discovered. Each one of these mechanisms provides multiple nodes for genetically or environmentally induced variation. Polymorphisms in alleles for COX-2, GAD, PGEsynthase, NR2, mGluR’s, FAK, paxillin, BDNF and trkB receptors could have profound influences on the actions of estradiol in discrete brain regions without compromising function in other brain regions. Moreover, epigenetic changes in associated genes in response to variance in maternal care (Cameron et al., 2008; Champagne, 2008), diet (McGowan et al., 2008) or stress (McEwen, 2008) could impact estrogen action during the critical period, resulting in enduring variation in the organizational substrate on which activational hormones will modulate adult physiology and behavior.
Fig. 4.
Multiple phenotypes due to multiple mechanisms. Estradiol simultaneously activates multiple cellular mechanisms in discrete brain regions during the sensitive period of sexual differentiation for feminization, defeminization and masculinization. The ultimate impact is a far greater number of potential phenotypes than would be achieved by a single unitary mechanism that mediated sex differences in physiology and behavior.
Challenges to the dogma
The value of the Organizational / Activational Hypothesis has been the ability to clearly frame questions and thereby challenge the predictions stemming from this construct. The primary challenge was made even prior to the formulation of the hypothesis and persists to this day, which is that under some circumstances males can be induced to show female typical behavior in adulthood, and likewise, normal females will mount other females or even mount males on occasion (Beach, 1948). The degree of behavioral plasticity and in which sex it predominates varies by species and strain (Goy and Goldfoot, 1975).(Goy and Goldfoot, 1975) In our laboratory, male Sprague Dawley rats treated with female hormones rarely if ever show lordosis behavior and likewise, females only occasionally exhibit male mounting behavior. Nonetheless, that there is plasticity in adult sexual behavior is not in question, but whether it challenges or even negates the Organizational / Activational Hypothesis is a topic of debate. A strict interpretation of the hypothesis insists that organizational events are permanent and therefore immutable, whereas a more liberal interpretation allows for organizational events to be enduring but ultimately reversible. Both interpretations have been demonstrated for behavior. Male guinea pigs can be induced to show lordosis if given pulsatile estradiol treatments over an extended period and tested repeatedly. However, these males never show proceptive behavior, an additional critical component of sexual behavior (Olster and Blaustein, 1989). Thus the sexual differentiation of lordosis is enduring but ultimately reversible whereas the sexual differentiation of proceptivity is permanent and apparently immutable.
Central to observations of dual sexuality in adult animals is the nature of the neural circuits controlling male versus female sexual behavior. Because male and female sexual behaviors are so different in motor patterns and hormonal dependence, it is often assumed that there are two distinct neural circuits that independently control each behavioral repertoire. And in an appealing analogy to the Mullerian and Wolffian duct systems that give rise to the female versus male reproductive tracts, respectively, masculinization could be seen as the retention of the male circuit and defeminization as the active degeneration (i.e. Mullerian Inhibiting Hormone) of the female circuit. The shortcoming of this analogy is the inability to actually identify separate male and female neural circuits. There are clear nodes that are more critical to one sex than the other, such that an intact and functioning POA is critical for male sexual behavior and the same is true for the VMN and female sexual behavior. But these two nuclei are intimately connected with each other as well as being part of a larger network of nuclei integrating sensory stimuli and motivational cues to coordinate a coherent behavioral response. Careful students of anatomy such as Newman, Simerly and De Vries have long suggested that there is only one neural network controlling sex behavior but it is differentially weighted toward expression of either male or female motor patterns in response to specific stimuli (De Vries and Simerly, 2002; Simerly, 2002). That this issue remains unresolved 50 years after the codifying of the Organizational / Activational Hypothesis is a testament to the complexity of the neural and hormonal control of reproduction.
Can studies on the mechanism of sexual differentiation provide any insight into this issue? At first blush the observation of clearly separate processes for masculinization and feminization favors the view that there are distinct circuitries for male versus female sexual behavior. For example, PGE2-induced masculinization of females results in adult animals that show perfectly normal male sex behavior under conditions of high testosterone, and perfectly normal female sex behavior following sequential estradiol and progesterone (Todd et al., 2005). The converse is also true, if normal masculinization in males is blocked by inhibiting COX-2 activity, those males will show no sex behavior in adulthood, either male or female. Thus, if one uses the Mullerian/Wolffian duct analogy, PGE2-masculinized females have both systems, whereas males treated neonatally with a COX inhibitor have neither system. But, as mentioned above, it is also possible to generate animals with a phenotype half-way between fully masculinized and fully feminized (Schwarz and McCarthy, 2008), and under some circumstances there is tremendous plasticity in adult sexual behavior. Does this mean that early organization of the neural substrate is not occurring? To answer this question, we must first identify what organization of the neural substrate means.
The purest examples of early organizational actions of gonadal steroids are arguably those involving differential cell death, and the two best examples of that are the larger SDN and smaller AVPV in males. Each of these subnuclei begin as a similarly sized collection of neurons in both sexes, and then neurons die selectively in one sex due to the lack or presence of estradiol, resulting in a significantly smaller nuclear volume. Cell death is permanent, and this is therefore a permanent organizational difference. Although even this seemingly irrefutable statement is challenged by the recent observation that continuing cell genesis around puberty contributes to the maintenance of the volumetric sex difference in both the SDN and AVPV (Ahmed et al., 2008). More importantly from the point of view of sexual differentiation of behavior, however, is that neither of these volumetric sex differences appears to have any relevance to sex behavior (the SDN may play a role in partner preference (Baum, 2006), whereas the AVPV is critical to the control of gonadotropin secretion from the anterior pituitary (Simerly, 2002), which is sexually dimorphic). This leaves the sexual differentiation of synaptic patterning as a potential mediator of male versus female sex behavior. As reviewed above, in the POA males have a 2- to 3-fold greater density of dendritic spine synapses, while in the VMN males have 2- to 3-fold more dendritic spines because the dendrites are longer and branch more frequently. These hormonally induced differences are enduring and levels of a marker of dendritic spines in the POA correlate with measures of masculine sex behavior in adults (Fig. 5). This correlation is intriguing in that it suggests a direct relationship between the number of dendritic spines and the magnitude of the expression of male sexual behavior. The more POA spine synapses, which are excitatory, the more intense the behavior. In other words, the system is weighted towards male behavior by increased excitatory input onto the critical POA neurons. Females still have plenty of POA dendritic spine synapses but simply not enough to put them over a threshold necessary for expressing male sex behavior (Fig. 6). Steroid-induced neuronal plasticity in the adult frequently involves induction of dendritic spine synapses (Woolley, 1999), and an important component of organization is likely a reduction in sensitivity of the adult brain to the sex-typic hormonal profile of the opposite sex and a retention of sensitivity to your own sex-typic hormonal profile. The idea that steroid sensitivity is organized is not new (Sodersten, 1984), but past focus has been on changes in steroid receptor levels and we are now proposing that perhaps the sensitivity is at the level of the neural plasticity, as opposed to the steroid receptors themselves. The threshold for activation of behavior is thereby determined by the previously organized sensitivity of neuronal plasticity and the threshold can be shifted by the intensity and duration of activational hormone treatment to induce plasticity. This interpretation could also help explain the anomalous behavior of some mutant mouse strains, such as that lacking the Trp2c channel (Kimchi et al., 2007). This mouse has altered vomeronasal organ function and females show very high levels of indiscriminant male sexual behavior. The threshold for expression of male sexual behavior appears to be much lower in mice to begin with, compared to rats, and it is possible that the distortion of vomeronasal organ function induces a brainstorm of electrical activity that reduces the threshold still lower by inducing excessive excitatory input to the existing POA dendritic spine synapses. Unfortunately there is currently no information available regarding the dendritic morphology of the mouse POA, making studies on sexual differentiation of the mouse brain and behavior difficult to interpret.
Fig. 5.
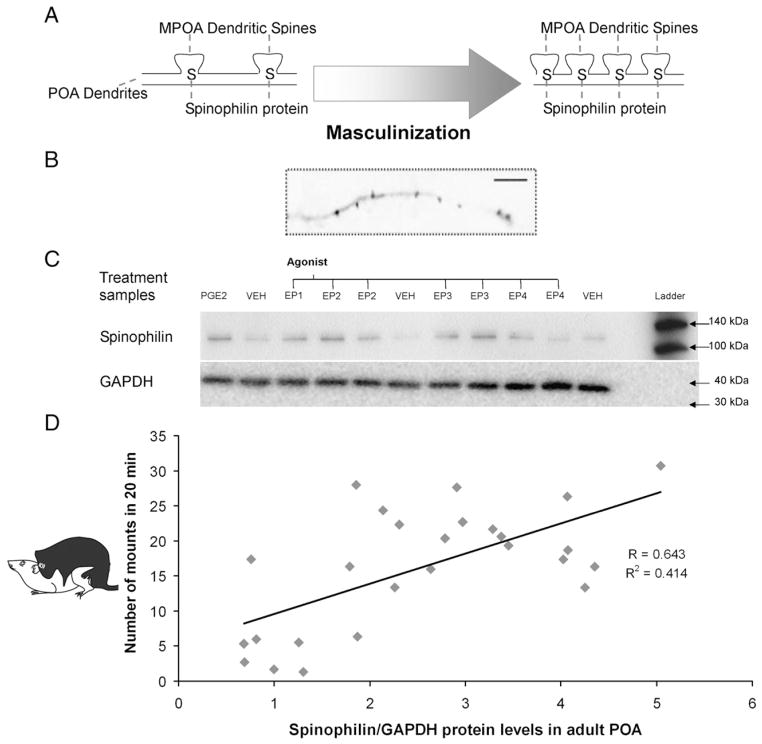
Synaptic profiles predict male behavior. Increases in spinophilin protein correlated with the density of dendritic spines on POA neurons and measures of adult male sexual behavior correlated with adult POA spinophilin protein. A) Stylized representation of spinophilin in the necks of dendritic spines. B) Image of POA cultured neuron visualized by immunoctyochemistry for spinophillin which is concentrated in spine-like protrusions (Amateau and McCarthy, 2004). C) Effect of neonatal treatment with EP receptor agonists on adult POA spinophilin protein assayed by western blot. Each lane represents the POA of one animal in a typical western immunoblot for spinophilin (above) and GAPDH loading control (below). POA spinophilin protein levels incrased 2–4 fold above vehicle (VEH) treated animals and equivalent to PGE2 treated animals (Wright et al., 2008). D) Regression of adult POA spinophilin protein levels and number of mounts in individual animals shows a significant correlation (p<0.001) between the two endpoints.
Fig. 6.
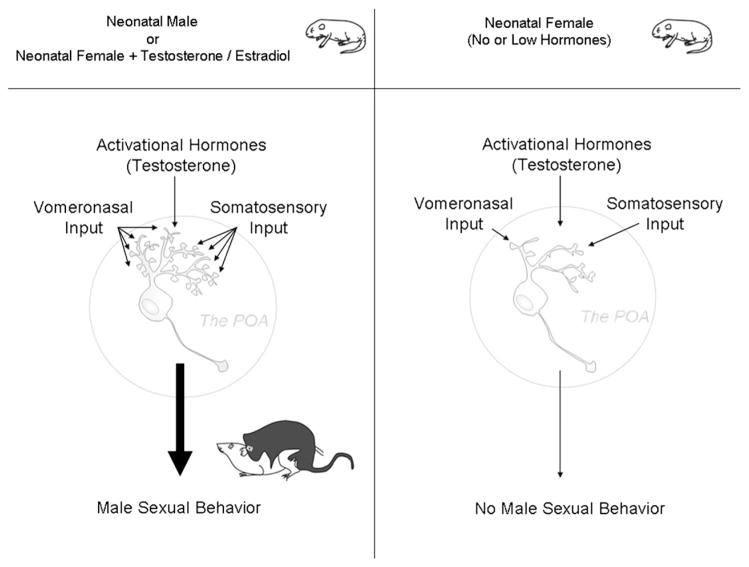
Neural circuit(s) controlling sex behavior. The question of whether there are two distinct neural circuits that separately control expression of male versus female sexual behavior, or a single neural circuit that is preferentially weighted towards one versus the other, remains unresolved. The observation of increased density of dendritic spines on POA neurons correlating with male sexual behavior is consistent with a single circuit that is weighted in favor of males in response to salient olfactory and somatosensory input. Similarly, the observation of decreased dendritic branching and fewer dendritic spine synapses in the VMN suggests a weighting in favor of female sexual behavior. But in both instances, the potential for synaptic plasticity and expression of opposite sex behavior still exists.
Studies of mice have also constituted another source of challenge to the dogma, the importance of genetic mediators as opposed to gonadal or steroidal mediators of sexual differentiation. The ability to generate mice that are genetically female (XX), but express the SRY gene on an autosome and thereby have testis and comparing them to genetic males (XY) lacking the SRY gene and thereby have ovaries, allows for discriminating between these two sources of biological determinism (Arnold, 2004). Many interesting behavioral and neuroanatomic endpoints are impacted by genetic sex, but when it comes to the differentiation of reproductive behavior, hormones still carry the day (De Vries et al., 2002). So in this instance, a wonderfully crafted challenge to the dogma was highly effective at demonstrating the validity of the dogma, while simultaneously highlighting that there are multiple other endpoints that exhibit sex differences, but are not necessarily sexually differentiated in the classic hormonally-mediated manner.
This same principle is borne out by attempts to shoe horn other sex differences in the adult brain into the Organizational / Activational Hypothesis. There has been a general blurring between the distinction of a sex difference and sexual differentiation, with the result that all sex differences are often assumed to be the product of hormonally mediated sexual differentiation unless proven otherwise (see for discussion (McCarthy and Konkle, 2005). This notion is partly predicated on the reasonable assumption that the male bias in gonadal steroidogenesis during the sensitive period would result in elevated testosterone and estradiol throughout the male brain, but in reality there have been no reports of sex differences in steroid levels in the developing telencephalon and a notable lack of sex differences in the hippocampus and cortex (Amateau et al., 2004). Circumstantial evidence supports the view that cells of the female hippocampus are capable of de novo steroidogenesis of estradiol from cholesterol (Prange-Kiel et al., 2003; Nunez and McCarthy, 2009) and this might serve the purpose of reducing, not producing sex differences in hippocampal development. However, there are still sex differences in the hippocampus, and they can be manipulated by exogenous estradiol. For instance, in direct contrast to the lack of evidence in the rates of cell genesis in the hypothalamus and preoptic area, there are significantly more new cells born in the male hippocampus during the first few days of life than the female (Zhang et al., 2008). Treating newborn females with estradiol increases the number of new cells, some of which will differentiate into glia. What has not yet been determined is whether endogenous estradiol is a critical regulator of this sex difference, and if so, how. Moreover, whether this sex difference adheres to the basic principles of the Organizational / Activational Hypothesis, or whether it is time to craft a new set of guidelines by which specific parts of the male and female brain develop, remains to be determined.
Gaps in our knowledge
Throughout this review we have attempted to highlight those places where there is a clear lack of information that precludes definitive conclusions from being reached. There is still a great deal to learn regarding the specific mechanisms of organizational actions of hormones on the developing brain and the consequences of this organization for adult function. But on a more global level there are two basic questions which constitute large gaps in our knowledge; 1) how are the organizational actions of steroids maintained across the lifespan?, and 2) how are the distinct cellular mechanisms of steroids, in particular estradiol, coordinated across multiple brain regions to generate a coherent neural network? Both of these are tractable experimentally but will require different approaches than those currently being used. Epigenetic modifications of critical genes is a reasonable candidate for organizing an enduring effect on a neural circuit that could, under specific circumstances, be at least partly un-done, and this is likely to be a fruitful future area of research. The coordination across brain regions requires a more integrated approach. This has already been achieved for the sexually dimorphic projection of the pBNST to the AVPV and the discovery of a target-derived diffusible factor from the former that draws in the axons of the latter (Ibanez et al., 2001). Similar things may be occurring between other sub-nuclei, but there is more than one way to wire a brain and it cannot be assumed the same principle applies broadly. Finally, the use of live imaging to monitor the process of hormonally-induced synaptogenesis in real time may further our ability to understand cell-to-cell communication during these critical developmental windows and provide evidence of more new tricks for an old, but still very lively dogma.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 Sep;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA. Sexual orientation and the size of the anterior commissure in the human brain. Proc Natl Acad Sci U S A. 1992;89:7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J Neurosci. 2002a;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendo. 2002b;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev, Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Wade J, Grisham W, Jacobs EC, Campagnoni AT. Sexual differentiation of the brain in songbirds. Dev Neurosci. 1996;18:124–136. doi: 10.1159/000111400. [DOI] [PubMed] [Google Scholar]
- Ayoub DM, Greenough WT, Juraska JM. Sex differences in dendritic structure in the preoptic area of the juvenile macque monkey brain. Science. 1983;219:197–198. doi: 10.1126/science.6849133. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Mammalian animal models of psychosexual differentiation: when is ‘translation’ to the human situation possible? Horm Behav. 2006;50:579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormones and Behavior. Paul B. Hoeber, Inc; New York: 1948. [Google Scholar]
- Beyer C, Feder HH. Sex steroids and afferent input: their roles in brain sexual differentiation. Annu Rev Physiol. 1987;49:349–364. doi: 10.1146/annurev.ph.49.030187.002025. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Burks SR, Wright CL, McCarthy MM. Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience. 2007;146 (3):1117–1127. doi: 10.1016/j.neuroscience.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W. The medial preoptic and anterior hypothalamic regions of the rhesus monkey: cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–350. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Davis AM, Grattan DR, McCarthy MM. Decreasing GAD neonatally attenuates steroid-induced sexual differentiation of the rat brain. Behav Neurosci. 2000;114:923–933. [PubMed] [Google Scholar]
- De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:91–96. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 1. Academic Press; New York: 2002. pp. 137–192. [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhler KD, Jarzab B, Sickmöller PM, Kokociñska D, Kaminski M, Gubala E, Achtelik W, Wagiel J. Influence of neurotransmitters on sexual differentiation of brain structure and function. Exp Clin Endocrinol. 1991;98 (2):99–109. doi: 10.1055/s-0029-1211106. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble JA, Karunadasa DK, Pape JR, Skynner MJ, Todman MG, Bicknell RJ, Allen JP, Herbison AE. Disruption of ephrin signaling associates with disordered axophilic migration of the gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:3142–3150. doi: 10.1523/JNEUROSCI.4759-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Goy RW, Goldfoot DA. Neuroendocrinology: animal models and problems of human sexuality. Arch Sex Behav. 1975;4:405–420. doi: 10.1007/BF01541724. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sexual dimorphism in the CNS and the role of steroids. In: Stowe TW, editor. CNS Neurotransmitters and Neuromodulators: Neuroactive Steroids. CRC Press; London: 1996. pp. 127–152. [Google Scholar]
- Harley V, Goodfellow P. The biochemical role of SRY in sex determination. Mol Reprod Dev. 1994;39:184–193. doi: 10.1002/mrd.1080390211. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Wallen K, Edwards DA. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 1986;370:21–28. doi: 10.1016/0006-8993(86)91100-5. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, De Jonge FH, Joosten RN, van den Poll N, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Bitran D, Nishita JK, Scaletta LL. Organizational and activational effects of dopamine on male sexual behavior. In: Ellis L, Ebertz L, editors. Males, Females and Behavior: Toward Biological Understanding. Greenwood Press; 1998. pp. 79–96. [Google Scholar]
- Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21:5652–5659. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzab B, Kokocinska D, Kaminski M, Gubala E, Achtelik W, Wagiel J, Dohler KD, Balthazart J. Hormones, Brain and Behavior in Vertebrates: I. Sexual Differentiation, Neuroanatomical Aspects, Neurotransmitters and Neuropeptides. Karger; Basel: 1990. Influence of neurotransmitters on sexual differentiation of the brain: relationship between the volume of the SDN-POA and functional characteristics; pp. 41–50. [Google Scholar]
- Jeong JK, Ryu BJ, Choi J, Kim DH, Choi EJ, Park JW, Park JJ, Lee BJ. NELL2 participates in formation of the sexually dimorphic nucleus of the pre-optic area in rats. J Neurochem. 2008;106:1604–1613. doi: 10.1111/j.1471-4159.2008.05505.x. [DOI] [PubMed] [Google Scholar]
- Kasai M, Mizumura K. Effects of PGE2 on neurons from rat dorsal root ganglia in intact and adjuvant-inflamed rats: role of NGF on PGE2-induced depolarization. Neurosci Res. 2001;41:345–353. doi: 10.1016/s0168-0102(01)00291-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann W, Andreasson K, Isakson P, Worley P. Cyclooxygenases and the central nervous system. Prostaglandins. 1997;54:601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behavior in the female mouse brain. Nature. 2007;448:1009–1015. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladosky W, Gaziri LCJ. Brain serotonin and sexual differentiation of the nervous system. Neuroendocrinology. 1970;6:168–174. doi: 10.1159/000121920. [DOI] [PubMed] [Google Scholar]
- Loffler KA, Koopman P. Charting the course of ovarian development in vertebrates. Int J Dev Biol. 2002;46:503–510. [PubMed] [Google Scholar]
- Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sexual dimorphism in ‘wiring pattern’ in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980;19:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male–female differences in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinolgy. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57 (Suppl 2):S11–15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendocrinol. 2002;14:1–16. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preopti area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y. Sexual dimorphism in synaptic organization in the amygdala and its dependence on neonatal hormone environment. Brain Res. 1981;211:31–38. doi: 10.1016/0006-8993(81)90029-9. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing GABA and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2009 Jan 23;158(2):623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olster DH, Blaustein JD. Progesterone facilitation of lordosis in male and female Sprague–Dawley rats following priming with estradiol pulses. Horm Behav. 1989;22:294–304. doi: 10.1016/0018-506x(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller M, Eipper B, Mains R, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic EphrinB–EphB receptor activation of the Rhogef Kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Morphological changes in the brains of adult male rats after neonatal castration. J Endocrinol. 1966;36:415–416. doi: 10.1677/joe.0.0360415. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Rage F, Lee BJ, Ma YJ, Ojeda SR. Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J Neurosci. 1997;17:9145–9156. doi: 10.1523/JNEUROSCI.17-23-09145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav. 2008;54:662–668. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009 Jan;25(1):19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sodersten P. Sexual differentiation: do males differ from females in behavioral sensitivity to gonadal hormones? Prog Brain Res. 1984;61:257–270. doi: 10.1016/S0079-6123(08)64440-4. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert DB, Konkle ATM, Zup SL, Schwarz JA, Shiroor C, Taylor M. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, van der Vaart PD, van der Shoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008:68. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Chen S, Wang C, Lee E. Focal adhesion kinase is required, but not sufficient, for the induction of long-term potentiation in dentate gyrus neurons in vivo. J Neurosci. 2003;15:4072–4080. doi: 10.1523/JNEUROSCI.23-10-04072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]