Abstract
A wide range of evidence points to a role for GLP-1 to regulate food intake. Anorectic effects of GLP-1 are most apparent when the peptide is administered directly into the central nervous system (CNS), but suppression of food intake has also been noted in some cases with peripheral administration. It is unclear which GLP-1 receptor (GLP-1r) population mediates the effects of plasma GLP-1, although direct actions to activate CNS neurons have been demonstrated. More recently several groups have demonstrated that GLP-1 can activate peripheral nerves in the hepatic portal vein to regulate glucose metabolism. To test the hypothesis that GLP-1 receptors on nerve terminals in the hepatic portal affect feeding behavior, we compared the effects of direct infusions into hepatic portal and jugular veins in rats. Jugular GLP-1 decreased food intake at doses as low as 10 μg from 0.5-4 hours into the dark cycle, whereas portal GLP-1 decreased food intake only at the highest dose tested (100 μg). The blockade of endogenous GLP-1 action before or during eating by infusing dH-Ex, GLP-1 receptor antagonist, into either jugular or portal vein did not cause any change in food intake during either the dark or light cycles. Taken together, these data suggest that while peripheral GLP-1 may be involved in the regulation of food intake, the key GLP-1 receptors are unlikely to be those associated with vagal afferent nerves innervating the hepatic portal vein.
Introduction
Glucagon-like peptide 1 (GLP-1) is a peptide hormone released from L cells of the small intestine and colon in response to enteric nutrient ingestion [1]. GLP-1 is also produced in a discrete set of neurons in the nucleus of the solitary tract [2]. After secretion into the circulation, GLP-1 is rapidly degraded by a dipeptidyl peptidase IV, which is widely expressed on capillary endothelial cells most prominently in the liver, lung and kidney [3, 4]. GLP-1 receptors (GLP-1r) are widely expressed in pancreatic islets, the gastrointestinal tract, lungs, kidneys, and in key regions of the nervous system [1, 5, 6]. In the brain the GLP-1r is expressed in the hindbrain, the hypothalamus and amygdyla [7], and recent evidence also indicates synthesis by vagal afferent nerves located in the nodose ganglia [5, 8].
One function proposed for GLP-1 is the regulation of food intake. GLP-1 potently reduces food intake when administrated either into brain [9-11] or peripherally [12-15] in rats. The effect of single peripheral administrations of GLP-1 produces only transient reductions in food intake [13-15] compared to what is observed after CNS administration. This is likely explained by the short half-life of the biologically active GLP-1 (∼ 2 min) in the circulation. Given the limited time intact GLP-1 resides in the circulation and the resulting low levels of GLP-1 as compared to other gut peptides, the key issue is whether endogenous GLP-1 could reduce food intake while being secreted from the intestine. Thus an important goal for understanding the actions of GLP-1 has been identifying key receptor populations that regulate food intake and glucose homeostasis.
Previous studies indicate that the hepatic portal region is innervated by afferent nerves that detect substrates and hormones in the circulation. For example, it has been shown that changes in plasma glucose and glucagon are detected in the hepatic portal vein and contribute to the regulation of food intake [16-18]. More recently, several lines of evidence also point to the hepatic portal region as a site for GLP-1 action. GLP-1 receptors exist on nerve terminals of the hepatic portal region, and participate in glycemic regulation following enteral administration of glucose in rats [5]. Intraportal administration of GLP-1 also activates electrical activity in hepatic vagal afferents and pancreatic vagal efferents in rats [8], and a ganglionic blocker suppresses the augmenting effect of portal GLP-1 on glucose-induced insulin secretion in rats [19]. Given these data supporting a role for hepatic portal GLP-1 receptors to participate in the regulation of glucose levels, we hypothesized that the hepatic portal GLP-1 receptors play a role in detecting changes in GLP-1 secretion from the gut to regulate food intake.
Materials and Methods
Animals
Male Long-Evans rats at 12 weeks of age (300 ∼350 g) obtained from Harlan (Indianapolis, IN, USA) were housed individually with free access to rodent chow diet and water in standard rat cages (11:11 light-dark cycle, 50-60% humidity, 25°C). After 1 week of acclimation, the rats were operated for catheterization of jugular vein or portal vein. All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Surgery
The rats were anesthesized with isoflurane and injected subcutaneously with buprenex analgesia (300 μg/kg). A midline laparotomy was performed, and silastic catheters (Technical Products, Inc., GA, USA; i.d. 0.508 mm, o.d. 0.940 mm) were inserted anterogradely into the hepatic portal vein or right jugular vein. The portal line was initiated in the superior mesenteric vein and positioned with the tip approximately 1.0-1.5 cm from the porta hepatis. The catheter exited through the subcutaneous tissue between the shoulder blades and attached to a vascular access harness (Instech Laboratories, Inc., PA, USA). The catheters were maintained with a daily flush of 10-20 μl of heparinized bacteriostatic saline for the first 10 days after the operation, and then every 3 day to ensure the patency. Experiments were initiated only when the rats completely regained the presurgical body weight (10∼14 days).
Experimental procedures
The rats were adapted to the experimental procedures every second day before each experiment. Rats were randomized to each group and the order of treatments were counter-balanced across subjects in each experiment. Food was removed 4 hours or overnight before lights out in the experiments performed during dark, or 4 hours before infusion of solutions in the experiments performed during light. Animals were habituated to the infusion room and apparatus for a full 2 hours before the infusion was begun. Rats had continuous access to water throughout the experiments. The vascular access harnesses attached to the catheter were connected to a syringe pump (model KDS220; KD Scientific, Inc., MA, USA) via a vascular access harness tether assembly kit (Instech Laboratories, Inc., PA, USA) and CoEx™ PE/PVC tubing (Instech Laboratories, Inc., PA, USA; 0.61 mm i.d., 1.60 mm o.d.) separated by a swivel above the cage cover. The food hoppers were filled with a small amount of fresh food for each experiment. The area around the food hopper and bedding were carefully examined at each measurement in order to detect any spillage of food. To block the activity of endogenous GLP-1, the GLP-1 antagonist, [desHis1,Glu8] exendin-4 (dH-Ex; American Peptides, Sunnyvale, CA, USA) or exendin-3 (9-39) (TOCRIS bioscience, Ellisville, MO, USA) was used.
1) Food intake studies during dark
In the experiments for effect of GLP-1 on food intake in either jugular or portal vein during dark, GLP-1 (1, 10, and 100 μg) in 0.5% bovine serum albumin/0.15 M saline (BSA/saline) or vehicle (BSA/saline) was infused into either to be jugular or portal vein at 7 μl/min starting 15 minutes before returning food hoppers and continued for 25 minutes after lights out. After the 40-min infusion of test substances, vehicle was infused at the same rate for another 30 minutes. The lines were detached from the vascular access harness after measuring 2h-food intake. The food intake was measured at 0.5, 1, 2, 4, 6, and 24 hours after returning food hopper. Each rat was treated with the different doses of GLP-1 within subjects.
In the experiments for effect of pre-blockade of endogenous GLP-1 receptors before eating on food intake in either jugular or portal vein during dark, dH-Ex (total 1, 10, and 100 μg) in BSA/saline or vehicle was infused into portal vein at 7 μl/min for 15 minutes before dark. The food intake was measured at 0.5, 1, 2, 4, 6, and 24 hours after returning food hopper. In the experiments for effect of blockade of endogenous GLP-1 receptors during the meal on food intake in either jugular or portal vein during dark, dH-Ex (200 ng and 100 μg), exedin-3 (9-39) (100 μg) in BSA/saline or vehicle was infused into either jugular or portal vein at 7 μl/min 10 minutes before returning food hoppers and continued to be infused for 30 minutes during dark. After the 40-min infusion of dH-Ex or vehicle, vehicle continued to be infused at the same rate for another 30 minutes. The lines were detached to the vascular access harness after measuring 2h-food intake. The food intake was measured at 0.5, 1, 2, 4, 6, and 24 hours after returning food hopper. Each rat was treated with the different doses of dH-Ex within subjects.
2) Food intake studies during light
The experiments during light were performed between 6 hours after lights on and 11 hours. dH-Ex (50 ng, 500 ng, 20 μg, and 50 μg) in BSA/saline or vehicle (BSA/saline) was infused at 7 μl/min 10 minutes before returning food hoppers and continued to be infused for 30 minutes during light. Each rat was treated with the different doses of dH-Ex within subjects. The food intake was measured at 30 minutes after returning food hopper.
Statistics
All data are expressed as mean ± SEM. The data from the dose-response experiments of dH-Ex on food intake during dark or light were analyzed using repeated two-way analysis of variance (ANOVA) followed by a Bonferroni's test with dose of drugs as a within-subject factor. Different intravenous route of administration was included as a between-subject factor. The data from the experiments testing GLP-1 effects on food intake over 22 hours were analyzed by using repeated two-way ANOVA followed by a Bonferroni's test. The data about 30-min and 4-h food intake were analyzed using two-way or one-way ANOVA followed by a Bonferroni's test.
Results
Dose-effect curves of portal versus jugular infusion of GLP-1 on food intake
To determine whether portal GLP-1 receptors play a role in the regulation of food intake, we compared the effect of infusion of GLP-1 into portal vein (portal GLP-1) on food intake with jugular vein. The infusion of 10 μg GLP-1 into jugular vein (jugular GLP-1) significantly decreased food intake while portal GLP-1 at the same dose did not cause significant changes (Figure 1A and B). The effect of jugular GLP-1 was more potent than portal GLP-1 when the percent changes in food intake relative to vehicle at each time point were compared to jugular GLP-1 (P<0.05; Figure 1C). There was no change in body weight after treatment with GLP-1 (1, 10, and 100 μg), consistent with no change in 22-h food intake when compared based on percentage of initial body weight (Jugular vehicle vs. 10 μg GLP-1, 3.18 ± 0.28 vs. 3.18 ± 0.25; Portal vehicle vs. 10 μg GLP-1, 2.97 ± 0.41 vs. 2.63 ± 0.21). The 30-min food intake was significantly reduced in a dose-dependent manner (Figure 2A). The significant suppression of food intake induced by jugular GLP-1 was more persistent by 4 hours after infusion compared to portal GLP-1 (by 2 hours) at 100 μg of GLP-1 (Figure 2B).
Figure 1.
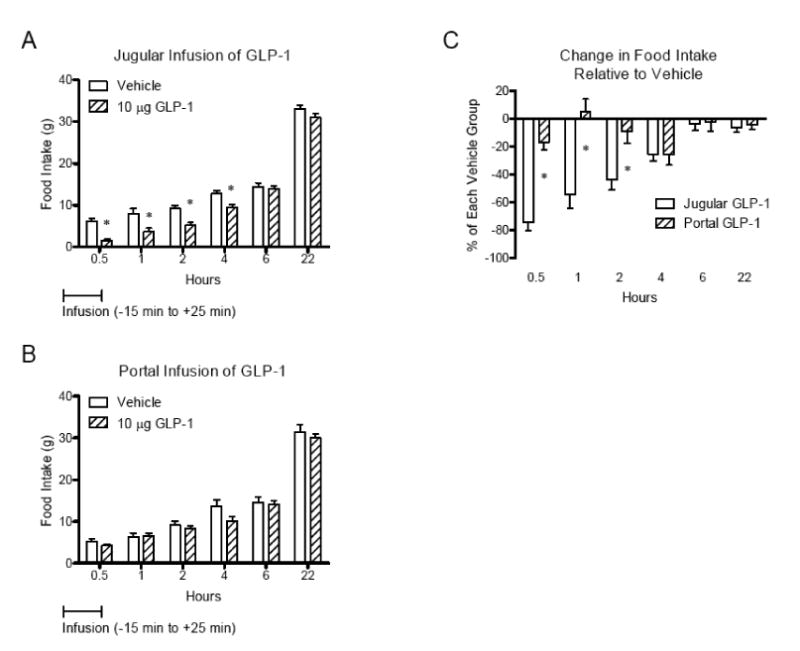
Effect of infusion of GLP-1 into portal (A) or jugular vein (B) on food intake. Comparison of the percent change in food intake relative to each vehicle between jugular and portal infusion of GLP-1 (C). The jugular vein- (n=6) or portal vein-catheterized rats (n=6) were infused with GLP-1 (10 μg) from 15 min before dark to 25 min after dark at 7 μl/min for 40 min after overnight fasting. Infusion of GLP-1 into jugular vein suppressed food intake more potently than portal vein. Data were expressed as mean ± SEM, and analyzed using repeated two-way ANOVA followed by a Bonferroni's test. *, P<0.05.
Figure 2.
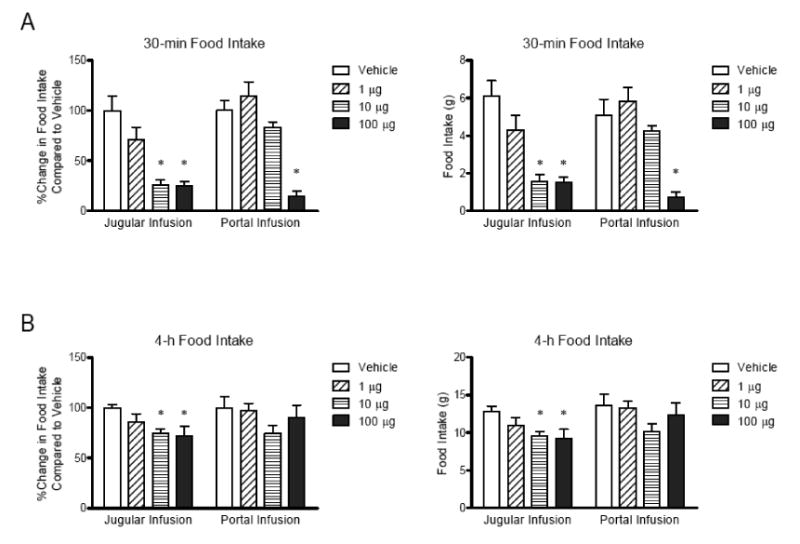
Comparison of the effect of jugular or portal infusion of GLP-1 on 30-min food intake (A) and 4-hour food intake (B). The jugular- or portal-catheterized rats were infused with GLP-1 (total 1, 10, and 100 μg) at 7 μl/min for total 40 minutes from 15 min before dark to 25 min after dark. The 30 min-food intake was measured at the end of GLP-1 infusion into jugular (n=6) or portal vein (n=6). The data were expressed as percent changes in food intake relative to vehicle or amount of food intake, and analyzed by two-way ANOVA followed by a Bonferroni's test. The jugular infusion of GLP-1 was significantly potent than portal infusion. *, P<0.05.
The effect of a GLP-1 receptor antagonist on food intake in either the jugular or portal vein
Previous studies have demonstrated effects of GLP-1r signaling on glucose regulation by blocking the receptor with peptide antagonists [5, 20]. To test the role of portal GLP-1r in tonic regulation of food intake, we infused dH-Ex (total 1, 10, and 100 μg) into the portal vein for 15 min before dark to see whether blockade of hepatic portal GLP-1 before eating would affect food intake in the following 30-minutes. There was no difference in food intake between animals given the GLP-1r antagonist or vehicle (Figure 3). We also infused dH-Ex into the portal or jugular veins at various doses beginning 10 min before returning food hopper for total 40 minutes during the dark (200 ng and 100 ug) or light (50 ng, 500 ng, 20 μg, and 50 μg) cycles in order to test whether blockade of GLP-1 receptors during the meal increased food intake. Similar to the pre-meal treatment with dH-Ex along, there was no difference in food intake when high or low doses of dH-Ex was infused into either portal or jugular vein during dark or light (Figure 4A and B). To test the specificity of the antagonist we infused a high dose (100 μg) of another commonly used GLP-1 receptor antagonist, Exendin-3 (9-39), into jugular and portal vein, but again observed no effect on subsequent food intake (data not shown). Taken together, these data indicate that blocking GLP-1 receptors in either the portal or jugular veins does not change food ingestion in rats.
Figure 3.
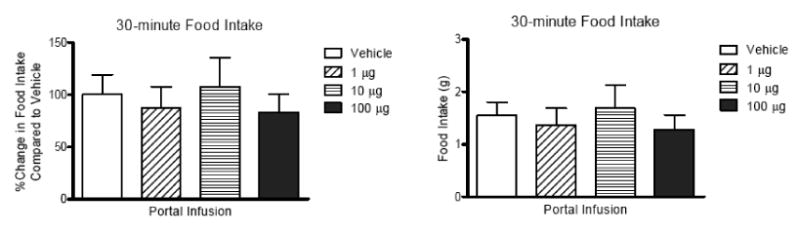
Effect of pre-blockade of hepatic portal GLP-1 receptors before eating on 30-min food intake during dark. The dH-Ex was infused into portal vein for 15 minutes before returning food hopper (n=10) and the weight of food hopper was measured at 30 minutes after dark. The data were expressed as percent changes in food intake relative to vehicle or amount of food intake. The pre-blockade of hepatic portal GLP-1 receptors did not significantly affect 30 min-food intake.
Figure 4.
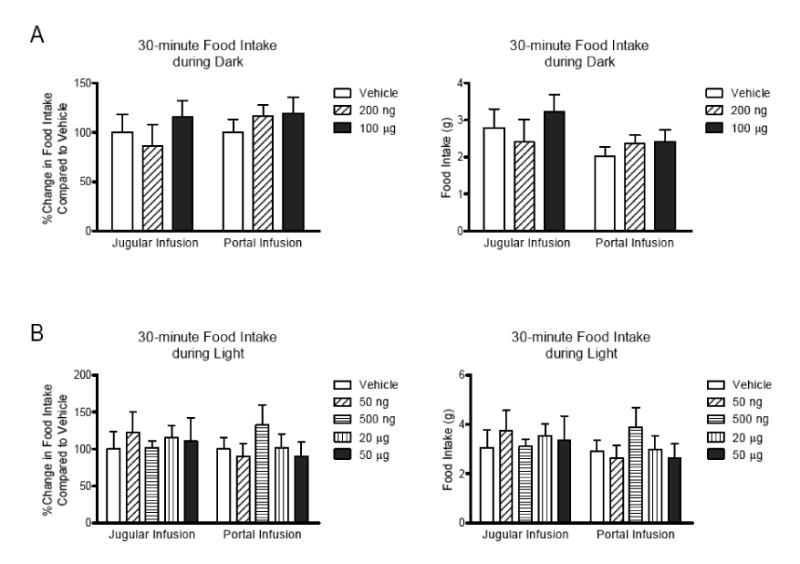
Effect of infusion of dH-EX into jugular or portal vein on 30-min food intake during dark and light. The dH-Ex was infused into either jugular or portal vein 10 minutes before returning food hopper and continued over total 40 minutes during dark or light. After finishing the infusion, the weight of food hopper was measured as 30 min-food intake. The data were expressed as percent changes in food intake relative to vehicle or amount of food intake. Both low and high doses of dH-Ex did not significantly change 30-min food intake during dark in rats infused into jugular (n=10) or portal vein (n=10) using repeated two-way ANOVA analysis (A). There was no significant effect of jugular (n=6∼8) or portal dH-Ex (n=6∼8) on food intake during light (B).
Discussion
The aim of this study was to determine whether GLP-1 receptors in the hepatic portal region play a role in regulating food intake. This goal was based on recent work demonstrating effects of portal GLP-1r signaling to regulate glucose metabolism [5, 20] and evidence that other compounds, such as glucagon and glucose suppress food intake when given into the portal vein [16-18]. In this study, we found that intrajugular infusion of GLP-1 actually decreased food intake more potently and persistently than intraportal infusion. Despite a significant action of peripheral GLP-1 to reduce food intake, portal and jugular infusion of two different GLP-1 receptor antagonists at various doses and in various food intake regimens showed no effect to increase food intake.
These data are surprising based on theoretical and experimental considerations. First, hepatic portal GLP-1 receptors are ideally suited to act as a monitor of GLP-1 secretion from the gut, and to integrate this signal with sensing of the nutrient composition of meals. The form of GLP-1 that activates the single GLP-1 receptor is extremely short lived in the plasma with a half life of approximately 1∼1.5 min [21, 22]. The result is that after meals, GLP-1 levels only rise 2 fold while other gut hormones such as GIP can rise as much as ∼10 fold [23]. However, hepatic portal GLP-1 receptors see much higher levels and larger fold changes since they see GLP-1 before it passes through the liver [5]. Second, good evidence links portal GLP-1r to activation of visceral afferent nerves. GLP-1r mRNA is expressed by neurons in the nodose ganglia, which innervate the viscera including the portal vein [5, 6]. In addition, hepatic portal infusion of GLP-1 results in increased firing of hepatic vagal afferents activity (Nakabayshi et al., 1996) that could signal satiety centers in the brainstem similar to other gut hormones such as CCK [24]. Finally, there is solid evidence linking stimulation of hepatic portal GLP-1 receptors to the regulation of glucose homeostasis likely via neural regulation of peripheral organs that regulate the production and disposal of glucose [5, 19]. Despite these strong circumstantial arguments, the current data simply provide no support to the hypothesis that hepatic portal GLP-1 receptors participate in the regulation of food intake.
The GLP-1 system has a number of key components. In addition to being produced in L-cells in the ileum, GLP-1 is also produced in a discrete population of neurons within the nucleus of the solitary tract [2]. GLP-1 receptors are also found in a number of sites in the CNS including regions linked to the regulation of food intake [7]. In fact, strong evidence links CNS GLP-1 receptors to the regulation of food intake. Administration of GLP-1 into the CNS potently inhibits food intake, and administration of GLP-1 antagonists stimulates feeding [11, 14, 25-28]. However, it is highly unlikely that GLP-1 administration into either jugular or hepatic portal vein causes significant activation of central GLP-1 receptors. However, there is good evidence that peripheral GLP-1 receptors can regulate food intake. GLP-1 and Exendin-4, a GLP-1r agonist, potently reduce food intake when given intravenously in humans and rats [12, 13, 29-32]. A key question from our data is where jugular administration of GLP-1 exerts its effect to regulate food intake. Given that jugular infusion is significantly more potent than hepatic portal infusion, it is unlikely that those receptors lie in the hepatic portal region. One potential explanation is that the key receptors mediating signals from intestinal L-cells are actually in the gut mucosa where they would be exposed to the highest possible levels of GLP-1. This is how the effects of CCK are thought to be mediated [33]. Greater amounts of GLP-1 might reach GLP-1r in the intestine when given in the central venous circulation rather than the portal vein because of avoidance of high rates of metabolism in the liver.
In the studies of the role of hepatic portal GLP-1 receptors in glucose homeostasis, blockade of GLP-1 receptors was more effective than administration of GLP-1 in demonstrating effects [5, 20]. One hypothesis raised from these findings is that hepatic portal GLP-1 receptors may be saturated even at low levels of circulating GLP-1, such that additional increases in GLP-1 have minimal effects. By extension, blockade of hepatic portal GLP-1 receptors have more demonstrable effects. This led us to posit that the best way to uncover effects of GLP-1 on food intake was to block its action specifically in the portal vein. However, even with use of multiple doses of two different GLP-1 antagonists in multiple feeding paradigms, we found no effect of a GLP-1 antagonist to increase food intake when given into the hepatic portal either before or during bouts of ingestion. Interestingly, we also failed to see an effect of GLP-1r blockade when the antagonist was delivered via the jugular vein, and again raises the question of the relevant receptor populations most critical to GLP-1 regulation of food intake.
In summary, the present studies do not support the hypothesis that hepatic portal GLP-1 receptors are key regulators of food intake. Future work will be required to identify which receptor populations are important for the actions of both endogenous GLP-1 and exogenous GLP-1 analogues such as exendin-4 to suppress food intake.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54890 and DK56863. We would like to thank Eileen E. Elfers for technical instruction of portal vein catheterization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16:97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- 3.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–7. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 4.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 5.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–73. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808–13. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 9.Davis HR, Jr, Mullins DE, Pines JM, Hoos LM, France CF, Compton DS, Graziano MP, Sybertz EJ, Strader CD, Van Heek M. Effect of chronic central administration of glucagon-like peptide-1 (7-36) amide on food consumption and body weight in normal and obese rats. Obes Res. 1998;6:147–56. doi: 10.1002/j.1550-8528.1998.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 10.Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140:244–50. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 11.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 12.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1695–706. doi: 10.1152/ajpregu.00870.2004. [DOI] [PubMed] [Google Scholar]
- 13.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50:2530–9. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 14.Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gomez R, Munoz RM, Eng J, Blazquez E. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism. 2000;49:709–17. doi: 10.1053/meta.2000.6251. [DOI] [PubMed] [Google Scholar]
- 15.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 16.Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264:R116–22. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- 17.Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80. doi: 10.1038/197079b0. [DOI] [PubMed] [Google Scholar]
- 18.Tordoff MG, Tluczek JP, Friedman MI. Effect of hepatic portal glucose concentration on food intake and metabolism. Am J Physiol. 1989;257:R1474–80. doi: 10.1152/ajpregu.1989.257.6.R1474. [DOI] [PubMed] [Google Scholar]
- 19.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1449–54. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 20.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–8. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- 21.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–31. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 22.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–70. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 23.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 24.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–7. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 25.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–6. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–9. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 27.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7--36) amide inhibits food and water intake in rats. American Journal of Physiology. 1996;271:R848–56. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 28.Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, Bernstein IL, Woods SC, Seeley RJ. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol. 1996;271:R1096–100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- 29.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 30.Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–6. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2004;36:867–76. doi: 10.1055/s-2004-826178. [DOI] [PubMed] [Google Scholar]
- 33.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007;7:570–4. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


