SUMMARY
Accumulation of unfolded protein within the endoplasmic reticulum (ER) lumen attenuates mRNA translation through activation of the protein kinase PERK and subsequent phosphorylation of eukaryotic initiation factor 2 on Ser51 of the alpha subunit (eIF2α). Genetic disruption of the PERK/eIF2α pathway in humans and mice produces severe pancreatic beta cell deficiency and post-natal lethality. To elucidate the role of eIF2α phosphorylation in beta cells, we have rescued the lethality of homozygous eIF2α Ser51Ala mice by expression of a loxP-flanked wild-type eIF2α transgene. Beta cell-specific transgene deletion to prevent eIF2α phosphorylation caused a severe diabetic phenotype due to heightened, unregulated proinsulin translation, defective intracellular trafficking of secretory and plasma membrane proteins, increased oxidative damage, reduced expression of stress response and beta cell-specific genes, and apoptosis. However, glucose intolerance and beta cell death in these mice were attenuated by antioxidant treatment. We conclude that phosphorylation of eIF2α coordinately attenuates mRNA translation, prevents oxidative stress, and optimizes ER protein folding to support insulin production in the beta cell. These findings that show increased proinsulin synthesis causes oxidative stress leading to beta cell failure may reflect events in the beta cell loss associated with insulin resistance in type 2 diabetes.
INTRODUCTION
The rough ER is a network of interconnected tubules, vesicles, and sacs that serves many specialized functions in the cell including calcium storage and gated release, biosynthesis and folding of membrane and secretory proteins, and production of lipids and sterols. Cells have a unique adaptive capability to adjust and coordinate the ER protein-folding capacity with the protein-folding load. One profound example of physiological fluctuations in the protein-folding load is the unique translational response of pancreatic beta cells to variations in blood glucose. Proinsulin synthesis imposes a heavy biosynthetic burden upon the beta cell as approximately 1 × 106 proinsulin molecules are synthesized per minute upon glucose stimulation (Schuit et al., 1988). Conditions that interfere with productive protein folding in the ER lumen result in the accumulation of unfolded or misfolded (herein referred to as misfolded) proteins, a condition known as ER stress. Accumulation of misfolded proteins within the lumen of the ER in beta cells occurs as a consequence of increased proinsulin translation, expression of inherently misfolded proinsulin, defects in adaptive response signaling pathways, and physiological stimuli including glucose, cytokines, lipids, and nitric oxide (Scheuner and Kaufman, 2008).
The unfolded protein response (UPR) is an adaptive signaling program that evolved to resolve the accumulation of misfolded proteins in the ER (Ron and Walter, 2007; Rutkowski and Kaufman, 2007). The UPR increases ER protein-folding capacity, decreases protein synthesis to reduce the folding demand, and enhances clearance of misfolded proteins. When these mechanisms fail to reestablish ER homeostasis, numerous death signaling pathways are activated (Kim et al., 2008). Recent studies suggest there is a close interrelationship between misfolded protein-mediated cell death and oxidative stress (Malhotra and Kaufman, 2007). During disulfide bond formation in the ER, electrons are transferred from cysteine residues through protein disulfide isomerase (PDI) and ER oxidoreductase 1 (ERO1) to reduce molecular oxygen and form hydrogen peroxide (Tu and Weissman, 2004). In addition, glutathione is consumed in the process of reducing mispaired disulfide bonds (Cuozzo and Kaiser, 1999) that may deplete the cellular glutathione pool that is required to neutralize reactive oxygen species (ROS) and prevent oxidation of cytosolic proteins. Therefore, cells with a high biosynthetic load, defective UPR, or inadequate ER-associated protein degradation (ERAD) are vulnerable to oxidative stress (Harding et al., 2003; Malhotra et al., 2008; Merksamer et al., 2008; Song et al., 2008). ER to mitochondria calcium crosstalk may also compromise mitochondrial function and induce oxidative stress in response to protein misfolding (Sharaf El Dein et al., 2009). The studies suggest the UPR has evolved as a protective mechanism to prevent oxidative stress associated with protein folding in the ER.
In metazoan cells, the UPR is signaled through three ER transmembrane sensors of unfolded protein: inositol-requiring 1α (IRE1α), activating transcription factor 6α (ATF6α), and PKR-like ER kinase (PERK) (Ron and Walter, 2007; Rutkowski and Kaufman, 2007). IRE1α has ER stress-regulated kinase/endoribonuclease activities that initiate unconventional splicing of the mRNA encoding the X box binding protein 1 (XBP1) transcription factor. ATF6α is a basic region/leucine zipper transcription factor that is released from the membrane by regulated proteolytic cleavage for trafficking to the nucleus. XBP1 and ATF6α act in concert to induce transcription of genes to enhance protein folding and degradation. However, to date, there is no direct evidence that either IRE1α/XBP1 or ATF6α are important for the function and/or viability of pancreatic beta cells in vivo (Lee et al., 2005; Wu et al., 2007; Yamamoto et al., 2007).
The third ER stress transducer, PERK, is a protein kinase that phosphorylates eIF2α at Ser51, thereby reducing the level of functional eIF2B to inhibit general mRNA translation (Hershey, 2000). The PERK/eIF2α pathway is not only responsible for mRNA translation attenuation, but also plays a key role in transcriptional control (Harding et al., 2000; Scheuner et al., 2001). Phosphorylation of eIF2α is required for the selective translation of some mRNAs, such as activating transcription factor 4 (Atf4) mRNA (Harding et al., 2003). It is proposed that ATF4 promotes cell survival by inducing expression of genes for amino-acid biosynthesis and transport, anti-oxidative stress responses, and protein folding and secretion (Harding et al., 2003).
Four mammalian protein kinases, PERK, GCN2, PKR, and HRI, phosphorylate eIF2α at Ser51 in response to different stress conditions (Sonenberg and Hinnebusch, 2009). However, the biological significance of PERK/eIF2α signaling is underscored by the observation that mutations in Perk reduce beta cell mass and cause neonatal insulin-dependent diabetes in humans (Delepine et al., 2000). Furthermore, null mutations in Perk and mutation at the Ser51 phosphorylation site in eIF2α cause beta cell deficiency and diabetes in murine models (Harding et al., 2001; Scheuner et al., 2005; Scheuner et al., 2001; Zhang et al., 2002). However, the mechanism whereby PERK-mediated translational control through eIF2a phosphorylation prevents beta cell failure is unknown. To elucidate the role of PERK/eIF2α signaling in beta cells, we have engineered mice with conditional homozygous eIF2α Ser51/Ala (herein, A/A) mutation. In the absence of eIF2α phosphorylation, mRNA translation was not repressed by low glucose or ER stress. The conditional loss of eIF2α phosphorylation and unrestricted translation in differentiated beta cells was sufficient to disrupt ER function and cause oxidative damage. The consequences were defective insulin trafficking from the ER to the Golgi complex and reduced expression of beta cell-specific transcription factors. These events lead to beta cell failure and apoptosis that result in severe glucose intolerance and hyperglycemia.
RESULTS
Ubiquitous expression of a floxed wild-type (wt) eIF2α transgene rescues lethality of homozygous eIF2αA/A mice
Beta cell-specific wild-type (wt) eIF2α expression restored beta cell mass and ultrastructure in A/A embryos affirming that beta cell deficiency in the homozygous mutant mice was due to an absence of eIF2α phosphorylation in beta cells and not another cell type that influences beta cell function (Figure S1). However, it did not prevent the postnatal lethality of A/A mutation (Table S1), suggesting neonatal viability requires eIF2α phosphorylation in cell types additional to the beta cell. To determine whether ubiquitous expression of wt eIF2α could prevent the postnatal lethality of homozygous A/A mice and study why beta cells fail in the absence of eIF2α phosphorylation, mice were derived that express wt eIF2α from a floxed wt eIF2α transgene (fTg) (Experimental Procedures and Supplemental Experimental Procedures). The transgene was designed to coordinately induce green fluorescence protein (EGFP) expression upon Cre recombinase-mediated deletion of the eIF2α coding region. Transgenic mice ubiquitously expressing fTg mRNA from a single copy transgene (Figure S2A–S2D) were bred with heterozygous S/A mice (Supplemental Experimental Procedures). Genotype and phenotype analyses of 1–2 month old offspring demonstrated that homozygous A/A mice carrying the fTg (A/A;fTg/0) were viable (Table S2), had normal body mass (Table S3), and were morphologically indistinguishable from control heterozygous S/A littermates (data not shown). Western blot analysis of islets revealed that total eIF2α protein expression was increased 2-fold in A/A;fTg/0 islets compared to S/A islets (Figure S2E–S2F). Glucose tolerance tests (GTT), insulin production, and ultrastructural analyses indicated that the beta cells from homozygous A/A mice harboring the fTg were functional and morphologically indistinguishable from wt S/S and heterozygous S/A mice (Figure 2C, 2E, Figure S3, and Figure S6).
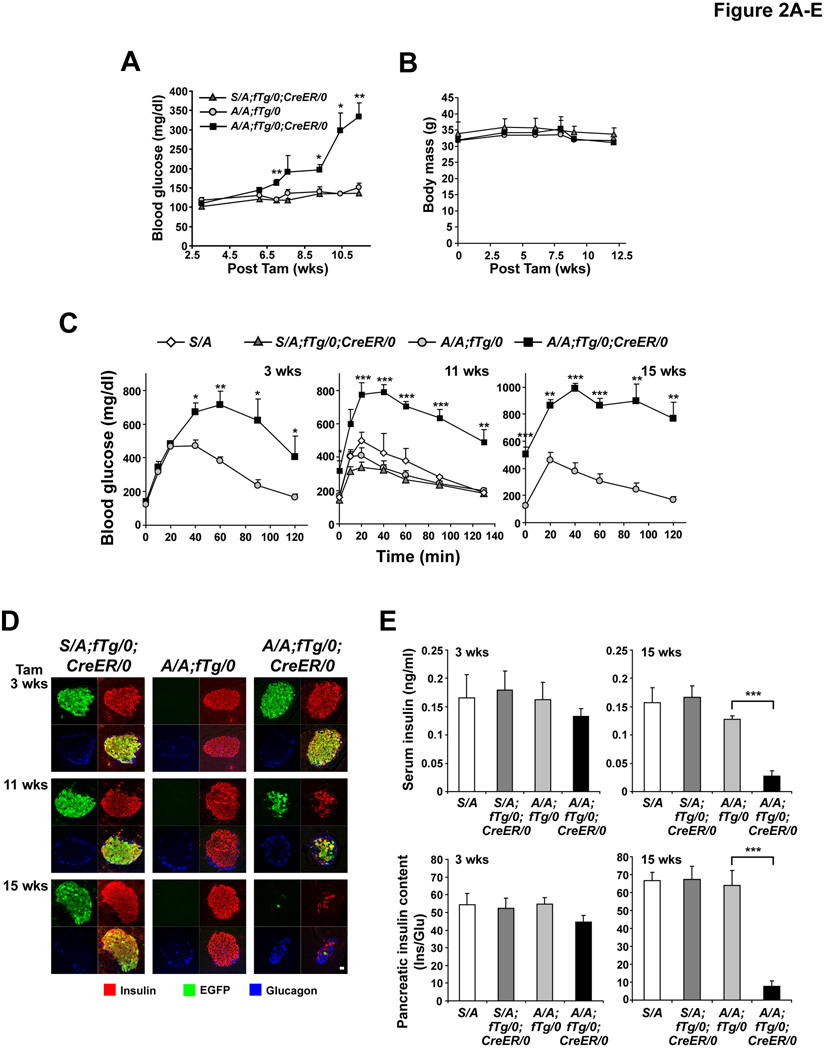
Figure 2. Beta cell-specific deletion of wt eIF2α fTg causes glucose intolerance due to reduced islet mass.
(A and B) At the indicated weeks after Tam injection, blood glucose (A) and body mass (B) measurements were performed in mice fasted for 5–6 hrs. n=5–6 mice per group. Significant differences between A/A;fTg/0 versus A/A;fTg/0;CreER/0 mice are indicated.
(C) GTTs were performed at 3–15 wks after Tam administration. n=5–6 mice per group. Significant differences between A/A;fTg/0 versus A/A;fTg/0;CreER/0 mice are indicated.
(D) Pancreatic tissue sections obtained from mice at the indicated times after Tam administration were triple immunostained for insulin, EGFP, and glucagon. Representative single channel fluorescence images are shown individually and merged (lower right image of each group). The scale bar represents 20 µm.
(E) Serum insulin levels and pancreatic insulin content were measured in mice at 3 and 15 wks after Tam injection. Insulin (Ins) measurements were normalized to glucagon (Glu). n=3–7 mice per group.
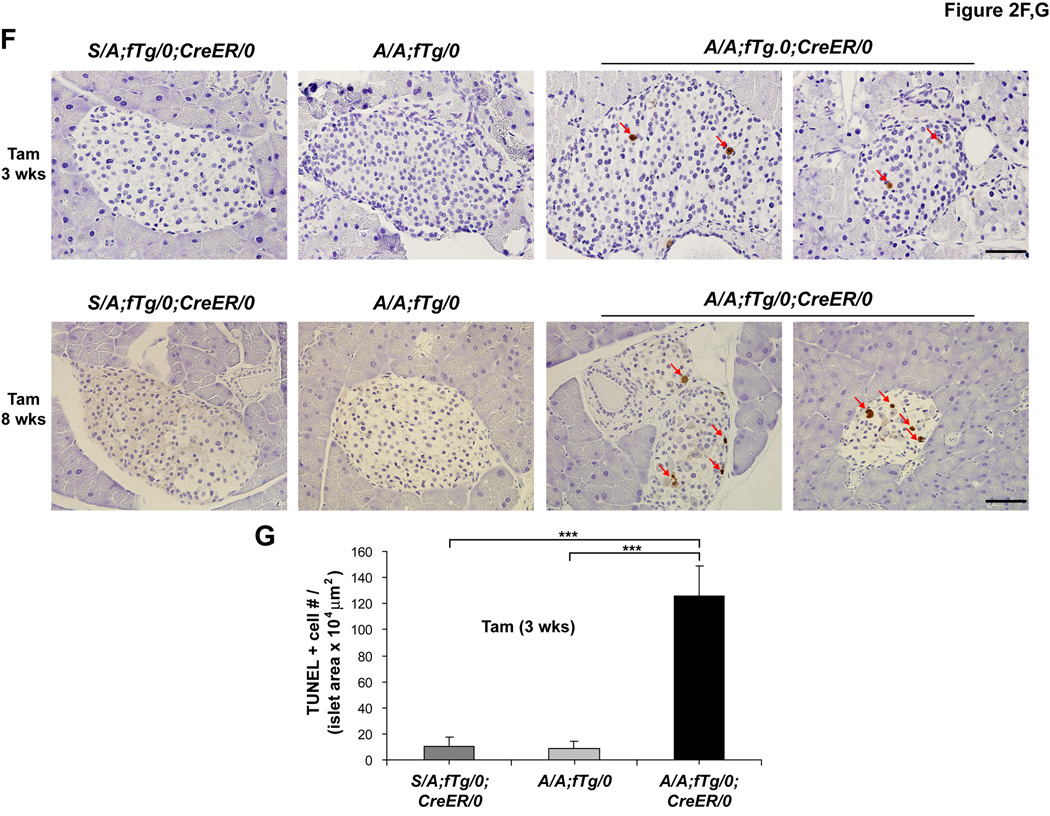
(F and G) TUNEL staining was performed on pancreatic sections obtained from mice at 3 and 8 wks after Tam injection and the number (#) of positive (+) cells per islet area was quantified. n = 5–7 mice per group. The scale bars represent 50 µm. Data are Mean ± SEM, (A–C), (E), and (G).
Deletion of the floxed wt eIF2α transgene in pancreatic beta cells is efficient
To study the role of eIF2α phosphorylation in pancreatic beta cells, the rescued A/A;fTg/0 mice were crossed with RIP–CreER (CreER/0) mice that express an estrogen receptor-Cre recombinase fusion protein (CreER) under control of the rat insulin II promoter (RIP) (Dor et al., 2004). Tamoxifen (Tam) administration in this system results in a rapid nuclear translocation of the CreER protein, permitting CreER-mediated recombination (Figure 1A). Triple immunostaining analyses of pancreatic sections for insulin, EGFP, and glucagon revealed that Tam treatment to delete the eIF2α coding sequence induced EGFP expression in over 95% of the beta cells in islets of S/A;fTg/0;CreER/0 mice (Figure 1B and data not shown).
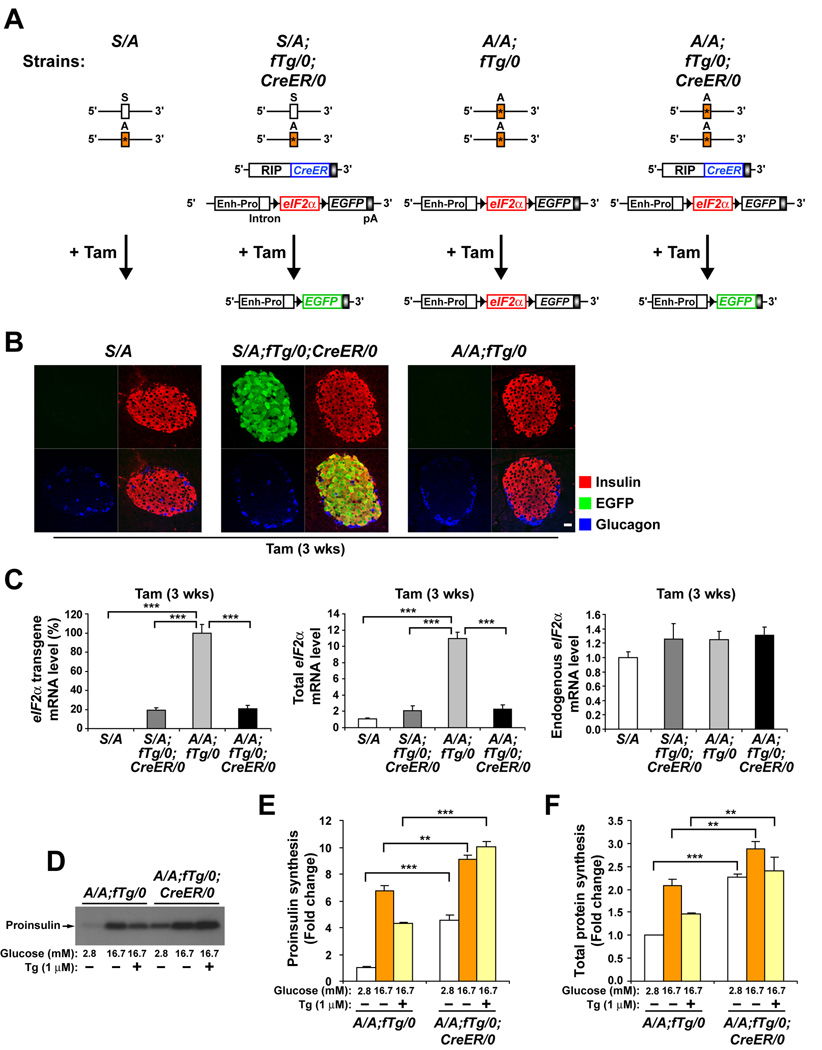
Figure 1. Ubiquitous expression of a floxed wt eIF2α transgene (fTg) in homozygous eIF2αA/A mice prevents lethality, preserves beta cell mass, and is required for glucose-regulated protein synthesis.
(A) Diagram depicts the four genotypes of mice used in these experiments. Heterozygous eIF2α S/A mice harbor Ser51Ala (*) mutation in exon 2 of one eIF2α allele. fTg/0 represents the floxed wt eIF2α transgene driven by the CMV- enhancer and chicken β-actin promoter (Enh-Pro). LoxP sequences (black arrowheads) allow excision of eIF2α coding sequence and coordinate expression of EGFP. CreER/0 represents the CreER recombinase transgene driven by the rat insulin II promoter (RIP). The deletion of fTg catalyzed by Tam treatment is represented.
(B) Tam-induced Cre recombinase deletion of wt eIF2α fTg is specific. Pancreatic tissue sections obtained from mice 3 wks after Tam administration were triple immunostained for EGFP, insulin, and glucagon and representative single channel fluorescence images are shown individually and merged (lower right image of each group). The scale bar represents 20 µm.
(C) Tam-induced Cre recombinase deletion of wt eIF2α fTg is efficient. The wt eIF2α fTg directs high-level conditional expression of wt eIF2α mRNA in beta cells. Tam was administered to all mice and after 3 wks total RNA was prepared from isolated islets. Results from quantitative RT-PCR analyses of transgenic, total, and endogenous eIF2α mRNAs are shown. n= 3 mice per group.
(D–F) eIF2α phosphorylation is required to attenuate protein synthesis in beta cells. Islets from tamoxifen-injected A/A;fTg/0 and A/A;fTg/0;CreER/0 mice were isolated and preincubated for 1 hr in Kreb’s buffer containing basal 2.8 mM glucose, followed by incuation for 1 hr in buffer containing 2.8 mM, 16.7 mM glucose, or 16.7 mM glucose and 1µM thapsigargin (Tg). Metabolic labeling with [35S] methionine and cysteine was performed during the last 20 min. of incubation as described in EXPERIMENTAL PROCEDURES. (D and E), Proinsulin synthesis was measured by immunoprecipitation from labeled extracts. (D) Immunoprecipitated samples were subjected to acrylamide gel electrophoresis and autoradiography. (E) Quantitation of labeled proinsulin by phosphorimaging is expressed as the fold change relative to A/A;fTg/0 islets incubated with 2.8 mM glucose. (F) Total protein synthesis was quantified by TCA precipitation of labeled extracts and results were normalized to total protein. n=3 samples per group. Data are Mean ± SEM, (C), (E), and (F).
The efficiency of Cre-mediated recombination was further characterized at the expression level using quantitative real-time RT-PCR of total islet RNA (Figure 1C). At 3 weeks after Tam injection, the amount of transgene-driven floxed eIF2α Tg mRNA was decreased to 20% in islets from both S/A;fTg/0;CreER/0 and A/A;fTg/0;CreER/0 mice compared to islets from A/A;fTg/0 mice that do not express CreER (Figure 1C, left panel). Similarly, although the amount of total eIF2α mRNA was increased 11-fold due to fTg expression, after Tam treatment the total amount of eIF2α mRNA was decreased to the level of endogenous eIF2αmRNA (Figure 1C, middle panel). Residual fTg mRNA detected in these islet preparations after Tam treatment may be due to fTg expression in other non-beta cell types of the islet preparations. The presence of fTg did not significantly alter mRNA expression from the endogenous eIF2α alleles (Figure 1C, right panel). These results collectively affirm that this CreER/loxP system specifically and efficiently removes the floxed wt eIF2α transgene in pancreatic beta cells.
eIF2α phosphorylation is required to attenuate protein synthesis in beta cells
To elucidate the impact of the eIF2α Ser51Ala mutation on regulation of protein synthesis, metabolic labeling was performed in islets treated with low glucose to repress translation, high glucose to stimulate translation (Itoh and Okamoto, 1980; Wicksteed et al., 2003), and thapsigargin (Tg) to induce ER stress, PERK activation, and eIF2α phosphorylation (Harding et al., 2000). When cultured in low glucose, proinsulin synthesis was increased 5-fold in A/A;fTg/0;CreER/0 islets compared to A/A;fTg/0 islets isolated at 10 weeks after Tam treatment (Figure 1D and 1E). Additionally, in high glucose, proinsulin translation in A/A;fTg/0;CreER/0 islets was significantly greater than in A/A;fTg/0 islets. Finally, thapsigargin partially inhibited glucose-stimulated proinsulin translation in A/A;fTg/0 islets, but not in A/A;fTg/0;CreER/0 islets (Figure 1D and 1E). These differences were also reflected in total protein synthesis (Figure 1F). Therefore, eIF2α phosphorylation-deficient beta cells exhibit an unrestricted, high rate of protein synthesis that is not repressed by low glucose or ER stress.
Glucose homeostasis and adult beta cell survival require translation attenuation
Tam administration to A/A;fTg/0 mice and to heterozygous S/A;fTg/0;CreER/0 mice did not alter fasting blood glucose levels up to 11 weeks because these mice retain the capacity for eIF2α phosphorylation in beta cells due to the floxed wt eIF2α transgene or wt eIF2α allele (S) (Figure 2A and 2C). In contrast, mild hyperglycemia in A/A;fTg/0;CreER/0 mice was detectable at ∼7 weeks after Tam treatment and became severe after 11 weeks (Figure 2A-2C). At 3 weeks after Tam injection, glucose clearance was abnormal in A/A;fTg/0;CreER/0 male mice and became increasingly more aberrant over 15 weeks (Figure 2C). As commonly observed in models of beta cell failure (Le May et al., 2006), the hyperglycemic phenotype of female A/A;fTg/0;CreER/ mice was milder than that observed in the male animals (Figure S3). These results indicate that a beta cell-specific defect in eIF2α phosphorylation compromises beta cell function and disrupts glucose homeostasis in adult mice.
To discern if loss of glycemic control was associated with a reduced beta cell mass, islet morphology, serum insulin levels, and pancreatic insulin content were evaluated at 3–15 weeks after Tam injection. Although there was not a significant difference at 3 weeks after Tam injection, after 6–15 weeks the number of cells that were immunoreactive with both insulin and EGFP antibodies was severely reduced in islets from A/A;fTg/0;CreER/0 mice compared to control mice, while there was no change in the mantle of glucagon-producing alpha cells (Figure 2D, third column, Figure 3D and Figure S4). Compared to control A/A;fTg/0 mice, pancreatic insulin content was decreased by ∼88% and blood insulin level was decreased by ∼78% in A/A;fTg/0;CreER/0 mice at 15 weeks after Tam injection (Figure 2E). These studies suggest that the beta cell mass of A/A;fTg/0;CreER/0 mice is reduced due to cell death.
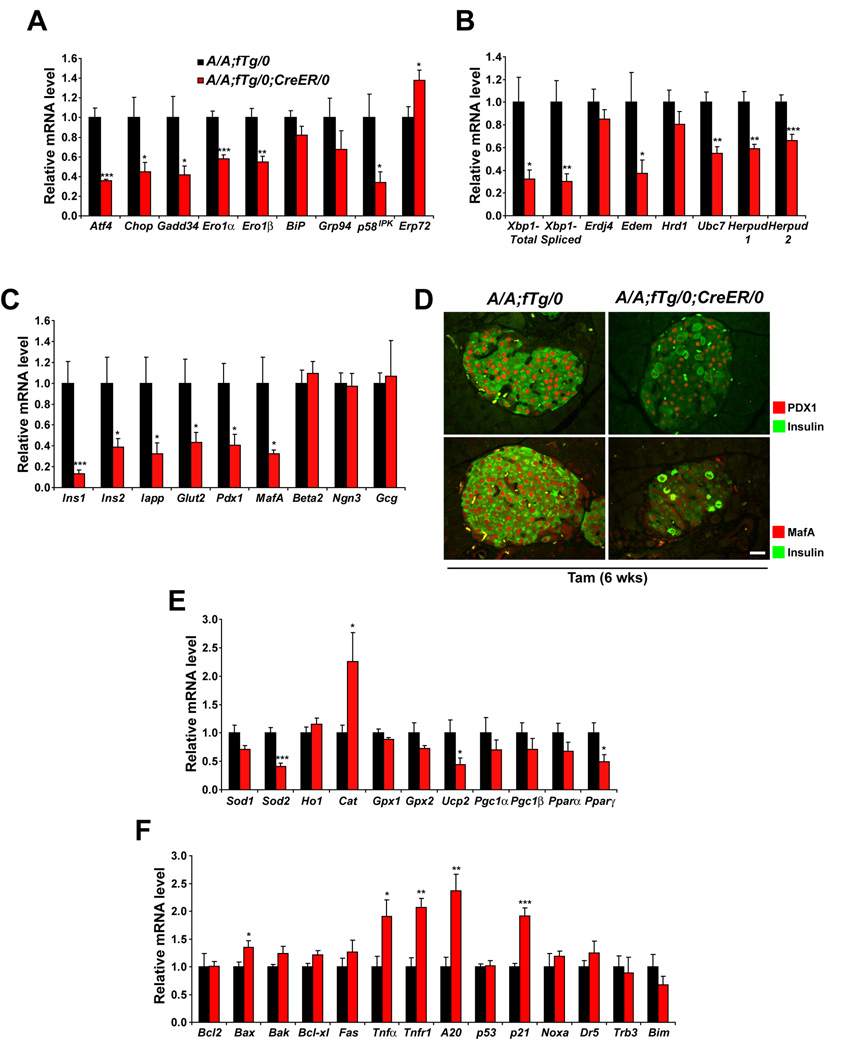
Figure 3. Translational control is required for optimal expression of UPR genes, antioxidant response genes, and beta cell-specific genes.
(A–C, E, and F) Total RNA was extracted from islets isolated from mice at 3 wks after Tam administration. Expression levels of mRNA were measured using quantitative RT-PCR. Data are Mean ± SEM, n= 5–6 mice per group.
(A) Genes regulated through eIF2α phosphorylation.
(B) Genes regulated through IRE1 α signaling and genes of ERAD machinery.
(C) Pancreatic beta or alpha cell-specific genes.
(D) PDX1, MafA, and insulin immunolabeling of pancreatic sections was conducted on samples isolated from mice at 6 wks after Tam administration. Representative merged images of PDX1 with insulin (upper panels) and MafA with insulin (lower panels) are shown. The scale bars represent 20 µm.
(E) Antioxidant response genes.
(F) Anti- and pro-apoptotic genes.
To analyze beta cell death more directly, TUNEL staining was employed. At 3 and 8 weeks after Tam injection, a significant number of apoptotic islet cells were detected in pancreata from A/A;fTg/0;CreER/0 mice, while TUNEL-positive cells were rarely detected within islets of S/A;fTg/0;CreER/0 or A/A;fTg/0 mice (Figure 2F and 2G). Therefore, beta cell apoptosis likely causes the development of, hypoinsulinemia, severe glucose intolerance, and hyperglycemia in these animals.
Translation attenuation is required for optimal expression of UPR genes, oxidative stress response genes, and beta cell-specific genes
To provide insight into the requirement for eIF2α phosphorylation in beta cells, we compared gene expression in islets from A/A;fTg/0;CreER/0 mice to A/A;fTg/0 mice. The expression of the majority of UPR genes, including Xbp1, was significantly decreased in islets from A/A;fTg/0;CreER/0 mice at 3 weeks after Tam treatment (Figure 3A and 3B). This is reminiscent of the defect in UPR gene induction observed in Perk-null and eIF2α A/A fibroblasts treated with pharmacological ER stress inducers (Calfon et al., 2002; Lu et al., 2004; Scheuner et al., 2001). We confirmed that the ER stress induction of a major proportion of UPR genes was also defective in homozygous eIF2α A/A immortalized hepatocytes (Figure S5). Therefore, optimal expression of many UPR-regulated genes apparently requires eIF2α phosphorylation in fibroblasts, hepatocytes, as well as beta cells in vivo. These findings indicate there may be defects in ER protein folding and/or degradation in eIF2α phosphorylation-deficient beta cells. In addition, these results indicate that ER stress is not necessarily associated with elevated UPR gene expression, especially when ER stress signaling pathways are defective.
Further analysis demonstrated that expression levels of the beta cell-specific transcription factors Pdx1 and MafA and their target genes Ins1, Ins2, Iapp, and Glut2 were significantly decreased in A/A;fTg/0;CreER/0 islets compared to A/A;fTg/0 islets (Figure 3C and 3D). In contrast, the expression levels of Beta2, Ngn3, and the alpha cell-specific gene Gcg (glucagon) were not altered in A/A;fTg/0;CreER/0 islets. In addition, the expression levels of the anti-oxidant response genes Sod2, Ucp2, and Pparγ were decreased in A/A;fTg/0;CreER/0 islets compared to A/A;fTg/0 islets (Figure 3E). Finally, the expression of Tnfα, Tnfr1, and A20 and p21Waf/Cip downstream in TNFα signaling were increased in A/A;fTg/0;CreER/0 islets (Figure 3F). Importantly, this indicates that unabated protein synthesis is sufficient to initiate a proinflammatory response in the islet. In summary, the gene expression analyses suggest that eIF2α phosphorylation may be required to regulate expression of genes that limit oxidative stress and preserve beta cell function.
ER distention and mitochondrial damage occur in the absence of translational control
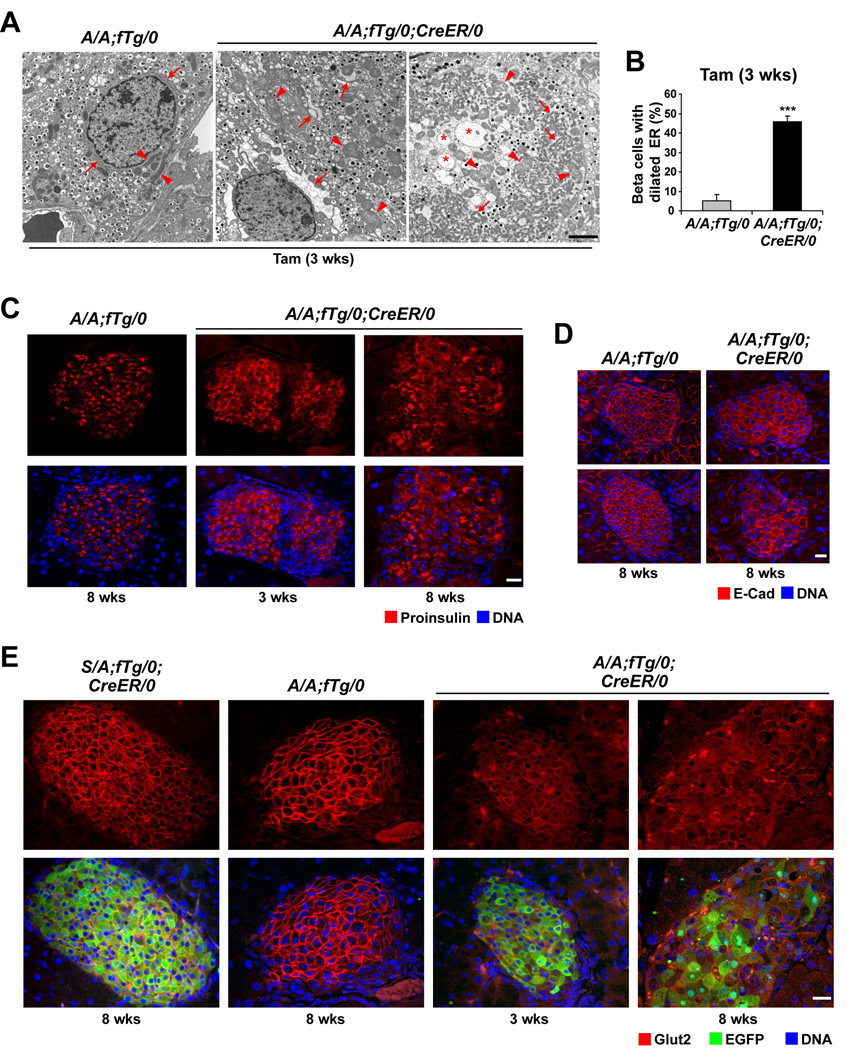
The increased protein synthesis and reduced expression of ER chaperones suggested the protein-folding load may exceed the protein-folding capacity of the ER, leading to ER stress in cells that lack eIF2α phosphorylation. Ultrastructural analysis of islets from A/A;fTg/0;CreER/0 mice at 3 weeks after Tam injection revealed a mixed population of beta cells with 46% (Figure 4B) exhibiting abnormal ER morphologies (arrows in Figure 4A and Figure S6A) indicative of ER stress (Scheuner et al., 2005). However, at 3 weeks after Tam treatment, the insulin granule content was not detectably reduced (Figure 4A, middle panel, and Figure S6A). At 11 weeks after Tam injection, more extensive abnormalities including ER distention, low electron density vesicular structures, and reduced granule number were observed, coincident with markedly diminished beta cell mass in the A/A;fTg/0;CreER/0 mice (Figure S6C and S6D). These findings indicate that ER distention and dysfunction precedes the loss of secretory granule content.
Figure 4. Translation attenuation is required for ER and mitochondrial integrity and proper protein trafficking.
(A and B) TEM was performed on pancreatic sections from A/A;fTg/0 and A/A;fTg/0;CreER/0 mice at 3 wks after Tam administration and representative images are shown. In the A/A;fTg/0 image, arrows indicate normal ER regions and arrowheads demark normal mitochondria. In A/A;fTg/0;CreER/0 images, arrows indicate regions of severe ER distention, asterisks indicate abnormal structures of low electron density, and arrowheads demark swollen mitochondria. The scale bars represent 2 µm. (B) The percentage of beta cells with dilated ER at 3 wks was quantified. Data are Mean ± SEM, n = 5–7 mice per group.
(C–E) Pancreata from mice at 3 and 8 wks after Tam administration were analyzed by immunofluorescence for proinsulin (C), E-Cad (D), and GLUT2 and EGFP (E). The scale bars represent 20 µm.
Interestingly, we observed abnormally large or oval mitochondria in beta cells from A/A;fTg/0;CreER/0 mice at 3 weeks after Tam treatment (arrow heads in Figure 4A, Figure S6A, and Figure S6B). Most of the beta cells that had distended ER in islets from transgene-deleted A/A;fTg/0;CreER/0 mice also displayed swollen mitochondria with disrupted cristae. In addition, some beta cells were extraordinarily enlarged (1 or 2 beta cells per islet in ∼40% of the islets within pancreata of A/A;fTg/0;CreER/0 mice, Figure 4A, third panel, and Figure S6B) with ER distention and mitochondrial swelling as observed in type 3B (cytoplasmic type) cell death (Clarke, 1990). The ultrastructural analyses demonstrate that translational control through eIF2α phosphorylation is essential to preserve the structural integrity of the ER and to prevent mitochondrial damage.
Proteins are mislocalized in the absence of eIF2α phosphorylation
The abnormal ER observed in eIF2α phosphorylation-deficient beta cells motivated us to analyze the subcellular localization of proinsulin, E-cadherin (E-cad), and GLUT2 as proteins that traffic the secretory pathway en route to secretory granules and the plasma membrane. Immunofluorescence analysis demonstrated that proinsulin was confined to the perinuclear area of beta cells in S/A;fTg/0;CreER/0 (data not shown) and A/A;fTg/0 pancreata, a distribution that corresponds to the Golgi apparatus (Zhu et al., 2002) (Figure 4C, left). In contrast, the localization of proinsulin in beta cells from A/A;fTg/0;CreER/0 mice at 3 and 8 weeks after Tam treatment was less restricted, where it appeared intracellularly, both diffusely and in large aggregates (Figure 4C, middle and right), possibly reflecting accumulation of proinsulin in an expanded ER compartment, as suggested by the ultrastructural analyses (Figure 4A). In addition, there was increased intracellular localization and reduced plasma membrane localization of both E-cad (Figure 4D) and Glut2 (Figure 4E) in beta cells from A/A;fTg/0;CreER/0 mice compared to S/A;fTg/0;CreER/0 and A/A;fTg/0 mice at 3 and 8 weeks after Tam treatment. These findings support the hypothesis that eIF2α phosphorylation is required to limit translation and prevent misfolding and/or retention of secretory and plasma membrane proteins in the ER lumen.
eIF2α phosphorylation is required to limit oxidative and nitrosative damage in pancreatic beta cells
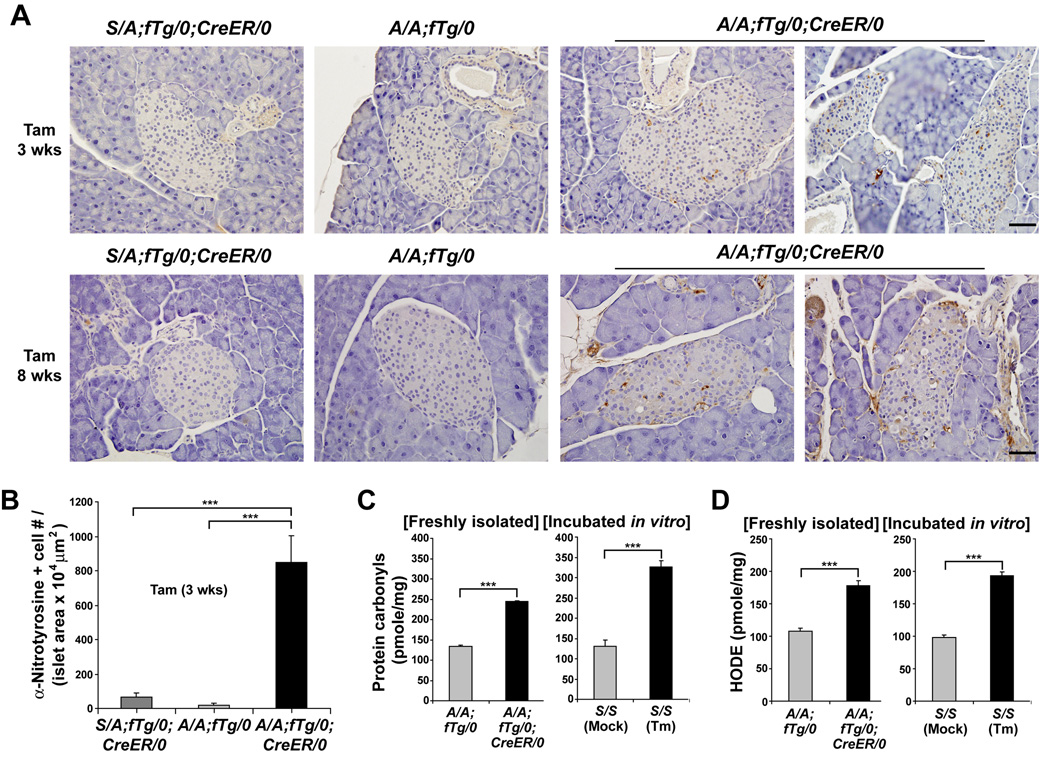
Since ultrastructural analyses suggested mitochondrial damage (Figure 4A) and gene expression analyses indicated a reduced antioxidant response (Figure 3E) in A/A;fTg/0;CreER/0 islets, we analyzed oxidative damage in control and mutant islets. Cellular protein damage due to oxidative stress can occur when superoxide reacts with nitric oxide leading to peroxynitrite modification of tyrosine residues. Anti-nitrotyrosine specific antibodies intensely stained islets of A/A;fTg/0;CreER/0 mutant pancreata compared to islets of A/A;fTg/0 and S/A;fTg/0;CreER/0 mice at 3 weeks after Tam injection (Figure 5A and 5B). Consistent with the nitrotyrosine immunostaining results, protein oxidation (carbonyls) and lipid peroxidation [hydroxyoctadecadienoic acid (HODE)] were significantly elevated in islet samples from A/A;fTg/0;CreER/0 mice compared to A/A;fTg/0 mice at a time prior to development of hyperglycemia (3 weeks after Tam treatment) (Figure 5C and 5D). In addition, direct treatment of wt S/S islets in vitro with tunicamycin (Tm) also increased protein oxidation and lipid peroxidation, indicating the accumulation of misfolded protein in the ER is sufficient to induce oxidative stress in the islets (Figure 5D). These data indicate that PERK-eIF2α signaling is required in pancreatic beta cells to limit oxidative damage that may result from excessive protein synthesis and accumulation of misfolded proteins in the ER. However, analysis of Atf4-null mice demonstrated that ATF4 is not required for beta cell function, suggesting that the beta cell requirement for eIF2α phosphorylation is not mediated through selective translation of Atf4 mRNA (See Supplemental Result and Figure S7).
Figure 5. Translation attenuation is required to prevent oxidative stress in beta cells.
(A and B) Immunohistochemistry was performed to detect nitrotyrosine in pancreatic sections from mice at 3 and 8 wks after Tam administration. Representative images and quantitation of the number (#) of nitrotyrosine-positive (+) beta cells per islet area are shown. n = 5–7 mice per group. The scale bars represent 50 µm.
(C and D) Protein carbonyls (C) and HODEs (D) were quantified in extracts from islets freshly isolated from mice at 3 wks after Tam injection. n= 7 mice per group. Islets were isolated from wt S/S mice and in vitro incubated in the absence or presence of tunicamycin (Tm, 2 µg/ml) for 5 hrs prior to analysis of oxidation products. Data are Mean ± SEM, (B–D).
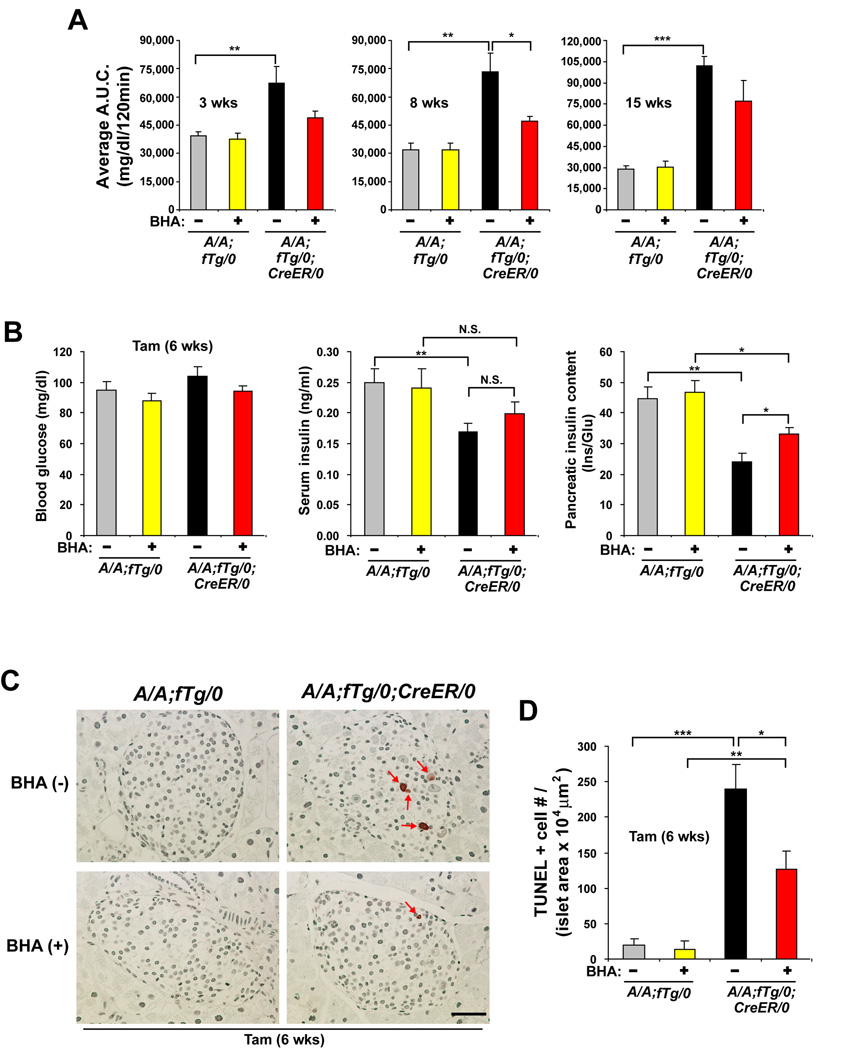
To assess the significance of oxidative stress in the beta cell failure that occurs in the absence of eIF2α phosphorylation, we analyzed the effect of antioxidant treatment in A/A;fTg/0;CreER/0 mice. Groups of A/A;fTg/0 and A/A;fTg/0;CreER/0 mice were fed with regular chow or chow supplemented with the antioxidant butylated hydroxyanisole (BHA) (Malhotra et al., 2008) just prior to and following Tam treatment. The early development of glucose intolerance in A/A;fTg/0;CreER/0 mice induced at 3–8 weeks after Tam treatment was reduced by BHA administration (Figure 6A and Figure S8). The protection persisted for 15 weeks, although the effect was less profound at later times. To determine how antioxidant treatment led to preserved glucose homeostasis in mice lacking phosphorylation of eIF2α in beta cells, we analyzed serum and pancreatic insulin levels and beta cell death. At 6 weeks after Tam treatment, the levels of serum and pancreatic insulin were modestly increased in A/A;fTg/0;CreER/0 mice fed BHA-containing chow compared to regular chow (Figure 6B). In addition, there was reduced apoptosis in islets from A/A;fTg/0;CreER/0 mice fed BHA-containing chow compared to regular chow (Figure 6C and 6D). These data indicate that deficiency of eIF2α phosphorylation in pancreatic beta cells causes oxidative damage-induced cell death that can be partially prevented by antioxidant treatment.
Figure 6. Antioxidant treatment preserves insulin production, reduces beta cell death and improves glucose intolerance in A/A;fTg/0;CreER/0 mice.
(A) GTTs were performed on mice fed chow supplemented with or without BHA for 3–15 wks after Tam treatment. The area under the curve (A.U.C.) from the GTTs was quantified. n=5–6 mice per group.
(B) Blood glucose (left panel), serum insulin (middle panel), and pancreatic insulin (right panel) were measured in A/A;fTg/0 and A/A;fTg/0;CreER/0 mice at 6 weeks after Tam injection with and without BHA feeding. Insulin measurements were normalized to glucagon (Glu). n=7–9 mice per group.
(C and D) TUNEL staining was performed on pancreatic sections obtained from mice at 6 wks after Tam injection and the number (#) of positive (+) cells per islet area was quantified. n = 7–9 mice per group. The scale bars represent 50 µm. Data are Mean ± SEM, (A), (B), and (D).
DISCUSSION
Phosphorylation of eIF2α at Ser51 is an evolutionarily conserved regulatory mechanism that efficiently attenuates the rate of translation in all eukaryotic cells, from protozoa to plants and humans. Thus, it is remarkable that mice harboring homozygous Ser51Ala mutation (A/A) at the phosphorylation site within eIF2α develop to birth, yet have embryonic beta cell failure and die perinatally (Scheuner et al., 2001). Through expression of a beta cell-specific wt eIF2α transgene we demonstrate that embryonic development of beta cell mass requires eIF2α phosphorylation. However, perinatal lethality was only rescued by ubiquitous expression of wt eIF2α. Through design of a conditional ubiquitously-expressed wt eIF2α transgene, we demonstrated that eIF2α phosphorylation is required for maintenance and function of differentiated beta cells. Our results demonstrate that Tam-induced beta cell-specific eIF2α fTg deletion in homozygous eIF2α Ser51Ala mice disrupts ER homeostasis, prevents polypeptide trafficking through the secretory pathway, causes mitochondrial damage and oxidative stress, depletes insulin-containing granules, and induces apoptosis in beta cells (Figure 7). These alterations were accompanied with reduced expression of UPR genes, antioxidative stress response genes, and beta cell-specific genes. The dire consequences of uncontrolled proinsulin synthesis in the absence of eIF2α phosphorylation bears a striking similarity to the beta cell failure that occurs in type 2 diabetes where the demand for insulin production is increased (Prentki and Nolan, 2006). We have described a unique model in which beta cell failure is associated with a primary increase in protein synthesis, which may recapitulate events that occur in beta cells as they increase proinsulin expression to compensate for insulin resistance.
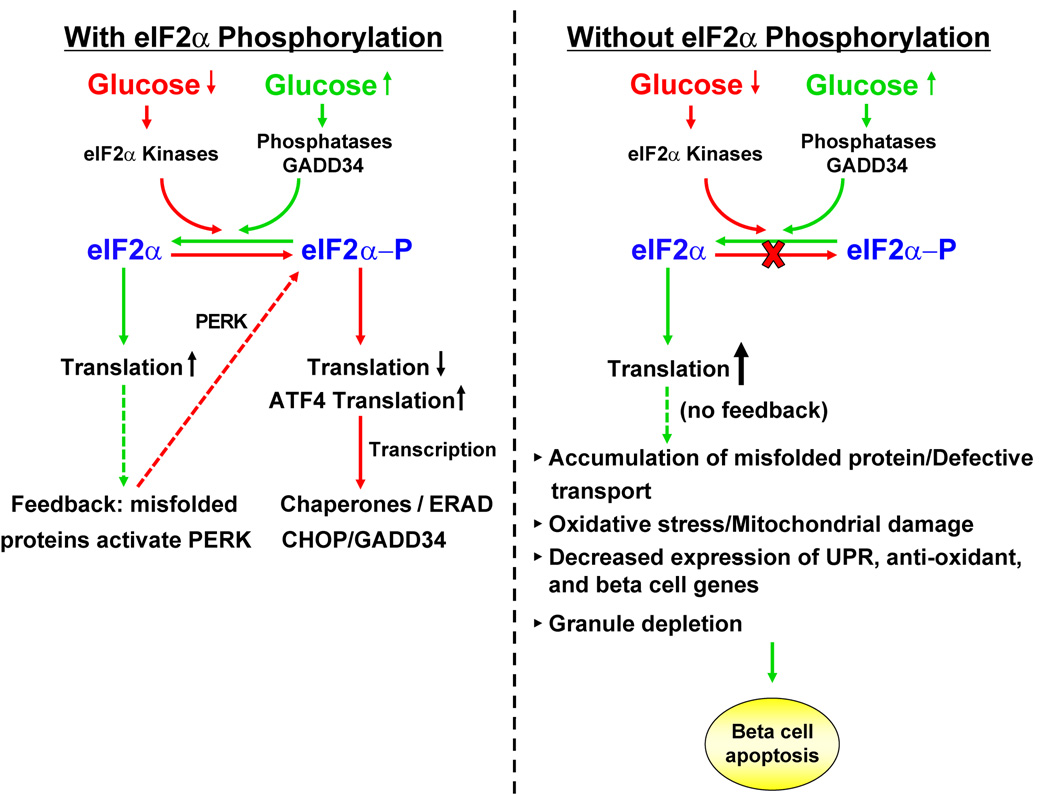
Figure 7. Mechanism for dysfunction and death of eIF2α phosphorylation-deficient beta cells.
(A) Fluctuations in glucose regulate eIF2α kinases and phosphatases to control eIF2α phosphorylation and the rate of mRNA translation in the beta cell. A feedback loop attenuates mRNA translation through PERK activation in response to misfolded proteins. Phosphorylation of eIF2α is required for translation of Atf4 mRNA that leads to transcriptional induction. Increased expression of GADD34 causes dephosphorylation of eIF2α to increase mRNA translation. The eIF2α A/A mutation prevents glucose repression of protein synthesis and causes unrestricted high rates of mRNA translation. Elevated protein synthesis leads to the accumulation of misfolded proteins in the ER. The absence of eIF2α phosphorylation reduces expression of UPR genes and antioxidant response genes, thereby exacerbating the protein-folding defect. Accumulation of misfolded proteins in the ER initiates production of ROS. ROS may inactivate beta cell-specific transcription factors, leading to insulin granule depletion. Beta cells ultimately succumb to apoptosis.
Compared to other cell types, beta cells have the unique ability to couple protein synthesis with changes in blood glucose. Recent studies suggest this regulation may be mediated through eIF2α phosphorylation (Gomez et al., 2008; Vander Mierde et al., 2007). Both eIF2α Ser51Ala mutation and Perk-deletion in beta cells causes a higher rate of translation that is not repressible under ER stress conditions (see Mol cell 2001, 7, 1153–1163, Figure 4, and our Figure 1D-1F). These findings indicate that PERK-mediated phosphorylation of eIF2α controls the maximal rate of translation under these conditions. However, under conditions of low glucose, translation was not repressed in islets from homozygous eIF2α Ser51Ala mice. This is in contrast to translation in Perk-/- islets that was repressed by low glucose (Harding et al., 2001). Therefore, although our studies show a requirement for eIF2α phosphorylation for translation attenuation in response to low glucose, it is unlikely that PERK is the sole eIF2α kinase responsible.
Our findings show that eIF2α phosphorylation is required for function and survival of differentiated beta cells and are consistent with the beta cell failure observed upon deletion of Perk in humans and mice (Delepine et al., 2000; Harding et al., 2001; Zhang et al., 2002). In contrast, analysis of murine beta cell-specific Perk deletion indicated that PERK is not required to maintain beta cell function (Zhang et al., 2006). It is possible that a partial reduction in eIF2α phosphorylation, as occurs in the deletion of any single eIF2α kinase gene, may not significantly affect protein synthesis to elicit a phenotype in beta cells. Alternatively, it is possible that beta cells adapt to Perk-deletion by compensatory mechanisms, possibly by increasing activity of an alternate eIF2α kinase(s). Additional mechanisms that could account for the differences between beta cell-specific Perk deletion and eIF2α Ser51Ala mutation, such as deletion efficiency and strain variation, are discussed in the Supplemental Discussion.
We have shown that unregulated protein synthesis is sufficient to initiate a series of events that leads to ROS production and beta cell failure. Multiple sources may contribute to ROS production in the absence of eIF2α phosphorylation. It is proposed that hyperglycemia and/or hyperlipidemia associated with insulin resistance contributes to ROS production in beta cells (Kajimoto and Kaneto, 2004; Robertson et al., 2007). We believe hyperglycemia is unlikely to initiate the ROS-dependent beta cell failure in our model because fed and fasted blood glucose levels were not significantly increased in the eIF2α fTg-deleted homozygous eIF2α A/A mice at 3 weeks after Tam injection (Figure 2A, 2C, and data not shown), a time at which increased oxidative damage was detected (Figure 5). Alternatively, ROS production may result from changes in gene expression that occur in the absence of eIF2α phosphorylation, such as reduced expression of genes encoding antioxidant functions (Sod2, Ucp2, and Pparγ) (Brand and Esteves, 2005; Jo et al., 2006) or increased expression of genes that function in TNFα signaling (Tnfα, Tnfr1, A20 and p21) that contribute to ROS production (O’Reilly, 2005; Xue et al., 2005). It is also possible that uncontrolled protein synthesis increases oxidative protein folding and/or protein misfolding, both of which may initiate ROS production (Harding et al., 2003; Malhotra et al., 2008; Merksamer et al., 2008; Teckman et al., 2004). Our ultrastructural analysis demonstrated mitochondrial swelling occurs in the eIF2α phosphorylation-deficient beta cells, suggesting altered mitochondrial function may play a role in ROS production. As oxidative stress is associated with beta cell failure in type 2 diabetes (Lowell and Shulman, 2005; Robertson et al., 2007), more extensive studies on the mechanism of ROS production in these mice should provide important insight into fundamental causes of beta cell failure in humans.
Ultrastructural analysis and immunolocalization of proinsulin, GLUT2, and E-cad revealed that in the absence of eIF2α phosphorylation, secretory proteins accumulate in the ER. This may be a consequence of uncontrolled protein synthesis or reduced protein folding and/or ERAD capacities as a consequence of decreased expression of UPR genes. Although it is surprising that both hepatocytes and beta cells require eIF2α phosphorylation for an intact UPR, this observation is consistent with earlier reports (Lu et al., 2004; Scheuner et al., 2001). Present studies are directed to understand why eIF2α phosphorylation is required for optimal UPR induction. Reduced expression of UPR genes could well contribute to the defective cargo export from the ER. For example, eIF2α phosphorylation-deficient beta cells have decreased expression of both Ero1α and Ero1β, which are required to maintain PDI in an active state to promote proper disulfide bond formation. Alternatively, eIF2α phosphorylation-deficient beta cells also express lower levels of mRNA encoding p58IPK, a cochaperone for BiP that protects cells from ER stress (Rutkowski et al., 2007). Indeed, p58IPK-knockout mice display pancreatic beta cell failure and diabetes (Ladiges et al., 2005).
Our studies show that eIF2α phosphorylation is required for beta cell function/survival. Analysis of mRNA expression and immunostaining demonstrated that eIF2α phosphorylation maintains expression of PDX1 and MafA, transcription factors necessary for beta cell differentiation, proliferation, and survival (Ackermann and Gannon, 2007; Ahlgren et al., 1998; Kaneto et al., 2008; Zhang et al., 2005). However, the Pdx1 and MafA genes do not contain any known ER stress-response elements (not shown) and are not known to be directly regulated by the UPR or eIF2α phosphorylation. It is possible that eIF2α phosphorylation promotes mRNA translation of a factor required to maintain differentiated beta cells. We have shown that ATF4, the most well characterized mRNA that requires eIF2α phosphorylation for translation, is not necessary for beta cell function (Figure S7). Therefore, selective translation of one or more alternate mRNAs may be required. Alternatively, eIF2α phosphorylation may be required to prevent excessive protein synthesis in the beta cell to limit protein misfolding and oxidative stress. Oxidative stress was previously implicated in decreasing expression of both PDX1 and MafA protein (Kaneto et al., 2008; Robertson et al., 2007). We attribute the loss of expression of beta cell specific-genes in the absence of eIF2α phosphorylation to decreases in PDX1 and MafA through one or more of these mechanisms.
We have described a conditional eIF2α Ser51Ala mouse model based on Cre recombinase-mediated excision of a supporting transgene that can be used to study the role of eIF2α phosphorylation in any tissue. This model will provide a platform for additional mechanistic studies on the role of eIF2α phosphorylation in many physiological processes, including metabolic disease, carcinogenesis and metastasis, hypoxia, infectious and inflammatory diseases, and functions of the nervous system.
EXPERIMENTAL PROCEDURES
Mouse strains and animal procedures
Details of founder analysis, strain interbreeding, and PCR genotyping are provided in Supplemental Experimental Procedures and Table S4. Heterozygous eIF2α Ser51Ala (S/A) mice were generated as previously described (Scheuner et al., 2005). Transgenic mice (β-Tg/0) expressing wt eIF2α (β-Tg) driven by the rat insulin II promoter (RIP) (Figure S1A) were derived as described in Supplemental Experimental Procedures. Conditional transgenic mice (fTg/0) that express excisable floxed wt eIF2α (fTg) in all tissues were derived from a plasmid construct (pCX-EGFP) containing cytomegalovirus enhancer, chicken beta-actin promoter, beta-actin intron, enhanced green fluorescence protein (EGFP), and a bovine globin polyadenylation signal (a gift of Dr. Masaru Okabe, Osaka University) (Okabe et al., 1997). Modifications were performed to flank the wt murine eIF2α cDNA with LoxP sequences and to position EGFP downstream of eIF2α (Figure 1A) as described in Supplemental Experimental Procedures. Mice (CreER/0) expressing an estrogen receptor-Cre recombinase fusion protein (CreER) under the control of the rat insulin II promoter (RIP) were kindly provided by Dr. Douglas A. Melton, Harvard University Medical Center (Dor et al., 2004).
Animals were housed at 21–23 °C with 12 hrs light and 12 hrs dark cycles in the Unit for Laboratory Animal Medicine (ULAM) at The University of Michigan with free access to water and standard rodent chow (LabDiet® Formulab Diet #5001). Butylated hydroxyanisole (BHA, Sigma)-containing chow was formulated by Research Diets, Inc. with 0.7% BHA in a base of LabDiet® Formulab Diet #5001. BHA diet study mice were housed pair-wise.
Deletion of the fTg in pancreatic beta-cells was induced by subcutaneous injection of tamoxifen (Tam, Sigma) dissolved in corn oil (20 mg/ml). Five doses of 8 mg Tam were administered over 2 weeks to 3–5 month old mice (Dor et al., 2004). In every experiment all genotypes of mice received Tam. Tam administration did not affect any of the parameters under analysis.
Blood glucose measurements and glucose tolerance testing (GTT) were performed in mice fasted 5–6 hr and serum and pancreatic insulin and glucagon content were measured in mice fasted overnight as described (Song et al., 2008). Measurements of body weight were performed between 3–5 pm.
All animal care and procedures were conducted according to the protocols and guidelines approved by The University of Michigan Committee on the Use and Care of Animals (UCUCA).
Islet isolation
Islets were isolated by intraductal collagenase P (Roche) perfusion, histopaque-1077 gradient (Sigma), and hand-picking (Sutton et al., 1986).
Gene expression studies
Semi-quantitative PCR by standard methods or quantitative real-time PCR were performed as previously described (Back et al., 2005; Song et al., 2008). Real-time PCR results were normalized to the levels of 18S rRNA or beta actin and Primer sequences are provided in Table S4.
Protein synthesis
Islets isolated from female mice (n=7 per genotype) at 10 wks after Tam injection were cultured for 22 hrs in RPMI 1640 medium containing 5.6 mM glucose, 10% fetal bovine serum, and 10 mM HEPES and pooled for selection of 80 size-matched islets per experimental condition. Glucose-stimulated protein synthesis was analyzed as previously described (Harding et al., 2001; Wicksteed et al., 2003). Analysis of proinsulin synthesis is described in the Supplemental Experimental Procedures.
Islet morphology, apoptosis, and oxidative damage
Pancreata were isolated, fixed with 10% buffered formalin or 4% paraformaldehyde, and embedded in paraffin. Sections were prepared and either stained with hematoxylin/eosin (H&E) for visualization of islet morphology by light microscopy or subjected to other histological procedures. TUNEL labeling was performed on tissue sections using ApopTag® Peroxidase In Situ Apoptosis Detection Kit (CHEMICON). Nitrated proteins were detected within islets of pancreatic sections with anti-nitrotyrosine antibody (Upstate) using Histostain®-plus Kit (2nd generation LAB-SA detection system, Zymed Laboratories). The sections were then counterstained by hematoxylin (Zymed Laboratories).
The measurements of protein carbonyls and HODEs in isolated islets were performed as previously described (Pennathur et al., 2005; Song et al., 2008).
Immunofluorescence
Immunofluorescence labeling was performed for detection of insulin, EGFP, glucagon, proinsulin, GLUT2, E-cad, PDX1, and MafA. Nuclear counterstaining with DAPI was performed in some instances. Multiple labeling procedures were performed serially and the following antibody combinations were utilized for antigen detections: Insulin - guinea pig anti-insulin antibody (Linco) and Texas Red-conjugated donkey anti-guinea pig secondary antibody (Jackson Immuno Research); EGFP - mouse anti-EGFP antibody (Clontech) and FITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, Inc.); Glucagon - rabbit anti-glucagon antibody (Linco) and Alexa Fluor 488- or 350-conjugated goat anti-rabbit secondary antibodies (Molecular Probes); Proinsulin or E-cad- mouse anti-proinsulin (HyTest Ltd.) or mouse anti-E-cad (BD Bioscience) and TRITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, Inc.); GLUT2, MafA, or PDX1- rabbit anti-GLUT2 (Santa Cruz), rabbit anti-MafA (Santa Cruz), or rabbit anti-PDX1 (Upstate) and Alexa 594-conjugated goat anti-rabbit secondary antibody (Molecular Probes).
Confocal and light microscope images were recorded digitally by camera and beta cell mass was measured using Image-Pro Plus software (Media Cybernetics) (Figure 2G, Figure 5B, and Figure 6D). All data were processed with Adobe Photoshop software (Mountain View).
Transmission electron microscopy (TEM)
TEM was performed on pancreas tissue as previously described (Scheuner et al., 2005). ER dilation was quantified (Figure 4B) at 7900X magnification and more than 150 beta cells for each genotype of mice were analyzed. Dilation (>0.3 µm diameter cisternae) visible more than 4 times in a cell was criteria for positive ER distention.
Statistical analysis
All data are presented as means ± SEM. Statistical significance of difference between groups was evaluated using a Student t-test or ANOVA one-way test (Tukey’s test). P < 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Supplemental Data include Supplemental Results, Supplemental Discussion, Supplemental Experimental Procedures, eight Figures, and four Tables.
ACKNOWLEDGEMENTS
We thank Janet Mitchell for assistance with manuscript preparation and the members of the Kaufman laboratory for critical input. Electron, confocal, and light microscopy were performed at the University of Michigan M.I.L. (Microscope and Image Analysis Lab). We thank Dotty Sorenson and Sasha Meshinchi of the M.I.L. for their expertise and assistance in these studies. We acknowledge The University of Michigan Transgenic Animal Model Core for preparation of transgenic mice.
This work was supported in part by NIH grant 5P60DK020572 and a Juvenile Diabetes Research Foundation Career Development Award (2-2003-149) (S.P), and NIH grants DK42394, HL52173, and HL057346 (R.J.K.). R.J.K. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol. 1999;1:130–135. doi: 10.1038/11047. [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J. 2008;410:485–493. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/-mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hershey JW. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, et al., editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; 2000. pp. 33–88. [Google Scholar]
- Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283:100–102. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- Jo SH, Yang C, Miao Q, Marzec M, Wasik MA, Lu P, Wang YL. Peroxisome proliferator-activated receptor gamma promotes lymphocyte survival through its actions on cellular metabolic activities. J Immunol. 2006;177:3737–3745. doi: 10.4049/jimmunol.177.6.3737. [DOI] [PubMed] [Google Scholar]
- Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–176. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Miyatsuka T, Kawamori D, Yamamoto K, Kato K, Shiraiwa T, Katakami N, Yamasaki Y, Matsuhisa M, Matsuoka TA. PDX-1 and MafA play a crucial role in pancreatic beta-cell differentiation and maintenance of mature beta-cell function. Endocr J. 2008;55:235–252. doi: 10.1507/endocrj.k07e-041. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. Embo J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA. Redox activation of p21Cip1/WAF1/Sdi1: a multifunctional regulator of cell survival and death. Antioxid Redox Signal. 2005;7:108–118. doi: 10.1089/ars.2005.7.108. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007;48:139–146. doi: 10.1007/s12013-007-0026-5. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS. The Role of p58IPK in Protecting the Stressed Endoplasmic Reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Kaufman RJ. The Unfolded Protein Response: A Pathway That Links Insulin Demand with {beta}-Cell Failure and Diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Mierde DV, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Schuit FC, In’t Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988;85:3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf El Dein O, Gallerne C, Deniaud A, Brenner C, Lemaire C. Role of the permeability transition pore complex in lethal inter-organelle crosstalk. Front Biosci. 2009;14:3465–3482. doi: 10.2741/3465. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Peters M, McShane P, Gray DW, Morris PJ. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986;42:689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- Teckman JH, An JK, Blomenkamp K, Schmidt B, Perlmutter D. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286:G851–G862. doi: 10.1152/ajpgi.00175.2003. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, Tsukamoto K, Beullens M, Kaufman RJ, Bollen M, Schuit FC. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology. 2007;148:609–617. doi: 10.1210/en.2006-1012. [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Alarcon C, Briaud I, Lingohr MK, Rhodes CJ. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. J Biol Chem. 2003;278:42080–42090. doi: 10.1074/jbc.M303509200. [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha Optimizes Long-Term Endoplasmic Reticulum Function to Protect Cells from Chronic Stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional Induction of Mammalian ER Quality Control Proteins Is Mediated by Single or Combined Action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002;99:10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include Supplemental Results, Supplemental Discussion, Supplemental Experimental Procedures, eight Figures, and four Tables.