Abstract
A series of artemisinin-vinyl sulfone hybrid molecules with the potential to act in the parasite food vacuole via endoperoxide activation and falcipain inhibition was synthesized and screened for antiplasmodial activity and falcipain-2 inhibition. All conjugates were active against the Plasmodium falciparum W2 strain in the low nanomolar range and those containing the Leu-hPhe core inhibited falcipain-2 in low micromolar range.
Keywords: antimalarial, artemisinin, FP-2, Vinylsulfone
Artemisinin, 1, a sesquiterpene lactone isolated from the Artemisia annua Chinese herb, and its analogues (e.g. artemether, 2, arteether, 3, and artesunate, 4) were a major breakthrough in malaria chemotherapy because they produce a very rapid therapeutic response, particularly against multidrug-resistant Plasmodium falciparum malaria.1,2 Despite the rapid clearance of parasites, the short half-lives of these compounds lead to many late recrudescences after monotherapy.3 Thus, artemisinin-based combination therapy (ACT) has now been recommended by the World Health Organisation as standard therapy for falciparum malaria.4
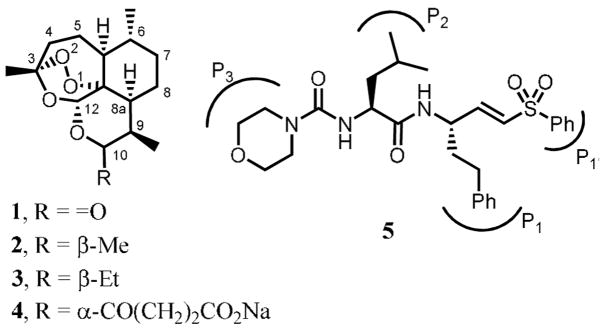
Cysteine proteases from malaria parasites are of particular interest as therapeutic targets due to their role in parasite development.5 P. falciparum expresses four cysteine proteases from the papain family known as falcipains, of which falcipain-2 (FP-2)6,7 and falcipain-3 (FP-3)7,8 are the most relevant as therapeutic targets. Peptidyl vinyl sulfones, e.g. 5, are potent irreversible inhibitors of falcipains, acting as Michael acceptors of the catalytic cysteine residue.9 Falcipain inhibitors have been shown to inhibit the development of cultured erythrocytic parasites by blocking the hydrolysis of host hemoglobin and to cure mice infected with lethal malaria infections.10
A concern regarding the use of protease inhibitors as antimalarials is that selection of drug-resistant mutants will eventually occur. Indeed, parasites resistant to a dipeptidyl vinyl sulfone have been selected in the laboratory, although this resistance was somewhat unstable.11 Thus, dipeptidyl vinyl sulfones are obvious candidates for combination antimalarial therapy as a strategy to retard the development of resistance. This information prompted us to design artemisinin-vinyl sulfone hybrid molecules with the potential to help prevent multi-drug resistance in P. falciparum malaria. It has recently been shown that hybrid molecules in which the two antimalarials are combined via a linker can offer an effective means of delivering these agents to the parasite site of action.12–14
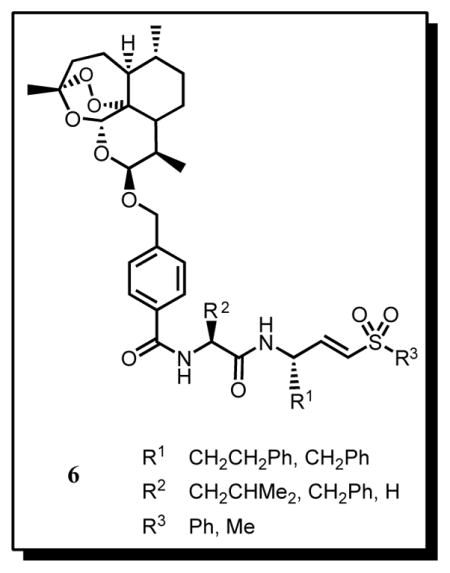
Structure-activity relationship (SAR) data for the inhibition of FP-2 reveals that peptidyl vinyl sulfones containing a Leu residue at the P2 position and a hPhe (homophenylalanine) at the P1 position, e.g. 5, are the most active, with IC50 values in the low-nM range.6,8,15,16 With this information in hand we designed hybrid molecules 6, in which the vinyl sulfone component is linked to the endoperoxide moiety via the N-terminus, using a 4-hydroxymethylbenzoic acid linker. In addition to the Leu-hPhe sequence, compounds 6 containing the Phe-hPhe and Phe-Phe moieties were also prepared based on the SAR against falcipains 16 and cruzain, 17 a related cysteine protease from Trypanosoma cruzi. The substituent at the P1′ position was a methyl or phenyl group.
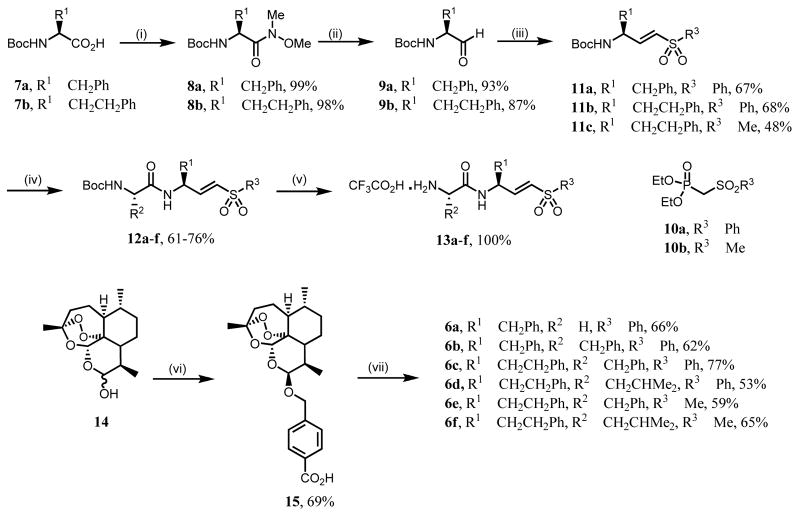
The synthesis of compounds 6 involved the preparation of aldehydes 9 containing the P1 residue, using Weinreb chemistry and the appropriate Nα-Boc-protected amino acids 7 (Scheme 1).18,19. The Horner-Wadsworth-Emmons reaction of 9 with the appropriate sulfones 10, prepared by oxidation of the corresponding sulfide with H2O2 in AcOH,18 afforded the amino acyl vinyl sulfones, 11. The Boc group was removed with trifluoroacetic acid (TFA) and the resulting trifluoroacetates were then reacted with the second Nα-Boc-protected amino acid and TBTU, to yield the Nα-Boc-protected dipeptidyl vinyl sulfones 12a–f. These were quantitatively deprotected to 13a–f with TFA. Finally, compounds 13 were converted in reasonable to good yields into the target compounds, 6a–f, by reaction with 15 (artelinic acid, synthesized from dihydroartemisinin, 14 20) and TBTU.21 The hybrid molecules 6a–f were isolated as single isomers as shown by the 1H-NMR spectra 21, which presented (i) only one singlet at δ ca. 5.4 ppm, corresponding to the H-12 signal and (ii) a small coupling constant for the H-10 signal at δ 4.9 ppm, J ca 4 Hz, indicative of a vicinal equatorial-axial coupling with H-9. This result, which is similar to that for precursor 15 (J = 3.7 Hz for the H-10 signal), is consistent with the β-isomer at C-10.
Scheme 1.
Reagents and conditions: (i) TBTU, TEA, HN(Me)OMe, DCM; (ii) LiAlH4, THF; (iii) 10a or 10b, NaH, THF; (iv) a) TFA, DCM; b) BocAAOH, TBTU, HOBt, TEA, DMF; (v) TFA, DCM; (vi) BF3.OEt2, HOCH2C6H4CO2H; (vii) 13a–f, TBTU, TEA, DMF, rt.
The semi-synthetic artemisinin derivatives 6a–f were screened for FP-2 inhibition and compared to dipeptidyl vinyl sulfones and E64 (Table 1). Selectivity assays were also carried out by testing compounds 6a–d against chabaupain-1 (CP-1), a cysteine protease from the murine parasite P. chabaudi.22 Inspection of the data in Table 1, shows that the Leu-hPhe sequence leads to a higher level of FP-2 inhibition than the Phe-hPhe (6d versus 6c and 6f versus 6e) or Phe-Phe counterparts (6c versus 6b), in line with the SAR for dipeptidyl vinyl sulfones.15,16 In contrast, a Phe residue at the P2 position is preferred for CP-1 inhibition (e.g. 6b and 6c), implying that this enzyme presents a different structural requirement for molecular recognition at this position. The presence of the endoperoxide moiety at the amino (P3) terminus of the dipeptide sequence decreases significantly the inhibitory potency. The IC50 ratio for compounds 6d, 12d and 5 is 1650:70:1, which suggests that voluminous groups at position P3 in dipeptidyl vinyl sulfones are deleterious for FP-2 inhibition. Finally, the effect of substituents at the P1′ position seems to be dependent on the dipeptide core. Interestingly, the reduction in activity observed when the phenyl group is exchanged for a methyl at P1′ in compounds with the Phe-hPhe moiety (6c versus 6e) is in line with that reported for Mu-Phe-hPhe vinyl sulfones.16
Table 1.
Effect of hybrid compounds 6, artemisinin (1), artelinic acid (15), dipeptidyl vinyl sulfones 5 and 12d and E64 on the inhibition of falcipain-2, chabaupain-1, and growth of P. falciparum W2 strain.
| Compound | R1 | R2 | R3 | IC50/μM |
IC50/nM |
|
|---|---|---|---|---|---|---|
| FP-2a | CP-1b | W2 P. falciparuma | ||||
| Artemisinin | -- | -- | -- | ND | ND | 12.0±1.97 |
| 15 | -- | -- | -- | ND | ND | 5.66±0.58 |
| 6a | CH2Ph | H | Ph | 16.5 | 56.8 | 4.09±0.13 |
| 6b | CH2Ph | CH2Ph | Ph | 22.4 | 0.40 | 2.27±0.73 |
| 6c | CH2CH2Ph | CH2Ph | Ph | 9.22 | 0.538 | 3.94±0.28 |
| 6d | CH2CH2Ph | CH2CHMe2 | Ph | 4.95 | 2.29 | 2.08±0.89 |
| 6e | CH2CH2Ph | CH2Ph | Me | 21.6 | ND | 4.81±0.12 |
| 6f | CH2CH2Ph | CH2CHMe2 | Me | 0.35 | ND | 4.21±0.56 |
| 12d | CH2CH2Ph | CH2CHMe2 | Ph | 0.21 | ND | >10000 |
| 5 | CH2CH2Ph | CH2CHMe2 | Ph | 0.003c | ND | 22.0d |
| E64 | -- | -- | -- | 0.084 | 0.009 | 1955±121 |
The antiplasmodial activity of compounds 6a–f was screened against the chloroquine-resistant W2 strain of P. falciparum (Table 1). All hybrids 6 displayed activity in the nM range, being more active than artemisinin and equipotent to artelinic acid, 15. This result strongly suggests that the endoperoxide pharmacophore is the major contributor to the antiplasmodial activity exerted by compounds 6. This hypothesis is further supported by the absence of swollen food vacuoles in trophozoites incubated with 6. We have previously shown that this specific abnormality, observed when parasites are incubated with dipeptidyl vinyl sulfones and E64, is indicative of a block in hemoglobin hydrolysis.23 The lack of a food vacuole abnormality for derivatives 6 can be explained by the relatively poor activity of hybrids against FP-2 and/or limited access to the food vacuole. Compounds 6a, 6e and 6f were also screened against 4 additional P falciparum strains with different phenotypes: FCR3 (atovaquone resistant), 3D7 (chloroquine-sensitive), V1/S (chloroquine and pyrimethamine resistant) and D6 (chloroquine sensitive, mefloquine resistant) (Table 2). The IC50 values show the superior activity of compounds 6a, 6e and 6f when compared to chloroquine and artemisinin against all strains.
Table 2.
IC50 values of compounds 6a, 6e and 6f, chloroquine and artemisinin against the W2, FCR3,3D7, VI/S and D6 P. falciparum strains.
| Compd | IC50/nM |
||||
|---|---|---|---|---|---|
| W2 | FCR3 | 3D7 | VI/S | D6 | |
| Chloroquine | 78.1±6.91 | 51.1±0.1 | 11.6±1.1 | 65.0±4.1 | 16.9±2.1 |
| Artemisinin | 12.0±1.97 | 5.38±0.54 | 9.71±4.18 | 4.24±0.54 | 14.5±1.2 |
| 6a | 4.09±0.13 | 1.75±0.32 | 3.11±0.72 | 1.59±0.11 | 4.71±0.12 |
| 6e | 4.81±0.12 | 2.00±0.02 | 2.50±1.51 | 2.25±0.24 | 4.75±0.37 |
| 6f | 4.21±0.56 | 1.89±0.10 | 2.48±0.41 | 1.65±0.11 | 4.96±0.66 |
In summary, a new class of hybrid molecules, 6, based on dipeptidyl vinyl sulfone and artemisinin cores has been synthesized and shown to display potent antiplasmodial activity against a panel of P. falciparum chloroquine-sensitive and multidrug-resistant strains, with IC50 values ranging from 2 to 5 nM. Despite the fact that these hybrids incorporate the structural elements required for falcipain inhibition (e.g. Leu residue at P2), they inhibited FP-2 only in the μM range. These results indicate that, although the artemisinin core or the linker may not be suitable for optimal enzyme binding, there is space to improve the bi-functional molecules as the SAR for both activities is better understood. The synthesis of novel hybrid molecules incorporating vinyl sulfone and artemisinin cores is underway.
Figure 1.
Structures of artemisinin 1, artemether, 2, arteeether, 3, sodium artesunate, 4, and a dipeptidyl vinyl sulfone, Mu-Leu-hPhe-VSPh, 5.
Acknowledgments
This work was supported by FCT (Portugal) and the National Institutes of Health; RC acknowledges FCT for the PhD grant SFRH/BD/30418/2006. PJR is a Doris Duke Charitable Foundation Distinguished Clinical Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Martinelli A, Moreira R, Cravo PVL. Mini-Rev Med Chem. 2008;8:201. doi: 10.2174/138955708783744092. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill PM, Posner GH. J Med Chem. 2004;47:2945. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner J, Ortmann R, Jomaa H, Schlitzer M. Angew Chem Int Ed. 2003;42:5274. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]
- 4.Nosten F, Brasseur P. Drugs. 2002;62:1315. doi: 10.2165/00003495-200262090-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sajid M, McKerrow JH. Mol Biochem Parasitol. 2002;120:1. doi: 10.1016/s0166-6851(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 6.Shenai BR, Sijwali PS, Singh N, Rosenthal PJ. J Biol Chem. 2000;275:29000. doi: 10.1074/jbc.M004459200. [DOI] [PubMed] [Google Scholar]
- 7.Dahl EL, Rosenthal PJ. Mol Biochem Parasitol. 2005;139:205. doi: 10.1016/j.molbiopara.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Sijwali PS, Shenai BR, Gut J, Singh N, Rosenthal PJ. Biochem J. 2001;360:481. doi: 10.1042/0264-6021:3600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Santos MMM, Moreira R. Mini-Rev Med Chem. 2007;7:1040. doi: 10.2174/138955707782110105. [DOI] [PubMed] [Google Scholar]; b) Chung JY, Bae YA, Na BK, Kong Y. Expert Opinion Ther Patents. 2005;15:995. [Google Scholar]
- 10.Olson JE, Lee GK, Semenov A, Rosenthal PJ. Bioorg Med Chem. 1999;7:633. doi: 10.1016/s0968-0896(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Rosenthal PJ. J Biol Chem. 2004;279:35236. doi: 10.1074/jbc.M404235200. [DOI] [PubMed] [Google Scholar]
- 12.Walsh JJ, Coughlan D, Heneghan N, Gaynor C, Bell A. Bioorg Med Chem Lett. 2007;17:3599. doi: 10.1016/j.bmcl.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Opsenica I, Opsenica D, Lanteri CA, Anova L, Milhous WK, Smith KS, Solaja BA. J Med Chem. 2008;51:6216. doi: 10.1021/jm8006905. [DOI] [PubMed] [Google Scholar]
- 14.Meunier B. Acc Chem Res. 2008;41:69. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 15.Shenai BR, Lee BJ, Alvarez-Hernandez A, Chong PY, Emal CD, Neitz RJ, Roush WR, Rosenthal PJ. Antimicrob Agents Chemother. 2003;47:154. doi: 10.1128/AAC.47.1.154-160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal PJ, Olson JE, Lee GK, Palmer JT, Klaus JL, Rasnick D. Antimicrob Agents Chemother. 1996;40:1600. doi: 10.1128/aac.40.7.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roush WR, Gwaltney SL, II, Cheng J, Scheidt KA, McKerrow JH, Hansell E. J Am Chem Soc. 1998;120:10994. [Google Scholar]
- 18.Scheidt KA, Roush WR, McKerrow JH, Selzer PM, Hansell E, Rosenthal PJ. Bioorg Med Chem. 1998;6:2477. doi: 10.1016/s0968-0896(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 19.Palmer JT, Rasnick D, Klaus JL, Brömme D. J Med Chem. 1995;38:3193. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill PM, Bishop LP, Storr RC, Hawley SR, Maggs JL, Ward SA, Park BK. J Med Chem. 1996;39:4511. doi: 10.1021/jm9604944. [DOI] [PubMed] [Google Scholar]
- 21.Compound 6d. To a solution of 15 (103.7 mg, 0.248 mmol) in DMF (2 ml) stirred at 0°C were added TBTU (86.3 mg, 0.258 mmol), Et3N (35 μl, 0.249 mmol) and a solution of 13d (129.6 mg, 0.245 mmol) and Et3N (35 μl, 0.249 mmol) in DMF (2 ml). The reaction was allowed to warm slowly to room temperature and monitored by TLC. After completion, the reaction mixture was diluted with AcOEt (25 ml) and then poured into saturated NaHCO3 (25 ml). The layers were separated and the aqueous layer was extracted twice with AcOEt (15 ml). The combined organic layers were treated with saturated NaHCO3, HCl 1N and brine and then dried over Na2SO4. The solvent was removed and the crude product was purified by column chromatography using AcOEt/hexane (1:1), to give 6d, 53 % (106.7 mg) yield, as a white solid, mp 102–104 °C. 1H NMR (400 MHz, CDCl3) δH (ppm) 7.89 (2H, m), 7.74 (2H, d, 8.4 Hz), 7.65 (1H, m), 7.56 (2H, t, 7.6 Hz), 7.40 (2H, d, 8.4 Hz), 7.20 (3H, m), 7.03 (2H, m), 6.93 (1 H, dd, 15.2, 5.2 Hz), 6.70 (1H, d, 8.4 Hz), 6.48 (1H, dd, 15.2, 1.6 Hz), 6.44 (1H, d, 8.0 Hz), 5.47 (1H, s), 4.96 (1H, d, 13.2 Hz), 4.93 (1H, d, 3.6 Hz), 4.74-4.65 (1H, m), 4.64-4.55 (1H, m), 4.58 (1H, d, 13.2 Hz), 2.71 (1H, m), 2.59 (2H, m), 2.40 (1H, m), 2.07 (1H, m), 2.02-1.73 (5H, m), 1.70-1.60 (5H, m), 1.57-1.25 (7H, m), 1.02-0.90 (12H, m). 13C NMR (100 MHz, CDCl3) δC (ppm) 171.5, 167.7, 145.4, 142,9, 140.3, 140.1, 133.6, 132.3, 130.9, 129.4, 128.6, 128.4, 127.7, 127.3, 127.1, 126.3, 104.2, 101.6, 88.1, 81.1, 69.1, 52.5, 52.2, 49.2, 44.3, 40.2, 37.4, 36.4, 35.6, 34.6, 31,8, 30.9, 26.2, 25.0, 24.7, 24.5, 22.9, 22.2, 20.3, 13.1. Anal. (C, H, N): Cal. for C46H58N2O9S: C, 67.73; H, 7.12; N, 3.44; Found: C, 67.58; H, 7.20; N, 3.35. ESI/MS (m/z): Cal. for C46H58N2O9S.Na+: 838.04; Found: 838.24.
- 22.Caldeira RL, Goncalves LMD, Martins TM, Novo C, Rosario LD, Domingos A. Exp Parasitol. 2009 doi: 10.1016/j.exppara.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal PJ. Exp Parasitol. 1995;80:272. doi: 10.1006/expr.1995.1033. [DOI] [PubMed] [Google Scholar]