Abstract
The high frequencies of both α+ thalassemia and the sickle cell trait (hemoglobin AS [HbAS]) found in many tropical populations are thought to reflect selection pressure from Plasmodium falciparum malaria. For HbAS, but not for α+ thalassemia, protection appears to be mediated by the enhanced phagocytic clearance of ring-infected erythrocytes. We have investigated the genotype-specific distributions of peripheral blood leukocyte populations in two groups of children living on the coast of Kenya: a group of healthy P. falciparum parasite-negative children sampled at cross-sectional survey during a period of low malaria transmission, and a group of children attending the hospital with acute malaria. We report distinctive distributions of peripheral blood myeloid dendritic cells and monocytes in children with α+ thalassemia and HbAS during healthy periods and disease, and suggest ways in which these might relate to the mechanisms of protection afforded by these conditions.
INTRODUCTION
Dendritic cells (DCs) are important in both the initiation of immune responses, and in the maintenance of peripheral tolerance. At steady state, DCs screen their environment, take up protein and cellular debris, and a proportion migrate into secondary lymphoid tissues. When DCs become activated by pathogens they migrate into draining lymph nodes or the spleen and mature to become powerful antigen-presenting cells that are capable of activating naive T cells.1 Two major DC subsets can be detected in the peripheral blood that have distinct but overlapping functions. Myeloid DCs (mDCs) express HLA DR, CD11c, and CD1c and are the main producers of interleukin-12 (IL-12), while plasmacytoid DCs (pDCs) express HLA DR, CD123, and blood dendritic cell antigen 2 (BDCA2), and are the main producers of interferon-α (IFN-α).2 Studies on rodent malaria suggest that during the early stages of infection, mDCs become activated and migrate into the T cell areas of the spleen to induce IFN-γ-producing T cells.3-5 There is now some evidence that malaria products such as glycosylphosphatidyl inositol and hemozoin may induce DC activation via engagement of toll-like receptor 2 (TLR2), TLR4, and TLR 9, respectively.6,7 In contrast, in vitro adhesion of Plasmodium falciparum–infected human erythrocytes to mDCs via CD36 can modulate mDC maturation and function whereby they fail to produce IL-12 and activate T cells but produce large amounts of IL-10 instead.8
We have recently found that there is no change in mDC or pDC frequencies during acute episodes of P. falciparum malaria, but that monocyte frequencies are increased (Urban BC and others, unpublished data). However, expression of HLA DR on mDCs and monocytes, but not on pDCs, was reduced, implying that myeloid cells may be functionally impaired during acute disease.9 In a recent study, Pichyangkul and others reported that peripheral blood pDC frequencies were reduced in Thai adults with acute malaria.6 Together, these results suggest that, consistent with differential expression of TLRs and other surface molecules, acute blood stage infection has different effects on pDC and mDC function.
In the course of immunologic studies in naturally exposed children living on the coast of Kenya, we were interested in investigating whether there were differences in peripheral blood DCs and monocyte populations during acute P. falciparum malaria in children with different hemoglobin (Hb) genotypes. Both sickle cell trait and α+ thalassemia are common on the coast of Kenya, where the allele frequencies for HbS and the common African 3.7-kb α gene deletion are 0.07 and 0.43 respectively.10 In vitro studies suggest that in HbAS, but not in α+ thalassemia, protection may be mediated by effects on red blood cell (RBC) parasite invasion and intracellular growth,11,12 and by the enhanced phagocytic clearance of P. falciparum ring-infected erythrocytes.13 Studies have shown that although children with HbAS are protected from both mild and severe malaria,14,15 protection by α+ thalassemia is largely limited to severe disease.16-19 In vivo, HbAS is associated with reduced parasite densities during intercurrent P. falciparum infections15,20 and enhanced acquired immunity,20-23 which suggests that HbAS probably protects against malaria infection per se due to increased parasite clearance and induction of antibodies. In contrast, the effect of α+ thalassemia appears to be largely restricted to the pathologic consequences of malaria,17 but the underlying mechanisms are less well understood. Although several studies showed increased binding of immunoglobulin to infected α-thalassemic erythrocytes,24,25 this phenomenon does not seem to reduce parasite density in children with heterozygote or homozygote α+ thalassemia. Interestingly, co-inheritance of α+ thalassemia and HbAS reverses the protective effect of HbAS, suggesting that these two mechanisms are not compatible.26
The hemoglobinopathies provide an opportunity to dissect the physiologic and immunologic mechanisms conferring protection against mild or severe malaria. In the course of immunologic studies in naturally exposed children living on the coast of Kenya, we were interested to investigate whether there are differences in the frequency of antigen presenting cells such as peripheral blood DCs and monocyte during acute P. falciparum malaria in children with different Hb genotypes. We show that in healthy Kenyan children both homozygous and heterozygous for α+ thalassemia are associated with reduced frequencies of mDCs and that monocyte frequencies are reduced in children with HbAS. These differences were also observed in children with α+ thalassemia who were recovering from an acute malaria episode, but not during the acute episode.
MATERIALS AND METHODS
Study population
Blood samples were collected from children living in the Ngerenya area of Kilifi District, Kenya who were under active surveillance for malaria as described in detail previously.10 We studied 148 and 164 children during cross-sectional surveys conducted during periods of low transmission in October 2003 and October 2004, respectively. Participants were selected on the basis of their Hb genotype. At the time of each survey, all children were examined clinically, and venous blood samples were collected for whole blood counts and to determine the presence of malaria parasites. Only those children who were negative for P. falciparum blood stage parasites by microscopy were considered. In August 2004, blood samples were collected from children attending the outpatient clinic at Kilifi District Hospital with mild, uncomplicated malaria (fever > 37.5°C, associated with a blood film positive for P. falciparum parasites and with no alternative explanation on careful clinical examination), and from children admitted to the wards with severe malaria, defined as deep acidotic breathing, coma (inability to respond to pain) or prostration (inability to breast feed or to sit unsupported) or severe anemia (Hb < 5 mg/dL). All participants of this study were invited to donate a convalescence blood sample 14 days after discharge from hospital. We analyzed data from all children who were typed for α+ thalassemia and who returned to the clinic for a follow-up visit (n = 27). We did not type children with acute malaria for the presence of HbS because in this group admission to hospital with acute malaria is extremely rare.15
The study was reviewed and approved by the Kenya Medical Research Institute/National Ethical Review Committee and the Oxford Tropical Research Ethical Committee. Written informed consent was obtained from all participants or their parents.
Reagents
The following antibodies were used in flow cytometry: phycoerythrin-Texas Red-x (ECD)–conjugated anti-CD3, anti-CD14, and anti-CD19, phycoerythrin-cyanin 5.1 (PC5)-conjugated anti-HLA DR, fluorescein isothiocyanate (FITC)–conjugated anti-human IgG1, phycoerythrin (PE)–conjugated anti-human IgG1 (all from Beckman Coulter Ltd., High Wycombe Buckinghamshire, United Kingdom), FITC-conjugated anti-CD11c (Serotec, Kidlington, Oxford, United Kingdom), FITC-conjugated anti-BDCA2, PE-conjugated anti-CD123 (Becton Dickinson, Oxford, Oxfordshire, United Kingdom), PE-conjugated anti-BDCA3, and PE-conjugated anti-CD1c (Miltenyi Biotec, Bergisch Gladbach, Germany). Optilyse C solution (Beckman Coulter Ltd.) was used according to the manufacturer's instructions.
Flow cytometry
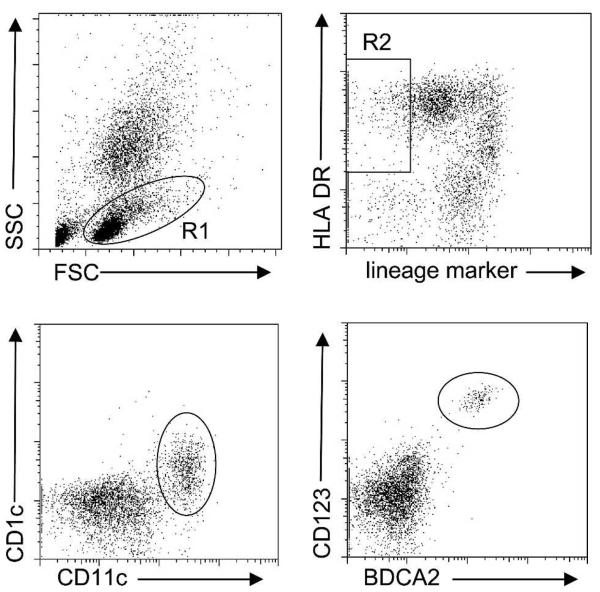
Venous blood samples (500 μL) were drawn into blood tubes containing EDTA (TekLab, Durham, United Kingdom). Aliquots of 50 μL were incubated for 30 minutes at 4°C with a cocktail containing the lineage markers anti-CD3 (T cells), anti-CD14 (monocytes), and anti-CD19 (B cells) antibodies to exclude T cells, monocytes, and B cells, as well as anti-HLA DR antibody to identify DCs as lineage marker negative cells that express HLA DR. Within the lineage-marker negative, HLA DR–positive cell population, DC subsets were identified as mDC using anti-CD11c and anti-CD1c antibodies, or as pDC using anti-BDCA2 and anti-CD123 antibodies.27 After lysis of erythrocytes, white blood cells (WBCs) were washed in phosphate-buffered saline and analyzed by flow cytometry (Epics II; Beckman Coulter Ltd.). For each sample, we acquired at least 1,000 lineage marker-negative, HLA DR–positive events. Flow cytometry data were analyzed blind to Hb genotype using FlowJo™ software (Tri Star Inc., San Carlos, CA). Lineage marker-negative cells were gated within the peripheral blood mononuclear cell (PBMC) gate, and HLA DR+ CD11c+ CD1c+ or HLA DR+ CD123+ BDCA2+ were counted as mDCs or pDCs, respectively (Figure 1). Monocytes were identified within the PBMC gate as CD14+ HLA DR+ cells. Absolute numbers of mDC, pDC, and CD14+ monocytes were calculated using whole blood counts.
Figure 1.
Frequencies of dendritic cell subsets in whole blood. Peripheral blood mononuclear cells (PBMCs) were gated in R1 to exclude granulocytes. Dendritic cell (DC) markers such as CD1c and CD11c defining myeloid DCs (mDCs) and CD123 and BDCA2 defining plasmacytoid DCs (pDCs) can be expressed on subsets of T cells, monocytes, and B cells. It is therefore necessary to exclude these cell populations from analysis using a cocktail of antibodies against lineage markers for these subsets (CD3 for T cells, CD14 for monocytes, and CD19 for B cells). Within the R1 gate (gates are indicated by the rectangle and ovals), DCs are then defined as lineage marker negative, HLA DR-positive cells (R2). Within the R2 gate, the different DC subsets are defined as CD11c- and CD1c-positive mDC or CD123- and blood dendritic cell antigen 2 (BDCA2)–positive pDC. The absolute number of mDCs or pDCs is then calculated from their percentage in PBMCs using white blood cell counts. SSC = side scatter; FSC = forward scatter.
Statistical analysis
We compared continuous data using the Spearman correlation, the Mann-Whitney U test, or the Kruskal-Wallis H test for trend. All data were analyzed with SPSS software (SPSS Inc., Chicago, IL). Weight-for-age and height-for-age z-scores were calculated using Epi-Info software (Centers for Disease Control and Prevention, Atlanta, GA).
RESULTS
Steady-state dendritic cell frequencies
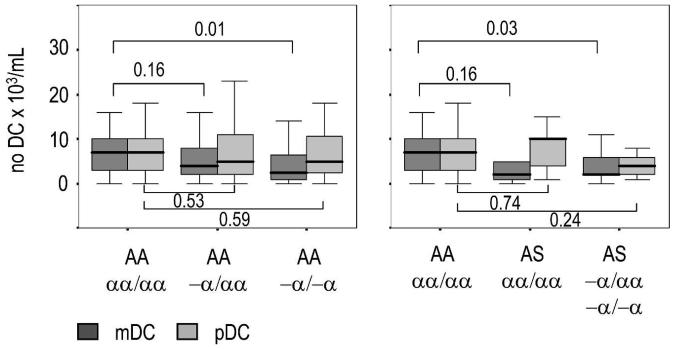
We found no significant between-genotype differences in age or total WBC counts among healthy Kenyan children involved in the cross-sectional survey (Table 1). As expected, RBC counts were significantly higher and hemoglobin concentrations were significantly lower in both heterozygotes and homozygotes for α+ thalassemia, reflecting the reduced volume and increased turnover of RBCs in such children.28 When we determined the frequency of DC subsets in the peripheral circulation (Figure 1), we found no correlation between the absolute number of peripheral blood mDCs, pDCs, or monocytes with age, WBC counts, or nutritional status (defined as weight-for-age and height-for-age z-scores) in our study population (Table 1), consistent with previous studies on total peripheral blood DC frequencies conducted both in Kilifi and in Caucasian children.9,29,30 However, the frequency of mDCs was reduced in α+ thalassemic children. The differences in mDC counts were greatest between children with normal hemoglobin and children homozygous for α+ thalassemia whether or not if they were also carriers for HbS (Figure 2). We observed no differences in the frequency of pDCs in either group. In contrast, the absolute number of monocytes was reduced in children with the HbAS genotype, although this effect was abrogated when co-inherited with α+ thalassemia (Table 1). These trends were confirmed in samples collected during the second cross-sectional survey, conducted in October 2004 (Table 2), indicating that the data reflect true differences in the steady-state frequency of myeloid cells in children with these two hemoglobinopathies. The pDC counts were increased in children with the HbAS genotype although this reached significance only in the second cross-sectional survey.
Table 1.
Cross-sectional survey in the study population in October 2003: differences by hemoglobin genotype*
| AA αα/αα |
AA −α/αα |
AA −α/−α |
P † | AS αα/αα |
AS −α/αα −α/−α |
P ‡ | |
|---|---|---|---|---|---|---|---|
| No. | 36 | 66 | 26 | 6 | 14 | ||
| Age (months) |
52.6 (35.6–72.3) |
53.3 (39.7–77.3) |
68.4 (50.5–79.3) |
0.116 | 63.9 (56.2–67.8) |
65.6 (54.0–83.0) |
0.311 |
| WBCs (106/mL) |
7.4 (6.15–10.2) |
7.9 (6.2–9.7) |
7.7 (6.6–9.1) |
0.991 | 6.55 (5.7–7.9) |
7.6 (6–8.7) |
0.373 |
| RBCs (109/mL) |
4.3 (3.9–4.5) |
4.4 (4.1–4.6) |
5.0 (4.7–5.2) |
< 0.001 | 4.5 (3.9–4.8) |
4.7 (4.4–4.8) |
0.001 |
| Hb (mg/dL) |
11.3 (10.8–11.9) |
10.6 (10.2–11.5) |
10.4 (9.8–10.9) |
0.003 | 11.3 (10.1–12.7) |
10.7 (10.4–11.9) |
0.575 |
| WAZ | −0.8 (−2.3 to 0.2) |
−1.1 (−1.5 to −0.6) |
−1.2 (−2.2 to −0.4) |
0.158 | −1.6 (−3.2 to −0.6) |
−0.7 (−3.2 to 0.3) |
0.811 |
| HAZ | −0.9 (−2.3 to 0.36) |
−0.91 (−3.0 to −0.1) |
−1.2 (−2.4 to 0) |
0.388 | −1.8 (−4.9 to 0.3) |
−0.43 (−4.3 to 1.7) |
0.681 |
| CD14 (× 103/mL) |
105 (43–156) |
62 (27–99) |
69 (36–92) |
0.117 | 24§ (14–60) |
77 (53–128) |
0.472 |
| pDCs (× 103/mL) |
7.0 (3.0–10.2) |
5.0 (2.0–10.5) |
6.1 (2.2–10.7) |
0.169 | 10.1 (4.5–12.5) |
3.5 (2–5.5) |
0.142 |
| mDCs (× 103/mL) |
7.0 (3.1–10.6) |
4.0 (2.4–7.8) |
2.5 (1.0–6.5) |
0.043 | 2.6 (0.6–10.0) |
2.5§ (1.5–6.5) |
0.065 |
WBCs = white blood cells; RBCs = red blood cells; Hb = hemoglobin; WAZ = weight-for-age z score; HAZ = height-for-age z score; pDCs = plasma cytoid dendritic cells; mDCs = myeloid dendritic cells. Values in parentheses are 25th and 75th percentiles.
By Kruskal-Wallis H test for trend; degrees of freedom (df) = 2.
By Kruskal-Wallis H test for trend; df = 2 comparing AA (αα/αα), AS (αα/αα), and AS (−α/αα or −α/−α).
P < 0.05 versus control, by Mann-Whitney U test.
Figure 2.
Boxplots of the absolute number of myeloid dendritic cells (mDCs) and plasmocytoid DCs (pDCs) in the peripheral circulation of healthy Kenyan children with different hemoglobin genotypes. The absolute number of mDCs was reduced in children with α+ thalassesmia independent of whether the children were carriers of hemoglobin S. Medians are indicated by the solid black lines within the boxes, which represent the 25th and 75th percentiles. Errors bars indicate the 5th and 95th percentiles. The P values (numbers above and below the brackets) were obtained by pairwise comparisons of DC numbers using the Mann-Whitney U test.
Table 2.
Cross-sectional survey of the study population in October 2004: differences in hemoglobin genotype*
| AA αα/αα |
AA −α/αα |
AA −α/−α |
P † | AS αα/αα |
AS −α/αα −α/−α |
P ‡ | |
|---|---|---|---|---|---|---|---|
| No. | 36 | 66 | 29 | 9 | 24 | ||
| Age (months) |
62.5 (52.1–88.0) |
72.3 (52.0–92.0) |
65.4 (47.6–89.0) |
0.636 | 72.6 (54.0–88.0) |
72.8 (50.2–93.0) |
0.747 |
| WBCs (106/mL) |
7.4 (5.2–9) |
8.4 (6.7–10) |
7.5 (6.5–8.8) |
0.149 | 8.0 (7.2–9.7) |
8.2 (6.6–10.4) |
0.186 |
| RBCs (109/mL) |
4.3 (4–4.6) |
4.4 (4.2–4.7) |
5.0 (4.7–5.2) |
< 0.001 | 4.4 (4.1–4.6) |
4.7 (4.5–5.1) |
0.001 |
| Hb (mg/dL) |
11.9 (11.3–12.5) |
11.0 (10.6–11.7) |
10.5 (10.1–11.2) |
< 0.001 | 11.2 (10.8–12.2) |
11.3 (10.7–11.6) |
0.03 |
| WAZ | −1.3 (−2.0 to −0.8) |
−1.5 (−2.0 to −0.9) |
−1.4 (−1.7 to −0.8) |
0.506 | −1.6 (−1.9 to −1.5) |
−1.9 (− 2.1 to −1.1) |
0.243 |
| HAZ | −1.7 (−2.2 to −0.7) |
−1.6 (−2.3 to −1.1) |
−1.2 (−1.8 to −0.9) |
0.172 | −1.6 (−2.0 to −1.2) |
−1.4 (−2.2 to −1.1) |
0.910 |
| CD14 (× 103/mL) |
119 (56–215) |
79 (37–127) |
84 (40–125) |
0.183 | 56§ (39–104) |
136 (119–165) |
0.376 |
| pDCs (×103/mL) |
7.5 (4.8–11.4) |
7.7 (4.6–12.3) |
9.8 (4.7–12.4) |
0.534 | 14.7§ (8.2–16.5) |
7.7 (3.5–12.3) |
0.089 |
| mDCs (×103/mL) |
11.7 (7.4–13.5) |
9.0 (6.5–12.0) |
7.4 (6.5–10.2) |
0.037 | 10.4 (5.5–12.6) |
8.8 (7.6–10.2) |
0.308 |
For definitions of abbreviations, see Table 1. Values in parentheses are 25th and 75th percentiles.
By Kruskal-Wallis H test for trend; degrees of freedom (df) = 2.
By Kruskal-Wallis H test for trend, df = 2 comparing AA (αα/αα), AS (αα/αα), and AS (−α/αα or −α/−α).
P < 0.05 versus AA (αα/αα), by Mann-Whitney U test.
Dendritic cell frequencies and acute malaria
Because of the unexpected differences in mDC frequencies that we observed in healthy Kenyan children with α+ thalassemia, we next analyzed data from children with acute malaria and during convalescence who had been genotyped for α+ thalassemia. Of these, 27 children had severe malaria whereas 12 children had with mild malaria. Although mDC counts increased in children with mild malaria compared with healthy controls or children with severe malaria (Urban BC and others, unpublished data), we did not distinguish between disease severity because of the relative small number of children homozygous for α+ thalassemia. However, children with severe disease show the same trend as the overall group of children (Table 3). As expected, all children had increased WBC counts and decreased RBC counts and hemoglobin levels during acute malaria. During convalescence, the WBC count in children homozygous for α+ thalassemia was near normal, whereas the WBC count in children with normal hemoglobin or heterozygous for α+ thalassemia remained increased. As we observed previously, monocyte frequencies were significantly increased during acute P. falciparum malaria, whereas the frequencies of mDCs and pDCs remained relatively constant, irrespective of hemoglobin genotype. Thus, we found no significant differences in DC and monocyte frequencies between wild-type or α+ thalassemic children during acute episodes of P. falciparum malaria (Table 4). In contrast, during convalescence the frequency of mDCs and pDCs was lower in children with α+ thalassemia, with the greatest differences between children with normal hemoglobin and homozygous for α+ thalassemia (αα/αα versus −α/−α; P = 0.014 for mDC and P = 0.004 for pDC, by Mann-Whitney U test). The frequency of monocytes remained elevated during convalescence from an acute malaria episode compared with the frequency in healthy children with the same hemoglobin genotype, but tended to be lower in children homozygous for α thalassemia (Table 4).
Table 3.
Comparisons of children with severe malaria and during convalescence*
| AA αα/αα |
AA −α/αα |
AA −α/−α |
P † | |
|---|---|---|---|---|
| No. | 12 | 11 | 4 | |
| Age (months) | 19.5 (10.4–32.3) | 22.1 (12.6–40.9) | 20.2 (19.9–29) | 0.354 |
| WBCs (106/mL) | ||||
| Acute | 13.5 (8.2–20.0) | 10.9 (6.9–20.0) | 8.4 (8.1–11.2) | 0.796 |
| Convalescent | 11.7 (10.3–16.1) | 9.9 (6.3–13.0) | 8.0 (7.7–8.4) | 0.067 |
| RBC (109/mL) | ||||
| Acute | 2.8 (2.0–4.2) | 3.1 (1.9–3.8) | 4.1 (3.8–4.1) | 0.413 |
| Convalescent | 3.4 (3.2–4.3) | 3.5 (3.2–3.9) | 4.4 (4.2–4.4) | 0.128 |
| Hb (mg/dL) | ||||
| Acute | 7.3 (5.0–9.8) | 7.7 (4.5–8.7) | 7.5 (7.4–8.0) | 0.948 |
| Convalescent | 9.3 (8.5–10.9) | 9.1 (8.9–9.8) | 9.0 (8.8–9.6) | 0.972 |
| CD14 (× 103/mL) | ||||
| Acute | 416 (119–616) | 263 (207–628) | 814 (152–756) | 0.978 |
| Convalescent | 530 (323–943) | 9.93 (380–1,070) | 204 (161–264) | 0.158 |
| pDC (× 103/mL) | ||||
| Acute | 4.2 (2.3–10.0) | 6.7 (4.0–11.3) | 4.2 (2.6–20.4) | 0.608 |
| Convalescent | 10.4 (6.6–25) | 14.5 (5.9–22.0) | 2 (1.8–2.5) | 0.022 |
| mDC (× 103/mL) | ||||
| Acute | 9.5 (7.5–12.7) | 9.6 (3.1–23.1) | 6.4 (4.8–24.8) | 0.928 |
| Convalescent | 17.0 (9.5–32.0) | 15 (12.7–24) | 5 (4.3–6.4) | 0.045 |
WBCs = white blood cells; RBCs = red blood cells; Hb = hemoglobin; pDCs = plasma cytoid dendritic cells; mDCs = myeloid dendritic cells. Values in parentheses are 25th and 75th percentiles.
By Kruskal-Wallis H test for trend; degrees of freedom = 2.
Table 4.
Comparisons of children with acute malaria and during convalescence*
| AA αα/αα |
AA −α/αα |
AA −α/−α |
P † | |
|---|---|---|---|---|
| No. | 14 | 17 | 8 | |
| Age (months) | 28.5 (11.4–37.1) | 43.8 (16.0–53.2) | 37.8 (23.1–65.0) | 0.135 |
| WBCs (106/mL) | ||||
| Acute | 11.1 (7.6–19.0) | 8.6 (5.5–14.8) | 8.3 (7.1–13.5) | 0.625 |
| Convalescent | 11.5 (9.8–16.1) | 9.4 (6.2–12.0) | 7.7 (5.3–8.1) | 0.031 |
| RBCs (109/mL) | ||||
| Acute | 2.9 (2.2–4.2) | 3.5 (2.2–4.2) | 4.1 (3.8–4.7) | 0.075 |
| Convalescent | 3.5 (3.2–4.1) | 3.7 (3.3–4.4) | 4.4 (4.2–4.8) | 0.016 |
| Hb (mg/dL) | ||||
| Acute | 8.1 (5.4–9.7) | 8.6 (5.2–9.8) | 7.8 (7.4–9.4) | 0.833 |
| Convalescent | 9.5 (8.7–10.5) | 9 (8.7–10.0) | 8.9 (8.1–9.6) | 0.602 |
| CD14 (× 103/mL) | ||||
| Acute | 421 (192–542) | 468 (244–540) | 811 (274–1,321) | 0.335 |
| Convalescent | 720 (369–925) | 610 (390–933) | 258 (162–395) | 0.046 |
| pDCs (× 103/mL) | ||||
| Acute | 4.0 (3.0–7.1) | 6.8 (4.1–11.5) | 7.5 (3.2–12.5) | 0.375 |
| Convalescent | 10.3 (6.6–19.0) | 9.8 (5.9–19.5) | 4.2 (2.1–7.1) | 0.021 |
| mDCs (× 103/mL) | ||||
| Acute | 10.0 (7.5–16.4) | 10.3 (6.6–25.6) | 11.3 (5.1–31.9) | 0.954 |
| Convalescent | 21.1 (10.8–76.0)‡ | 13.3 (7.1–17.9) | 6.2 (3.5–11.5) | 0.010 |
For definitions of abbreviations, see Table 1. Values in parentheses are 25th and 75th percentiles.
By Kruskal-Wallis H test for trend, degrees of freedom = 2.
Values due to extreme outliers; median (range) when these are excluded: 10.6 (7.0–13.9).
DISCUSSION
We have shown that the frequency of peripheral blood mDCs was reduced in a group of healthy children with α+ thalassemia who live in a malaria-endemic area on the coast of Kenya. Although this observation remains unexplained, the most likely possibilities are reduced mDC production in the bone marrow or chronic activation and enhanced retention of mDCs in the spleen. The first explanation seems unlikely because the effect we describe here was specific to myeloid cells. Although we observed a trend towards reduced frequencies in monocytes, this was not significant, possibly due to an overall greater variability of monocyte counts. However, RBCs are part of the physiologic microenvironment of both mDCs and monocytes, and it is possible that their homeostasis may be affected by the membrane changes that typically occur in mutant RBCs. The half life of RBCs from subjects with α+ thalassemia is reduced through oxidative membrane damage and loss of CD35.28,31,32 It seems feasible, therefore, that the enhanced removal of damaged RBCs in the spleen induces recruitment of peripheral blood mDCs and monocytes, either directly or indirectly through cytokine and/or chemokine gradients. This hypothesis is supported by the observation that children with α+ thalassemia have reduced numbers of both mDCs and pDC when convalescing from a malaria episode compared with children with normal hemoglobin, but have normal numbers during an acute attack. In other infectious diseases, the number of mDC and pDC is reduced during the acute attack,33-35 whereas in acute malaria DC subsets remain constant or are increased9 (Urban BC and others, unpublished data). Furthermore, activated lymphocytes leave the peripheral circulation and migrate into lymph nodes and spleen during acute malaria.36 Our observation indicates that activation of mDCs and pDCs during acute malaria may be faster or more profound in children with α+ thalassemia than in children with normal hemoglobin. Subsequent prolonged activation and migration into the spleen during convalescence from acute malaria may subsequently enhance acquired immune responses to infected RBC antigens including the induction of antibodies against variant surface antigens.24,25
The acquired reduction of CD35 on the surface of thalassemic RBCs may provide a further explanation for our observation.31 CD35 expressed on RBCs binds immune complexes that are then removed from CD35 by macrophages in the liver and spleen without destruction of RBCs.37 Due to the reduced levels of CD35 on RBCs in children with α+ thalassemia, circulating immune complexes could be increased for a prolonged period of time after acute malaria. Recent studies have shown that P. falciparum glycosylphosphatidylinositol (GPI) can bind to TLR2 and TLR4 expressed on mDCs and monocytes,7,38 whereas a component of schizont lysate as well as hemozoin can bind to TLR9 and activate pDCs.6,39 Immune complexes containing malarial antigen including GPI and hemozoin might activate DCs by cross-linking Fc receptors and TLRs,40 resulting in prolonged activation and migration of mDCs and pDCs into the spleen and lymph nodes, a hypothesis that could be tested by monitoring the concentration of immune complexes in parallel with peripheral blood DC counts in children with α+ thalassemia in longitudinal studies.
In contrast, children with the HbAS genotype had significantly reduced numbers of monocytes, but this effect was reversed when children were also carriers of α+ thalassemia. In addition, reduced mDC frequencies and increased pDC frequencies were seen in HbAS children in one cross-sectional survey; however, these observations were not reproduced in the other cross-sectional survey. Although enhanced removal of RBCs with HbAS may lead to increased recruitment of monocytes into the spleen, we have to assume that membrane changes of RBCs and the accompanying effect on myeloid cells differ for different hemoglobinopathies. In this context, it is interesting that co-inheritance of α+ thalassemia and HbS has a negative epistatic effect on the protection against malarial disease mediated by either variant alone.26 The concentration of the mutant βS chain in RBCs with HbAS is reduced when co-inherited with α+ thalassemia. This may be due to a higher affinity of the α-globin chain for the normal β chain, which could result in less pronounced changes of RBC morphology.41 In any case, in vitro studies of monocyte and mDC function after exposure to RBCs of different hemoglobin genotypes may help to elucidate the underlying mechanism(s).
Could enhanced turnover or recruitment of myeloid cells into the spleen in children with hemoglobinopathies at steady state contribute to protection against malaria? Infection with P. falciparum would occur on the background of enhanced splenic reactivity with respect to antigen presentation and T cell activation, which could explain the results of previous studies that have identified increased cellular and humoral immune responses during episodes of acute malaria in children with HbAS compared with children with HbAA.23,42-44 Larger longitudinal studies will be required to elucidate the relationships between the hemoglobinopathies, immunologic processes, and the severity in P. falciparum malaria.
Acknowledgments
This study is published with permission from the Director of the Kenyan Medical Research Institute. We thank the children and parents for their participation in the study, and the field research team and clinical and medical officers at the Centre for Geographic Medicine Research Coast for their assistance.
Financial support: This study was supported by the Wellcome Trust via grants awarded to Britta C. Urban, Kevin Marsh, and Thomas N. Williams.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–717. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 3.Seixas E, Cross C, Quin S, Langhorne J. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur J Immunol. 2001;31:2970–2978. doi: 10.1002/1521-4141(2001010)31:10<2970::aid-immu2970>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Perry JA, Rush A, Wilson RJ, Olver CS, Avery AC. Dendritic cells from malaria-infected mice are fully functional APC. J Immunol. 2004;172:475–482. doi: 10.4049/jimmunol.172.1.475. [DOI] [PubMed] [Google Scholar]
- 5.Leisewitz AL, Rockett KA, Gumede B, Jones M, Urban B, Kwiatkowski DP. Response of the splenic dendritic cell population to malaria infection. Infect Immun. 2004;72:4233–4239. doi: 10.1128/IAI.72.7.4233-4239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, Stewart VA, Hasegawa H, Looareesuwan S, Shanks GD, Miller RS. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 7.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 9.Urban BC, Mwangi T, Ross A, Kinyanjui S, Mosobo M, Kai O, Lowe B, Marsh K, Roberts DJ. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood. 2001;98:2859–2861. doi: 10.1182/blood.v98.9.2859. [DOI] [PubMed] [Google Scholar]
- 10.Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. Am J Clin Nutr. 2004;80:1604–1610. doi: 10.1093/ajcn/80.6.1604. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA. 1978;75:1994–1997. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–703. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 13.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 14.Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 15.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, Marsh K. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen SJ, O'Donnell A, Alexander ND, Alpers MP, Peto TE, Clegg JB, Weatherall DJ. alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci USA. 1997;94:14736–14741. doi: 10.1073/pnas.94.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CR, Maitland K. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 18.Mockenhaupt FP, Ehrhardt S, Gellert S, Otchwemah RN, Dietz E, Anemana SD, Bienzle U. Alpha(+)-thalassemia protects African children from severe malaria. Blood. 2004;104:2003–2006. doi: 10.1182/blood-2003-11-4090. [DOI] [PubMed] [Google Scholar]
- 19.Williams TN, Maitland K, Bennett S, Ganczakowski M, Peto TE, Newbold CI, Bowden DK, Weatherall DJ, Clegg JB. High incidence of malaria in alpha-thalassaemic children. Nature. 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 20.Le Hesran JY, Personne I, Personne P, Fievet N, Dubois B, Beyeme M, Boudin C, Cot M, Deloron P. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int J Epidemiol. 1999;28:793–798. doi: 10.1093/ije/28.4.793. [DOI] [PubMed] [Google Scholar]
- 21.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 22.Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, Wambua S, Kortok M, Snow RW, Marsh K. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191:1631–1638. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 24.Luzzi GA, Merry AH, Newbold CI, Marsh K, Pasvol G, Weatherall DJ. Surface antigen expression on Plasmodium falciparum-infected erythrocytes is modified in alpha- and beta-thalassemia. J Exp Med. 1991;173:785–791. doi: 10.1084/jem.173.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams TN, Weatherall DJ, Newbold CI. The membrane characteristics of Plasmodium falciparum-infected and -uninfected heterozygous alpha(0)thalassaemic erythrocytes. Br J Haematol. 2002;118:663–670. doi: 10.1046/j.1365-2141.2002.03610.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams TN, Mwangi TW, Wambua S, Peto TE, Weatherall DJ, Gupta S, Recker M, Penman BS, Uyoga S, Macharia A, Mwacharo JK, Snow RW, Marsh K. Negative epistasis between the malaria-protective effects of α+-thalassaemia and the sickle cell trait. Nat Genet. 2005;37:1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 28.Rees DC, Williams TN, Maitland K, Clegg JB, Weatherall DJ. Alpha thalassaemia is associated with increased soluble transferrin receptor levels. Br J Haematol. 1998;103:365–369. doi: 10.1046/j.1365-2141.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 29.Hagendorens MM, Ebo DG, Schuerwegh AJ, Huybrechs A, van Bever HP, Bridts CH, de Clerck LS, Stevens WJ. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin Exp Allergy. 2003;33:633–639. doi: 10.1046/j.1365-2222.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- 30.Vakkila J, Thomson AW, Vettenranta K, Sariola H, Saarinen-Pihkala UM. Dendritic cell subsets in childhood and in children with cancer: relation to age and disease prognosis. Clin Exp Immunol. 2004;135:455–461. doi: 10.1111/j.1365-2249.2003.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cockburn IA, Mackinnon MJ, O'Donnell A, Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC, Rowe JA. A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria. Proc Natl Acad Sci USA. 2004;101:272–277. doi: 10.1073/pnas.0305306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrier SL, Rachmilewitz E, Mohandas N. Cellular and membrane properties of alpha and beta thalassemic erythrocytes are different: implication for differences in clinical manifestations. Blood. 1989;74:2194–2202. [PubMed] [Google Scholar]
- 33.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 34.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 35.Pichyangkul S, Endy TP, Kalayanarooj S, Nisalak A, Yongvanitchit K, Green S, Rothman AL, Ennis FA, Libraty DH. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol. 2003;171:5571–5578. doi: 10.4049/jimmunol.171.10.5571. [DOI] [PubMed] [Google Scholar]
- 36.Hviid L, Theander TG, Abdulhadi NH, Abu-Zeid YA, Bayoumi RA, Jensen JB. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. Eur J Immunol. 1991;21:1249–1253. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- 37.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev. 2001;180:100–111. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 41.Brittenham G, Lozoff B, Harris JW, Mayson SM, Miller A, Huisman TH. Sickle cell anemia and trait in southern India: further studies. Am J Hematol. 1979;6:107–123. doi: 10.1002/ajh.2830060203. [DOI] [PubMed] [Google Scholar]
- 42.Abu-Zeid YA, Abdulhadi NH, Theander TG, Hviid L, Saeed BO, Jepsen S, Jensen JB, Bayoumi RA. Seasonal changes in cell mediated immune responses to soluble Plasmodium falciparum antigens in children with haemoglobin AA and haemoglobin AS. Trans R Soc Trop Med Hyg. 1992;86:20–22. doi: 10.1016/0035-9203(92)90422-9. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Zeid YA, Theander TG, Abdulhadi NH, Hviid L, Saeed BO, Jepsen S, Jensen JB, Bayoumi RA. Modulation of the cellular immune response during Plasmodium falciparum infections in sickle cell trait individuals. Clin Exp Immunol. 1992;88:112–118. doi: 10.1111/j.1365-2249.1992.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayoumi RA, Abu-Zeid YA, Abdulhadi NH, Saeed BO, Theander TG, Hviid L, Ghalib HW, Nugud AH, Jepsen S, Jensen JB. Cell-mediated immune responses to Plasmodium falciparum purified soluble antigens in sickle-cell trait subjects. Immunol Lett. 1990;25:243–249. doi: 10.1016/0165-2478(90)90122-7. [DOI] [PubMed] [Google Scholar]